Abstract
Effects of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) oxidation were evaluated for magnetic bio-nanocomposites of nanocrystalline cellulose (NCC). The magnetic bio-nanocomposites were prepared using NCC and nanomagnetite (NM) to produce organic compounds. The NCC was oxidized by sodium bromide (NaBr), sodium hypochlorite (NaOCl), and TEMPO. The oxidized NCC (ONCC) was characterized by Fourier-transform infrared spectroscopy (FTIR) and conductometry. The bio-nanocomposite particles from the NCC and the ONCC were prepared via in situ precipitation of iron salts from alkaline solution. The resultant bio-nanocomposites were coated on the surface of kraft paper. The magnetic bio-nanocomposite and coated papers were characterized using scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray diffraction (XRD), and vibrating sample magnetometry (VSM). The results revealed that the carboxyl groups were introduced successfully on the NCC surface. The bio-nanocomposite particles showed good dispersion in the surface of the papers. The saturation magnetizations of the coated papers with magnetic bio-nanocomposites of NCC and ONCC were 10.9 and 14.57 emu/g, respectively. The magnetic coated papers exhibited a superparamagnetic behavior. The strengths, apparent density, and air resistance of the coated paper with the ONCC bio-nanocomposites increased in comparison to the NCC coated sample, while the water absorption diminished due to the TEMPO-oxidation.
Download PDF
Full Article
Characterization of Magnetic Bio-Nanocomposites of Nanocrystalline Cellulose (NCC) Coated on Paper Surface
Shaghayegh Rezanezhad,* Noureddin Nazarnezhad, Hossein Resalati, and Seyed Majid Zabihzadeh
Effects of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) oxidation were evaluated for magnetic bio-nanocomposites of nanocrystalline cellulose (NCC). The magnetic bio-nanocomposites were prepared using NCC and nanomagnetite (NM) to produce organic compounds. The NCC was oxidized by sodium bromide (NaBr), sodium hypochlorite (NaOCl), and TEMPO. The oxidized NCC (ONCC) was characterized by Fourier-transform infrared spectroscopy (FTIR) and conductometry. The bio-nanocomposite particles from the NCC and the ONCC were prepared via in situ precipitation of iron salts from alkaline solution. The resultant bio-nanocomposites were coated on the surface of kraft paper. The magnetic bio-nanocomposite and coated papers were characterized using scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray diffraction (XRD), and vibrating sample magnetometry (VSM). The results revealed that the carboxyl groups were introduced successfully on the NCC surface. The bio-nanocomposite particles showed good dispersion in the surface of the papers. The saturation magnetizations of the coated papers with magnetic bio-nanocomposites of NCC and ONCC were 10.9 and 14.57 emu/g, respectively. The magnetic coated papers exhibited a superparamagnetic behavior. The strengths, apparent density, and air resistance of the coated paper with the ONCC bio-nanocomposites increased in comparison to the NCC coated sample, while the water absorption diminished due to the TEMPO-oxidation.
DOI: 10.15376/biores.17.3.4607-4622
Keywords: Nano crystalline cellulose; NCC; Magnetic paper; Bio-nanocomposite; Oxidation; TEMPO
Contact information: Faculty of Natural Resources, Wood and Paper Science Department, Sari Agricultural Sciences and Natural Resource University, P.O. Box 737, Sari 4818168984 Iran;
* Corresponding author: rezanezhad.sh@gmail.com
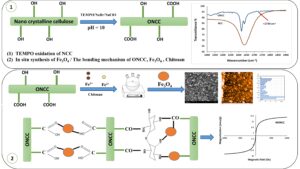
GRAPHICAL ABSTRACT

INTRODUCTION
Environmental considerations have led to extensive research and development of organic and natural materials (Lin et al. 2012; Sibaja et al. 2015; Babaei-Ghazvini et al. 2020). Materials based on cellulose polymer are important in this regard (Isogai et al. 2011). Cellulose has been considered as the most abundant biopolymer on the planet. Cellulose has attracted interest due to its interesting properties such as strengthening features, low cost, biodegradability, renewability, environmental friendliness, and versatility (Abe et al. 2007). Different cellulose derivatives have been produced and used. In the derivation, the cellulose structure is modified and the cellulose hydroxyl groups are replaced with other functional groups (Yang et al. 2001; Chen et al. 2006). These processes lead to the production of cellulosic derivatives, which provide a wide range of applications. One of the most important derivatives of cellulose is nanocellulose (Nechyporchuk et al. 2016). There are different types of nanocellulose, each of which differs in size, production method, surface chemistry, and source (Carlsson et al. 2014). In general, nanocellulose is available in two main forms, nanofibrillated cellulose (NFC) and nanocrystalline cellulose (NCC), which differ in their isolation and shape (Wang et al. 2007; Liu et al. 2015; Nechyporchuk et al. 2016; Haniffa et al. 2017; Nypelö 2020). Nanocrystalline cellulose is produced by chemical methods (Xu et al. 2014; Haniffa et al. 2017). Nanocrystalline cellulose is one of the most important natural nano materials due to its environmental compatibility, biodegradability, abundance, high physical strength and stiffness, large surface area, high aspect ratio, and nanometer width (Chen et al. 2011; Dufresne 2012; Zhang et al. 2016; Faradilla et al. 2017; Chen et al. 2020).
Nanocellulose and metal bio-nanocomposites have been produced for different applications (Biliuta and Coseri 2016; Salama et al. 2018). The metal in the bio-nanocomposites can be iron and its derivatives such as iron oxide (magnetite). Magnetite nanoparticles are the most important practical particles for preparing magnetic nanocomposites (Nypelö 2020). Magnetic composites of cellulose have created new definitions of smart and environmentally friendly materials (Chen et al. 2020). Many researchers have studied the nature, different synthesis methods, applications, and characteristics of magnetic cellulose materials including recyclable medical catalysts (Xiong et al. 2013), magnetic-optical transparent films (Li et al. 2013), the magnetic absorption of oil (Chin et al. 2014), functional composites of cellulose (Liu et al. 2011; Wu et al. 2011), magnetic aerogels, the purification of water and oil recovery (Olsson et al. 2010; Rashid et al. 2017), security adhesives on paper (Fragouli et al. 2012), drug delivery (Kaco et al. 2017; Zhu et al. 2022), storage of information, security cards and electro-magnetic shielding (Marchessault et al. 1992; Mashkour et al. 2011). The bio-nanocomposites of NCC and nanomagnetite (NM) have been evaluated by many researchers for different applications (Anirudhan and Rejeena 2013; Nypelö et al. 2014; Cao et al. 2015; Liu et al. 2015; Zhou et al. 2015; Ren et al. 2017; Zhu et al. 2022).
Multiple function bio-nanocomposites can be produced for storage of information, magnetic shielding, and security applications of paper through coating NCC and NM bio-nanocomposites on the surface of paper. Nevertheless, the NM acts as a filler in paper and reduces the physical and mechanical properties of paper due to the diminished hydrogen bonding between the fibers (Mo et al. 2017). The mechanical properties can be improved via the selective oxidation process. Functional groups can be replaced on the surface of the NCC through chemical modification (Eyley and Thielemans 2011). Oxidation of cellulose fibers by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) has been extensively used for chemical modification to introduce sodium carboxyl groups into the cellulose micro fibril surfaces (Habibi et al. 2006; Biliuta and Coseri 2016; Du et al. 2016; Haniffa et al. 2017). TEMPO is a water-soluble chemical catalyst, and TEMPO oxidation is a selective method that is used to oxidize aqueous and non-aqueous polysaccharide solutions. The amount of available carboxyl groups will grow through the oxidation treatment. The selective oxidation reaction changes the carbon 6 of primary hydroxyl groups on the surface of the crystalline cellulose micro fibrils (Saito and Isogai 2004; Puangsin et al. 2013; Meng et al. 2016), where negatively charged carboxyl groups are introduced onto the micro fibrils (Isogai et al. 2011).
In this study, the NCC was oxidized using TEMPO, and the effect of the oxidation process was evaluated on the NCC. Magnetic bio-nanocomposites were produced based on NM, NCC, and oxidized NCC (ONCC). The magnetic bio-nanocomposites were coated on the surface of paper. The effect of the TEMPO oxidation was investigated relative to the magnetization capacity of the coated papers. The results of the oxidation process, the bio-nanocomposite synthesis, and the properties of the coated paper were characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), Fourier-transform infrared spectroscopy (FTIR), vibrating sample magnetometry (VSM), and X-ray diffraction (XRD).
EXPERIMENTAL
Materials
Hardwood kraft fibers (KFs) were provided from the wood and paper industry (Mazandaran Co., Sari, Iran). The iron dichloride tetrahydrate (FeCl2∙4H2O, 98.0% purity), iron trichloride hexahydrate (FeCl3∙6H2O, 98.0% purity), ammonium hydroxide solution (NH4OH, 28.0%), hydrochloric acid (HCl, 37.0%), sodium bromide (NaBr, 99%) with a molecular weight of 102.9 (g/mol), sodium hypochlorite solution (NaOCl, 6-14% active chlorine), and epichlorohydrin (C3H5ClO) were purchased from Merck (Kenilworth, NJ). The chitosan (C6H11NO4) with a medium molecular weight (190,000 to 310,000 Da), TEMPO (98.0%), and sodium hydroxide (NaOH, 97.0%) were purchased from Sigma-Aldrich (St. Louis, MO). The NCC was purchased from Nano Novin Polymer Co. (Gorgan, Iran).
Methods
Preparation of the KFs and handsheet
The KF sheets were soaked in deionized water for 24 h. The kraft pulp was treated mechanically using a laboratory refiner (PFI mill – IDM test, Spain) to a freeness level of 300 CSF. The KF handsheet had a basis weight of 120 g/cm2 and was synthesized according to the TAPPI standard T205 (2002).
TEMPO-oxidation
The TEMPO-oxidation was performed according to the methods outlined by Habibi et al. (2006). Briefly, 1 g of NCC (based on the dry weight) was soaked in 100 mL of deionized water, after which 15 mg of TEMPO, 165 mg of NaBr (per gram of NCC), and 10 mmol of NaOCl were added to the suspension slowly. The pH of the suspension was elevated to 10 by adding NaOH 0.5 mmol at room temperature. The NCC was oxidized for 2 h. The oxidation was terminated by adding methanol (1 mL), and the pH of the suspension was adjusted to 7 by adding 0.5 M HCl.
Production of the magnetic bio-nanocomposite
The synthesis of the magnetic bio-nanocomposite of NCC (MNCC) and ONCC (MONCC) was performed using the method of Cao et al. (2015). The process was performed for NCC and ONCC. The NCC (1 g) were dispersed in 200 mL of 1.5 wt.% aqueous suspension followed by FeCl2∙4H2O (0.5 g), FeCl3∙6H2O (1 g), chitosan (0.6 g), and epichlorohydrin (2% per the dry weight of the NCC) under a nitrogen flow. Next, NH4OH was added until a pH of 11 was reached under continuous stirring at a temperature of 40 °C for 30 min. Finally, the magnetic fiber was washed with distilled water.
Coating method
For coating the papers, 1 g of the magnetic bio-nanocomposites was immersed in 20 mL of deionized water. The coating suspension was spread on the surface of the papers with a metal roller. The coated papers were placed on oven at 30 °C and pressurized in plastic frames for 24 h.
Determination of the mechanical paper properties
The tensile strength, tear strength, air resistance, apparent density, and water absorption properties of the papers were measured according to the TAPPI standards T494 (2001), T414 (2004), T460 (2006), T220 (2001), and T441 (2013), respectively. The tests of mechanical properties of papers were carried out three times for each sample.
Carboxyl content measurement
The carboxyl content of the ONCC was determined according to the methods described by Habibi et al. (2006) using conductimetric titration. 0.05 g of ONCC was added in 15 mL of HCl 0.01 M and stirred for 10 min. Then, the mixture was titrated by NaOH 0.01 M. The amount of carboxyl groups was calculated according to Eq. 1,
(1)
where C is the carboxyl concentration (mmol), V1 and V0 represent the consumption of NaOH (mL), CNaOH shows the concentration of NaOH, and m is the dry weight of NCC (mg).
FTIR analysis
The infrared spectra of the NCC and ONCC were measured using a Cary 630 FTIR spectrometer (Agilent Technologies, Santa Clara, CA, USA). The spectra were measured with a spectral width ranging from 600 to 4000 cm-1 at a resolution of 2 cm-1.
XRD analysis
The XRD analysis was performed using a Philips X’Pert modular powder diffractometer (MPD) (Almelo, Netherlands). The samples were scanned with Cu Kα = 1.5406 Å radiation at a diffraction angle (2θ) ranging from 5° to 80° with a step size of 1°/min.
AFM analysis
Atomic force microscopy was employed to determine the size of the NM and NCC particles. The AFM analysis was performed via an Easyscan2 Flex AFM probe microscope (Nanosurf, Liestal, Switzerland) in air.
SEM and EDX analysis
The morphology of the NM particles and the coated papers was examined via SEM analysis. The SEM micrographs of the samples were taken using an SNE-4500 scanning electron microscope (SEC, Suwon, Korea). The samples were covered with a thin layer of gold via sputtering. The EDX analysis was used to determine the chemical composition analysis and the elemental mapping was conducted with the SNE-4500 electron microscope equipped with an EDX system. The operating voltage was 30 kV.
Magnetic measurements
The magnetization of the samples was measured via VSM using a Meghnatic Daghigh Kavir company device (Kashan, Iran) in an applied magnetic field sweeping between ±10000 Oe.
RESULTS AND DISCUSSION
FTIR of the TEMPO-Oxidized NCC
Figure 1 indicates the FTIR of the NCC and ONCC. The broad peaks near 1059.8 and 3311 to 3415 cm-1 correspond to the C–O–C stretching vibration plus the free O–H groups and hydroxyl stretching vibration (Sundar et al. 2010; Liu et al. 2015; Biliuta et al. 2017). The peak around 2900 cm-1 matches the C–H stretching groups (Liu et al. 2015; Haniffa et al. 2017). The peaks around 1633 cm-1 in the NCC and 1637 cm-1 in the ONCC are attributed to the glucosidic linkages between the cellulose units (Liu et al. 2015). The TEMPO-oxidized NCC exhibited a new peak at 1737 cm-1 corresponding to the C=O stretching vibration of carboxyl groups (Habibi et al. 2006; Saito et al. 2006). As can be seen in Fig. 1, the intense peak around 3300 to 3400 cm-1 in the NCC diminished due to the oxidation process. This peak relates to the hydroxyl groups in the NCC. This confirms the oxidation of the hydroxyl groups and their conversion to carboxyl groups.
Fig. 1. The FTIR spectra of the NCC and ONCC samples
One of the most important factors in evaluating the oxidation reaction intensity is the amount of carboxyl groups produced. When NCC is treated with NaClO, NaBr, and TEMPO in aqueous solution, the hydroxyl group of carbon 6 is changed to the carboxyl group selectively (Saito and Isogai 2004; Puangsin et al. 2013; Haniffa et al. 2017). The amount of carboxyl groups depends on the method of oxidation and the NaClO dosage. The concentrations of NaClO and carboxyl contents of the TEMPO-oxidized NCC determined by conductimetric titration are reported in Table 1. The untreated NCC had the lowest amount of carboxyl groups, and the NaClO treatment elevated the carboxyl content (Saito and Isogai 2004; Djafari et al. 2018). Therefore, it can be concluded that the amount of available carboxyl groups was increased through the oxidation treatment (Puangsin et al. 2013). The amount of carboxyl groups in the NCC treated with NaClO in the presence of TEMPO was 0.8 mmol/g.
Table. 1. Conductimetry and Carboxyl Contents of the NCC, MNCC and MONCC
AFM, SEM, and EDX Analysis
The surface morphology and particle size of the magnetite nanoparticles, the fibers, and the magnetic fibers were observed via AFM and SEM micrographs. Figure 2a illustrates the SEM micrograph of the NM. The cubic shape of the NM particles may be because of the accumulation of particles (Rezanezhad et al. 2022).
Fig. 2. SEM images of the a) magnetite nanoparticles, b) surface of the paper coated with the magnetic bio-nanocomposite NCC, c) surface of the paper coated with the magnetic bio-nanocomposite ONCC. The EDX graphs of the d) Fe3O4 nanoparticles, e) surface of the paper coated with the magnetic bio-nanocomposite NCC, f) surface of the paper coated with the magnetic bio-nanocomposite ONCC
Figures 2b and 2c show the surface of the kraft papers coated with the MNCC and the MONCC. The nanocomposites and the fibers can be seen clearly on the micrographs. These images show that the magnetic bio-nanocomposites bonded to the fibers. The results of the EDX analysis of the NM, the papers coated with the magnetic bio-nanocomposite before the oxidation process, and the papers coated with the magnetic bio-nanocomposite after the oxidation process are shown in Fig. 2e, 2d, and 2f, respectively. As can be seen in the NM sample, the Fe element had the highest content. The Fe element values of the samples before and after oxidation were 78% and 82%, respectively. The increase in the amount of the Fe element suggests more precipitation of NM in the magnetic bio-nanocomposite after oxidation.
The frequency of the NM and NCC particle size was measured by AFM. Figures 3a, 3b, and 3c reveal the AFM image, particle size distribution diagram, and three-dimensional (3D) topography of the nanomagnetite. The NM particle size was within the range of 1 nm to 85.1 nm. Figures 3d, 3e, and 3f depict the image, particle size distribution diagram, and 3D topography of the NCC. The particle size of the NCC was within the range of 1 nm to 93.2 nm.
Fig. 3. The a) AFM image, b) 3D topography, and c) frequency of the particle diameter distribution diagram of the nanomagnetite. The d) AFM image, e) 3D topography, and f) frequency of the particle diameter distribution of the NCC
Strength Properties of the Coated Papers by Magnetic Bio-Nanocomposites
The tensile and tear strengths of the control (kraft paper) and coated papers were investigated. The diagrams of the tensile and tear index values can be seen in Fig. 4. The tensile and tear index diagrams indicated that the control paper had the highest strength. However, the paper strength diminished substantially after it was coated with magnetic bio-nanocomposites. The NM functions as a filler in the paper structure and reduces the strength of the paper. The reaction sites increased due to the TEMPO oxidation, which introduced carboxyl groups onto the NCC. As a result, the bonding between the fibers increased and the strength of the paper increased. The strength of the coated papers increased due to the mechanical interaction between the magnetic bio-nanocomposite and the fibers on the surface of paper. This formed hydrogen and covalent bonding between the coating compositions such as the NCC, NM, chitosan, and the hydroxyl groups of the fiber surface.
Fig. 4. Tensile and tear index values of the control and coated paper by magnetic bio-nanocomposites
Figure 5 shows results for apparent density and related paper properties. The apparent density of the control (kraft paper) and coated papers can be seen in Figs. 5a. Figure 5b shows the corresponding results for air resistance and water absorption. The apparent density of paper is one of its basic physical properties that affects its other physical and mechanical properties. The strength of paper has been found to increase by enhancing the apparent density (Maurer and Kearney 1998). The apparent density of the paper is obtained by calculating the ratio of the basis weight to the thickness of the paper. The apparent density of the paper indicates the volume of paper filled with fibrous and non-fibrous components such as fillers (Fišerová et al. 2007). The deposition of the coating solution that contained magnetic bio-nanocomposites of the NCC and ONCC onto the cellulose substrate of the papers augmented the thickness. Furthermore, the magnetic particles were deposited in the empty spaces among the fibers in the paper. The SEM micrographs indicated the deposition of the magnetic bio-nanocomposite coating layer on the surface of the fibers, as well as the entry into the paper structure and the deposition in the empty space among the fibers. This precipitation formed a coating layer on the surface of papers. The thickness of the coating layer depends on the nature of the polymer and the amount of soluble solids (Guillaume et al. 2010). The basis weight increased as the paper was coated and the thickness increased. The apparent density increased as the basis weight increased. The coating surface on the paper increased due to the small size and high specific surface area of the NM and NCC.
Fig. 5. The a) apparent density and b) air resistance and water absorption values of the control and coated papers
Figure 5b illustrates the air resistance values of the observed samples. The Gurley test measures the air volume that passes through the paper, where a longer time represents greater resistance of the paper (Scott et al. 1997). The air resistance values of the coated papers were higher compared to the control sample. The air resistance was also higher in the paper that was coated with the ONCC magnetic bio-nanocomposite as compared to the non-oxidized sample. Coating creates a layer on the surface of the paper, bonds with the fibers, and enhances the apparent density and air resistance of the paper (Gällstedt et al. 2005).
One of the disadvantages of paper is its low water resistance, which may interfere with some of its applications, such as in insulating papers and oil absorption in printing. As a result, it is essential to control the water absorption of paper. According to the results, the highest and lowest water absorption values were related to the uncoated sample (control) and the paper coated with the ONCC magnetic bio-nanocomposite, respectively. In a paper network, water molecules are absorbed by the fibers as well as the inter-fiber empty space. Coating of composites impregnate the paper structure and fill the pores to form a layer on the surface of the paper. The oxidation process increases the carboxyl groups and enhances the reactivity between the fibers and the ONCC magnetic bio-nanocomposite. The oxidation process also boosts the bonding, thereby reducing the reactive points that can react with the water molecules. The contact angle of water droplets with the paper surface increased due to the coating with magnetic bio-nanocomposite. Therefore, the surface of paper tends to be hydrophobic (Marvizadeh et al. 2017). Water molecules bond with hydroxyl and carboxyl groups introduced by oxidation and reduce the water absorption capacity.
XRD Analysis
Figure 6 displays XRD patterns of the NM, NCC, and papers coated with magnetic bio-nanocomposites of NCC and ONCC. The NM had diffraction peaks at approximately 35°, 41°, 62°, 74°, and 87°. These diffraction peaks referred to the magnetite nanoparticles with a cubic crystalline structure (Long et al. 2009; Small and Johnston 2009; Dimitrov et al. 2013). The diffraction pattern of the NCC revealed three intense diffraction peaks at approximately 2θ = 16°, 22.41°, and 26.34°, which are assigned to the native cellulose (Mashkour et al. 2011; Wu et al. 2011; Cao et al. 2015). The peak at approximately 2θ = 22° corresponded to the cellulose І structure and indicates the presence of crystalline phases of NCC (Cao et al. 2015). Diffraction peaks for the paper coated with magnetic bio-nanocomposites of the NCC and ONCC emerged at approximately 16°, 22.5°, 35°, 41°, 50°, 62°, and 74°, referring to both the NCC and NM (Long et al. 2009; Rashid et al. 2017). The diffraction peak at approximately 2θ = 32° indicated a hematite structure (Dimitrov et al. 2013). The XRD patterns indicated that the intensity of the NM peaks decreased by reducing the amount of precipitated NM. Păcurariu et al. (2015) confirmed that the intensity of the XRD peaks diminished by decreasing the NM value in the samples. Thus, the intensity of the XRD peaks of the paper coated with the ONCC magnetic bio-nanocomposite was higher than that of the paper coated with the NCC magnetic bio-nanocomposite.
Fig. 6. The XRD patterns of the NM, NCC without treatment, NCC magnetic bio-nanocomposite, and ONCC magnetic bio-nanocomposite
Magnetic Properties
The magnetic behavior of the samples was investigated via vibrating sample magnetometry at room temperature. Figure 7 indicates the saturation of magnetization (Ms) of the NM, the NCC, the paper coated with the NCC magnetic bio-nanocomposite, and the ONCC magnetic bio-nanocomposite. The Ms values for the NM, NCC, paper coated with NCC magnetic bio-nanocomposite, and ONCC magnetic bio-nanocomposite were 23, 0.12, 10.9, and 14.57 emu/g, respectively. The Ms values of the MNCC and MONCC were both lower compared to the NM particles, because of the reduction in the content of the NM loaded onto the fiber. Furthermore, the Ms increased in the TEMPO-oxidized NCC. The reactivity of the NCC after the oxidation treatment was high due to the carboxyl functional groups in its structure. Carboxyl groups act as a linkage point for Fe3O4 and hydroxyl groups in the cellulose chain (Biliuta and Coseri 2016). The Fe3+ and Fe2+ are absorbed onto the NCC due to the negative charge of carboxyl group in NCC. As a result, the Ms increased due to the rise in the content of iron salts absorbed onto the NCC. The samples exhibited superparamagnetic behavior, as confirmed by the absence of coercivity (Hc), remanence (Mr), and the ferromagnetic behavior (Xiong et al. 2013; Biliuta and Coseri 2016).
Fig. 7. The hysteresis loops of the NM, NCC without treatment, NCC magnetic bio-nanocomposite, and ONCC magnetic bio-nanocomposite
CONCLUSIONS
- Magnetic bio-nanocomposites of nanocrystalline cellulose (NCC) and oxidized NCC (ONCC) were successfully prepared by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidation and in-situ synthesis.
- The magnetic analysis revealed that the saturation magnetization of the paper coated with the NCC magnetic bio-nanocomposite was enhanced due to the TEMPO-oxidation.
- The strength properties of the coated paper increased by increasing the carboxyl groups and bonding between the fibers as well as bonding between the paper and the bio-nanocomposite.
- The water absorption properties of the coated papers diminished due to the coating and surface smoothness.
ACKNOWLEDGMENTS
The authors are grateful to the Sari Agricultural Science and Natural Resources University for providing equipment support.
REFERENCES CITED
Abe, K., Iwamoto, S., and Yano, H. (2007). “Obtaining cellulose nanofibers with a uniform width of 15 nm from wood,” Biomacromolecules 8(10), 3276-3278. DOI: 10.1021/bm700624p
Anirudhan, T. S., and Rejeena, S. R. (2013). “Selective adsorption of hemoglobin using polymer-grafted-magnetite nanocellulose composite,” Carbohydrate Polymers 93(2), 518-527. DOI: 10.1016/j.carbpol.2012.11.104
Babaei-Ghazvini, A., Cudmore, B., Dunlopa, M. J., Acharya, B., Bissessur, R., Ahmed, M., and Whelan, W. M. (2020). “Effect of magnetic field alignment of cellulose nanocrystals in starch nanocomposites: Physicochemical and mechanical properties,” Carbohydrate Polymers 247, article no. 116688. DOI: 11668810.1016/j.carbpol.2020.116688
Biliuta, G., and Coseri, S. (2016). “Magnetic cellulosic materials based on TEMPO – oxidized viscose fibers,” Cellulose 23(6), 3407-3415. DOI: 10.1007/s10570-016-1082-z
Biliuta, G., Sacarescu, L., Socoliuc, V., Iacob, M., Gheorghe, L., Negru, D., and Coseri, S. (2017). “Carboxylated polysaccharides decorated with ultrasmall magnetic nanoparticles with antibacterial and MRI properties,” Macromolecular Chemistry and Physics 218(10), 1-9. DOI: 10.1002/macp.201700062
Cao, S.-L., Xu, H., Li, X.-H., Lou, W.-Y., and Zong, M.-H. (2015). “Papain@magnetic nanocrystalline cellulose nanobiocatalyst: A highly efficient biocatalyst for dipeptide biosynthesis in deep eutectic solvents,” ACS Sustainable Chemistry and Engineering 3(7), 1589-1599. DOI: 10.1021/acssuschemeng.5b00290
Carlsson, D. O., Hua, K., Forsgren, J., and Mihranyan, A. (2014). “Aspirin degradation in surface-charged TEMPO-oxidized mesoporous crystalline nanocellulose,” International Journal of Pharmaceutics 461(1-2), 74-81. DOI: 10.1016/j.ijpharm.2013.11.032
Chen, X., Burger, C., Fang, D., Zhang, L., Hsiao, B. S., and Chu, B. (2006). “X-ray studies of regenerated cellulose fibers wet spun from cotton linter pulp in NaOH/thiourea aqueous solution,” Polymer 47(8), 2839-2848. DOI: 10.1016/j.polymer.2006.02.044
Chen, W., Yu, H., Liu, Y., Zhang, M., and Chen, P. (2011). “Isolation and character-ization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process,” Cellulose 18(2), 433-442. DOI: 10.1007/s10570-011-9497-z
Chen, L., Sharma, S., Darienzo, R. E., and Tannenbaum, R. (2020). “Decoration of cellulose nanocrystals with iron oxide nanoparticles,” Materials Research Express 7, article no. 055003. DOI: 10.1018/2053-1591/ab8a82
Chin, S. F., Romainor, A. N. B., and Pang, S. C. (2014). “Fabrication of hydrophobic and magnetic cellulose aerogel with high oil absorption capacity,” Materials Letters 115, 241-243. DOI: 10.1016/j.matlet.2013.10.061
Dimitrov, K. V., Herzog, M., and Nenkova, S. (2013). “Fe3O4 modification of microcrystalline cellulose for composites materials,” American Journal of Chemistry 3(5), 140-147. DOI: 10.5923/j.chemistry.20130305.04
Djafari, S. R. P., Ranjbar, J., and Rasooly, E. G. (2018). “Eco-friendly superabsorbent polymers based on carboxymethyl cellulose strengthened by TEMPO-mediated oxidation wheat straw cellulose nanofiber,” Carbohydrate Polymers 197, 565-575. DOI: 10.1016/j.carbpol.2018.06.008
Du, C., Liu, M., Li, B., Li, H., Meng, Q., and Zhan, H. (2016). “Cellulose nanocrystals prepared by persulfate one-step oxidation of bleached bagasse pulp,” BioResources 11(2), 2017-4024. DOI: 10.15376/biores.11.2.4017-4024
Dufresne, A. (2012). Nanocellulose: From Nature to High Performance Tailored Materials, De Gruyter, Berlin, Germany.
Eyley, S., and Thielemans, W. (2011). “Imidazolium grafted cellulose nanocrystals for ion exchange applications,” Chemical Communications 47(14), 4177-4179. DOI: 10.1039/C0CC05359G
Fišerová, M., Gigac, J., and Boháček, S. (2007). “Application of pre-treated sugar beet pulp in paper manufacture,” Cellulose Chemistry and Technology 41(4), 283-289.
Fragouli, D., Bayer, I. S., Corato, R. D., Brescia, R., Bertoni, G., Innocenti, C., Gatteschi, D., Pellegrino, T., Cingolani, R., and Athanassiou, A. (2012). “Superparamagnetic cellulose fiber networks via nanocomposite functionalization,” Journal of Materials Chemistry 22(4), 1662-1666. DOI: 10.1039/c1jm14755b
Faradilla, R. H. F., Lee, G., Arns, J.-Y., Roberts, J., Martens, P., Stenzel. M. H., and Arcot, J. (2017). “Characteristics of a free-standing film from banana pseudostem nanocellulose generated from TEMPO-mediated oxidation,” Carbohydrate Polymers 174, 1156-1163. DOI: 10.1016/j.carbpol.2017.07.025
Gällstedt, M., Brottman, A., and Hedenqvist, M. S. (2005). “Packaging-related properties of protein- and chitosan-coated paper,” Packaging Technology and Science 18(4), 161-170. DOI: 10.1002/pts.685
Guillaume, C., Schwab, I., Gastaldi, E., and Gontard, N. (2010). “Biobased packaging for improving preservation of fresh common mushrooms (Agaricus bisporus L.),” Innovative Food Science and Emerging Technologies 11(4), 690-696. DOI: 10.1016/j.ifset.2010.05.007
Habibi, Y., Chanzy, H., and Vignon, M. R. (2006). “TEMPO-mediated surface oxidation of cellulose whiskers,” Cellulose 13(6), 679-687. DOI: 10.1007/s10570-006-9075-y
Haniffa, M. A. C. M., Ching, Y. C., Chuah, C. H., Ching, K. Y., Nazri, N., Abdullah, L. C., and Nai-Shang, L. (2017). “Effect of TEMPO-oxidization and rapid cooling on thermo-structural properties of nanocellulose,” Carbohydrate Polymers 173, 91-99. DOI: 10.1016/j.carbpol.2017.05.084
Isogai, A., Saito, T., and Fukuzumi, H. (2011). “TEMPO-oxidized cellulose nanofibers,” Nanoscales 3(1), 71-85. DOI: 10.1039/C0NR00583E
Kaco, H., Baharin, K. W., Zakaria, S., Chia, C. H., Sajab, M. S. Jaafar, S. N. S., and Gan, Y. S. (2017). “Preparation and characterization of Fe3O4/regenerated cellulose membrane,” Sains Malaysiana 46(4), 623-628. DOI: 10.17576/jsm-2017-4604-15
Li, Y., Zhu, H., Gu, H., Dai, H., Fang, Z., Weadock, N. J., Gou, Z., and Hu, L. (2013). “Strong transparent magnetic nanopaper prepared by immobilization of Fe3O4 nanoparticles in a nanofibrillated cellulose network,” Journal of Materials Chemistry A 1(48), 15278-15283. DOI: 10.1039/C3TA12591B
Lin, N., Huang, J., and Dufresne, A. (2012). “Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review,” Nanoscale 4(11), 3274-3294. DOI: 10.1039/C2NR30260H
Liu, S., Zhou, J., and Zhang, L. (2011). “In situ synthesis of plate-like Fe2O3 nanoparticles in porous cellulose films with obvious magnetic anisotropy,” Cellulose 18(3), 663-673. DOI: 10.1007/s10570-011-9513-3
Liu, K., Nasrallah, J., Chen, L., Huang, L., and Ni, Y. (2015). “Preparation of CNC-dispersed Fe3O4 nanoparticles and their application in conductive paper,” Carbohydrate Polymers 126, 175-178. DOI: 10.1016/j.carbpol.2015.03.009
Long, Z., Li, H. F., Yang, X., and Liang, H. N. (2009). “Study on preparation and characterization of magnetic paper with bleached chemical pulp,” in: Proceedings of the 2nd International Congress on Image and Signal Processing, Tianjin, China, pp. 4131-4244.
Marchessault, R. H., Rioux, P., and Raymond, L. (1992). “Magnetic cellulose fibers and paper: Preparation, processing and properties,” Polymer 33(19), 4024-4028. DOI: 10.1016/0032-3861(92)90600-2
Marvizadeh, M. M., Oladzadabbasabadi, N., Nafchi, A. M., and Jokar, M. (2017). “Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide,” International Journal of Biological Macromolecules 99, 1-7. DOI: j.ijbiomac.2017.02.067
Mashkour, M., Tajvidi, M., Kimura, T., Kimura, F., and Ebrahmi, G. (2011). “Fabricating unidirectional magnetic papers using permanent magnets to align magnetic nanoparticles covered natural cellulose fibers,” BioResources 6(4), 4731-4738. DOI: 10.15376/biores.6.4.4731-4738
Maurer, H. W., and Kearney, R. L. (1998). “Opportunities and challenges for starch in the paper industry,” Starch 50(9), 396-402. DOI: 10.1002/(SICI)1521-379X (199809)50:9<396::AID-STAR396>3.0.CO;2-8
Meng, Q., Fu, S., and Lucia, L. A. (2016). “The role of heteropolysaccharides in developing oxidized cellulose nanofibrils,” Carbohydrate Polymers 144, 187-195. DOI: 10.1016/j.carbpol.2016.02.058
Mo, Z., Gou, H., Wang, Y., Yin, H., Yang, C., and Song, J. (2017). “Surface modification of graphene oxide sheets on magnetic particles for magnetic paper,” Journal of Alloys and Compounds 695(C), 2525-2531. DOI: 10.1016/j.jallcom.2016.11.157
Nechyporchuk, O., Belgasem, M. N., and Bras, J. (2016). “Production of cellulose nanofibrils: A review of recent advances,” Industrial Crops and Products 93, 2-25. DOI: 10.1016/j.indcrop.2016.02.016
Nypelö, T., Rodriguez-Abreu, C., Rivas, J., Dickey, M. D., and Rojas, O. J. (2014). “Magneto-responsive hybrid materials based on cellulose nanocrystals,” Cellulose 21(4), 2557-2566. DOI: 10.1007/s10570-014-0307-2
Nypelö, T. (2020). “Magnetic cellulose: Does extending cellulose versatility with magnetic functionality facilitate its use in devices?,” Journal of Materials Chemistry C 10, 805-818. DOI: 10.1039/d1tc02105b
Olsson, R.T., Samir, M. A. S. A., Salazar-Alvarez, G., Belova, L., Ström., V., Berglund, L. A., Ikkala, O., Nogués, J., and Gedde, U. W. (2010). “Making flexible magnetic aerogel and stiff magnetic nanopaper using cellulose nanofibrils as templates,” Nature Nanotechnology 5, 584-595. DOI: 10.1038/nnano.2010.155
Păcurariu, C., Tăculescu, E.-A., Ianoş, R., Marinică, O., Mihali, C.-V., and Socoliuc, V. (2015). “Synthesis and characterization of ɣ-Fe2O3/SiO2 composites as possible candidates for magnetic paper manufacture,” Ceramics International 41(1B), 1079- 1085. DOI: 10.1016/j.ceramint.2014.09.031
Puangsin, B., Yang, Q., Saito, T., and Isogai, A. (2013). “Comparative characterization of TEMPO-oxidized cellulose nanofibril films prepared from non-wood resources,” International Journal of Biological Macromolecules 59, 208-213. DOI: 10.1016/j.ijbiomac.2013.04.016
Rashid, M., Ghafur, M. A., Sharafat, M. K., Minami, H., Miah, M. A. J., and Ahmad, H. (2017). “Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method,” Carbohydrate Polymers 170, 72-79. DOI: 10.1016/j.carbpol.2017.04.059
Ren, S., Zhang, X., Dong, L., Lei, T., Teng, Z., Song, K., Sun, X., and Wu, Q. (2017). “Cellulose nanocrystal supported superparamagnetic nanorods with aminated silica shell: Synthesis and properties,” Journal Materials Science 52, 6432-6441. DOI: 10.1007/s10853-017-0878-z
Rezanezhad, S., Nazarnezhad, N., Resalati, H., and Zabihzadeh, S. M. (2022). “The use of gluconic acid as an additive in magnetic paper,” BioResources 17(1), 342-354. DOI: 10.15376/biores.17.1.342-354
Salama, A., Etri, S., Mohamed, S. A. A., and El-Sakhawy, M. (2018). “Carboxymethyl cellulose prepared from mesquite tree: New source for promising nanocomposite materials,” Carbohydrate Polymers 189, 138-144. DOI: 10.1016/j.carbpol.2018.02.016
Saito, T., and Isogai, K. (2004). “TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions,” Biomacromolecules 5(5), 1983-1989. DOI: 10.1021/bm0497769
Saito, T., Nishiyama, Y., Putaux, J.-L., Vignon, M., and Isogai, A. (2006). “Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose,” Biomacromolecules 7(6), 1687-1691. DOI: 10.1021/bm060154s
Scott, W. E., Abbott, J. C., and Trosset, S. (1997). Properties of Papers: An Introduction, TAPPI Press, Atlanta, GA.
Sibaja, B., Culbertson, E., Marshall, P., Boy, R., Broughton, R. M., Solano, A. A., Esquivel, M., Parker, J., De La Fuent, L., and Auad, M. L. (2015). “Preparation of alginate-chitosan fibers with potential biomedical applications,” Carbohydrate Polymers 134, 598-608. DOI: 10.1016/j.carbpol.2015.07.076
Small, A. C., and Johnston, J. H. (2009). “Novel hybrid materials of magnetic nanoparticles and cellulose fibers,” Journal of Colloid and Interface Science 331(1), 122-126. DOI: 10.1016/j.jcis.2008.11.038
Sundar, S. T., Sain, M. M., and Oksman, K. (2010). “Characterization of microcrystalline cellulose and cellulose long fiber modified by iron salt,” Carbohydrate Polymers 80(1), 35-43. DOI: 10.1016/j.carbpol.2009.10.072
TAPPI T205 (2002). “Forming hand-sheets for physical tests of pulp,” TAPPI Press, Atlanta, GA.
TAPPI T220 (2001). “Physical testing of pulp handsheets,” TAPPI Press, Atlanta, GA.
TAPPI T414 (2004). “Internal tearing resistance of paper (Elmendorf-type method),” TAPPI Press, Atlanta, GA.
TAPPI T441 (2013). “Water absorptiveness of sized (non-bibulous) paper, paperboard, and corrugated fiberboard (Cobb test),” TAPPI Press, Atlanta, GA.
TAPPI T460 (2006). “Air resistance of paper (Gurley method),” TAPPI Press, Atlanta, GA.
TAPPI T494 (2001). “Tensile properties of paper and paperboard (using constant rate of elongation apparatus),” TAPPI Press, Atlanta, GA.
Wang, B., Sain, M., and Oksman, K. (2007). “Study of structural morphology of hemp fiber from the micro to the nanoscale,” Applied Composite Materials 14(2), 89-103. DOI: 10.1007/s10443-006-9032-9
Wu, W.-B., Jing, Y., Gong, M.-R., Zhou, X.-F., and Dai, H.-Q. (2011). “Preparation and properties of magnetic cellulose fiber composites,” BioResources 6(3), 3396-3409. DOI: 10.15376/biores.6.3.3396-3409
Xiong, R., Lu, C., Wang, Y., Zhou, Z., and Zhang, X. (2013). “Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposites with catalytic and antibacterial activity,” Journal of Materials Chemistry A 1(47), 14910-14918. DOI: 10.1039/C3TA13314A
Xu, Q., Li, W., Cheng, Z., Yang, G., and Qin, M. H. (2014). “TEMPO/NaBr/ NaClO-mediated surface oxidation of nanocrystalline cellulose and its microparticulate retention system with cationic polyacrylamide,” BioResources 9(1), 994-1006. DOI: 10.15376/biores.9.1.994-1006
Yang, G., Zhang, L., Han, H., and Zhou, J. (2001). “Cellulose/casein blend membranes from NaOH/urea solution,” Journal of Applied Polymer Science 81(13), 3260-3267. DOI: 10.1002/app.1781
Zhang, K., Sun, P., Liu, H., Shang, S., Song, J., and Wang, D. (2016). “Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods,” Carbohydrate Polymers 138, 237-243. DOI: 10.1016/j.carbpol.2015.11.038
Zhou, W., Ma, W., Li, C., Pan, J., and Dai, X. (2015). “Well-designed multihollow magnetic imprinted microspheres based on cellulose nanocrystals (CNCs) stabilized Pickering double emulsion polymerization for selective adsorption of bifenthrin,” Chemical Engineering Journal 276, 249-260. DOI: 10.1016/j.cej.2015.04.084
Zhu, X., Tong, J., Lan, H., and Pan, D. (2022). “Fabrication of polyethyleneimine-functionalized magnetic cellulose nanocrystals for the adsorption of diclofenac sodium from aqueous solutions,” Polymers 14, 720-732. DOI: 10.3390/polym14040720
Article submitted: January 11, 2022; Peer review completed: April 25, 2022; Revised version received: May 1, 2022; Accepted: June 12, 2022; Published: June 14, 2022.
DOI: 10.15376/biores.17.3.4607-4622
