Abstract
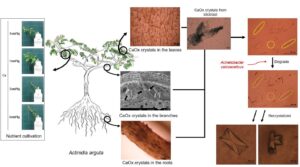
The presence, distribution, and morphology of calcium oxalate (CaC2O4, CaOx) crystals were observed in the stunted bodies of Actinidia arguta (A. arguta) vines, and their ability of microorganisms to degrade CaOx crystals was analyzed. Leaves, branches, and roots of stunted A. arguta vines were collected. In the roots, CaOx crystal bundles were distributed in the form of dotted lines. In the branches, CaOx crystal bundles were observed blocking and accumulating in the vessels. In the leaves, CaOx crystal bundles were observed in a net-like entanglement. Therefore, CaOx crystals present in the roots, branches, and leaves hinder the growth. A hydroponic cultivation with different calcium (Ca) concentrations showed that the growth of A. arguta was best at a Ca concentration of 1 cmol+/kg; at a Ca concentration of 2 cmol+/kg, the edges of the leaves began to dry out after 7 days; and at a Ca concentration of 4 cmol+/kg, the entire leaves died within 14 days. From this study, it was concluded that CaOx crystals hindered the growth of A. arguta, and the Acinetobacter calcoaceticus (A. calcoaceticus) strain was effective in degrading CaOx crystals. Therefore, the Ca concentration in the soil of the cultivation site should be managed at less than 2 cmol+/kg.
Download PDF
Full Article
Calcium Oxalate Crystals and the Optimal Growing Conditions for Actinidia arguta
Ree Keun Kong,a Jun Ho Goh,a Kyung Rok Won,a Hyeon Woo Shin,a Byung Il Jang,b Jong Wan Kim,c Chun Sik Kim,d and Hee Seop Byeon a,e,*
The presence, distribution, and morphology of calcium oxalate (CaC2O4, CaOx) crystals were observed in the stunted bodies of Actinidia arguta (A. arguta) vines, and their ability of microorganisms to degrade CaOx crystals was analyzed. Leaves, branches, and roots of stunted A. arguta vines were collected. In the roots, CaOx crystal bundles were distributed in the form of dotted lines. In the branches, CaOx crystal bundles were observed blocking and accumulating in the vessels. In the leaves, CaOx crystal bundles were observed in a net-like entanglement. Therefore, CaOx crystals present in the roots, branches, and leaves hinder the growth. A hydroponic cultivation with different calcium (Ca) concentrations showed that the growth of A. arguta was best at a Ca concentration of 1 cmol+/kg; at a Ca concentration of 2 cmol+/kg, the edges of the leaves began to dry out after 7 days; and at a Ca concentration of 4 cmol+/kg, the entire leaves died within 14 days. From this study, it was concluded that CaOx crystals hindered the growth of A. arguta, and the Acinetobacter calcoaceticus (A. calcoaceticus) strain was effective in degrading CaOx crystals. Therefore, the Ca concentration in the soil of the cultivation site should be managed at less than 2 cmol+/kg.
DOI: 10.15376/biores.19.1.1602-1616
Keywords: Actinidia arguta; Calcium oxalate crystals; Acinetobacter calcoaceticus
Contact information: a: College of Agriculture & Life Science, Gyeongsang National University, Jinju, 52828, Korea; b: ByProm. Co., Ltd., Geochang-gun, Gyeongsangnam–do, Republic of Korea, 50110; c: Agricultural corporation Okyeong Co., Ltd., Jinju-si, Gyeongsangnam–do, Republic of Korea, 52711; d: Okryong-myeon, Gwangyang-si, Jeollanam-do, Republic of Korea, 57702; e: Institute of Agriculture and Life Science,Gyeongsang National University, Jinju, 52828, Korea;
* Corresponding author: hsbyeon@gnu.ac.kr
GRAPHICAL ABSTRACT
INTRODUCTION
As the average life expectancy of humans increases and living standards gradually improve, interest in healthy living is growing. Recently, the antioxidant activity and whitening effect of pollen derived from Actinidia arguta (A. arguta) have been recognized, and it is used as a health food and cosmetic material; as a consequence, the value of A. arguta as a honey plant has increased and the demand is increasing (Hong et al. 2014; Park 2016). The A. arguta, which belongs to the family Actinidiaceae, is a deciduous broad-leaved vine that is resistant to cold and pests and can be cultivated in all regions of South Korea. It has the advantage of ripening faster than Actinidia deliciosa (A. deliciosa, kiwi), so both the demand for A. arguta and its cultivation area are increasing (Latocha and Olszewska-Kaczyńska 2003; Kim et al. 2014; Drzewiecki et al. 2016; Kim et al. 2017; Kim et al. 2018). A. deliciosa, which has been recognized as a health food, has started to die in South Korea since 2009, and as of 2015, some areas such as Haenam-gun have lost 90% of the 500 hectares of kiwi cultivation, reducing the area to 500 hectares.
Sacheon City in South Korea recognized the seriousness of the problem and conducted a survey with farmers growing A. deliciosa. It revealed that 13.8% of the farms were experiencing withering of the plants, and 98.6% of the farms had a Ca concentration of 2 cmol+/kg or more, indicating high Ca concentration in the soil. Farmers growing A. arguta were also concerned with the problem of dieback of A. deliciosa, and thought that the Ca concentration in the soil of the planting site would affect the growth of A. arguta (Jang 2014). Actinidiaceae vines absorb large amounts of Ca, resulting in the production and accumulation of CaOx crystals in the plant body (Horner et al. 2007), so it was predicted that CaOx crystals generated by excessive Ca fertilization would be present in various places such as vessels in the xylem, hindering growth due to the obstruction of nutrient and water movement through the vessels. Reducing the Ca content in the soil optimizes the growth of the plantation soil.
When the CaOx crystals present in all parts of the A. arguta are decomposed, the space in the vessels of xylem or other vessels to move elements could be secured to move nutrients and water. Microorganisms utilize several forms of phosphorus compounds for their growth, and some microorganisms are known to have the ability to dissolve insoluble phosphate, providing a source of soluble phosphorus for plants in soil and water systems, as well as soluble phosphorus essential for the growth of other microorganisms (Nautiyal et al. 2000). Microorganisms capable of degrading poorly soluble phosphate are expected to degrade CaOx crystals, a poorly soluble salt produced by the combination of Ca and oxalate, which are expected to be present in A. arguta vines.
This study observed the presence, distribution, and morphology of CaOx crystals in the roots, branches, and leaves of the poorly grown A. arguta vine, analyzed their content, and cultivated them with different Ca contents to observe the growth situation and verify the ability of microorganisms to degrade CaOx crystals in the branches of the A. arguta vine.
EXPERIMENTAL
Materials
To analyze the anatomical form and location of CaOx crystals in the body of A. arguta vines, roots, branches, and leaves were collected from poorly grown vines harvested from a farm (146-1 Unam-gil, Okryong-myeon, Gwangyang-si, Jeollanam-do). The growth status of A. arguta was determined by the following three criteria. Firstly, more than 20% of the total fruit produced per A. arguta vine with an age of 20 to 28 years should weigh 8 grams or less. Secondly, the total yield should be 50 kg or less. Thirdly, the diameter at breast height should be 15 cm or less. Five A. arguta vines that met the above three criteria were selected and used in this experiment.
Distribution Location and Morphology of CaOx Crystals in the Body
Roots, branches, and leaves of the A. arguta vine were collected to analyze the location, distribution form, and content of CaOx. The experiment was conducted as follows:
CaOx crystals in the roots
The fine roots that grew in the same direction as the collected branches extended were collected within 20 cm of the ground surface, washed and completely immersed in sterilized distilled water, and photographed by the camera (PowerShot A650IS, Canon Inc., China) under a light microscope (Olympus CX31, Japan) at a total magnification was x600, with the camera magnification being x6, the eyepiece magnification being x10, and the objective magnification being x10, from the root tip to 10 mm. Each photograph was stitched together to distinguish and observe the distribution of CaOx crystals.
CaOx crystals in the branches
The branch samples with 2 years of age within 1 cm in diameter were collected. The samples were dried at 40 °C for 48 h and at 60 °C for 48 h, dehumidified in a desiccator with silica gel, a desiccant, and then gold-coated on the cross-sectional surface and observed with a field-emission scanning electron microscope (FE-SEM, JEOL (JSM-7610F), Japan).
CaOx crystals in the leaves
Leaf samples were collected from the fifth leaf with a clear petiole from branches grown on nutrient solution with the composition presented in Table 1. Because leaves are difficult to observe by light microscope due to tissue components and chloroplasts, a microscopic analysis method was developed. Young leaves were decolorized for 6 h after immersion in a mixture of 5.5% sodium hypochlorite and 0.3% sodium hydroxide (decolorizing solution); the decolorizing solution was removed using sterile distilled water. The entire leaf was photographed with adaxial side using a light microscope at a total magnification was x600, and each photo was arranged like a mosaic to complete the entire leaf, and the overall distribution of CaOx crystals in the leaf was checked.
Identification of CaOx Crystals
Samples prepared by collecting 1 cm of branches and cut into several pieces in the direction of the fibers were mixed well with 5 mL of sterile distilled water in a 15 mL cornical tube and incubated for 30 min. Next, 100 μL of CaOx crystals precipitated on the floor were taken with an autopipette (LABMATE LMP200, HTL, Poland), placed on silicon paper and dried at 24 ℃. The samples were analyzed and identified by X-ray fluorescence spectrometer (XRF, Bruker AXS (S8 TIGER), USA) and multipurpose X-ray diffractometer (XRD, Bruker (D8 Advance A25 Plus), USA).
Evaluation of the Growth Based on Ca Concentration
Since there are no nutrient formulas for A. arguta, a new solution was prepared based on the literature. The nutrients uptake amount was calculated by referring to the nutrient composition table analyzed by G. S. Smith et al. (1987) for the young leaves grown for 20 weeks of A. arguta. The previously calculated uptake amount was converted by the medium composition conversion method suggested by Yamazaki (1982) to prepare a nutrient composition table. For the growth and development of A. arguta at different Ca concentrations, the plants were hydroponically cultivated in nutrient solution medium with different Ca concentrations of 0 to 4 cmol+/kg, and the growth was observed for up to14 days. As the samples grown at the nutrient solution with 4 cmol+/kg of Ca concentration died within 14 days, the observation period was set.
Table 1. Composition of the Nutrient Solution for Pure Hydroponics
Validation of CaOX Crystals Resolution by Microbial Strains Treatment
The ability to dissolve poorly soluble compounds, such as calcium oxalate, has been associated with the following microorganism species: Acinetobacter sp., Bacillus sp., Pseudomonas sp., Streptomyces sp., Aspergillus sp., Penicillium sp. (Lee et al. 2012). Among them, A. calcoaceticus was used. The strain for degrading CaOx crystals, which is a poorly soluble salt, was inspired by microorganisms that degrade poorly soluble phosphate, and the strain A. calcoaceticus BYS-2, patent number KACC 91429P, was used as a strain with the ability to solubilize phosphate, a poorly soluble salt (Jang, Patent No. 10-0908412, 2009). To confirm the resolution of the CaOx crystals, 15 mL of distilled water was added to the CaOx crystals extracted from the branches of A. arguta vine, followed by 1 mL of the culture of the A. calcoaceticus strain, and the appearance of the crystals immediately after treatment and after 3 days was observed and photographed under a light microscope at a total magnification was x600 to evaluate the resolution of the A. calcoaceticus strains over time.
RESULTS AND DISCUSSION
Distribution of CaOx in the Roots
Figures 1 and 2 show the distribution of CaOx crystals in the roots. As is identified in the Fig. 1, in the thimble-shaped tip of the roots of the A. arguta, countless CaOx crystals were distributed as dotted lines. The bundles of CaOx crystals were highly variable in shape, with some being very large, some being small, some being parallel to the root direction, some showing deviation in direction, and some being individual crystals without bundles of CaOx crystals. When the roots were examined closely under a microscope, they appeared as black dotted lines on the plant cells in the vessel area, similar to the appearance of bacterial wilt (Fig. 1-A). Bacterial wilt caused by Pseudomonas solanacearum formerly called Ralstonia solanacearum, causes the bacteria to become densely packed within the cells from root to stem, creating sticky liquid that blocks the movement of nutrients and water. Plants appear to be normal in the morning when the air humidity is high, and they turn blue and eventually die when the humidity decreases in the afternoon (Cho et al. 2000).
Fig. 1. Distribution of calcium oxalate (CaOx) crystals inside the roots of A. arguta. The first image is focused on the inside of the root, and the second image is focused on the surface of the root, showing that the CaOx crystals have not been ejected outside the root. To compare the two photos, the same area is labeled with alphabetized and lowercase letters for comparison. (I) The left side shows well-aligned bundles of CaOx crystals distributed inside the tissue (A), irregularly distributed crystals in the tissue (B), and irregular distribution of isolated crystals in the tissue (C). (II) CaOx crystals in the area of (a), (b), (c) are faint or nearly invisible, indicating that the CaOx crystals are inside the root
Fig. 2. Roots infected with bacterial wilt by Pseudomonas solanacearum (A), roots of A. arguta with many calcium oxalate (CaOx) crystals (B). When observed under a microscope (A) cells are densely packed along the vessels of the root as shown in the black dotted line, (B) CaOx is densely filled as the black dotted line along the duct of the root.
Although CaOx crystals (Fig. 1-B) are not bacterial infections and thus do not produce the phaseolotoxin, coronatine, or sticky liquid produced by bacteria, their physical bulk may be a limiting factor to some extent in the movement of water (Shin et al. 2004; Ko et al. 2002). The findings of Li et al (2014) suggested that high Ca in rhizospheric solution may cause Ca toxicity to reduce plant growth rates and forming tiny yellowish or gold spots in the cell wall of fruits. Therefore, the roots exposed to the soil with high Ca concentration may produce excessive CaOx crystals, and the crystals may hinder the smooth movement of nutrients. So they may be associated with the symptoms of drying and wilting of the leaves at the edges, away from the roots, resulting in stunted growth or death of A. arguta vines.
Distribution of CaOx Crystals in the Branches
CaOx crystals in the branches were present in various places, including the vessels in xylem, and they were particularly abundant in xylem (Fig. 3). In the branches, the crystals in the xylem were quite densely packed, and accumulation in vessels was also observed. The crystals found around the vessels were partly singular, one to several crystals, or clusters of several crystals. Coté (2009) reported that CaOx crystals have a wide variety of shapes, including rounded, plate-like, needle-like, and spiky like a chestnut bur. This study observed needle-like shapes with both ends pointed and slender, as well as deformed shapes that were destroyed or damaged during sample preparation. As shown in Fig. 3, CaOx crystals were observed (a) blocking the vessels and (b) embedded in the vessels and blocking it.
Fig. 3. Formation of calcium oxalate (CaOx) crystals on the cross-sectional surface in A. arguta stem
According to Paiva (2019), CaOx crystals as Ca reserve is not functional in most situations because plants die by Ca starvation even with a plentiful presence of such crystals. Also, plants do not have an excretory system providing a way to eliminate excess Ca. This means that these crystals are created and then they would accumulate in the vessels. Therefore, it is thought that these crystals hinder the movement of nutrients and water through the vessels and impair metabolic activity, resulting in stunted growth of the entire body.
Distribution of CaOx Crystals in the Leaves
It is difficult to observe the distribution of CaOx crystals in detail when observing leaves under a light microscope due to tissue components and chloroplasts. A leaf decolorization method was developed, and the distribution of CaOx crystals could be clearly observed. The entire leaf before and after decolorization was photographed while observing it with an optical microscope, and the photos were put together like a mosaic to complete the entire leaf.
Fig. 4. Microscopic observation of A. arguta leaves after decolorization; A : The leaves before decolorization, B : The leaves after decolorization; A and B 1-3 : The leaves that are magnified at the same place
As shown in Fig. 4-B, there were countless bundles of CaOx crystals in the A. arguta leaf, and the bundles were composed of countless crystals. In B-1, the net-like entanglement is identified inside the leaf. In B-2 and B-3, magnified bundles of CaOx crystals can be seen under magnification, and individual crystal structures were clearly apparent. The CaOx crystal bundles distributed in the leaf veins are predicted to have an adverse effect on crop growth by hindering the smooth movement of nutrients supplied by the roots and the photosynthesis of the leaves.
Identification of CaOx Crystals
This study observed the distribution form of CaOx crystals in high density at each part of an A. arguta vine, and investigated their impact. The CaOx crystals in the A. arguta varied in size from one to several hundred μm, were mainly needle-shaped with pointed ends, and were frequently observed inside the roots, leaves, and stems in the form of bundles accumulated in the idioblasts. When the idioblasts are damaged, the CaOx crystals existing inside are ejected to outside (Fig. 5). The CaOx crystals isolated from the A. arguta were analyzed by XRF, which showed the highest content of Ca within the crystals, and XRD, which confirmed that the CaOx crystals from the idioblast were CaOx crystals by matching 100% with calcium oxalate hydrate (Fig. 6). The results of the distribution form of CaOx crystals by XRD in this study looked similar to the pattern of CaOx crystals analyzed by Gao et al . (2014), Shang et al. (2013), and Murray et al. (2016).
Fig. 5. Calcium oxalate (CaOx) crystals primarily observed in the body of Actinidia arguta A: CaOx Crystals, B: CaOx crystals erupting from idioblast, C: CaOx crystals in root, D: CaOx crystals in leaf, E: CaOx crystals in stem
Growth of A. arguta vine Based on Ca Concentration
To derive the appropriate Ca concentration for the growth of the A. arguta, a nutrient solution medium for A. arguta was prepared and employed during growth (Table 1). The results showed that at Ca concentrations of 4 cmol+/kg and above, the whole leaves began to dry out after 7 days and died within 14 days. At Ca concentrations above 2 cmol+/kg, leaf margins began to dry out after 7 days but did not die until 14 days. The best growth condition was observed at 1 cmol+/kg (Fig. 7).
Fig. 6. Analysis of calcium oxalate (CaOx) crystals extracted from A. arguta by multipurpose X-ray diffractometer and X-ray fluorescence spectrometer
Jang (2014) also calculated the mortality rate of A. deliciosa in 139 orchards (area 502,280 m2) of 115 farms in Sacheon City, Gyeongnam, South Korea, as the area of the orchards in the cultivated area compared to the area of the orchards in the cultivated area, and found that 69,442 m2 were subject to die-off in 56 orchards of 49 farms, resulting in a mortality rate of 13.8%. The soil analysis of the mortalized orchards showed that 89.2% of the farms had a Ca concentration of more than 4 cmol+/kg, and 98.6% of the farms had a Ca concentration of more than 2 cmol+/kg, indicating a high Ca concentration in the soil. Li et al. (2014) showed that Ca is very rich in the soil of Karst regions, three times more than in acid soils and, particularly in Europe, Asia, North and Central America and Caribbean, biodiversity and agricultural production have been threatened by the high Ca content in soil. Wang et al. (2023) also reported that the high levels of Ca2+ are harmful to plant cells. Therefore, it is predicted that damage caused by excessive Ca may occur in the long term in areas with a Ca concentration of more than 2 cmol+/kg.
Fig. 7. Growth status of Actinidia arguta by the different calcium concentrations
Quantity of CaOx Crystal Bundles per Ca Treatment Concentration
The number of CaOx crystal bundles was counted in the leaves of A. arguta vines grown with different Ca concentrations.
Fig. 8. The quantity of calcium oxalate (CaOx) bundles of decolorized leaves in each calcium treatment level
After decolorizing the A. arguta leaves, CaOx crystal bundles distributed in an area of the camera with 1,500 μm × 1,130 μm (width × length) were counted in three replicates, and the average value was obtained. As a result, the number of CaOx crystal bundles was 37.7 in the non-Ca-treated group. The number of CaOx crystal bundles produced in the leaves increased with increasing Ca concentration, with 37.7 CaOx crystal bundles at 0 cmol+/kg, 100.3 CaOx crystal bundles at 1 cmol+/kg, 138.0 CaOx crystal bundles at 2 cmol+/kg, and 147.7 CaOx crystal bundles at 4 cmol+/kg (Fig. 8). The distribution of CaOx crystal bundles generated in the leaves increased as the concentration of Ca increased, as shown in Fig. 9.
Fig. 9. The distribution of calcium oxalate (CaOx) bundles of decolorized leaves in each calcium treatment level; * Calcium concentration in nutrient solution for hydroponic cultivation
Decomposition of CaOx Crystals
The results from the above experiments showed that CaOx crystals were produced and accumulated in the roots, branches, and leaves of the A. arguta vine. The CaOx crystals extracted from the A. arguta vines after treatment with the A. calcoaceticus culture were acicular in shape with pointed ends, and they were cut into several fragments after 3 days (Fig. 10). Based on the arrangement of the CaOx crystals in the sample before and after treatment, it was determined that the A. calcoaceticus culture treatment caused the CaOx crystals to degrade. The decomposition continued, and when the distilled water was evaporated, a new form of crystal structure was formed (Fig. 11). Paula et al. (2023) found that the CaOx crystallization is influenced by phytate and other inositol-phosphates and vary in shape. In addition, Ghate et al. (2021) reported that A. calcoaceticus was effective in oxalate degradation. These results suggest that treatment with A. calcoaceticus cultures can dissolve CaOx crystals leached from plant debris in soil, and that plant uptake of fungal metabolites can inhibit crystal formation within the plant body.
Fig. 10. Decomposition of calcium oxalate (CaOx) crystals over time after treatment with Acinetobacter calcoaceticus strains. In the photograph after 3 days, the solid lines indicate no change in the CaOx crystals, and the dotted lines indicate that the CaOx crystals were degraded.
Fig. 11. Recrystallized form of calcium oxalate (CaOx) after evaporation
CONCLUSIONS
- Numerous calcium oxalate (CaOx) crystals were present in a variety of forms in the roots, branches, and leaves of the Actinidia arguta vine. In the roots, CaOx crystal bundles were distributed like dotted lines, and excessive production is thought to limit the movement of nutrients by increasing their physical volume. In the branches, CaOx crystals were observed in various places such as the vessels in the xylem, and they accumulated in vessels. In the leaves, countless CaOx crystal bundles were observed in a net-like tangle, and individual crystal structures were clearly observed.
- When the nutrient solution medium was prepared and cultivated, the best growth was observed at a Ca concentration of 1 cmol+/kg, while the edges of the leaves began to dry out after 7 days at a Ca concentration of 2 cmol+/kg or more, and the leaves died within 14 days at a Ca concentration of 4 cmol+/kg or more. Therefore, it is recommended that the soil of the cultivation site should be managed at a Ca concentration of less than 2 cmol+/kg to maintain an adequate amount of CaOx crystals that accumulate in the body and not interfere with growth.
- Treatment with A. calcoaceticus cultures was found to have an effect on degradation of CaOx crystals. This result suggested that A. calcoaceticus culture treatment may degrade CaOx crystals.
ACKNOWLEDGMENTS
This study was carried out with the support of ‘R&D Program for Forest Science Technology (Project No. 2021305A00-2121-AD02)’ provided by Korea Forest Service (Korea Forestry Promotion Institute).
REFERENCES CITED
Coté, G. G. (2009). “Diversity and distribution of idioblasts producing calcium oxalate crystals in Dieffenbachia seguine (Araceae),” American Journal of Botany 96(7), 1245-1254. DOI: 10.3732/ajb.0800276
Gao, J., Xue, J. F., Xu, M., Gui, B. S., Wang, F. X., and Ouyang, J. M. (2014). “Nanouric acid or nanocalcium phosphate as central nidus to induce calcium oxalate stone formation; A high-resolution transmission electron microscopy study on urinary nanocrystallites,” International Journal of Nanomedicine 9, 4399-4409. DOI: 10.2147/IJN.S66000
Ghate, S. D., Shastry, R. P., and Rekha, P. D. (2021). “Rapid detection of oxalotrophic endophytic bacteria by colony PCR from Colocasia esculenta and Remusatia vivipara,” Ecological Genetics and Genomics 21, article 100102. DOI: 10.1016/j.egg.2021.100102
Hong, I. P., Woo, S. O., Han, S. M., Yeo, J. H., Cho, M. L., Ju, W. T., Sim, H. S., Choi, Y. S., Kim, H. K., Lee, M. L., and Lee, M. Y. (2014). “Morphology and antioxidant activity in pollens of Korean oak and darae (Actinidia arguta),” J. Apic 29(2), 137-142.
Horner, H. T., Healy, R. A., Ren, G., Fritz, D., Klyne, A., Seames, C., and Thornburg, R. W. (2007). “Amyloplast to chromoplast conversion in developing ornamental tobacco floral nectaries provides sugar for nectar and antioxidants for protection,” Am. J. Bot. 94, 12-24. DOI: 10.3732/ajb.94.1.12
Jang, B. I. (2009). Patent No. 10-0908412. Korea Intellectual Property Office. DOI: 10.8080/1020090001318
Jang, B. I. (2014). “The report for the result after putting in technology to prevent mortality of Actinidia deliciosa,” Sacheon Agricultural Technology Center.
Kim, C. W., Oh, S. I., Kim, M. J., and Park, Y. K. (2014). “Optimal harvest time by the seasonal fruit quality and ripening characteristics of hardy kiwifruit in Korea,” J. Korean For. Soc. 103, 353-358. DOI: 10.14578/jkfs.2014.103.3.353
Kim, M. J., Chae, D. H., Kwon, Y. H., Kwack, Y. B., and Kwak, Y. S. (2018). “Occurrences of major diseases and pests on ‘Goldone’, ‘Redvita’, ‘Garmrok’, new cultivars of kiwifruit,” Res. Plant Dis. 24(2), 123-131. DOI: 10.5423/RPD.2018.24.2.123
Kim, G. H., Jung, J. S., and Koh, Y. J. (2017). “Occurrence and epidemics of bacterial canker of kiwifruit in Korea,” Plant Pathol. J. 33(4), 351-361. DOI: 10.5423/PPJ.RW.01.2017.0021
Ko, S. J., Lee, Y. H., Cha, K. H., Park, K. B., Park, I. J., and Kim, Y. C. (2002). “An improved method for testing pathogenicity of Pseudomonas syringae pv. actinidiae causing bacterial canker of kiwifruit,” Res. Plant Dis 8(4), 250-253. DOI: 10.5423/RPD.2002.8.4.250
Latocha, P., and Olszewska-Kaczyńska, I. (2003). “Preliminary morphological, chemical and sensory analysis of fruit of different Actinidia genotypes (Actinidia Lindl.),” Annals of Warsaw Agricultural University. Horticulture (Landscape Architecture) 24, 91-104.
Lee, K. K., Mok, I. K., Yoon, M. H., Kim, H. J., and Chung, D. Y. (2012). “Mechanismas of phosphate solubilization by PSB (phosphate-solubilizing bacteria) in soil,” Korean J. Soil Sci. Fert. 45(2), 169-176. DOI: 10.7745/kjssf.2012.45.2.169
Li, W., Duan, H., Chen, F., Wang, Z., Huang, X., Deng, X., and Liu, Y. (2014). “Identification of quantitative trait loci controlling high calcium response in Arabidopsis thaliana,” PLoS ONE 9(11), article e112511. DOI: 10.1371/journal.pone.0112511
Murray, B. M., Meredith F., Sabrina E. K., and Ludmiaal A. (2016). “Solubility, structure, and morphology in the co-precipitation of cadmium and zinc with calcium-oxalate,” Journal of Colloid and Interface Science 486, 309-315. DOI: 10.1016/j.jcis.2016.09.079
Nautiyal, C. S., Bhadauria, S., Kumar, P., Lal, H., Mondal, R., and Verma, D. (2000). “Stress induced phosphate solubilization in bacteria isolated from alkaline soils,” FERMS Microbiol Lett 182, 291-296. DOI: 10.1111/j.1574-6968.2000.tb08910.x
Paiva, E. A. S. (2019). “Are calcium oxalate crystals a dynamic calcium store in plants?,” New Phytol 223, 1707-1711. DOI: 10.1111/nph.15912
Park, Y. K. (2016). “Nutritional compositions of new hardy kiwi fruit (Actinidia arguta) cultivars as honey plant,” Journal of Apiculture 31(3), 233-238. DOI:10.17519/apiculture.2016.09.31.3.233
Shang, Y. F., Xu, M., Zhang, G. N., and Ouyang, J. M. (2013). “Concave urinary crystallines: Direct evidence of calcium oxalate crystals dissolution by citrate, in vivo,” Bioinorganic Chemistry and Applications 2023, 8. DOI: 10.1155/2013/637617
Shin, J. S., Park, J. K., Kim, G. H., Park, J. Y., Han, H. S., Jung, J. S., Hur, J. S., and Koh, Y. J. (2004). “Identification and ecological characteristics of bacterial blossom blight pathogen of kiwifruit,” Res. Plant Dis 10(4), 290-296. DOI: 10.5423/RPD.2004.10.4.290.
Smith, G. S., Asher, C. J., and Clark, C. J. (1987). Kiwifruit Nutrition. Diagnosis of Nutritional Disorders, new edition, Agpress Communications Ltd., Wellington, New Zealand. ISBN 0-9597693-0-7
Wang, T., Chen, X., Ju, C., and Wang, C. (2023). “Calcium signaling in plant mineral nutrition: From uptake to transport,” Plant Comm 4, article 100678. DOI: 10.1016/j.xpic.2023.100678
Yamazaki, K. (1982). “Whole book for hydroponics,” Hakutomo, Japanese Journal of Soil Science and Plant Nutrition 53(4), article 276. DOI: 10.20710/dojo.534_276
Article submitted: December 12, 2023; Peer review completed: January 3, 2024; Revised version received and accepted: January 15, 2024; Published: January 22, 2024.
DOI: 10.15376/biores.19.1.1602-1616
