Abstract
Fungal spoilage of bread can be a great problem; however, it can be explored as a producer of enzymes. The fungi were isolated from breads, and their activity for α-amylase production was planned. The results identified nine fungi on spoiled breads. Aspergillus fumigatus occurred with 85% frequency, followed by other isolates. Starch yeast (SY), white flour (WF), and black flour (BF) were applied as substrates for α-amylase activity using fungal isolates. The SY was the best, followed by WF and BF for α-amylase activity. Using SY, A. niger showed the greatest potency for α-amylase (7.67 U/mL) unlike Monilia sitophila, which reflected low α-amylase activity (2.69 U/mL). Using WF, A. fumigatus reflected high amylase activity (5.76 U/mL) while A. niger, A. terreus, and Penicillium expansum showed less activity (5.12 U/mL, 4.41 U/mL, and 3.56 U/mL, respectively). The temperature 30 °C and pH 6 were the optimum for α-amylase activity by A. niger, A. fumigatus, and P. chrysogenum, using the three media, but α-amylase activity of A. fumigatus at 40 °C was higher than at 20 °C. At the ninth day of incubation, the maximum α-amylase activity was reported using SY, while at the twelfth day, maximum activity was reported using WF and BF.
Download PDF
Full Article
Bread Spoilage Fungi as Creators of α‑Amylase Using Two Types of Wheat Flour
Aisha M. H. Al-Rajhi,a Hanan Moawad,b Mohamed M. Alawlaqi,b Hashim R. Felemban,c,d and Tarek M. Abdel Ghany e,*
Fungal spoilage of bread can be a great problem; however, it can be explored as a producer of enzymes. The fungi were isolated from breads, and their activity for α-amylase production was planned. The results identified nine fungi on spoiled breads. Aspergillus fumigatus occurred with 85% frequency, followed by other isolates. Starch yeast (SY), white flour (WF), and black flour (BF) were applied as substrates for α-amylase activity using fungal isolates. The SY was the best, followed by WF and BF for α-amylase activity. Using SY, A. niger showed the greatest potency for α-amylase (7.67 U/mL) unlike Monilia sitophila, which reflected low α-amylase activity (2.69 U/mL). Using WF, A. fumigatus reflected high amylase activity (5.76 U/mL) while A. niger, A. terreus, and Penicillium expansum showed less activity (5.12 U/mL, 4.41 U/mL, and 3.56 U/mL, respectively). The temperature 30 °C and pH 6 were the optimum for α-amylase activity by A. niger, A. fumigatus, and P. chrysogenum, using the three media, but α-amylase activity of A. fumigatus at 40 °C was higher than at 20 °C. At the ninth day of incubation, the maximum α-amylase activity was reported using SY, while at the twelfth day, maximum activity was reported using WF and BF.
DOI: 10.15376/biores.18.3.5908-5923
Keywords: Breads; Amylolytic; Hydrolytic enzymes; Mycobiota; Storing condition
Contact information: a: Department of Biology, College of Science, Princess Nourah bint Abdulrahman University P.O. Box 84428, Riyadh 11671, Saudi Arabia; b: Biology Department, College of Science, Jazan University, Jazan 82817, Saudi Arabia; c: Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah 22254, Saudi Arabia; d: Special Infectious Agents Unit-BSL3, King Fahd Medical Research Center, King Abdulaziz University, Jeddah 21362, Saudi Arabia; e: Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo 11725, Egypt; *Corresponding authors: tabdelghany.201@azhar.edu.eg (T.M.A.); amoalrajhi@pnu.edu.sa(AMHA)
GRAPHICAL ABSTRACT

INTRODUCTION
Amylases are starch-hydrolyzing enzymes derived from different sources of living organisms (Abu et al. 2005). There are different types of amylases, from which are α-amylases that catalyze the breakdown of internal linkages of α-1,4-glycosidic in starch molecules to simple molecules, including maltose, glucose, and maltotriose (Anto et al. 2006; Rajagopalan and Krishnan 2008). Microbial amylases are being explored more due to greater simplicity of large-scale manufacturing compared to plant and animal amylases and their relevance in ensuing use at industry level (Ali et al. 2023; Premalatha et al. 2023).
Amylases from fungi are widely applied for the formulation of oriental foods (Sidar et al. 2023). According to several studies, fungi such as Aspergillus niger may synthesise a variety of enzymes to hydrolyse complex polysaccharides into simple compounds, which are then utilized for development and reproduction (Abdelghany and Bakri 2019; Al Abboud et al. 2022; Bakri et al. 2022; Al-Rajhi et al. 2022a, 2022b). Several investigations tried bread waste as raw substrates for enzyme creations, including protease and glucoamylase, utilizing different microorganisms such as Aspergillus sp. (Melikoglu et al. 2015), Thermomyces sp. (Cerda et al. 2016), Monascus purpureus (Haque et al. 2016), and Rhizopus oryzae (Benabda et al. 2019). Because of numerous advantages, such as availability and cost, bread waste is an effective component for production of enzymes.
α-Amylases addition to bakery products promotes enhancing the acceptance of consumers to these products (Ait Kaki El-Hadef El-Okki et al. 2017). Some studies recommended the addition of fungal α-amylases as safe additives (Goesaert et al. 2005). According to a review report of Movahedpour et al. (2022), microbial α-amylases contribute to the success of several industries such as fermentation, food, textile, detergent, paper, distilling, brewing, and pharmacological processes. The fermentation rate of wheat dough was accelerated as a result of α-amylase addition that provides easy degradable sugars like maltose and malto-oligosaccharides required for yeast growth (Struyf et al. 2017). Aspergillus fumigatus was isolated and showed a great source of amylase (Singh et al. 2014), these enzyme can be cost efficiently utilized in textile wet processing. The activity of microbial enzymes is critically affected by physical and nutritional factors as well as varies according to microbial creator (Deshmukh et al. 2011). As mentioned in earlier studies, foods with high carbohydrate content in addition to other constituents make them ideal substrates for microbial development and enzyme synthesis (Ravindran and Jaiswal 2016; Sigüenza-Andrés et al. 2022).
Although some fungi are considered toxic and unsafe, they are widely used in many industrial applications. This overlap baffled many non-specialists in the field of mycology, which prompted mycologists to explain this mystery. Earlier reports indicated that the fungal secondary metabolites, as well as enzymes production may be regulated by exogenous compounds and minerals (Abdelghany 2006). To avoid the mycotoxigenic properties of Aspergillus niger, the used strains in the biotechnological industry have been developed via mutagenesis and genes deletion responsible for toxins production (Susca et al. 2014). Also, Frisvad et al. (2018) mentioned that the industrial strains of A. oryzae are not able to synthesize toxins because of the existence of deactivating mutations in the cluster of genes. So, some strains used in the enzyme production are selected based on the deleted genes of cyclopiazonic acid and aflatoxin production.
Aspergillus oryzae, A. awamori, and A. niger as a fungal source of α-amylase contribute to a relatively large commercial production of α-amylase (Goto et al.1998), while Penicillium chrysogenum and A. fumigatus are also applied for the same purpose but in the solid state fermentation practices (Goto et al.1998; Singh et al. 2014, 2021). Large quantities of α-amylase have been created from mesophilic or thermophilic fungi (Kamaraj et al. 2020). Constituents of bread encourage fungi growth and thereof are proliferated at many stages of bread manufacture, including production, packaging, and storage. Fungi represent the major causative of bread spoilage are able to be developed under an excessive variation of circumstances (Melini and Melini 2018). The aim of this investigation was to isolate some fungi from two types of breads its activity for α-amylase production with some optimization conditions.
EXPERIMENTAL
Collection, Contents of Breads Samples, and Fungal Contamination Processing
Two common types of white bread (WB) and black bread (BB) were collected from a bakery market in Egypt. According to the procedures of Cheesbrough (2008), the contents of breads including ash, protein, and carbohydrate were estimated. Where the protein content was determined via detection of total nitrogen (N) by Kjeldahl method, where the protein content was expressed as N x 6.25. Bread content of ash was assessed via burning of five g of bread porcelain crucible (pre-weighed known) for 6 h at 600 ℃ in a muffle furnace. The summation of bread contents including ash (%), protein (%), moisture (%), and % fat (%) was calculated. Then the total of carbohydrates was calculated as 100-bread contents. The collected breads were taken in sterile plastic bags to the lab, where they were kept under shade and humidity conditions. These conditions encourage and stimulate the growth of any fungal spores that filled on the breads from the bakery environment. They were checked daily for 6 days until fungal spoilage appeared. Via sterile insulated needle, the developed colonies on the spoilt bread were carefully transferred to Potato Dextrose Agar (PDA), and then incubated for 5 days at 28 °C to develop the fungal colonies.
Identification of Fungi
The developed fungal colonies were purified via transferring several times on PDA medium until pure colonies of fungal isolates were obtained. The macro- and micro-morphological fungal characteristics were examined to identify the fungal isolates according to identification keys (Raper and Fennell 1973; Domsch et al. 1981; Samson et al. 1981; Barnett and Hunter 1998). The fungal isolation frequency (Fr) of species was recorded using the following formula:
Amylase Activity Detection
Amylase activity of fungal isolates was detected via cultivation on three substrate media including starch yeast (SY) that composed of 5.0 g starch, 2.0 g yeast extract, 1.0 g KH2PO4, 0.5 g MgSO4.7H2O, and 1 L of water), wheat white flour (WF) (2%) used for white bread, and wheat black flour (BF) (2%) used for black bread. All fungal isolates were inoculated in flasks containing 100 mL of each medium, and then incubated under shaking conditions for 8 days at 28 °C. The fungal metabolized culture was filtered, and then centrifuged to obtain clear supernatants that were analyzed for amylase activity (Nouadri et al. 2010).
Amylase Activity Assay
According to Miller (1959), the approach of detecting reducing sugars produced by enzymatic hydrolysis of raw starch with dinitrosalicylic acid (DNS) was used to quantify the amylase activity. According to Singh et al. (2014), the quantity of enzyme producing 1 μmol of glucose (as reducing sugar) per minute under typical test circumstances was considered one unit of amylase hydrolyzing substrate (starch). On the sample tube, 1 mL of suspended starch in 1% of 0.1 M acetate buffer (pH 5.0) and 0.5 mL of crude enzyme were added. The tubes were placed in a boiling water bath for 5 min to halt the reaction, and then centrifuged for 5 min at a speed of 3000 revolutions per minute. Eight mL of distilled water was added to them after they had been steeped in boiling water for 5 min. At 550 nm using a spectrophotometer, the optical density (OD) values of the sample and control tubes were read against the blank tube. Absorbance values of the tubes containing control and sample were subtracted, and the OD values of the reducing sugars produced by the enzyme were recorded. Additionally, using the glucose standard graph previously published by the DNS at 550 nm, the enzyme activity was estimated as units/mL (Nouadri et al. 2010). The quantity of enzyme that liberated one μmol of reduced sugar per minute was identified as one unit of amylase activity.
Effect of Different Incubation Period on Amylase Activity
Three fungal isolates based on the greater ability to amylase production were selected for studying the effect of different conditions on the enzyme activity. Amylase activity of the selected fungal isolates was estimated at different incubation periods (3, 6, 9, 12, and 15 days) using the three substrates media SY, WF, and BF. Two fungal mycelial discs (6 mm diameter) were inoculated in flasks containing media, and then incubated at 28 °C. At the end of the incubation, amylase activity was estimated as described previously (Saleem and Mohsen 2014).
Effect of Different Temperatures and pH on Amylase Activity
Amylase activity of the three potent fungal isolates was estimated at different temperatures (10, 20, 30, 40, and 50 °C) using the three substrates media SY, WF, and BF. Two fungal mycelial discs (6 mm diameter) were inoculated in flasks containing media, and then incubated for 8 days. At the end of the incubation, amylase activity was estimated as described previously. Amylase activity of the three potent fungal isolates was estimated at determined at different pH ranging from 3 to 8. Two buffers were applied to adjusting the pH (20 mM of acetate buffer with pH 3-5), and 20 mM of sodium phosphate buffer with pH 5-8 as mentioned in case of temperature, but the inoculated isolates were incubated at 30 °C.
Statistical Evaluation
The tests were performed in 3 replicates to analyze the standard deviation (SD). The variance was planned by the SPSS ver. 22.0 software (version 14, IBM Corp., Armonk, NY, USA).
RESULTS AND DISCUSSION
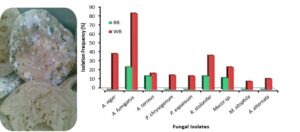
Two types of bread spoiled with fungi were used as a source of fungi-producing amylase. Some contents of the two unspoiled breads were estimated including ash, protein and carbohydrate. Ash, protein, and carbohydrate contents of white bread were 0.87, 4.43, and 72.54% compared to the content of black bread that include 1.98, 11.54, and 64.12%, of ash, protein, and carbohydrate, respectively (Table 1). These differences may affect the contamination and the ability of fungi to invade the bread. The quality of bread dough depends on these contents, as mentioned previously (Unachukwu and Nwakanma 2018). Nine fungal isolates including Aspergillus niger, Aspergillus fumigatus, Aspergillus terreus, Penicillium chrysogenum, Penicillium expansum, Rhizopus stolonifer, Mucor sp., Monilia sitophila, and Alternaria alternata were isolated from two contaminated breads, including white bread and black bread. In the current investigation, most of the fungal isolates were isolated from white bread while black bread was less contaminated. These results may explain the base flour content involved in bread making. Moulds are the reason for the most spoilages of bread and are considered costly and a serious problem for bakery products. Therefore, the application of preservatives is an important means to minimize spoilage and certify safety (Suhr and Nielsen 2004). According to findings of Olempska-Beer et al. (2006), the major bread spoilage molds are R. stolonifer, Mucor sp., Aspergillus sp., Penicillium sp., and M. sitophila. Production of various hydrolytic enzymes was associated to bread spoilage by these fungi that will facilitate them to use the nutrients of bread product. Ability of these fungi to propagate and then cause bread spoilage may be due to their capability to secrete the required enzymes to hydrolyze the constituents of bread. The capability of various moulds isolated from bread is commercially applied for enzyme production (Olempska-Beer et al. 2006). In recent study, Owolabi et al. (2023) isolated Aspergillus flavus from millet flour, this fungus characterized with high potential of amylase production. In the present study, A. fumigatus represent the most detected spoilage isolates with high frequency (85%) followed by A. niger, R. stolonifer, Mucor sp., A. terreus, P. chrysogenum, P. expansum, A. alternate, and M. sitophila in white bread. A. fumigatus, A. terreus, R. stolonifera, and Mucor sp. were detected while other fungi were not detected in black bread (Fig. 2). In another report, Rhizopus sp. represents the most fungal spoilage followed by Mucor sp., Aspergillus sp., and Penicillium sp. in bread (Nirmala 2016).
Table 1. Nutritional Properties of White and Black Breads
Fig. 1. White bread (WB) and black bread (BB); different samples including control sample not exposed to fungal contamination (1) and samples exposed to fungal contamination (2 through 4)
Fig. 2. Isolation frequency of fungal isolates from white bread (WB) and black bread (BB)
The isolated fungi Aspergillus niger, Aspergillus fumigatus, Aspergillus terreus, Penicillium chrysogenum, Penicillium expansum, Rhizopus stolonifer, Mucor sp., Monilia sitophila, and Alternata alternata were tested for amylase activity using starch yeast (SY), white flour (WF), and black flour (BF) (Fig. 3). The SY was a suitable substrate for amylase activity followed by WF and BF for all fungal isolates. A. niger was the most potent for amylase activity (7.67 U/mL), while M. sitophila showed weak amylase activity (2.69 U/mL) using SY. In contrast, A. fumigatus was the best producer of amylase (5.76 U/mL) compared to other fungi, such as A. niger (5.12 U/mL), A. terreus (4.41 U/mL), and P. expansum (3.56 U/mL), using WF. According to previous study, several species of Aspergillus and Rhizopus were applied as a source of α-amylase (Anto et al. 2006; Gupta et al. 2008). Different activities of hydrolytic enzymes were reported depending on the growth medium as mentioned using fungal isolates from bread including A. niger and R. stolonifer (Ahaota et al. 2010). A. niger and A. terreus were characterized as high amylase producers in a recent study (Ünal et al. 2022).
Fig. 3. Amylase activity of fungal isolates cultivated on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
The highest producers of fungal isolates for amylase were screened for enzyme productivity at different temperatures, from 10 to 50 °C (Figs. 4 through 6). It is clear that 30 °C was the optimum temperature for amylase activity by the three fungal isolates A. niger (Fig. 4), A. fumigatus (Fig. 5), and P. chrysogenum (Fig. 6), using the three substrates media SY, WF, and BF. The amylase activity increased with increasing temperature up to 30 °C, then decreased with temperature increment up to 50 °C; but for A. fumigatus, amylase activity at 40 °C (7.56, 7.34, and 5.65 U/mL using SY, WF, and BF, respectively) and 50 °C (7.05, 6.89, and 4.90 U/mL using SY, WF, and BF, respectively) was more than activity at 20 °C (6.11, 5.56, and 4.08 U/mL using SY, WF, and BF, respectively) (Fig. 5) unlike A. niger (Fig. 4). For P. chrysogenum, at 40 °C the amylase activity using SY and WF was higher that activity at 20 °C; however, a sharp decrease in amylase activity was observed at 50 °C (1.26, 1.56, and 1.22 U/mL using SY, WF, and BF, respectively) (Fig. 6). Enzyme activity was inhibited at high temperature, possibly due to the creation of metabolic heat in the growth media. Irfan et al. (2012) studied the optimization of conditions either environmental or nutritional for the α-amylase production by A. niger and Rhizopus oligosporus, where the optimum incubation period was 4 days and the optimum temperature was 30 °C (Irfan et al. 2012). A. fumigatus revealed the best amylase activity at 35 °C (Singh et al. 2014), possibly due to its thermophile properties (Abdelghany et al. 2019).
Fig. 4. Amylase activity of A. niger at different temperatures on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
Fig. 5. Amylase activity of A. fumigatus at different temperatures on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
Fig. 6. Amylase activity of P. chrysogenum at different temperatures on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
The optimum incubation period for amylase activity of A. niger, A. fumigatus, and P. chrysogenum was at the ninth day using SY substrate medium, where the activity was 7.65, 7.55, and 6.26 U/mL, respectively (Figs. 7 through 9). The maximum activity of amylase was obtained at 12 days using WF and BF using the three fungi A. niger (Fig. 7), A. fumigatus (Fig. 8), and P. chrysogenum (Fig. 9). At the 3rd day of the incubation period, negligible activities of amylase were observed compared to other incubation periods for the three fungal isolates. The less activity of amylase at 3rd day may be due to little growth of fungi, while less activity at 15th day may due to the decline or exhaustion of amylase substrate. According to Abdelwahab (2015), prolonged cultivation durations caused the culture of Aspergillus sp. to produce fewer enzymes due to lack of nutrients and the development of other by-products in the media.
Fig. 7. Amylase activity of A. niger at different incubation periods on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
Fig. 8. Amylase activity of A. fumigatus at different incubation periods on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
Fig. 9. Amylase activity of P. chrysogenum at different incubation periods on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
The effect of pH on the amylase activity of A. niger, A. fumigatus, and P. chrysogenum was investigated also using the three substrate media (Figs. 10 through 12). The optimum pH for amylase activity of the three fungi was 6. The acidic conditions pH ≤ 6 were favourable comparing to the alkaline pH for amylase activity of the three fungi using all substrates media. For instance, at pH 6, the amylase activity of A. niger was 7.64, 5.15, and 3.25 U/mL at pH 6, while it was 7.39, 5.06, and 3.16 U/mL at pH 5 compared to the activity 5.71, 4.80, and 2.55 U/mL at pH 7 using SY, WF, and BF (Fig. 10). Similar findings are obtained by Bellaouchi et al. (2021), who mentioned that the initial pHs 5 and 6 exhibited the high production level of amylase by A. niger. Other earlier reports indicated that the acidic pH was the optimal for amylases activity from A. niger (Mitidieri et al. 2006). A. oryzae exhibited maximum amylase activity at pH 6 (Balakrishnan et al. 2021). Almuhayawi et al. (2023) found that amylase activity of Rhizopus stolonifera increased with increasing pH value but the maximum activity was between 4 and 6.
Fig. 10. Amylase activity of A. niger at different pH on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
Fig. 11. Amylase activity of A. fumigatus at different pH on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
Fig. 12. Amylase activity of P. chrysogenum at different pH on three mediums: starch yeast (SY), white four (WF), and black flour (BF)
CONCLUSIONS
- The obtained findings indicated that white bread was a good reservoir and encouraged the growth and development of several spoiled fungi, compared to black bread.
- The high frequency of the occurred fungi was associated with Aspergillus fumigatus followed by A. niger, R. stolonifer, Mucor sp., A. terreus, P. chrysogenum, P. expansum, A. alternate, and M. sitophila in white bread
- A. niger represented the highest potential contrasting to Monilia sitophila for α-amylase activity using SY medium, while great α-amylase activity was associated A. fumigatus, compared to A. niger, A. terreus and P. expansum using WF
- As the optimum incubation period increased, α-amylase increased but depended on the substrate used (At ninth day using SY, while at twelfth day using WF and BF).
- However 30 °C was the optimum temperature for α-amylase activity, but high temperatures (40 and 50 °C) induced the α-amylase activity compared to low temperature (20 °C) using A. fumigatus
ACKNOWLEDGMENTS
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R217), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES CITED
Abd El-Ghany, T. M. (2006). “Metabolic regulation of fungal reproduction and their secondary metabolites,” Al-Azhar Bull. Sci. 17(1), 87-102. DOI: 10.21608/absb.2006.14728
Abdelghany, T. M., Alawlaqi, M. M., Shater, A. R. M., and Al Abboud, M. A. (2019). “Congo red biosorption with live and dead biomass of thermophilic Aspergillus fumigatus,” The Egyptian Journal of Experimental Biology (Botany) 15(1), 1-6. DOI: 10.5455/egyjebb.20181206084342
Abu, E. A., Ado, S.A., and James, D. B. (2005). “Raw starch degrading amylase production by mixed culture of Aspergillus niger and Saccharomyces cerevisiae grown on sorghum pomace,” African Journal of Biotechnology 4(8), 785-790.
Ahaota, N. N., Ogueke, C. C., and Ahaota, I. (2010). “Hydrolytic enzymes of moulds involved in bread spoilage,” New York Science Journal 3(11), 27-36.
Ait Kaki El-Hadef El-Okki A., Gagaoua, M., Bourekoua, H., Hafid, K., Bennamoun, L., Djekrif-Dakhmouche, S., El-Hadef El-Okki, M., and Meraihi, Z. (2017). “Improving bread quality with the application of a newly purified thermostable α-amylase from Rhizopus oryzae FSIS4,” Foods 6(1), article 1. DOI: 10.3390/foods6010001
Al Abboud, M. A., Al-Rajhi, A. M. H., Shater, A.-R. M., Alawlaqi, M. M., Mashraqi, A., Selim, S., Al Jaouni, S. K., and Abdelghany, T. M. (2022). “Halostability and thermostability of chitinase produced by fungi isolated from salt marsh soil in subtropical region of Saudi Arabia,” BioResources 17(3), 4763-4780. DOI: 10.15376/biores.17.3.4763-4780
Ali, I., Sultan, S., Tahir Mahmood, R., Tariq, M., Shamim, Z., Mushtaq, A. and Asiri, M. (2023). “Production and characterization of α-amylase from indigenously Isola-ted Streptomyces sp.,” BioResources 18(1), 6-18. DOI: 10.15376/biores.18.1.6-18
Almuhayawi, M. S, Hassan, E. A., Alkuwaity, K. K., Abujamel, T. S., Mokhtar, J. A., Niyazi, H. A., Almasaudi, S. B., Alamri, T. A., Najjar, A. A. and Zabermawi, N. M. (2023). “Enzymatic-based hydrolysis of digested potato peel wastes by amylase producing fungi to improve biogas generation,” Catalysts 13(5), article 913. DOI:10.3390/catal13050913
Anto, H., Trivedi, U. B., and Patel, K. C. (2006). “Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate,” Bioresource Technology 97(10), 1161-1166. DOI: 10.1016/j.biortech.2005.05.007
Bakri, M. M., Al-Rajhi, A. M. H., Abada, E., Salem, O. M. A., Shater, A-R., Mahmoud, M. S., and Abdel Ghany, T. M. (2022). “Mycostimulator of chitinolytic activity: Thermodynamic studies and its activity against human and food-borne microbial pathogens,” BioResources 17(3), 4378-4394. DOI: 10.15376/biores.17.3.4378-4394
Balakrishnan, M., Jeevarathinam, G., Kumar, S. K., Muniraj, I., and Uthandi, S. (2021). “Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes,” BMC Biotechnology 21(1), 33. DOI:10.1186/s12896-021-00686-7.
Barnett, H. L., and Hunter, B. B. (1998). Illustrated Genera of Imperfect Fungi, 4th edition, American Phytopathological Society Press, Saint Paul, MN, USA.
Bellaouchi, R., Abouloifa, H., Rokni, Y., Hasnaoui, A., Ghabbour, N., Hakkou, A., Bechchari, A. and Asehraou, A. (2021). “Characterization and optimization of extracellular enzymes production by Aspergillus niger strains isolated from date by-products,” J Genet Eng Biotechnol. 19(1), 50. DOI: 10.1186/s43141-021-00145-y.
Benabda, O., M’hir, S., Kasmi, M., Mnif, W., and Hamdi, M. (2019). “Optimization of protease and amylase production by Rhizopus oryzae cultivated on bread waste using solid-state fermentation,” Journal of Chemistry, 2019, 1-9. DOI: 10.1155/2019/3738181
Cerda, A., El-Bakry, M., Gea, T., and Sánchez, A. (2016). “Long term enhanced solid-state fermentation: Inoculation strategies for amylase production from soy and bread wastes by Thermomyces sp. in a sequential batch operation,” Journal of Environmental Chemical Engineering, 4(2), 2394-2401. DOI: 10.1016/j.jece.2016.04.022
Cheesbrough, N. (2008). “Mould growth on cake,” Biscuit Maker and Plant Baker 14, 961-964.
Deshmukh, K. D., Taur, S. A., Cherekar, M. N., Kothari, M. N., and Pathak, A. P. (2011). “Process optimization, purification and characterization of glucoamylase from different Sorghum varieties,” Journal of Chemical and Pharmaceutical Research 3(2), 732-737.
Domsch, K. H., Gams, W., and Anderson, T. (1980). Compendium of Soil Fungi, Academic Press (London) Ltd., London, UK.
Frisvad, J. C., Møller, L. L. H., Larsen, T. O., Kumar, R., and José Arnau, J. (2018). “Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei,” Applied Microbiology and Biotechnology 102, 9481-9515. DOI: 10.1007/s00253-018-9354-1
Goesaert, H., Brijs, K., Veraverbeke, W. S., Courtin, C. M., Gebruers, K., and Delcour, J. A. (2005). “Wheat flour constituents: how they impact bread quality, and how to impact their functionality,” Trends in Food Science and Technology 16(1-3), 12-30. DOI: 10.1016/j.tifs.2004.02.011
Goto, C. E., Barbosa, E. P., Kistner, L. C., Moreira, F. G., Lenartovicz, V., and Peralta, R. M. (1998). “Production of amylase by Aspergillus fumigatus utilizing alpha-methyl-D-glycoside, a synthetic analogue of maltose, as substrate,” FEMS Microbiology Letters 167(2), 139-143. DOI: 10.1111/j.1574-6968.1998.tb13219.x
Haque, M. A., Kachrimanidou, V., Koutinas, A., and Lin, C. S. K. (2016). “Valorization of bakery waste for biocolorant and enzyme production by Monascus purpureus,” Journal of Biotechnology 231, 55-64. DOI: 10.1016/j.jbiotec.2016.05.003
Irfan, M., Nadeem, M., and Syed, Q. (2012). “Media optimization for amylase production in solid state fermentation of wheat bran by fungal strains,” Journal of Cell and Molecular Biology 10(1), 55-64.
Kamaraj, M., and Subramaniam, D. (2020). “Optimization of experimental variables for the production of α- amylase by Aspergillus oryzae using rice bran,” International Journal of Advanced Research in Engineering and Technology 11(6), 113-126. DOI: 10.34218/IJARET.11.6.2020.011
Melikoglu, M., Lin, C. S. K., and Webb, C. (2015). “Solid state fermentation of waste bread pieces by Aspergillus awamori: Analysing the effects of airflow rate on enzyme production in packed bed bioreactors,” Food and Bioproducts Processing 95, 63-75. DOI: 10.1016/j.fbp.2015.03.011
Melini, V., and Melini, F. (2018). “Strategies to extend bread and GF bread shelf-life: from sourdough to antimicrobial active packaging and nanotechnology,” Fermentation 4(1), article 9. DOI: 10.3390/fermentation4010009
Miller, G. L. (1959). “Use of dinitrosalicylic acid reagent for determination of reducing sugar,” Journal of Analytical Chemistry 31, 426-428. DOI: 10.1021/ac60147a030
Mitidieri, S., Martinelli, A. H. S., Schrank, A., and Vainstein, M. H. (2006). “Enzymatic detergent formulation containing amylase from Aspergillus niger: A comparative study with commercial detergent formulations,” Bioresour. Techno. 97(10), 1217-1224. DOI: 10.1016/j.biortech.2005.05.022
Movahedpour, A., Asadi, M., Khatami, S. H., Taheri‐Anganeh, M., Adelipour, M., Shabaninejad, Z., Ahmadi, N., and Mousavi, P. (2022). “A brief overview on the application and sources of α‐amylase and expression hosts properties in order to production of recombinant α‐amylase,” Biotechnology and Applied Biochemistry 69(2), 650-659. DOI: 10.1002/bab.2140
Nirmala, R., Pathmanathan, S., and Selvaratnam, S. (2016). “Study on fungi associated with spoilage of bread,” International Journal of Advanced Research in Biological Sciences 3(4), 165-167.
Nouadri, T., Meraihi, Z., Shahrazed, D. D., and Leila, B. (2010). “Purification and characterization of the α-amylase isolated from Penicillium camemberti PL21,” African Journal of Biochemistry Research 4(6), 155-162.
Olempska-Beer, Z. S., Merker, R. I., Ditto, M. D., and DiNovi, M. J. (2006). “Food-processing enzymes from recombinant microorganisms – A review,” Regulatory Toxicology and Pharmacology 45(2), 144-158. DOI: 10.1016/j.yrtph.2006.05.001
Owolabi, O., E., Olaniyi, O. O., and Akinyosoye, F. A. (2023). “Catalytic properties of purified alpha amylase from Aspergillus flavus cultivated on low-cost agricultural substrate,” Revista Facultad Nacional de Agronomía Medellín 76(1), 10213-10225. DOI: 10.15446/rfnam.v76n1.100842
Premalatha, A., Vijayalakshmi, K., Shanmugavel, M., and Rajakumar, G. S. (2023). “Optimization of culture conditions for enhanced production of extracellular α-amylase using solid-state and submerged fermentation from Aspergillus tamarii MTCC5152,” Biotechnol. Appl. Biochem. 70(2), 835-845. DOI: 10.1002/bab.2403.
Rajagopalan, G., and Krishnan, C. (2008). “Alpha-amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate,” Bioresour Technolology 99(8), 3044-3050. DOI: 10.1016/j.biortech.2007.06.001
Raper, K. B., and Fennell, D. I. (1973). The Genus Aspergillus, Krieger Publishing Company, Huntington, New York, NY, USA.
Saleem, A., and Mohsen, K. H. E. (2014). “Production of amylase by fungi isolated from legume seeds collected in Almadinah Almunawwarah, Saudi Arabia,” Journal of Taibah University for Science 8(2), 90-97. DOI: 10.1016/j.jtusci.2013.09.002
Samson, R. A., Hoekstra, E. S., Frisvad, J. C., and Filtenborg, O. (1981). Introduction to Food-Borne Fungi, Centraalbureau voor Schimmelcultures, Institute of the Royal Netherlands Academy of Arts and Sciences, Netherlands.
Sigüenza-Andrés, T., Pando, V., Gómez, M., and Rodríguez-Nogales, J. M. (2022). “Optimization of a simultaneous enzymatic hydrolysis to obtain a high-glucose slurry from bread waste,” Foods 11(12), article 1793. DOI: 10.3390/foods11121793
Singh, S., Singh, S., Bali, V., Sharma, L., and Mangla, J. (2014). “Production of fungal amylases using cheap, readily available agriresidues, for potential application in textile industry,” BioMed Research International 2014, article ID 215748. DOI: 10.1155/2014/215748
Singh, S., Mangla, J., and Singh, S. (2021). “Evaluation of Aspergillus fumigatus NTCC1222 as a source of enzymes for detergent industry,” Resources, Environment and Sustainability 5, article ID 100030. DOI: 10.1016/j.resenv.2021.100030
Suhr, K. I., and Nielsen, P. V. (2004). “Effect of weak acid preservatives on growth of bakery product spoilage fungi at different water activities and pH values,” International Journal of Food Microbiology 95, 67-78. DOI: 10.1016/j.ijfoodmicro.2004.02.004
Susca, A., Proctor, R. H., Butchko, R. A. E., Haidukowski, M., Stea, G., Logrieco, A., and Moretti, A. (2014). “Variation in the fumonisin biosynthetic gene cluster in fumonisin-producing and nonproducing black Aspergilli,” Fungal Genet Biol 73, 39-52. DOI: 10.1016/j.fgb.2014.09.009
Unachukwu, M. N., and Nwakanma, C. (2018). “The fungi associated with the spoilage of bread in Enugu state,” International Journal of Current Microbiology and Applied Sciences 4(1), 989-995.
Ünal, A., Subaşı, A. S., Malkoc, S., Ocak, İ., Korcan, S. E., Yetilmezer, E., Yurdugül, S., Yaman, H., Şanal, T., and Keçeli, A. (2022). “Potential of fungal thermostable alpha amylase enzyme isolated from hot springs of Central Anatolia (Turkey) in wheat bread quality,” Food Bioscience 45, article ID 101492. DOI: 10.1016/j.fbio.2021.1014
Article submitted: June 12, 2023; Peer review completed: July 8, 2023; Revised version received and accepted: July 10, 2023; Published: July 17, 2023.
DOI: 10.15376/biores.18.3.5908-5923
