Abstract
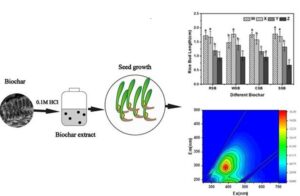
Positive or negative plant growth effects due to soluble organic biochar compounds are not well understood. Increasing quantities (0 g, 0.5 g, 2.5 g, and 5 g) of four different biochars were extracted with 0.1 M HCl, which were separated and marked as W, X, Y, and Z. Corn and rice seeds were treated with biochar extract solution. The rice straw Y and Z solutions positively affected corn germination, while the cotton straw Z solution negatively affected it. When differences were present, the biochar extract solutions always caused reduction in rice seed germination. The rice straw and cotton straw solutions tended to increase, the wheat straw Y and Z solutions tended to decrease, while little differences were evident in terms of the effects of the Spartina alterniflora solution on corn bud and root length when compared to the control. Increasing the biochar solution extract treatments reduced the rice bud and root length when compared to the control, especially with solution Z. Organic compounds, e.g., triethyl phosphate, 2,4-bisphenol, were present in the solutions, which likely promoted seed growth at lower concentrations. Determining the presence of biochar organic moieties helped with designing biochars for enhanced seed germination, growth, and crop productivity.
Download PDF
Full Article
Biochar Extract Compounds Alter Germination and Growth of Crop Seed
Jingwen Ma,a,b Guixiang Quan,a Jinlong Yan,a,* James A. Ippolito,c Liqiang Cui,a,* Hui Wang,a Tianjing Qiu,a and Yu Sun a
Positive or negative plant growth effects due to soluble organic biochar compounds are not well understood. Increasing quantities (0 g, 0.5 g, 2.5 g, and 5 g) of four different biochars were extracted with 0.1 M HCl, which were separated and marked as W, X, Y, and Z. Corn and rice seeds were treated with biochar extract solution. The rice straw Y and Z solutions positively affected corn germination, while the cotton straw Z solution negatively affected it. When differences were present, the biochar extract solutions always caused reduction in rice seed germination. The rice straw and cotton straw solutions tended to increase, the wheat straw Y and Z solutions tended to decrease, while little differences were evident in terms of the effects of the Spartina alterniflora solution on corn bud and root length when compared to the control. Increasing the biochar solution extract treatments reduced the rice bud and root length when compared to the control, especially with solution Z. Organic compounds, e.g., triethyl phosphate, 2,4-bisphenol, were present in the solutions, which likely promoted seed growth at lower concentrations. Determining the presence of biochar organic moieties helped with designing biochars for enhanced seed germination, growth, and crop productivity.
DOI: 10.15376/biores.17.3.4151-4166
Keywords: Biochar; Extract solution; Germination rate; Corn; Rice
Contact information: a: School of Environmental Science and Engineering, Yancheng Institute of Technology, No. 211 Jianjun East Road, Yancheng 224051 China; b: School of Chemistry and Chemical Engineering, Yancheng Institute of Technology, No. 211 Jianjun East Road, Yancheng 224051 China; c: Department of Soil and Crop Sciences, Colorado State University, Fort Collins, CO 80523 USA;
* Corresponding authors: yjlyt4788@126.com; lqcui8@hotmail.com
GRAPHICAL ABSTRACT

INTRODUCTION
Biochar is a solid porous product, with an alkaline pH, that contains necessary plant nutrients and has the ability to sorb and retain nutrients due to its cation exchange properties (Lehmann 2007). These characteristics make biochar potentially useful for overall crop growth and production purposes (Ochiai et al. 2021). However, these properties likely do not affect seed germination and initial plant growth to the extent that the organic compounds present within biochar might. This area of research needs further exploration, with focus on the mechanisms and effects of biochar extract solutions on seed germination and growth (Sun et al. 2017).
The initial research in this area, performed by a variety of scientists, focused its attention on the presence of organic compounds in the biochar extract. For example, Lin et al. (2012) showed that relatively low pyrolysis temperatures (less than 450 °C) favored the creation of Acacia saligna wood chip biochar extracts that contained low molecular weight neutral and humic-acid-like components, while greater pyrolysis temperatures produced lower quantities of soluble organic compounds. Graber et al. (2010) studied compounds present in low temperature biochars (less than 500 °C) and observed the presence of low molecular weight organic acids, phenols, aromatic hydrocarbons, and alkanes. A subsequent study by Graber et al. (2014) found that biochar water-soluble organic carbon components contained a variety of small molecular organic acids and humic acid-like macromolecular organic compounds. Similarly, Quan et al. (2020) showed that fulvic and humic acid-like substances were released as the primary water-soluble products from biochar over time.
More specific research focused on biochar use and its soluble components, in terms of improving seed germination and root elongation, has been performed. Ahmad et al. (2012) showed that oak wood biochar, applied at 5% (w/w) in a Pb-contaminated soil, helped to improve lettuce seed germination and root length by 360% and 189%, respectively, as compared to the control; concomitantly, the biochar addition contributed to biologically active soil carbon. Lou et al. (2016) suggested that aqueous biochar extracts can contain organic moieties that increase crop yield and quality. The authors studied extracts from wheat straw and maize stalk biochars and observed the presence of aliphatic compounds, ketones, quinones, and aromatic humic substances; humic substances have been linked to promoting root growth (Eyheraguibel et al. 2008). Yang et al. (2020) quantified organic moieties released from biochar dissolved organic carbon phases and observed aliphatic, fulvic, and humic acid-like compounds; these compounds have been shown to stimulate seed germination (Zhang et al. 2020). Phoungthong et al. (2018) suggested that biochar leachate composition, and its effect on wheat seed germination, was dependent on the pyrolysis temperature; root growth appeared stimulated with extracts from biochars produced between a temperature in the range of 400 to 500 °C, yet root growth was reduced when the pyrolysis temperatures were increased to a range of 500 to 900 °C. However, the exact opposite effect on seedling growth and the pyrolysis temperature was observed by Kong et al. (2021). Fregolente et al. (2021) created hydrochar extracts via a phosphoric acid treatment and observed elevated bioavailable lignin-derived compounds. These compounds appeared to enhance corn seedling growth. However, others studying corn growth have found no significant seed germination and root elongation responses due to the presence of several different biochars or their organic compounds (Free et al. 2010).
Even given the above literature, biochar characteristics greatly vary as related to feedstock and pyrolysis temperature, and thus the mechanisms of biochar extract solutions on plant growth continue to remain unclear (Ippolito et al. 2020). In order to close the knowledge gap on this subject, biochars were prepared at the same temperature using four different feedstocks. Biochar solutions, using increasing biochar quantities extracted with 0.1 M HCl, were utilized to determine corn and rice germination, bud and root elongation, and seedling physiological indices. The novelty of this research lies in the comparison between seeds that have a true seed coat, i.e., corn, or a fused seed coat, i.e., rice, in the presence of the compounds found in the biochar extracts.
EXPERIMENTAL
Biochar Production and Extract Solution Preparation
Biochars were produced from rice straw (RSB), wheat straw (WSB), cotton straw (CSB), and Spartina alterniflora (SSB). All the feedstocks were air-dried and crushed with a pulverizer (FW80, Taisite Instrument Company, Tianjin, China). Then the powder was passed through a 2 mm sieve and pyrolyzed at a temperature of 450 °C with 10 °C min-1 under a continuous flow of N2 gas for 4 h. The ash content of the RSB, WSB, CSB, and SSB were determined as 35, 22, 11, and 18%, respectively.
After biochar creation, the biochar extract solutions were prepared by placing either 0 (W), 0.5 (X), 2.5 (Y), or 5 g (Z) of each biochar and 75 mL of a 0.1 M HCl solution in 125 mL plastic bottles, then shaking at 180 rpm at room temperature (25 °C) for 24 h. The supernatant was separated from the biochars via centrifugation at 10,000 rpm, then filtered twice through a glass fiber filter (Hill et al. 2019). The extract was used immediately for the seed germination and vigor test (as outlined below), with the remaining extract stored at a temperature of 4 °C for additional characterization and organic moiety analyses within one week. The extracted biochar was stored for subsequent organic functional group determination.
Seed Germination Rate, Bud, and Root Length Determination
The seed germination, bud, and root growth were determined using 50 seeds as a single replicate, with three replicates used for each biochar extract solution. The rice and corn seeds were randomly placed in petri dishes, and then 10 mL of the biochar extract solutions, adjusted to a pH of 6.5 using 0.1 M NaOH, was added. The seeds were incubated in a constant temperature incubator for 96 h at a temperature of 25 °C. After incubation, the number of germinated seeds was recorded, and all seedlings were measured with a vernier caliper to determine both the bud and root length.
Characterization of the Biochar Extract Solutions
The properties of the biochar extract solution were determined by the following methods recommended by the International Biochar Initiative, using three replicates of each biochar (Table 1) (IBI 2012). The solution was filtered through a glass fiber filter, and then the pH was measured using a glass electrode (pHS-3C, LEICI, Shanghai, China). Magnesium and calcium concentrations were determined by flame atomic adsorption spectrophotometry (FAAS) (TAS-990F, Beijing Purkinje General Instrument Co., Ltd., Beijing, China) (Ndour et al. 2008). The total nitrogen was determined via the alkaline potassium persulfate digestion UV spectrophotometric method. The total phosphorus was determined via the ammonium molybdate method followed by UV spectrophotometry analysis (TU-1901, Beijing Purkinje General Instrument Co., Ltd. China) (Liu et al. 2019).
The extract solutions were pretreated, and the organic phases present were determined as follows: 2 g of NaCl was added to 50 mL of the biochar extract solution and completely dissolved in a separatory funnel. Then, 4 mL of n-hexane was added, and the funnel was shaken via hand for 5 min followed by standing for 10 min. Then, the organic compounds in the aqueous phase was released from the bottom of the separatory funnel into a beaker. The organic compounds in the aqueous phase was extracted again following the same procedure. Afterwards, the organic phase was passed through a funnel containing anhydrous sodium sulfate to remove the water. The organic phase was subsequently transferred to a rotary evaporator (RE-52A, Shanghai Yarong Biochemical Instrument Factory, Shanghai, China) with N2 gas blown across the liquid phase until 1 mL remained, and then the organic phases present in the solution were quantified via a Thermo Trace DSQ II gas chromatography-mass spectrometer (GC-MS) (TRACE 1310- ISQ, Thermo Fisher Scientific, Waltham, MA) equipped with a TG-5MS capillary column (30 m, 0.25 mm i.d., 0.25 µm film thickness). The column temperature was initially 80 °C for 1 min, and then it increased to 350 °C at a rate of 10 °C/min. The carrier gas was helium at a flow rate of 1 mL/min, and the injection volume was 1 µL; the injector was operated in the split mode after sample injection (Cui et al. 2017a).
A portion of the biochar extract solution was placed in a 10 mm quartz cuvette. Then, the biochar extract solution organic moiety chemical characteristics were detected via three-dimensional excitation-emission matrix spectrophotometry (3DEEM) (Aqualog, HORIBA Scientific, Kyoto, Japan) with an excitation spectral range (λEx) of 240 to 340 nm and emission spectral range of 213 to 450 nm; the width of the excitation and emission slits were both 5 nm, and the exposure time was 0.5 s.
Seedling Malondialdehyde (MDA) Content
Malondialdehyde (MDA) is the final decomposition product of the membrane lipid peroxidation (Morales and Munné-Bosch 2019). Malondialdehyde can loosen the bridge bonds between cellulose molecules, can inhibit protein synthesis, and thus can be used as a marker for oxidation stress. The seedling MDA content was determined according to the thiobarbituric acid (TBA) developing method (Shang et al. 2019). After the germination and bud length characteristics were determined, 1.0 g of fresh bud was placed in a mortar, 2 mL of 10% trichloroacetic acid and approximately 0.1 g of quartz sand was added, and the bud was ground with a pestle. Next, 8 mL of 10% trichloroacetic acid was added, followed again by grinding. The homogenate was placed into a 50 mL centrifuge tube and then centrifuged at 6000 rpm for 10 min. Next, 2 mL of the supernatant and 2 mL of a 0.6% thiobarbituric acid solution were added to another centrifuge tube, boiled for 15 min in a water bath, cooled, and centrifuged again. The MDA in the supernatant was quantified with a UV spectrophotometer (TU-1901, Beijing Purkinje General Instrument Co., Ltd. China) at 450, 532, and 600 nm.
Extracted Biochar Functional Groups Determination
The biochar functional groups, before and after extraction, were determined utilizing pulverized biochar and KBr powder to create pellets. The pellets were then analyzed for the presence of functional groups via ATR-FTIR (NEXUS-670, NICOLET, Thermo Nicolet Corporation, Waltham, MA) from 4000 cm-1 to 400 cm-1 with a resolution of 1.0 cm-1.
Statistical Analysis
The data were expressed as the means ± standard error of the mean. Significant differences between the treatments were determined using analyses of variance (ANOVA) at a p-value less than 0.05, and when present, the differences between the means were determined using a Tukey post-hoc test. All statistical analyses were carried out using SPSS, version 22.0 (SPSS Institute, USA) and Origin 9.0 (Origin Lab, USA).
RESULTS AND DISCUSSION
Characterization of the Biochar Extract Solutions
The basic properties of the biochar extract solution, averaged across the three extracts replicates for a given biochar type, are shown in Table 1. After extraction with 0.1 M HCl (pH = 1.08), the biochar solution pH values ranged from 1.55 to 3.74. All the biochar extract pH values were greater than the initial extract solution pH, likely due to the alkaline materials created during pyrolysis. In a biochar meta-analyses review, Ippolito et al. (2020) showed that as the pyrolysis temperature increased, the biochar pH increased. The authors also showed that biochar pH was greater than or equal to 8.5 when the pyrolysis temperature was greater than 400 °C; in the current study, the pyrolysis temperature was 450 °C. Increasing the pyrolysis temperature increases the ash content and concomitantly the biochar pH, as Ca and Mg carbonate and hydroxide phases are present within the ash (Yuan et al. 2011; Ippolito et al. 2020). This information also helps explain the relatively elevated Ca and Mg concentrations within the biochar extracts. The total organic C, N, and P contents in all the extracts were in-line with the values published by Ippolito et al. (2020).
Table 1. Basic Properties of Biochar Extract Solutions (mg∙L-1)
The Effect of the Different Biochar Extract Solutions on the Corn and Rice Seed Germination, Bud, and Root Length
Various biochars and increasing biochar quantities in the extract solutions, and the subsequent effects of the extract solution on corn and rice germination rate (Table 2), bud, and root length, are shown in Fig. 1. The RSB biochar extract solutions Y and Z increased corn germination compared to the control, while CSB extract Z decreased the corn germination relative to the other solutions; all the WSB and SSB extract solutions had no effect on corn germination (Table 2). Results similar to those by RSB solutions Y and Z were found by Hille and Ouden (2005), who applied a phytotoxin with either granulated or powdered pine biochars to seedlings. The authors noted a positive germination response when the biochar was introduced to the solutions containing a relatively greater toxin concentrations compared to lower concentrations. Results similar to the WSB and SSB treatments were also found by Free et al. (2010), who used sludge-, corn stover-, and wood-biochar (at 550 °C) as well as by Kamara et al. (2014) who used corn stalk biochar, with all biochars applied to corn seeds; both sets of researchers observed no effect on corn germination. The RSB and CSB extract solution Y increased the corn bud length compared to the control, while WSB extract solution Z decreased the bud length over compared to the control; all other extract solutions, when compared to the control, affected the bud length similarly (Fig. 1a). The RSB extract solution X and Y, the CSB extract solutions Y and Z, and the SSB extract solution X increased the root length compared to the control (Fig. 1b). However, the WSB extract solutions Y and Z both decreased the root length compared to the control.
When the biochar extract effects were evident for rice germination, they were always expressed as a negative response as compared to the control (Table 2). A similar response was observed for the rice bud and root length (as shown in Fig. 1c and Fig. 1d). Often, extract Y from all biochars, and always extract Z from all biochars, reduced the bud and root length when compared to the control. Obviously, rice seeds did not prefer solutions extracted with greater biochar quantities compared to corn seeds.
Fig. 1. The effect of the biochar extract solution [from rice straw (RSB), wheat straw (WSB), cotton straw (CSB), and Spartina alterniflora (SSB) biochars] on corn and rice growth [corn seed bud length (a), root length (b), and rice seed bud length (c), root length (d)] Note: different lower-case letters above error bars (standard deviation of the mean; n = 3; p-value less than 0.05, determined via a Tukey post-hoc test) indicate significant differences between the same biochar yet extracted with different biochar to solution ratios [Figure legends: 0 (W), 0.5 (X), 2.5 (Y), or 5 g (Z) of biochar to 75 mL of 0.1 M HCl]
Rice seeds are a fusion of the seed coat and embryo, while corn seeds contain a seed coat that protects the embryo. The lack of a true seed coat in rice likely did not protect the seed from the adverse effects of the compounds present within the biochar extracts. A similar response was reported by Oh et al. (2012), who suggested that pyrolysis may or may not remove substances toxic to seed germination. Thus, the results from the current study suggested that biochar and its extracts, and the potential detrimental effects, should be considered prior to its addition to certain seeds within cropping systems.
Table 2. Effect of Biochar Extract Solution on Corn and Rice Seed Germination Percentage
Different lower-case letters above error bars (standard deviation of the mean; n=3; p<0.05, determined via a Tukey post-hoc test) indicate significant differences between the same biochar yet extracted with different biochar to solution ratios.
Organic Moieties Present in the Biochar Extract Solutions
The biochar extract solutions contained a variety of water-soluble organic phases, including triethyl phosphate (C6H15PO4), 2,4-bis (1,1-dimethylethyl)-phenol (C15H24O), and alkanes (CnH2n+2 species), as determined by GC-MS (as shown in Table 3). Other studies have shown that the hydrophilic-fraction of biochar extract solutions primarily contains simple organic acids, carbohydrates, phenols, amino acids, and amino sugars, while the hydrophobic-fraction consists of hydrocarbons, fatty acids, nucleic acids, and quinones (Sun et al. 2021). These compounds may play positive or negative roles in terms of seed germination and plant growth. In the current study, the biochars contained various organic moieties, with those higher molecular weight compounds potentially promoting plant growth, as shown by (Henner et al. 1999). Li et al. (2015) found that corn stover biochar water extracts contained polycyclic aromatic hydrocarbon (PAH)-like compounds, e.g., 2-ring (naphthalene) and 4-ring (chrysene) PAHs; these compounds improved tomato germination rates at relatively low doses but inhibited germination at higher doses.
Table 3. List of Organic Compounds in Biochar Extract Solutions as Determined via Gas Chromatography-Mass Spectrometer (GC-MS)
Three-Dimensional Excitation-Emission Matrix Spectrophotometry Analysis
The biochar extract solution soluble organic compound spectral characteristics, as detected using the three-dimensional excitation-emission matrix (3DEEM), are presented in Fig. 2. The fluorescence intensity suggests that these biochar extract solutions primarily contained humic- and fulvic-like acids, and a tryptophan-like compound in the region of λEm/λEx = (220 to 370 nm) /(330 to 470 nm) (as shown in Table 4). However, these organic moieties were present to lesser or greater extents (based on the fluorescence intensity) depending on the biochar type. The organic moiety disparities present are most likely due to the feedstock choice, as shown by Rajapaksha et al. (2019), who used the same excitation technique.
The 3DEEM not only shows the change of the fluorescence intensity, but it also shows the change in the peak position varying across all four biochars (Table 4). The change in the peak position, with respect to changing the biochar type, suggests alterations in the organic moieties present. However, the biochar soluble organic compounds were primarily derived from humic acid-like compounds. Although this technique cannot distinguish the specific compound present, humic acid-like substances are aromatic, and these aromatic compounds could have played a role in the corn and rice seed germination, bud, and root length observations.
Fig. 2. Three-dimensional fluorescence spectrum of the water-soluble organic matter in the biochar extract solution (A: RSB, rice straw biochar; B: WSB, wheat straw biochar; C: CSB, cotton straw biochar; D: SSB, Spartina alterniflora biochar)
Table 4. Characteristics of Organic Matter Components in Different Biochars Extract Solutions
Others have shown that relatively high concentrations of biochar extracts inhibited seed vitality, primarily due to relatively high organic compound concentrations present (Xia et al. 2020). These authors contended that the antagonistic effect on seed vitality was likely due to the presence of bio-oil, i.e., aromatic compounds, in the biochar, creating biological toxicity. Oppositely, Fregolente et al. (2021) found that the benzene derivatives and phenolic compounds, i.e., both aromatic compounds, present in the biochars were responsible for stimulating the shoot and root elongation.
Malondialdehyde (MDA) Content of the Seed Generation
Malondialdehyde is one of the major products of lipid peroxidation, and its accumulation reflects the degree of damage from oxidative stress (Jia et al. 2019). Malondialdehyde is typically employed as a general indicator to evaluate the extent of lipid peroxidation resulting from oxidative stress, which can be related to antioxidant enzyme activity. The MDA content in the corn or rice buds, as a function of biochar extract type, is presented in Fig. 3. In general, the MDA content of the corn and rice buds usually increased as the biochar extract ratio increased. In the RSB and CSB X, Y, and Z extract solutions, the MDA content in corn bud was increased by 24.6% to 62.9% compared to the control (Fig. 3a). However, the corn bud MDA content decreased with WSB Z solution and with the increasing SSB solutions, compared to the control. In the rice buds, the MDA content increased by 2.8% to 59.9% in all biochar extract solutions Y and Z, compared to the control (Fig. 3b). This result suggests that greater oxidative stress was present with greater biochar extract solution treatments.
In general, in both corn and rice, the MDA content remained relatively low in the presence of low biochar extract solution concentrations. As suggested by others, this may indicate that the reactive oxygen species, i.e., indicators of oxidative stress, were effectively eliminated by antioxidant enzymes (Li et al. 2013) or helped induce systemic resistance to oxidative stress (Graber et al. 2010). In addition, in general, in both the corn and rice, the MDA content was relatively elevated in the presence of high biochar extract solution concentrations. This may have been due to the increased presence of aromatic organic moieties that lead to oxidative stress. Similarly, Li et al. (2015) found that MDA content and antioxidant enzymatic activity in tomato seedling leaves and roots increased as the biochar extract solution concentrations increased, potentially due to the presence of PAHs. It is interesting to note that the work from Li et al. (2015) suggested that the toxicity to seedlings may be alleviated as the plants mature.
Fig. 3. The effect of the biochar extract solution [from rice straw (RSB), wheat straw (WSB), cotton straw (CSB), and Spartina alterniflora (SSB) biochars] on the content of malondialdehyde (MDA) in buds of corn (a) and rice (b). Note: different lower-case letters above error bars (standard deviation of the mean; n = 3; p-value less than 0.05, determined via a Tukey post-hoc test) indicate significant differences between the same biochar yet extracted with different biochar to solution ratios [Figure legends: 0 (W), 0.5 (X), 2.5 (Y), or 5 g (Z) of biochar to 75 mL of 0.1 M HCl]
Biochar Functional Groups
The presence of biochar surface functional groups in fresh or extracted biochars, as determined via Fourier-transform infrared spectroscopy (FTIR), are presented in Fig. 4a through 4d. In general, peak comparisons between the fresh and extracted biochars varied greatly, which likely indicated a stripping of the biochar-borne surface organic functional groups with subsequent accumulation of the biochar extracts. This observation further supports the findings presented above with respect to the presence of organic moieties in biochar extracts.
Table 5 outlines the FTIR peak assignments, which are presented in Fig 4a through 4d. Specifically, the rice, wheat, cotton, and S. alterniflora biochars and extracted biochars had peaks at 3430, 2858, 1586, and 1150 cm-1, yet the peak intensities were different and additional peaks were present in the fresh biochars. Fresh biochars contained more functional groups, especially from 673 to 1673 cm-1, which were lost in the extracted biochars. Various peaks, e.g., 1558 and 1409 cm-1 in RSB cm-1, 1409, 871, and 755 cm-1 in WSB, 1411, 1012, 869, and 750 cm-1 in CSB, and 1074, 863, 809, and 754 cm-1 in SSB, were present in fresh biochars but lost in extracted biochars. The peak located at 1037 to 1078 cm-1 was typically greater in the extracted biochars, likely due to the C-O-C or alcohol -OH group enhancement on the biochar surface. The peak at 3430 cm-1 was the result of the -OH stretching vibration of phenolic hydroxyl or aliphatic groups, with this peak becoming more pronounced in extracted biochars; a similar phenomenon was found with the peak at 1579 to 1612 cm-1, which is likely due to increased aromatic C=C stretching on the extracted biochar (Parshetti et al. 2012). Choudhary et al. (2017) found similar shifts between the fresh and washed Eucalyptus bark biochar; a rightward shift (10 cm-1) and enhancement of the 1621 cm-1 peak, the disappearance of peaks 1412 and 1370 cm-1, and suppression of the 874 cm-1 peak. The authors also noted a new peak located at 1384 cm-1, which corresponded to carboxylate C=O stretching in the fresh biochar, potentially indicating a carboxylic acid compound in the extracted biochar.
Table 5. Band Assignments in the Fourier-transform Infrared (FTIR) Spectra of the Fresh and Extracted Biochars
Fig. 4. The changes in the fresh and extracted (E+) biochar functional groups (Note: RSB = rice straw biochar (a), WSB = wheat straw biochar (b), CSB = cotton straw biochar (c), SSB = Spartina alterniflora biochar (d))
CONCLUSIONS
- Increasing the amounts of biochar in the extracts tended to improve the corn seed bud and root length; however, the opposite was observed in rice seeds. This was likely a function of the corn seeds containing a seed coat, and thus the seed embryo was protected from growth-inhibiting organic compounds in the biochar extracts.
- A variety of the organic moieties present in the biochar extract solutions were quantified via FTIR and 3DEEM analysis, e.g., triethyl phosphate, 2,4-bisphenol, alkanes, and other humic-like aromatic compounds, which likely influenced the corn and rice bud and root growth.
- It was possible that the presence of PAHs may have enhanced or inhibited seed growth at either low or high concentrations, respectively, with MDA analysis supporting this contention.
- Determining the presence of the biochar organic moieties may help with designing biochars for enhanced seed germination, growth, and crop productivity.
ACKNOWLEDGMENTS
The chemical property analysis and the FTIR, SEM, GC-MS, and 3D-EEM analysis, were performed in the Analytical and Testing Center of Yancheng Institute of Technology. This work was supported by the National Natural Science Foundation of China under Grant No. 21677119 and Grant No. 41501339.
REFERENCES CITED
Ahmad, M., Lee, S. S., Yang, J. E., Ro, H.-M., Lee, Y. H., and Ok, Y. S. (2012). “Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil,” Ecotoxicology and Environmental Safety 79, 225-231. DOI: 10.1016/j.ecoenv.2012.01.003
Chen, T., Zhang, Y., Yan, J., Ding, C., Yin, C., and Liu, H. (2015). “Heterogeneous photodegradation of mesotrione in nano α-Fe2O3/oxalate system under UV light irradiation,” RSC Advances 5(17), 12638-12643. DOI: 10.1039/c4ra11871e
Choudhary, B., Paul, D., Singh, A., and Gupta, T. (2017). “Removal of hexavalent chromium upon interaction with biochar under acidic conditions: Mechanistic insights and application,” Environmental Science and Pollution Research 24, 16786-16797. DOI: 10.1007/s11356-017-9322-9
Cimò, G., Kucerik, J., Berns, A. E., Schaumann, G. E., Alonzo, G., and Conte, P. (2014). “Effect of heating time and temperature on the chemical characteristics of biochar from poultry manure,” Journal of Agricultural and Food Chemistry 62(8), 1912-1918. DOI: 10.1021/jf405549z
Cui, L., Chen, T., Quan, G., Xiao, B., Ma, Y., Pan, M., Liu, Y., Liu, B., Yin, C., Yan, J., et al. (2017a). “Renewable material-derived biochars for the efficient removal of 2,4-dichlorophen from aqueous solution: Adsorption/desorption mechanisms,” BioResources 12(3), 4912-4925. DOI: 10.15376/biores.12.3.4912-4925
Cui, L., Chen, T., Ding, C., Li, Z., Yan, J., Liu, Y., Niu, X., Chen, A., and Yang, W. (2017b). “Spatial distribution of total halogenated organic compounds (TX), adsorbable organic halogens (AOX), and heavy metals in wetland soil irrigated with pulp and paper wastewater,” Chemical Speciation & Bioavailability 29(1), 15-24. DOI: 10.1080/09542299.2016.1252692
Cui, L., Yin, C., Chen, T., Quan, G., Ippolito, J., Liu, B., Yan, J., Ding, C., Hussain, Q., and Umer, M. (2019). “Remediation of organic halogen- contaminated wetland soils using biochar,” Science of the Total Environment 696, 1-9. DOI: 10.1016/j.scitotenv.2019.134087
Eyheraguibel, B., Silvestre, J., and Morard, P. (2008). “Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize,” Bioresource Technology 99(10), 4206-4212. DOI: 10.1016/j.biortech.2007.08.082
Fan, Q., Sun, J., Chu, L., Cui, L., Quan, G., Yan, J., Hussain, Q., and Iqbal, M. (2018). “Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar,” Chemosphere 207, 33-40. DOI: 10.1016/j.chemosphere.2018.05.044
Free, H. F., McGill, C. R., Rowarth, J. S., and Hedley, M. (2010). “The effect of biochars on maize (Zea mays) germination,” New Zealand Journal of Agricultural Research 53(1), 1-4. DOI: 10.1080/00288231003606039
Fregolente, L. G., Santos, J. V. d., Vinci, G., Piccolo, A., Moreira, A. B., Ferreira, O. P., Bisinoti, M. C., and Spaccini, R. (2021). “Insights on molecular characteristics of hydrochars by 13C-NMR and off-line TMAH-GC/MS and assessment of their potential use as plant growth promoters,” Molecules 26(4), 1-16. DOI: 10.3390/molecules26041026
Graber, E. R., Harel, Y. M., Kolton, M., Cytryn, E., Silber, A., David, D. R., Tsechansky, L., Borenshtein, M., and Elad, Y. (2010). “Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media,” Plant Soil 337, 481-496. DOI: 10.1007/s11104-010-0544-6
Graber, E., Tsechansky, L., Lew, B., and Cohen, E. (2014). “Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe,” European Journal of Soil Science 65(1), 162-172. DOI: 10.1111/ejss.12071
Henner, P., Schiavon, M., Druelle, V., and Lichtfouse, E. (1999). “Phytotoxicity of ancient gaswork soils. Effect of polycyclic aromatic hydrocarbons (PAHs) on plant germination,” Organic Geochemistry 30(8, Part 2), 963-969. DOI: 10.1016/S0146-6380(99)00080-7
Hill, R. A., Hunt, J., Sanders, E., Tran, M., Burk, G. A., Mlsna, T. E., and Fitzkee, N. C. (2019). “Effect of biochar on microbial growth: A metabolomics and bacteriological investigation in E. coli,” Environmental Science & Technology 53(5), 2635-2646. DOI: 10.1021/acs.est.8b05024
Hille, M., and Ouden, J. (2005). “Charcoal and activated carbon as adsorbate of phytotoxic compounds – A comparative study,” Oikos 108(1), 202-207. DOI: 10.1111/j.0030-1299.2005.13482.x
IBI (2012). Standardized Product Definition and Product Testing Guidelines for Biochar that is Used in Soil, International Biochar Initiative, Canandaigua, NY.
Ippolito, J. A., Cui, L., Kammann, C., Wrage-Mönnig, N., Estavillo, J. M., Fuertes-Mendizabal, T., Cayuela, M. L., Sigua, G., Novak, J., Spokas, K., et al. (2020). “Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review,” Biochar 2, 421-438. DOI: 10.1007/s42773-020-00067-x
Jia, W., Wang, C., Ma, C., Wang, J., Sun, H., and Xing, B. (2019). “Mineral elements uptake and physiological response of Amaranthus mangostanus (L.) as affected by biochar,” Ecotoxicology and Environmental Safety 175, 58-65. DOI: 10.1016/j.ecoenv.2019.03.039
Kamara, A., Kamara, A., Mansaray, M. M., and Sawyerr, P. A. (2014). “Effects of biochar derived from maize stover and rice straw on the germination of their seeds,” American Journal of Agriculture and Forestry 2(6), 246-249. DOI: 10.11648/j.ajaf.20140206.12
Kong, L., Zhang, X., Wang, X., Han, M., Shan, Q., Jin, C., and Tian, X. (2021). “Effect of temperature on PTEs deportment and ecological risks of the biochars obtained from sewage sludge,” Environmental Science and Pollution Research 29, 14733-14742. DOI: 10.1007/s11356-021-16859-y
Lehmann, J. (2007). “A handful of carbon,” Nature 447, 143-144. DOI: 10.1038/447143a
Li, Q., Kang, S., Wang, N., Li, Y., Li, X., Dong, Z., and Chen, P. (2017). “Composition and sources of polycyclic aromatic hydrocarbons in cryoconites of the Tibetan Plateau glaciers,” Science of the Total Environment 574, 991-999. DOI: 10.1016/j.scitotenv.2016.09.159
Li, Y., Shen, F., Guo, H., Wang, Z., Yang, G., Wang, L., Zhang, Y., Zeng, Y., and Deng, S, (2015). “Phytotoxicity assessment on corn stover biochar, derived from fast pyrolysis, based on seed germination, early growth, and potential plant cell damage,” Environmental Science and Pollution Research 22, 9534-9543. DOI: 10.1007/s11356-015-4115-5
Li, Y., Zhang, S., Jiang, W., and Liu, D. (2013). “Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L,” Environmental Science and Pollution Research 20, 1117-1123. DOI: 10.1007/s11356-012-1054-2
Lin, Y., Munroe, P., Joseph, S., and Henderson, R. (2012). “Migration of dissolved organic carbon in biochars and biochar-mineral complexes,” Pesquisa Agropecuária Brasileira 47(5), 677-686. DOI: 10.1590/S0100-204X2012000500007
Liu, B., Cai, Z., Zhang, Y., Liu, G., Luo, X., and Zheng, H. (2019). “Comparison of efficacies of peanut shell biochar and biochar-based compost on two leafy vegetable productivity in an infertile land,” Chemosphere 224, 151-161. DOI: 10.1016/j.chemosphere.2019.02.100
Lou, Y., Joseph, S., Li, L., Graber, E., Liu X., and Pan, G. (2016). “Water extract from straw biochar used for plant growth promotion: An initial test,” BioResources 11(1), 249-266. DOI: 10.15376/biores.11.1.249-266
Mia, S., Dijkstra, F., and Singh, B. (2017). “Aging induced changes in biochar’s functionality and adsorption behavior for phosphate and ammonium,” Environmental Science & Technology 51(15), 8359-8367. DOI: 10.1021/acs.est.7b00647
Morales, M., and Munné-Bosch, S. (2019). “Malondialdehyde: Facts and artifacts,” Plant Physiology 180(3), 1246-1250. DOI: 10.1104/pp.19.00405
Ndour, N. Y. B., Baudoin, E., Guissé, A., Seck, M., Khouma, M., and Brauman, A. (2008). “Impact of irrigation water quality on soil nitrifying and total bacterial communities,” Biology and Fertility of Soils 44, 797-803. DOI: 10.1007/s00374-008-0285-3
Ochiai, S., Iwabuchi, K., Itoh, T., Watanabe, T., Osaki, M., and Taniguro, K. (2021). “Effects of different feedstock type and carbonization temperature of biochar on oat growth and nitrogen uptake in coapplication with compost,” Journal of Soil Science and Plant Nutrition 21, 276-285. DOI: 10.1007/s42729-020-00359-y
Oh, T.-K., Shinogi, Y., Chikushi, J., Lee, Y.-H., and Choi, B. (2012). “Effect of aqueous extract of biochar on germination and seedling growth of lettuce (Lactuca sativa L.),” Journal of the Faculty of Agriculture, Kyushu University 57(1), 55-60. DOI: 10.5109/22048
Parshetti, G. K., Hoekman, S. K., and Balasubramanian, R. (2012). “Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches,” Bioresource Technology 135, 683-689. DOI: 10.1016/j.biortech.2012.09.042
Phoungthong, K., Zhang, H., Shao, L.-M., and He, P. (2018). “Leaching characteristics and phytotoxic effects of sewage sludge biochar,” Journal of Material Cycles and Waste Management 20, 2089-2099. DOI: 10.1007/s10163-018-0763-0
Quan, G., Fan, Q., Zimmerman, A. R., Sun, J., Cui, L., Wang, H., Gao, B., and Yan, J. (2020). “Effects of laboratory biotic aging on the characteristics of biochar and its water-soluble organic products,” Journal of Hazardous Materials 382, 1-9. DOI: 10.1016/j.jhazmat.2019.121071
Rajapaksha, A. U., Ok, Y. S., El-Naggar, A., Kim, H., Song, F., Kang, S., and Tsang, Y. F. (2019). “Dissolved organic matter characterization of biochars produced from different feedstock materials,” Journal of Environmental Management 233, 393-399. DOI: 10.1016/j.jenvman.2018.12.069
Shang, Y., Liu, S., Chen, Y., Liu, L., Tang, X., Qin, Z., and Liao, J. (2019). “Effects of antioxidant system and soluble protein in Dongying wild soybean seedling under cadmium stress,” Journal of Sichuan Agricultural University 37(1), 15-21. DOI: 10.16036/j.issn.1000-2650.2019.01.003
Sun, J., Drosos, M., Mazzei, P., Savy, D., Todisco, D., Vinci, G., Pan, G., and Piccolo, A. (2017). “The molecular properties of biochar carbon released in dilute acidic solution and its effects on maize seed germination,” Science of the Total Environment 576, 858-867. DOI: 10.1016/j.scitotenv.2016.10.095
Sun, Y., Xiong, X., He, M., Xu, Z., Hou, D., Zhang, W., Ok, Y. S., Rinklebe, J., Wang, L., Tsang, D. C. W. (2021). “Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review,” Chemical Engineering Journal 424, 1-14. DOI: 10.1016/j.cej.2021.130387
Xia, H., Yu, D., Zhu, Q., Liu, X., Li, Q., Wang, S., and Ding, W. (2020). “Effect of different biochar extracts on seed germination and seedling fluorescence of Brassica campestris,” Journal of Agricultural Science and Technology 22(3), 31-37. DOI: 10.13304/j.nykjdb.2019.0051
Yang, F., Zhang, Q., Jian, H., Wang, C., Xing, B., Sun, H., and Hao, Y. (2020). “Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol,” Journal of Hazardous Materials 396, 1-11. DOI: 10.1016/j.jhazmat.2020.122598
Yuan, J.-H., Xu, R.-K., and Zhang, H. (2011). “The forms of alkalis in the biochar produced from crop residues at different temperatures,” Bioresource Technology 102(3), 3488-3497. DOI: 10.1016/j.biortech.2010.11.018
Zhang, P., Huang, P., Xu, X., Sun, H., Jiang, B., and Liao, Y. (2020). “Spectroscopic and molecular characterization of biochar-derived dissolved organic matter and the associations with soil microbial responses,” Science of the Total Environment 708, 1-11. DOI: 10.1016/j.scitotenv.2019.134619
Article submitted: February 25, 2022; Peer review completed: May 7, 2022; Revised version received: May 11, 2022; Accepted: May 14, 2022; Published: May 18, 2022.
DOI: 10.15376/biores.17.3.4151-4166
