Abstract
Extracts from the inner stem bark of Berberis vulgaris were analyzed for their antioxidant activity using the 1,1-dipheny-2-picrylhydrazyl (DPPH) method and compared with ascorbic acid (AA) and butylated hydroxytoluene (BHT). The most active extracts were analyzed for their chemical composition using gas chromatography-mass spectrometry. Acetone extract was found to be the most active as an antioxidant agent at 98.61%, which was higher than the value of vitamin C (93.03%) at the concentration of 0.16 mg/mL. The major components identified in the acetone extract were tetracosanoic acid, methyl ester (26.36%), followed by phthalic acid, diisooctyl ester (20.93%), 1,2-bis(trimethylsiloxy) ethane (10.26%), and 1,2-benzendicarboxylic acid, diisononyl ester (8.70%). The dissolved water:methanol (1:1 v/v) partitioned from acetone extract afforded 12 fractions; among them, fraction F11 was found to have good antioxidant activity (95.41%) at the concentration of 0.16 mg/mL. The major compounds identified in F11 were N-methyl-4-(hydroxybenzyl)-1,2,3,4-tetrahydroisoquinoloine (28.82%), 9-α-hydroxy-17β-(trimethylsilyl-oxy)-4-anderostene-3-methyloxime (13.97%), ribitol, pentaacetate (9.76%), 1-methyl-4-[4,5-dihydroxyphenyl]-hexahydropyridine (6.83%), and 2-ethylacridine (4.77%).
Download PDF
Full Article
Chemical Composition and Antioxidant Activity of Extracts from the Inner Bark of Berberis vulgaris Stem
Seyyed Khalil Hosseinihashemi,a,* Hamidreza Anooshei,a Hamed Aghajani,a and Mohamed Z. M. Salem b
Extracts from the inner stem bark of Berberis vulgaris were analyzed for their antioxidant activity using the 1,1-dipheny-2-picrylhydrazyl (DPPH) method and compared with ascorbic acid (AA) and butylated hydroxytoluene (BHT). The most active extracts were analyzed for their chemical composition using gas chromatography-mass spectrometry. Acetone extract was found to be the most active as an antioxidant agent at 98.61%, which was higher than the value of vitamin C (93.03%) at the concentration of 0.16 mg/mL. The major components identified in the acetone extract were tetracosanoic acid, methyl ester (26.36%), followed by phthalic acid, diisooctyl ester (20.93%), 1,2-bis(trimethylsiloxy) ethane (10.26%), and 1,2-benzendicarboxylic acid, diisononyl ester (8.70%). The dissolved water:methanol (1:1 v/v) partitioned from acetone extract afforded 12 fractions; among them, fraction F11 was found to have good antioxidant activity (95.41%) at the concentration of 0.16 mg/mL. The major compounds identified in F11 were N-methyl-4-(hydroxybenzyl)-1,2,3,4-tetrahydroisoquinoloine (28.82%), 9-α-hydroxy-17β-(trimethylsilyl-oxy)-4-anderostene-3-methyloxime (13.97%), ribitol, pentaacetate (9.76%), 1-methyl-4-[4,5-dihydroxyphenyl]-hexahydropyridine (6.83%), and 2-ethylacridine (4.77%).
Keywords: Berberis vulgaris inner stem bark; Acetone extract; Fraction; GC/MS
Contact information: a: Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran; b: Department of Forestry and Wood Technology, Faculty of Agriculture (EL-Shatby), Alexandria University, Alexandria, Egypt; *Corresponding author: hashemi@kiau.ac.ir
INTRODUCTION
The barberry (Berberis vulgaris L., family Berberidaceae) plant grows in Asia and Europe. Various parts of this plant, including the roots, bark, leaves, and fruit, have been used extensively for medicinal purposes and are known for possessing antiarrhythmic and sedative effects in Iranian traditional medicine (Fatehi et al. 2005; Javadzadeh and Fallah 2012). B. vulgaris was macerated along with Foeniculum vulgare in Ancient Egypt to ward off pestilent fevers (Chevallier 2001), and was also used in Catawba for peptic ulcer disease.
Barberry is extensively used as a food additive, and the juice is recommended to cure cholecytitis (Zargari 1983). The B. vulgaris plant is a shrub, growing approximately 1 to 3 m tall, with spiny, yellow wood, and obviate leaves bearing pendulous yellow flowers succeeded by oblong red berries (Zargari 1983; Amin 1991; Ciulei et al. 1993; Dewick 2002; Damaschin and Analiza 2006).
The pharmacological, biochemical, and anti-cancer effects of berberine (isoquinoline alkaloid present in roots, rhizome, and outer bark) were reported in several studies concerning the importance of medicinal plant species in the Berberis genus (B. aquifolium, B. vulgaris, B. aristata, etc.) (Marinova et al. 2000; Kim et al. 2003; Mahata et al. 2011; Wu et al. 2011). Based on previous reports, the majority of the medical and toxic properties of barberries are related to the different alkaloids existing in the different parts of the plant (Končić et al. 2010; Javadzadeh and Fallah 2012). Barberry crude extract and the active alkaloid, berberine, have been promising for the treatment of hepatic oxidative stress, Alzheimer’s disease, and idiopathic male factor infertility (Abd El-Wahab et al. 2013). The ethanolic extract of dried powdery roots can be regarded as non-toxic, as it does not inhibit the growth of normal peripheral blood mononuclear cells that can induce cancer cells (Abd El-Wahab et al. 2013). Anthocyanins and barberry fruit extract have been found to have inhibitory effects on capillary permeability (Cohen-Boulakia et al. 2000) and epidermal growth factor (Meiers et al. 2001), as well as anticholinergic and antihistaminergic effects (Tomosaka et al. 2008).
Barberry fruits phenolic compounds, including anthocyanins and carotenoid pigments, have antioxidant and cytoprotective activities (Sabir 1971; Shamsa et al. 1999; Zhou and Mineshita 2000; Freile et al. 2003; Mahady et al. 2003; Kuo et al. 2004; Han and Lee 2005; Tomosaka et al. 2008). Extracts from different parts (bark, fruits) of B. vulgaris have been studied for their antibacterial and antifungal activities (Jain and Kar 1971; McCartney 1989; Parekh and Chanda 2005; Ghareeb et al.2013; Mahmoudvand et al. 2014). A larger number of these plants and their isolated constituents have beneficial therapeutic effects, such as a reduction in hepatitis C virus (HCV) symptoms, anti-oxidant, anti-inflammatory, anti-cancer, anti-microbial, and immunomodulatory activities (Huffman 2003; Ghareeb et al. 2013).
Barberry fruits have been reported to have a strong antioxidant capacity (Özgen et al. 2012). There were several studies related to the antioxidant activities of extracts from B. vulgaris. The ethanolic extracts from the roots, twigs, and leaves provide some antioxidant activities, which was determined to be caused by the scavenging effect on the DPPH free radical; the antioxidant activity was well correlated with the content of main plant antioxidants, phenols, and flavonols (Končić et al. 2010). The leaves and fruit have good antioxidant activity, which was revealed when using a DPPH free radical scavenging assay, and probably involves the high flavonoid content (Hadaruga et al. 2010).
To the best of our knowledge, there have been no reports on the chemical composition of the inner bark of the B. vulgaris stem extract. However, the chemical composition of this part has been reported. Previous phytochemical analyses of the root or stem bark extract of B. vulgaris has yielded the presence of protoberberines, bisbenzyl-isoquinoline alkaloids (berbamine, tetrandrine, chondocurine berberine, (-)-tejedine, jatrorrhizine, columbamine, berberubine, oxicanthine, palmatine, vitamin C, resin, and tannins), and flavonoids (quercetin and kaempferol) (Akhtar et al. 1978; Akhter et al. 1979; Dewick 1993; Ivanovska and Philipov 1996; Suau et al. 1998; Fatehi et al. 2005; Damaschin and Analiza 2006; Aghbashlo et al. 2008). Therefore, this novel approach investigated the antioxidant activity of some extracts, as well as the fractions of the stem inner bark extracts of B. vulgaris and compared them with ascorbic acid (AA) and butylated hydroxytoluene (BHT). The most active extract was analyzed for its chemical composition using gas chromatography-mass spectrometry (GC/MS).
EXPERIMENTAL
Plant Materials
Fresh stems of B. vulgaris were collected from Siahbishe, Chalous, and Mazandaran, Iran in May of 2013. The plant material was identified by Khosrow Ashrafi, Assistant Professor, Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran, and a voucher specimen was deposited in the Herbarium College of Agricultural and Natural Resources, Karaj Branch, Islamic Azad University, Karaj, Iran. The inner bark was separated from the stems and air-dried to achieve an 8.0% moisture content.
Extraction and fractionation
The inner bark of stems were cut into small pieces and chopped using a laboratory electrical rotary mill to obtain bark flour. The flour size was between 40 and 60 mesh. Approximately 50 g of this flour was placed into the 5 extraction thimbles, and then five independent extracted using pure acetone (300 mL in a 500-mL balloon) and a Soxhlet-type apparatus for 8 h. The combined extract was concentrated using a Heidolph Laborota 4001 rotary-evaporator apparatus (at 40 °C to reach total solvent evaporation) for approximately 15 min. Then, the extracts were collected, dried over anhydrous sodium sulphate, and stored at 4 °C until further analysis. The solid extractive weight was 2.72 g. Subsequently, 2.0 g of the solid extractives were dissolved in water:methanol (1:1 v/v) where the marc or the residue was discarded then the supernatant poured into a separatory funnel, followed by the addition 50 mL of n-hexane. The mixture was shaken by hand for 10 min. Half a gram of the water:methanol aqueous supernatant extractive was used for column chromatography with silica gel, Merck KGaA 64271 Darmstadt, Germany. The 12 fractions were labeled F1 to F12 (Fig. 1). The 3 × 10 mL eluent volume was used in the chromatographic separation for each solvent.
Fig. 1. Isolation scheme of active constituents of B. vulgaris from the water:methanol extract of inner stem bark.
Free radical scavenging activity by DPPH assay
The free radical scavenging activities of the acetone and water:methanol extracts, as well as the fractions from F1 to F12 of the stem inner bark powders, were determined using the 1,1-dipheny-2-picrylhydrazyl (DPPH) method (Karau et al. 2013). For this method, a stock solution was prepared by dissolving 2.4 mg of DPPH free radical in 100 mL of methanol. The stock solution was stored at 20 °C. The working solution was prepared by diluting the DPPH stock solution with methanol. Then, 1250 μL of the working solution was combined with 250 μL of the methanol extract from the medicinal plant (1 mg/mL). Serial dilutions were carried out with the stock solutions (1 mg/mL) of tested extract to obtain concentrations of 0.005, 0.01, 0.02, 0.04, 0.08, and 0.16 mg/mL. The experiment was performed in triplicates, and the average absorbance was recorded for each concentration. The reaction mixture was mixed for 10 s and left to stand at room temperature in a dark place for 30 min. The absorbance was measured at 517 nm, using a UV scanning spectrophotometer. Ascorbic acid (AA) and butylated hydroxytoluene (BHT) were used as the reference standards and were dissolved in methanol to make the stock solutions with the same concentration (1 mg/mL). The control samples were prepared with the same volume of solution, without test compounds and the referenced standards. Pure methanol (Sigma-Aldrich, Germany) was used as a blank. The DPPH free radical scavenging activity (%) was calculated using the following equation,
Inhibition = 100(Ac – As)/Ac (1)
where the percentage inhibition value was calculated from the absorbance of the control, Ac, and of the sample, As.
The controls contained all the reaction reagents except the extract or positive control substance. The values are presented as the means of triplicate analyses.
Analysis of extracts
Gas chromatography-mass spectrometry (GC/MS) analysis of the acetone and F11 extracts were performed using split mode (30:1 and 10:1) injection. One microlitre of the silylated extract, 30 μL N,O-bis-(trimethylsilyl)triflouroacetamide (BSTFA) + 1% trimethylchlorosilane (TMCS) reagent, and approximately 30 μL of pyridine were run on a HP 6890 (Hewlett Packard, USA) gas chromatograph fitted with a cross-linked 5.0% PH ME siloxane HP-5 capillary column (dimensions: 30 m x 0.25 mm, 0.50 μm coating thickness) and coupled with a model 5975B mass detector. The GC/MS operation conditions were as follows: injector temperature 250 °C; transfer line 290 °C; oven temperature program 50 to 250 °C (5 °C/min); carrier gas: He at 1.4 mL/min; mass spectra: electron impact (EI+) mode 70 eV with a mass range of 40 to 450 m/z; and ion source temperature at 250 °C. Individual components were identified using Wiley 275 L and NIST05 mass database matching and by comparing the retention times and mass spectra of constituents with published data (Julian and Konig 1988; Adams 1995, 2001). Retention indices (RI) were determined with reference to a homologous series of normal alkanes, using the following formula (Kovats 1958),
RI = 100 [(n + (N–n) × log t1R (x) – log t1R (Cn)/log t1R (CN) – log t1R (Cn)] (2)
where RI is the retention index of the compound of interest, t1R is the net retention time (tR–t0), t0 is the retention time of solvent (dead time), tR is the retention time of the compound of interest, Cn and CN are the number of carbons in the n-alkanes eluting immediately before and after the compound of interest, and N and n are the number of carbon atoms in the n-alkane eluting immediately before and after the compound of interest.
Statistical analysis
Data of antioxidant activity were statistically analyzed using the SPSS program.
RESULTS AND DISCUSSION
Antioxidant Activity
Statistically, there were significant differences among the treatments (F1→F12, water:methanol, acetone, BHT, vitamin C, and their concentrations (Tables 1 and 2).
The acetone extracts exhibited high antioxidant activity overall (Fig. 2). The lowest antioxidant activity, 91.66%, was observed at the concentration of 0.005 mg/mL, which was higher than for vitamin C (88.93%) at the same concentration. The highest activity came from acetone extract (98.61%) at 0.16 mg/mL, which was also higher than the value for vitamin C (93.03%) at the same concentration. The same trend was observed with the reference, BHT.
Additionally, among the assayed fractions, F11 and F12 showed high antioxidant activity at 95.41% and 93.65%, respectively, when the concentration was 0.16 mg/mL. Looking at Fig. 2, the more polar compounds seem to have the higher antioxidant activity, because following the extractions with non-polar solvents the fraction F11 retains an antioxidant activity comparable to acetone fraction.
Previously, the hexane fraction of the crude methanol extract from the shade dried plant material was the most active fraction, with an IC50 of 72 μg/mL (Kolář et al. 2010), in the case of inhibition with acetylcholinesterase. Berberine and methanolic barberry crude extract showed a significant reduction in their antioxidant abilities and radicals scavenging effects, especially on hydroxyl and DPPH radicals (El-Sayed et al. 2011). Furthermore, B. vulgaris extract, as well as berberine chloride, inhibited DPPH oxidation in the range of 13 to 46% more than control level (Abd El-Wahab et al. 2013).
Leaves, fruit, and stem extracts of B. vulgaris were reported as being active in the DPPH radical-scavenging assay, but the active constituents involved were not determined (Heinrich et al. 2005). However, Cannabisin G and (±)–lyoniresinol were responsible for the antioxidant activity of B. vulgaris root bark extract (Tomosaka et al. 2008).
Table 1. Statistical Analysis of the Effect of Treatments and Concentrations on the Antioxidant Activity of Inner Bark Extractives of B. vulgaris
Table 2. Mean±SD of the Antioxidant Activity (%) as Affected by Different Treatments of Inner Bark Extractives of B. vulgaris and Comparing them with BHT and Vitamin C
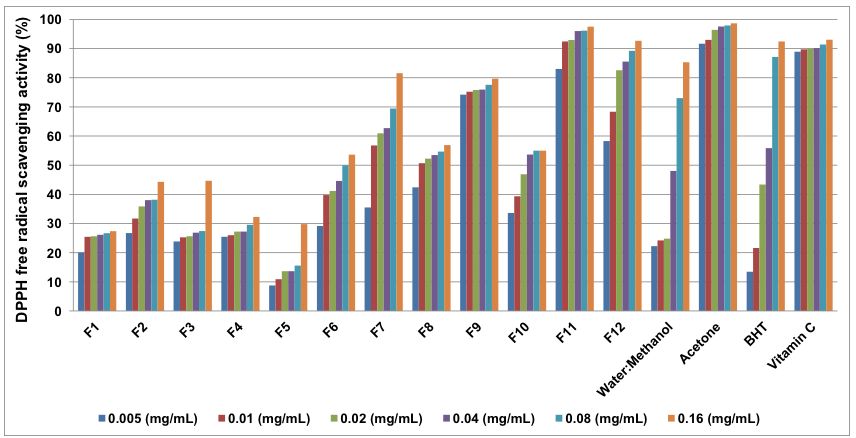
Fig. 2. The antioxidant activity of extracts from the inner bark of B. vulgaris
Inner Bark Extract
The suggestable chemical constituents identified in the acetone extract are presented in Table 3. The acetone extract of the fresh stem inner bark of B. vulgaris exhibited a yellowish extract with a pleasant aroma, and a yield of 8.0% (v/w). Ten components, comprising 100% of the inner bark extract, were identified. The major components of the inner bark extract were tetracosanoic acid, methyl ester (26.36%), phthalic acid, diisooctyl ester (20.93%), 1,2-bis(trimethylsiloxy)ethane (10.26%), and 1,2-benzenedicarboxylic acid, diisononyl ester (8.70%). Among the identified components, three of the components comprising 55.99% of the extract were fatty acids (aliphatic compounds), others included: sesquiterpene (5.22%) two aromatic amines (8.36%), benzoxepine derivatives (7.04%), oxetane compounds (6.80%), ammoxidized hydroquinone derivatives (10.26%), and ketone compound (6.34%).
Table 3. The Suggestable Chemical Composition of Acetone Inner Stem Bark Extract of B. vulgaris
Analyses of the extract showed that predominantly volatile organic compounds in the form of fatty acid esters were found. Two-(4-methylphenyl)-3-(1-hydroxyhexyl) oxetane is an oxetane derivative that is used in medicinal chemistry. Early studies in rats involving 3,3‐diethyloxetane and other simple oxetanes revealed their anesthetic, sedative, and anticonvulsant properties (Wuitschik 2008). For the coptidis, the use of an alternative biosynthesis route with proline, phenylalanine, catechollactate, and 2-mono-isobutyrin was proposed to synthesize its major bioactive alkaloids. It was observed that different fatty acids might be needed to modulate the biosynthesis of the bioactive secondary metabolites of medicinal herbs (Teo et al. 2011).
In addition, sesquiterpene, epi-ligulyl oxide, and derivatives from ammoxidized hydroquinone, i.e., 1,2-bis(trimethylsiloxy)ethane, were detected in barberry bark extracts for the first time. The epi-ligulyl oxide compound has been characterized in agarwood and eaglewood oil as a novel compound (Naf et al. 1992; Mei et al. 2008).
The suggestable chemical constituents identified in F11 are presented in Table 4. The major compounds were N-methyl-4-(o-hydroxybenzyl)-1,2,3,4-tetrahydroiso-quinoline (28.82%), 9-α-hydroxy-17β-(trimethylsilyloxy)-4-androstene-3-methyloxime (13.97%), ribitol, pentaacetate (9.76%), 1-methyl-4-[4,5-dihydroxyphenyl]-hexahydro-pyridine (6.83%), and 2-ethylacridine (4.77%). Most of the identified compounds were derived from isoquinoline alkaloids and one compound (ribitol, pentaacetate) was derived from carbohydrates.
Table 4. The Suggestable Chemical Composition of F11 Inner Stem Bark Extract of B. vulgaris
The alkaloid fraction has been found in some species of Berberis in both the stem and bark ethanolic extracts (Rocha et al. 2005). In the review article by Imanshahidi and Hosseinzadeh (2008), the most important constituents found in the stem bark of the plant were the isoquinoline alkaloids, acanthine, berbamine, berberine, columbamine, jatrorrhizine, and magnoflorine. Berberine and berbamine are the most biologically active compounds and are widely distributed in almost all Berberis species (Gorval and Grishkovets 1999; Dev 2006; Rashmi et al. 2008).
CONCLUSIONS
- The extracts from the inner stem bark of Berberis vulgaris exhibited high antioxidant activity and the values were higher than the standard antioxidant compounds (vitamin C and BHT).
- The suggestable major components identified in the acetone extract by GC/MS were tetracosanoic acid, methyl ester, phthalic acid, diisooctyl ester, 1,2-bis(trimethyl-siloxy)ethane, and 1,2-benzenedicarboxylic acid, diisononyl ester.
- Water:methanol (1:1 v/v) was divided into 12 fractions: Among these, fraction F11 exhibited high antioxidant activity and contained the following suggestable main chemical compounds by GC/MS: N-methyl-4-(o-hydroxybenzyl)-1,2,3,4-tetrahydro-isoquinoline, 9-α-hydroxy-17β-(trimethylsilyloxy)-4-androstene-3-methyloxime, ribitol, pentaacetate, 1-methyl-4-[4,5-dihydroxyphenyl]-hexahydropyridine, and 2-ethylacridine.
- It can be concluded that the extracts from the inner bark of Berberis vulgaris possess high antioxidant activity and most of the identified compounds are isoquinoline alkaloids.
ACKNOWLEDGMENTS
The authors are grateful to the Islamic Azad University, Karaj Branch for their financial support of the project. The authors would like to thank Dr. Irani, Dr. Ghare-vaysi, Dr. Mirabi, and Mr. Miri (Research of Islamic Azad University, Ghaemshahr Branch) for the use of their laboratory equipment, and special thanks to Mrs. Azimi (operator of GC/MS apparatus) for helping with the injection of test samples.
REFERENCES CITED
Abd El-Wahab, A. E., Ghareeb, D. A., Sarhan, E. E. M., Abu-Serie, M. M., and El Demellawy, M. A. (2013). “In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: Antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects,” BMC Complementary and Alternative Medicine 13(1), 218. DOI: 10.1186/1472-6882-13-218
Adams, R. P. (1995). Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry, 1st Edition, Allured Publishing Corp., Carol Stream, IL.
Adams, R. P. (2001). Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 3rd Edition, Allured Publishing Corp., Carol Stream, IL.
Aghbashlo, M., Kianmehr, M. H., and Samimi-Akhijahani, H. (2008). “Influence of drying conditions on the effective moisture diffusivity, energy of activation and energy consumption during the thin-layer drying of Berberis fruit (Berberidaceae),” Energy Conversion and Management 49(10), 2865-2871. DOI: 10.1016/j.enconman.2008.03.009
Akhtar, N., Naqvi, H., and Hussain, F. (1978). “Biochemical inhibition (allelopathy) exhibited by Cenchrus ciliaris and Chrysopogon aucheri,” Pakistan Journal of Forestry 28(4), 194-200.
Akhter, M. H., Sabir, M., and Bhide, N. K. (1979). “Possible mechanism of antidiarrheal effect of berberine,” Indian Journal of Medical Research 70, 233-241.
Amin, G. H. (1991). Popular Medicinal Plants of Iran, Health Ministry Press, Tehran, Iran.
Chevallier, A. (2001). The Encyclopedia of Medicinal Plants: A Practical Reference Guide to More Than 550 Key Medical Plants & Their Uses, Reader’s Digest, Pleasantville, NY.
Ciulei, I., Grigorescu, E., and Stănescu, U. (1993). Plante Medicinale, Fitochimie şi Fitoterapie (Medicinal herbs, Phytochemistry and Phytotherapy), vol. I, II, Ed. Medicală, Bucureşti, pp. 384-390.
Cohen-Boulakia, F., Valensi, P. E., Boulahdour, H., Lestrade, R., Dufour-Lamartinie, J. F., Hort-Legrand, C., and Behar A. (2000). “In-vivo sequential study of skeletal muscle capillary permeability in diabetic rats: Effect of anthocyanosides,” Metabolism 49(7), 880-885. DOI: http://dx.doi.org/10.1053/meta.2000.6754
Dev, S. (2006). A Selection of Prime Ayurvedic Plants Drugs, Ancient-Modern Concordance, Anamaya Publishers, New Delhi, India.
Damaschin, N., and Analiza şi, V. V. (2006). “Standardizarea unor forme farmaceutice homeopate,” (Standardization of homeopathic medicinal forms), Institutul Naþional de Farmacie, Chiºinãu, p. 25-32.
Dewick, P. M. (1993). “Isoflavonoids,” in: The Flavonoids. Advances in Research since 1986, Harborne, J. B. (ed.), Chapman and Hall, London, pp. 117-238.
Dewick, P. M. (2002). Medicinal Natural Products: A Biosynthetic Approach, John Wiley & Sons, Chichester, West Sussex, UK.
El-Sayed, M., Ghareeb, D. A., Sarhan, E. M., and Khalil, A. A. (2011). “Therapeutic bio-screening of the bioactive extracted ingredients of Berberis vulgaris, berberine,” Functional Plant Science and Biotechnology 5 (Special Issue 1), 63-68.
Fatehi, M., Saleh, T. M., Fatehi-Hassanabad, Z., Farrokhfal, K., Jafarzadeh, M., and Davodi, S. (2005). “A pharmacological study on Berberis vulgaris fruit extract,” Journal of Ethnopharmacology 102(1), 46. DOI: 10.1016/j.jep.2005.05.019
Freile, M. L., Giannini, F., Pucci, G., Sturniolo, A., Rodero, L., Pucci, O., Balzareti, V., and Enriz, R. D. (2003). “Antimicrobial activity of aqueous extracts and of berberine isolated from Berberis heterophylla,” Fitoterapia 74(7-8), 702-705. DOI: 10.1016/S0367-326X(03)00156-4
Ghareeb, D. A., Abd El-Wahab, A. E., Sarhan, E. E. M., Abu-Serie, M. M., and El Demellawy, M. A. (2013). “Biological assessment of Berberis vulgaris and its active constituent, berberine: Antibacterial, antifungal and anti-hepatitis C virus (HCV) effect,” Journal of Medicinal Plants Research 7(21), 1529-1536. DOI: 10.5897/JMPR13.4443
Gorval, L. M., and Grishkovets, V. L. (1999) “Alkaloids of some species of the genus Berberis introduced into the Crimea,” Chemistry Nature Compounds 35(2), 223-224.
Hadaruga, D. I., Hadăruga, N. G., Rivis, A., Costescu, C., Ordodi, V. L., and Ardelean, A. (2010). “Berberis vulgaris extract/beta-cyclodextrin nanoparticles synthesis and characterization,” Revista de Chimie 61(7), 669-675.
Han, Y., and Lee, J. H. (2005). “Berberine synergy with amphotericin B against disseminated candidiasis in mice,” Biological and Pharmaceutical Bulletin 28(3), 541-544. DOI: 10.1248/bpb.28.541
Heinrich, M., Leonti, M., Nebel, S., Peschel, W., Pieroni, A., Smith, F., Rivera, D., Obon, C., Inocencio, C., Verde, A., et al. (2005). “Understanding local Mediterranean diets: A multidisciplinary pharmacological and ethnobotanical approach,” Pharmacology Research 52(4), 353-366. DOI: 10.1016/j.phrs.2005.06.005
Huffman, M. (2003). “Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants,” Proceedings of the Nutrition Society 62(02), 371-381. DOI: 10.1079/PNS2003257
Imanshahidi, M., and Hosseinzadeh, H. (2008). “Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent,” Phytotherapy Research 22(8), 999-1012. DOI: 10.1002/ptr.2399
Ivanovska, N., and Philipov, S. (1996). “Comparative study on the immunological activity of a series of isoquinoline alkaloids,” Phytotherapy Research 10(1), 62-65. DOI: 10.1002/(SICI)1099-1573(199602)10:1<62::AID-PTR777>3.0.CO;2-Q
Jain, S. R., and Kar, A. (1971). “The antibacterial activity of some essential oils and their combinations,” Planta Medica 20(4), 118-123. DOI: 10.1055/s-0028-1099675
Javadzadeh, S. M., and Fallah, S. R. (2012). “Therapeutic application of different parts Berberis vulgaris,” International Journal of Agricultural Crop Science 4(7), 404-408.
Julian, D., and Konig, W. A. (1988). The Atlas of Spectral Data of Sesquiterpene Hydrocarbons, E. B. Verlag, Hamburg, Germany.
Karau, G. M., Njagi, E. N. M., Machocho, A. K., Wangai, L. N., and Kamau, P. N. (2013). “Phytonutrients, minerals and in vitro antioxidant capacity of leaf and stem bark powders of Senna spectabilis,” Journal of Pharmacognosy and Phytochemistry 2(2), 51-59.
Kim, T. S., Kang, B. Y., Cho, D., and Kim, S. H. (2003). “Induction of interleukin-12 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid, deviates CD4+ T cells from a Th2 to a Th1 response,” Immunology 109(3), 407-414. DOI: 10.1046/j.1365-2567.2003.01673.x
Kolář, D., Wimmerová, L., and Kádek, R. (2010). “Acetylcholinesterase and butyrylcholinesterase inhibitory activities of Berberis vulgaris,” Phytopharmacology 1(1), 7-11. DOI: 10.1.1.207.9096
Končić, M. Z, Kremer, D., Karlović, K., and Kosalec, I. (2010). “Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat,” Food and Chemical Toxicology 48(8-9), 2176-2180. DOI: 10.1016/j.fct.2010.05.025
Kovats, E. (1958). “Characterization of organic compounds by gas chromatography, Part 1. Retention, indices of aliphatic halides, alcohols, aldehydes, and ketones,” Helvetica Chimica Acta 41(7), 1915-1932. DOI: 10.1002/hlca.19580410703
Kuo, C., Chi, C., and Liu, T. (2004). “The anti-inflammatory potential of berberine in-vitro and in-vivo,” Cancer Letters 203(2), 127-137. DOI: 10.1016/j.canlet.2003.09.002
Mahata, S., Bharti, A. C., Shukla, S., Tyagi, A., Husain, S. A., and Das, B. C. (2011). “Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells,” Molecular Cancer 10(4), 39. DOI: 10.1186/1476-4598-10-39
Mahady, G. B., Pendland, S. L., Stoia, A., and Chadwick, L. R. (2003). “In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis,” Phytotherapy Research 17(3), 217-221. DOI: 10.1002/ptr.1108
Mahmoudvand, H., Mousavi, S. A. A., Sepahvand, A., Sharififar, F., Ezatpour, B., Gorohi, F., Dezaki, E. S., and Jahanbakhsh, S. (2014). “Antifungal, antileishmanial, and cytotoxicity activities of various extracts of Berberis vulgaris (Berberidaceae) and its active principle berberine,” ISRN Pharmacology2014, 302436. DOI: 10.1155/2014/602436
Marinova, E. K., Nikolova, D. B., Popova, D. N., Gallacher, G. B., and Ivanovska, N. D. (2000). “Suppression of experimental autoimmune tubulointerstitial nephritis in BALB/c mice by berberine,” Immunopharmacology 48(1), 9-16. DOI: 10.1016/S0162-3109(99)00189-7
McCartney, A. C. S. (1989). Practical Medical Microbiology, 14th Edition, Collee, J. G., Fraser, A. G., Marmion, B. P., and Simmons, A. (eds.), Churchill Livingstone, New York, pp. 161-181.
Mei, W. L., Zeng, Y. B., Wu, J., Cui, H. B., and Dai, H. F. (2008). “Chemical composition and anti-MRSA activity of the essential oil from Chinese eaglewood,” Journal of Chinese Pharmaceutical Sciences 17(5), 225-229.
Meiers, S., Kemény, M., Weyand, U., Gastpar, R., von Angerer, E., and Marko, D. (2001). “The anthocyanidins cyaniding and delphinidin are potent inhibitors of the epidermal growth factor receptor,” J. Agric. Food Chem. 49(2), 958-962. DOI: 10.1021/jf0009100
Naf, R., Velluz, A., Busset, N., and Gaudin, J. M. (1992). “New nor-sesquiterpenoids with 10-epi-eudesmane skeleton from agarwood (Aquilaria agallocha Roxb.),” Flavour & Fragrance Journal 7(6), 295-298. DOI: 10.1002/ffj.2730070602
Özgen, M., Saraçoğlu, O., and Geçer, E. N. (2012). “Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L.) fruits,” Horticulture, Environment, & Biotechnology 53(6), 447-451. DOI: 10.1007/s13580-012-0711-1
Parekh, J. N. R., and Chanda, S. (2005). “Preliminary screening of some folkloric plants from Western India for potential antimicrobial activity,” Indian Pharmacology Journal 37(6), 408-409. DOI: 10.4103/0253-7613.19085
Rashmi, P. J., Rajasekaran, A., and Pant, J. (2008). “The genus Berberis Linn.: A review,” Pharmacognosy Reviews 2(4), 369-385.
Rocha, L. G., Almeida, J. R. G. S., Macedo, R. O., and Barbosa-Filho, J. M. (2005). “A review of natural products with antileishmanial activity,” Phytomedicine 12(6-7), 514-535. DOI: 10.1016/j.phymed.2003.10.006
Sabir, M. (1971). “Study of some pharmacological actions of berberine,” Indian Journal of Physiology & Pharmacology 15(3), 111-132.
Shamsa, F., Ahmadiani, A., and Khosrokhavar, R. (1999). “Antihistaminic and anticholinergic activity of barberry fruit (Berberis vulgaris) in the guinea-pig ileum,” Journal of Ethnopharmacology 64(2), 161-166. DOI: http://dx.doi.org/10.1016/S0378-8741(98)00122-6
Suau, R., Rico, R., Lopez-Romero, J. M., Najera, F., and Cuevas, A. (1998). “Isoquinoline alkaloids from Berberis vulgaris subsp. Australis,” Phytochemistry 49(8), 2545-2549. DOI: 10.1016/S0031-9422(98)00121-6
Teo, C. C., Tan, S. N., Hong Yong, J. W., Ra, T., Liew, P., and Ge, L. (2011). “Metabolomics analysis of major metabolites in medicinal herbs,” Analytical Methods 3(3), 2898-2908. DOI: 10.1039/C1AY05334E
Tomosaka, H., Salim, A. A., Keller, W. J., Chai, H., and Kinghorn, A. D. (2008). “Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry),” Phytotherapy Research 22(7), 979-981. DOI: 10.1002/ptr.2443
Wuitschik, G. (2008). Oxetanes in Drug Discovery, Ph.D. dissertation, Swiss Federal Institute of Technology in Zurich, German: Eidgenössische Technische Hochschule Zürich.
Wu, Y., Li, J. Q., Kim, Y. J., Wu, J., Wang, Q., and Hao, Y. (2011). “In vivo and in vitro antiviral effects of berberine on influenza virus,” Chinese Journal of Integrative Medicine 17(6), 444-452. DOI: 10.1007/s11655-011-0640-3
Zargari, A. (1983). Medicinal Plants, Tehran University Press, Tehran, Iran.
Zhou, H., and Mineshita, S. (2000). “The effect of berberine chloride on experimental colitis in rats in vivo and in vitro,” Journal of Pharmacology & Experimental Therapeutics 294(3), 822-829.
Article submitted: August 5, 2015; Peer review completed: September 28, 2015; Revised version received and accepted: October 6, 2015; Published: October 13, 2015.
DOI: 10.15376/biores.10.4.7958-7969
