Abstract
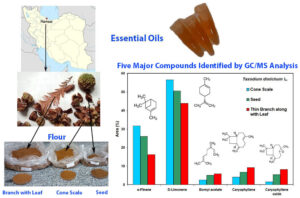
The essential oils extracted from the cone scale (CS), seed (SE), and thin branch with leaf (BL) of Taxodium distichum harvested during the winter season were analyzed by gas chromatography-mass spectrometry. Up to 37 components were identified, accounting for more than 96% of the total oil volume in all samples. Monoterpenes (CS 93.4%, SE 85.2%, and BL 72.8%) represented the major constituents of the essential oils, followed by smaller quantities of sesquiterpenoids. Monoterpene hydrocarbons (MH) dominated, with limonene (CS 56.5%, SE 50.5%, and BL 43.8%) and α-pinene (CS 31.7%, SE 26.1%, and BL 16.2%) being the main constituents. Representative minor constituents in the CS oil are camphene (1.3%) and bornyl acetate (2.6%); in the SE oil, β-myrcene (1.7%) and bornyl acetate (5.1%) were found; and in BL essential oil, β-myrcene (3.1%) and bornyl acetate (6%). Sesquiterpene hydrocarbons (CS 4.6%, SE 7.8%, and BL 12.9%) were the other major subclasses of components, with caryophyllene (CS 4.1%, SE 6.8%, and BL 9.3%) as the main constituent. The only oxygen-containing sesquiterpene found was caryophyllene oxide (CS 1.5%, SE 5.5%, and BL 8.3%). The compounds could be of great interest in food, cosmetics, and pharmaceutical applications.
Download PDF
Full Article
Chemical Composition and Content of Essential Oil from Cultivated Bald Cypress (Taxodium distichum L.)
Seyyed Khalil Hosseinihashemi,a,* Sayed Khosrow Hosseinashrafi,a,* Mehrnoush Kelkian,b Zohreh Shafighi,c and Luiz Claudio Almeida Barbosa d
The essential oils extracted from the cone scale (CS), seed (SE), and thin branch with leaf (BL) of Taxodium distichum harvested during the winter season were analyzed by gas chromatography-mass spectrometry. Up to 37 components were identified, accounting for more than 96% of the total oil volume in all samples. Monoterpenes (CS 93.4%, SE 85.2%, and BL 72.8%) represented the major constituents of the essential oils, followed by smaller quantities of sesquiterpenoids. Monoterpene hydrocarbons (MH) dominated, with limonene (CS 56.5%, SE 50.5%, and BL 43.8%) and α-pinene (CS 31.7%, SE 26.1%, and BL 16.2%) being the main constituents. Representative minor constituents in the CS oil are camphene (1.3%) and bornyl acetate (2.6%); in the SE oil, β-myrcene (1.7%) and bornyl acetate (5.1%) were found; and in BL essential oil, β-myrcene (3.1%) and bornyl acetate (6%). Sesquiterpene hydrocarbons (CS 4.6%, SE 7.8%, and BL 12.9%) were the other major subclasses of components, with caryophyllene (CS 4.1%, SE 6.8%, and BL 9.3%) as the main constituent. The only oxygen-containing sesquiterpene found was caryophyllene oxide (CS 1.5%, SE 5.5%, and BL 8.3%). The compounds could be of great interest in food, cosmetics, and pharmaceutical applications.
DOI: 10.15376/biores.19.1.751-765
Keywords: Taxodium distichum; Cone scale; Seed; Thin branches; Leaf; Essential oil content; Chemical composition; GC/MS
Contact information: a: Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran; b: Manager of Arya Herb Gene Biotechnology, Tehran, Iran; c: Department of Horticultural Sciences, Karaj Branch, Islamic Azad University, Karaj, Iran; d: Department of Chemistry, Universidade Federal de Minas Gerais, Av. Pres. Antônio Carlos, 6627, Campus Pampulha, CEP 31270-901, Belo Horizonte, MG, Brazil;
*Corresponding author: hashemi@kiau.ac.ir; khosrowashrafi@gmail.com
GRAPHICAL ABSTRACT

INTRODUCTION
Taxodium distichum is known as bald cypress and distributed in the eastern United States from Maryland and Illinois south into Florida and Central Texas (Middleton and McKee 2004). The plant species are spread as plantations in different regions, such as in wet or poor drainage soil lands of Guilan and Mazandaran, north of Iran, because the trees grow remarkably well in almost any soil including heavy, compacted, or poorly drained areas (Abdelsalam et al. 2019). Such topics have been studied by many researchers for different purposes (Jaimand and Rezaei 2003; Rostamabadi et al. 2010, 2013; Eslamdoust et al. 2014, 2015a, 2015b). The T. distichum tree is cultivated in urban areas as an ornamental and timber tree (Drazic and Batos 2002; Ninic-Todorovic and Ocokoljic 2002; Sijacic-Nikolic et al. 2010; Abdelsalam et al. 2019). The essential oil composition of different plant parts of T. distichum were investigated earlier from various geographic regions (Odell 1912; Liangfeng et al. 1995; El Tantawy et al. 1999; Flamini et al. 2000; Jaimand and Rezaee 2003; Abou Dahab et al. 2007; Ogunwande et al. 2007; Adams et al. 2012; Su et al. 2013; Starks et al. 2014).
The leaves and cones of the species are particularly rich in essential oils, and they were used as folk medicine to treat skin, gastro-intestinal, respiratory, inflammation, and infections (Ramos et al. 1984; Cortés-Arroyo et al. 2011).
The important uses of essential oils include food, perfumery, cosmetic, cleaning, sanitary, and pharmaceutical industries, as well as in medicine (Bartels 2001; Manach et al. 2004; Kaminaga et al. 2006). Furthermore, the essential oils of many plant species have been reported to possess useful biological, pharmacological, and therapeutic activities (Barclay and McKersie 1994; Garcıa et al. 2003; Adesina 2005; Lattanzio et al. 2006; Mustafa and Verpoorte 2007; Khadem and Marles 2010).
The most abundant chemical compounds in the volatile oil of T. distichum seed cones previously reported by Odell (1912) included dextro pinene (85%), along with dextro limonene (5%), carvone (3%), tricyclic sesquiterpene (3%), and a “pseudoterpene alcohol” (2%).
In the study conducted by Jaimand and Rezaei (2003), the time lag collection of T. distichum cone essential oil was considered. The cones were harvested in mid-summer (August 2000), and three fractions were collected at different times (10, 60, and 120 min) using the hydrodistillation method with a Clevenger-type apparatus. Subsequently, the chemical constituents were identified using gas chromatography (GC) and GC/mass spectrometry (MS) devices. These analyses showed that the major constituents in the first, second, and third fractions were α-pinene, 1-terpineol, and β-caryophyllene, in variable quantities.
Đapić and Ristić (2017) stated that in the chromatogram of cones extract (absolute ethanol) of coppery-red bald cypress collected in January 2015 in the Futoški Park, Novi Sad, Serbia revealed the presence of 53 compounds, of which 33 compounds were identified. Thus, the extract contained oxygenated monoterpenes (12.4%), sesquiterpenes (5.2%), oxygenated sesquiterpenes (17.4%), diterpenes (1.2%), and oxygenated diterpenes (30.9%), while the amount of retinoic acid was 0.3%. Monoacylglycerols were detected in the amount of 4.3%. The most abundant compounds of cones extract were: caryophyllene oxide (14.3%), 6,7-dehydro-ferruginol (12.5%), bornyl acetate (11.0%), 6-deoxy-taxodione (9.5%), and trans-caryophyllene (4.2%).
Furthermore, the cone essential oil of T. distichum grown in Egypt has been characterized to contain α-pinene (87.3%), thujopsene (3.7%), and minor quantities (< 2%) of myrcene, β-pinene, and limonene, which exhibit effective antimicrobial, antispasmodic, and anti-inflammatory properties (El Tantawy et al. 1999). Ogunwande (2007) conducted an analysis of the cytotoxic effects of T. distichum leaves and cone oils collected from Nigeria. Their findings revealed that the predominant compounds were α-pinene (60.5%) and thujopsene (17.6%) in the cones, while the leaves contained thujopsene (27.7%), pimara-8(14),15-diene (13.1%), widdrol (12.8%), and β-caryophyllene (11.4%).
Considering the great variation in the composition of T. distichum essential oil reported so far, and that there are few studies on the plant collected in Iran, the present work aimed to expand the previous studies mentioned above, trying to obtain and transmit a better knowledge of the volatiles from seeds, cones, and branch with leaf of this industrially important bald cypress, harvested for the first time in winter.
EXPERIMENTAL
Plant Material
Female cones and thin branch along with leaf of three individual plants representing the local population of cultivated T. distichum from Ramsar city, Mazandaran province in Iran stretched between 36º53′11.68″ N and 50º34′11.68″ E were harvested in December 2022. The weather is rainy and snowy in late autumn and winter. Regarding information about weather provided from synoptic station in Ramsar, the maximum and minimum temperature are 32.6 °C and -3 °C, respectively, and the average yearly raining is 1107 mL (Saeb et al. 2011). The location of sampling was in the zero height in 0.01 probability level.
The collected materials (female cones and thin branch along with leaf) were air-dried in shade at an ambient temperature about 23 ± 2 ºC. The plant part of T. distichum, including female cones, was cut into small pieces to separate seeds from cone scale, and then the plant parts chopped to obtain lignocellulosic flour. The particle size was between 30- and 40-mesh. The plant material was identified by the author (Sayed Khosrow Hosseinashrafi), Assistant Professor, Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran, and a voucher specimen was deposited in the Herbarium of College of Agriculture and Natural Resources of Islamic Azad University, Karaj Branch, Karaj, Iran, under the code 5242.
Isolation of Essential Oils
Air-dried cone scale, seed, and thin branch along with leaf from T. distichum (200 g for each harvested in winter (December 2022) were chopped, then poured separately into 2500-mL round bottom all-glass flasks that contained 1500 mL distilled water. Finally, test materials were hydrodistilled for 3 h using a clevenger-type apparatus in accordance with the method of Hosseinihashemi et al. (2023a, 2023b, 2023c).
The collected oil samples were dried over anhydrous sodium sulphate (Na2SO4), stored in sealed 2-mL plastic vials, and kept in a refrigerator at 4 ℃ until GC/MS analysis. The essential oil content was expressed as 1 mL per 100 g based on the dry weight of the plant material.
Essential Oils Analysis
The oil obtained from T. distichum cone scale, seed, and thin branch (with leaves) were analyzed by gas chromatography-mass spectrometry (GC-MS). Accordingly, 100 μL of each oil were dried over anhydrous sodium sulfate salt and dissolved with 900 μL of hexane and the sample was injected on a GC Agilent-7890A coupled to a MS Agilent 5975C mass spectrometer detector (Agilent Technologies, Palo Alto, CA, USA). For the analyses, a HP-5MS cross-linked capillary column (30 m long and 0.25 mm internal diameter, 0.25 μm film thickness) was used.
The GC/MS operation conditions were as follows: Helium was the carrier gas with a flow rate of 1 mL/min; injector temperature of 260 °C; transfer line of 270 °C; oven temperature program of 60 °C for 4 min, 3 °C/min to 100 °C for 2 min, then 4 °C/min to 250 °C for 5 min; carrier gas was He at 1 mL/min; the intrinsic energy that hits the sample in the MS system was 70 eV. The split ratio of the sample was 50:1 with a split flow (column flow) of 1 mL/min, with a total chromatographic run time of 52 min as reported by Barzegari et al. (2023).
The compounds were identified by comparing their mass spectra with data from two mass spectrometric libraries (Wiley 275 L, 1998 and NIST-05), mass database matching, and by comparing the retention times with published data (Julian and König 1988; Adams 2001). The retention indices (RI) were determined with reference to a homologous series of normal alkanes (C9 to C23) as proposed by Kovàts (1958).
The percentages of all identified components were calculated based on their corresponding peak areas, with the total area of all peaks in the chromatogram considered as 100%.
RESULTS AND DISCUSSION
The Yield of Cone Scale, Seed, and Thin Branch Along with Leaf Essential Oils
The yield of essential oils from the cone scale, seed, and thin branch along with leaf of T. distichum was determined by hydrodistillation and expressed as a percentage in relation to the dry weight of the plant parts. Hydrodistillation of T. distichum gave light-orange oils with yields of 1 mL/100 g, based on the dry weight of cone scale, seed, and thin branch along with leaf.
GC/MS Analysis of Essential Oils from Cone Scale, Seed, and Thin Branch Along with Leaf
Figures A1, A2, and A3 show the chromatograms of the essential oils of T. distichum cone scale, seed, and thin branch along with leaf obtained from Ramsar station and analyzed in the HP-5MS column.
The chemical components identified in the essential oils from cone scale, seed, and branch along with leaf of T. distichum are presented in Tables 1, 2, and 3. The GC-MS profiling revealed the cone scale, seed, and branch along with leaf of T. distichum contained 14, 18, and 37 volatile and bioactive components, representing 99.9, 99.5, and 96.2% of the oil volume of mentioned parts, respectively, in winter (December 2022). Among the classes, monoterpene hydrocarbons were most abundant (90.6, 79.9%, and 65.8%, respectively), followed by sesquiterpene hydrocarbons (4.6, 7.8, and 12.9%, respectively), oxygenated monoterpenes (2.8, 5.3, and 6.9%, respectively), and oxygenated sesquiterpene (1.6, 5.8, and 9.1%, respectively) (Table 4). Among the monoterpene hydrocarbons, limonene (56.5, 50.5, and 43.8%, respectively) and α-pinene (31.7, 26.1, and 16.2%, respectively) were the major compounds. The minor monoterpene hydrocarbons detected in cone scale oil were camphene (1.3%), β-pinene (0.6%), tricyclene (0.3%), and α-terpinolene (0.2%), in seed oil were β-myrcene (1.7%), camphene (0.9%), β-pinene (0.4%), and tricyclene (0.3%), but in branch along with leaf were β-myrcene (3.1%), camphene (1.0%), β-pinene (0.6%), α-terpinolene (0.6%), tricyclene (0.3%), and γ-terpinene (0.1%) (Tables 1, 2, and 3).
Among the sesquiterpene hydrocarbons, caryophyllene (4.1, 6.8, and 9.3%, respectively) was the main components in cone scale, seed, and branch along with leaf oils. The minor sesquiterpene hydrocarbons identified in cone scale and seed oils were α-humulene (0.5 and 0.9%, respectively), while, in branch along with leaf oil were (-)-β-bourbonene (0.03%), longifolene (0.04%), α-muurolene (0.04%), and γ-cadinene (0.05%).
Table 1. Essential Oil Composition of T. distichum Cone Scale that Harvested in Winter
KI exp and KI lit: experimental and literature Kovats indices, respectively, on HP-5MS column in reference to n-alkanes; RT: retention time
Caryophyllene oxide as a main oxygenated sesquiterpene was identified in cone scale, seed, and branch along with leaf oils (1.5, 5.5, and 8.3%, respectively) (Tables 1, 2, and 3).
In general, the results of the study show that the qualitative and quantitative composition of the CS and SE oils collected from Ramsar, Mazandaran province, Iran was similar, whilst the qualitative and quantitative composition of the BL oil was different.
The monoterpene hydrocarbons of both cone scale and seed oils were characterized by higher amounts (90% of cone scale, 79% of seed) than that of BL (65.8%). Most of the identified compounds in the cone scale were also found in the seed, but the seed oil contained two times more sesquiterpene hydrocarbons and oxygenated sesquiterpenes (almost 4%). These compounds were higher in the thin branch along with leaf oil.
The high content of α-pinene among the monoterpene, in the cone scale oil, is in agreement with the earlier reports of samples analyzed in Egypt, Iran, Nigeria, and India (El Tantawy et al. 1999; Flamini et al. 2000; Jaimand and Rezaei 2003; Ogunwande et al. 2007; Adams 2012; Padalia et al. 2016), and caryophyllene oxide among the sesquiterpenoids, is in agreement with the earlier report of sample analyzed by Jaimand and Rezaei (2003) in Iran.
According to the literature, the composition of examined T. distichum essential oil to compare contents of seven major components, e.g., tricyclene, α-pinene, β-pinene, myrcene, limonene, α-terpineol, and caryophyllene oxide, the plant essential oils from different geographic regions were subjected to the hierarchical cluster analysis (Padalia et al. 2016).
The major oxygenated monoterpene in this study was bornyl acetate (2.6% for CS, 5.1% for SE, and 6.0% for BL); additionally, the major sesquiterpene hydrocarbon was caryophyllene with the above-mentioned percentage that is similar to the results in study of Ogunwande et al. (2007) on T. distichum cone oil. Caryophyllene is widely researched and developed in the pharmaceutical, food, and cosmetics industries because it provides benefits in all three sectors (Kubo et al. 1996; Yin et al. 2019).
Table 2. Essential Oil Composition of T. distichum Seed that Harvested in Winter
KI exp and KI lit: experimental and literature Kovats indices, respectively, on HP-5MS column in reference to n-alkanes; RT: retention time
In the current investigation, the analysis revealed the presence of specific compounds in varying percentages in the CS, SE, and BL harvested during winter. Limonene, α-pinene, caryophyllene, caryophyllene oxide, and bornyl acetate were identified at 56.5%, 50.5%, and 43.8%; 31.7%, 26.1%, and 16.2%; 4.1%, 6.8%, and 9.3%; 1.5%, 5.5%, and 8.3%; and 2.6%, 5.1%, and 6.0%, respectively. The corresponding values with bold emphasis can be found in Tables 1, 2, and 3.
Sandaracopimaradiene and abietatriene as diterpene hydrocarbons and ferruginol as abietane oxygenated diterpene were found in the seed oil with minor amounts; ferruginol was only present in the cone scale oil with minor amounts, but sandaracopimaradiene, abieta-8,11,13-triene, sandaracopimarinal, and ferruginol were also present in the cone scale oil with minor amounts, where diterpenes also observed in the leaves, cones, and branches oil of T. distichum analyzed by Flamini et al. (2000) and Ogunwande et al. (2007).
The cone essential oil had a monoterpene hydrocarbon limonene in the percentage of 18.7% (Flamini et al. 2000). T. distichum cones belong to the Mediterranean Basin, where climate is mild with rainy winters and hot and dry summers and showed that α-pinene was present in 71.3%.
The predominant compound in the majority of the essential oils analyzed was α-pinene, while limonene was the next compound present in the analyzed samples (Đapić and Ristić 2017). In contrast, according to the information about weather provided from synoptic station in Ramsar, the weather is rainy and snowy in late autumn and winter (Saeb et al. 2011).
Table 3. Essential Oil Composition of T. distichum Thin Branch Along with Leaf that Harvested in Winter
KI exp and KI lit: experimental and literature Kovats indices, respectively, on HP-5MS column in reference to n-alkanes; RT: retention time
Table 4. Classification of the Identified Chemical Components of the T. distichum Cone Scale, Seed, and Branch Along with Leaf Essential Oil by GC/MS Data Analysis
The oils chemical composition of bald cypress in Italy had obviously higher content in limonene; more oxygenated monoterpenes, and sesquiterpene hydrocarbons (Flamini et al. 2000), while the oils from Nigeria contained much lower α-pinene level but had higher thujopsene content (Ogunwande et al. 2007). Thujopsene was not reported in the sample from Italy (Su et al. 2013) and limonene was not reported in the sample from Iran (Jaimand and Rezaee 2003).
The composition of essential oils in feminine cones, leaves, and branches of bald cypress, as identified by Flamini et al. (2000), revealed high concentrations of α-pinene (53.7% to 79.7%) and limonene (3.7% to 18.7%). Conversely, samples collected from China, as analyzed by Liangfeng et al. (1995), featured caryophyllene oxide (41.67%) as the predominant constituent, accompanied by significant proportions of bornyl acetate (6.24%), perilla ketone (5.45%), and α-asarone (5.39%).
Additionally, Odell (1912) reported that the major constituents of the seed cones of T. distichum were α-pinene (85.0%), d-limonene (5.0%), and carvone (3.0%). Comparatively, the data from our current study indicates that while the primary components of the volatile oils of T. distichum remain consistent, their quantities vary significantly.
According to the previous data (Howard et al. 1988; Kordali et al. 2006; Norouzi-Arasi et al. 2006), essential oils of other plant species possessing caryophyllene oxide as a major constituent are toxic. Acroptilon repens volatile oils containing 36.6% of caryophyllene oxide and 10% of caryophyllene inhibited the growth of Gram-positive bacteria (Norouzi-Arasi et al. 2006). This sesquiterpene oxide is toxic to ants and inhibits the growth of ant-associated fungi (Howard et al. 1988). The essential oils isolated from Artemisia species are toxic and contain caryophyllene oxide as a major component in which they showed a high mortality to granary weevil (Kordali et al. 2006).
CONCLUSIONS
- The GC-MS analysis of the essential oil of Taxodium distichum winter cone scale, seed, and thin branch along with leaf revealed the presence of 14, 18, and 37 compounds, of which all compounds were identified.
- The identification of chemical composition of the plant cone scale, seed, and thin branch along with leaf essential oil collected from northern Iran showed the presence of limonene, α-pinene, and caryophyllene in higher amounts in winter cone scale (December 2022); limonene, α-pinene, bornyl acetate, and caryophyllene in higher amounts in winter seed, while limonene, α-pinene, caryophyllene, caryophyllene oxide, and bornyl acetate in higher amounts in winter thin branch along with leaf.
- The constituents of the cone scale, seed, and thin branch along with leaf oils were as follows: monoterpene hydrocarbons (90.6, 79.9, and 65.8%, respectively), oxygenated monoterpenes (2.8, 5.3, and 6.9%, respectively), sesquiterpene hydrocarbons (4.6, 7.8%, and 12.9%, respectively), oxygenated sesquiterpenes (1.6, 5.8, and 9.1%, respectively), and diterpenes (0.4, 0.7, and 1.4%, respectively).
- Monoterpene hydrocarbons were the most abundant class of compounds, then sesquiterpene hydrocarbons, followed by oxygenated sesquiterpenes.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran. The LCAB thanks the Brazilian Research Council (CNPq) and Federal University of Minas Gerais for support.
REFERENCES CITED
Abdelsalam, N. R., Salem, M. Z., Ali, H. M., Mackled, M. I., Mervat, E.-H., Elshikh, M. S., and Hatamleh, A. A. (2019). “Morphological, biochemical, molecular, and oil toxicity properties of Taxodium trees from different locations,” Ind. Crop. Prod. 139, Article ID 111515. DOI: 10.1016/j.indcrop.2019.111515
Abou Dahab, M. A., El-Bahr, M. K., Taha, H. S., Habib, A. M., Bekheet, S. A., Gabr, A. M. M., and Refaat, A. (2007). “Cytotoxic activity of Taxodium calli extracts on rat liver cells,” J. Appl. Sci. Res. 3(12), 1987-1996.
Adams, R. P., Arnold, M. A., King, A. R., and Denny, G. C. (2012). “Geographic variation in the leaf essential oils of Taxodium (Cupressaceae),” Phytologia 94(1), 53-70.
Adams, R. P. (2012). “Seasonal variation in the leaf essential oil of Taxodium distichum (Cupressaceae),” Phytologia 94(1), 91-102.
Adams, R. P. (2001). Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 3rd Edition, Allured Publishing Corp., Carol Stream, IL, USA.
Adesina, S. K. (2005). “The Nigerian Zanthoxylum; chemical and biological values. Review,” Afr. J. Tradit. Complement. Altern. Med. 2(3), 282-301. DOI: 10.4314/ajtcam.v2i3.31128
Barclay, K. D., and McKersie, B. D. (1994). “Peroxidation reactions in plant membranes: Effects of free fatty acids,” Lipids 29(12), 877-883. DOI: 10.1007/BF02536256
Bartels, D. M. E. (2001). “Targeting detoxification pathways: an efficient approach to obtain plants with multiple stress tolerance?,” Trends Plant Sci. 6(7), 284-286. DOI: 10.1016/s1360-1385(01)01983-5
Barzegari, F., Hosseinihashemi, S. K., and Baseri, H. (2023). “Chemical composition and antioxidant activity of extracts from the fruit, leaf, and branchlet of Cupressus arizonica Greene,” BioResources 18(1), 19-38. DOI: 10.15376/biores.18.1.19-38
Cortés-Arroyo, A. R., Domínguez-Ramírez, A. M., Gómez-Hernández, M., Medina López, J. R., and López-Muñoz, F. J. (2011). “Antispasmodic and bronchodilator activities of Taxodium mucronatum Ten leaf extract,” Afr. J. Biotechnol. 10(1), 54-64. DOI: 10.5897/AJB10.1018
Dapić, N., and Ristić, M. (2017). “Chemical profile of Taxodium distichum winter cones,” Acta Periodica Technologica 48(48), 77-83. DOI: 10.2298/APT1748077D
Drazic, D., and Batos, B. (2002). “Mocvarni cempres Taxodium distichum (L.) Rich,” in: 7th Symposium on Flora of Southeastern Serbia and Neighbouring Regions, Dimitrovgrad, Serbia, pp. 195.
El Tantawy, M. E., El Sakhawy, F. S., El Sohly, M. A., and Ross, S. A. (1999). “Chemical composition and biological activity of the essential oil of the fruit of Taxodium distichum L. Rich growing in Egypt,” J. Essent. Oil Res. 11(3), 386-392. DOI: 10.1080/10412905.1999.9701161
Eslamdoust, J., Sohrabi, H., Hosseini, S. M., and Moradi, Z. (2015a). “Assessment of different methods of form factor determination for volume estimation of planted Populus deltoids and Taxodium distichum trees (Klodeh region-Mazandaran province),” Iranian J. Appl. Ecol. 4(12), 67-75. DOI: 10.18869/acadpub.ijae.4.12.67
Eslamdoust, J., Sohrabi, H., Hosseini, S. M., and Naseri, B. (2015b). “Biomass factor (BF) in various components and biomass expansion factor (BEF) for Alnus subcordata, Taxodium distichum and Populus deltoides afforested,” Iranian J. Forest. Poplar Res. 22(3), 540-549.
Eslamdoust, J., Sohrabi, H., and Hosseini, S. M. (2014). “Evaluation of growth characteristics of Populus deltoides and Taxodium distichum trees using stem analysis,” J. Nat. Eco. Iran 5(3), 51-59.
Flamini, G., Luigi, C., and Morelli, I. (2000). “Investigation of the essential oil of feminine cones, leaves and branches of Taxodium distichum from Italy,” J. Essent. Oil Res. 12(3), 310-312. DOI: 10.1080/10412905.2000.9699523
García, E., Chacón, J. L., Martínez, J., and Izquierdo, P. M. (2003). “Changes in volatile compounds during ripening in grapes of aire ́n, macabeo and chardonnay white varieties grown in La Mancha region (Spain),” Food Sci. Technol. Int. 9(1), 33-41. DOI: 10.1177/1082013203009001006
Hosseinihashemi, S. K., Hosseinashrafi, S. K., Barzegari, F., Baseri, H., Tajeddini, D., Torabi Tooranposhti, H., Jalaligoldeh, A., and Sheikh Mohammadi, F. (2023a). “Chemical composition of essential oil from female cones of Cupressus arizonica Greene,” Nat. Prod. Res. 37(14), 2408-2414. DOI:10.1080/14786419.2022.2152021
Hosseinihashemi, S. K., Barbosa, L. C. A., and Kermani, R. (2023b). “α-Pinene- and trans-caryophyllene-rich volatile female cones oil of Taxodium distichum L. from Northern Iran,” J. Essent. Oil Plant Compos. 1(3), 192-197. DOI: 10.58985/jeopc.2023.v01i03.24
Hosseinihashemi, S. K., Barzegari, F., Baseri, H., and Barbosa, L. C. A. (2023c). “Powerful antioxidants within Cupressus arizonica Greene female cones and leaves essential oil,” Nat. Prod. Res., 1-10. DOI: 10.1080/14786419.2023.2278157
Howard, J. J., Cazin, J., Jr., and Wiemer, D. F. (1988). “Toxicity of terpenoid deterrents to the leaf-cutting ant Atta cephalotes and its mutualistic fungus,” J. Chem. Ecol. 14, 59-69. DOI: 10.1007/BF01022531
Jaimand, K., and Rezaee, M. B. (2003). “Time lag collection of essential oil by hydrodistillation and identification of chemical constituents on Taxodium distichum (L.),” Iranian J. Med. Arom. Plant Res. 18(1), 77-88.
Julian, D., and Konig, W. A. (1988). The Atlas of Spectral Data of Sesquiterpene Hydrocarbons, E. B. Verlag, Hamburg, Germany.
Kaminaga, Y., Schnepp, J., Peel, G., Kish, C. M., Ben-Nissan, G., Weiss, D., Orlova, I., Lavie, O., Rhodes, D., Wood, K., et al. (2006). “Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation,” J. Biol. Chem. 281(33), 23357-23366. DOI: 10.1074/jbc.M602708200
Khadem, S., and Marles, R. J. (2010). “Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies,” Molecules 15(11), 7985-8005. DOI: 10.3390/molecules15117985
Kordali, S., Aslan, I., Calmasur, O., and Cakir, A. (2006). “Toxicity of essential oils isolated from three Artemisia species and some of their major components to granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae),” Ind. Crop. Prod. 23, 162-170. DOI: 10.1016/j.indcrop.2005.05.005
Kováts, E. (1958). “Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones,” Helv. Chim. Acta. 41(7), 1915-1932. DOI: 10.1002/hlca.19580410703
Kubo, I., Chaudhuri, S. K., Kubo, Y., Sanchez, Y., Ogura, T., Saito, T., Ishikawa, H., and Haraguchi, H. (1996). “Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides,” Planta Med. 62(5), 427-430. DOI: 10.1055/s-2006-957932
Lattanzio, V., Lattanzio, V. M. T., and Cardinali, A. (2006). “Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects,” in: Phytochemistry: Advances in Research, F. Imperato (Ed.), Trivandrum -695 023, Research Signpost, Kerala, India, pp. 23-67.
Liangfeng, Z., Yonghua, L., Baoling, L., Biyao, L., and Wenlian, Z. (1995). Aromatic Plants and Essential Constituents (Supplement 1), South China Institute of Botany, Chinese Academy of Sciences, Hong Kong, Hai feng Publishing Co., China.
Manach, C., Scalbert, A., Morand, C., Remesy, C., and Jimenez, L. (2004). “Polyphenols: Food sources and bioavailability,” Am. J. Clin. Nutr. 79(5), 727-747. DOI: 10.1093/ajcn/79.5.727
Middleton, B. L., and McKee, K. L. (2004). “Use of a latitudinal gradient in bald cypress (Taxodium distichum) production to examine physiological controls of biotic boundaries and potential responses to environmental change,” Glob. Ecol. Biogeogr. 13(3), 247-258. DOI: 10.1111/j.1466-822X.2004.00088.x
Mustafa, N., and Verpoorte, R. (2007). “Phenolic compounds in Catharanthus roseus,” Phytochem. Rev. 6(2), 243-258. DOI: 10.1007/s11101-006-9039-8
Ninic-Todorovic, J., and Ocokoljic, M. (2002). “Varijabilnosttaksodijuma (Taxodium distichum (L.) Rich.),” in: 7th Symposium on Flora of Southeastern Serbia and Neighbouring Regions, Dimitrovgrad, Serbia, pp. 125.
Norouzi-Arasi, H., Yavari, I., Chalabian, F., Kiarostami, V., Ghaffarzadeh, F., and Nasirian, A. (2006). “Chemical constituents and antimicrobial activities of the essential oil of Acroptilon repens (L.) DC,” Flavour Fragr. J. 21(2), 247-249. DOI: 10.1002/ffj.1568
Odell, A. F. (1912). “The oil of the southern cypress,” J. Am. Chem. Soc. 34(6), 824-826. DOI: 10.1021/ja02207a011
Ogunwande, I. A., Olawore, N. O., Ogunmola, O. O., Walker, T. M., Schmidt, J. M., and Setzer, W. N. (2007). “Cytotoxic effects of Taxodium distichum oils,” Pharm. Biol. 45(2), 106-110. DOI: 10.1080/13880200601112901
Padalia, R. C., Verma, R. S., Chauhan, A., Goswami, P., and Chanotiya, C. S. (2016). “Compositional and enantiomeric analysis of the essential oil of Taxodium distichum from India,” Nat. Prod. Commun. 11(3), 419-422. DOI: 10.1177/1934578X1601100325
Ramos, A. R., Escamilla, E. M., Calderon, J., and Rodriguez, B. (1984). “8β-Hydroxypimar-15-en-19-oic acid from Taxodium mucronatum,” Phytochem. 23(6), 1329-1330. DOI: 10.1016/S0031-9422(00)80454-9
Rostamabadi, A., Tabari, M., and Sayad, E. (2013). “Influence of Alnus subcordata, Populus deltoides and Taxodium distichum on poor drainage soil, northern Iran,” Ecopersia 1(3), 207-218.
Rostamabadi, A., Tabari, M., Salehi, A., Sayad, E., and Salehi, A. (2010). “Comparison of nutrition, nutrient return and nutrient retranslocation between stands of Alnus
subcordata and Taxodium distichum in Tashbandan, Amol (Mazandaran),” J. Wood Forest Sci. Technol. 17(1), 65-78.
Saeb, K., Kakouei, A., Jafari Hajati, R., Pourshamsian, K., and Babakhani, B. (2011). “Investigating the effect of height on essential oils of Urtica diocia L. (Case study: Ramsar, Mazandaran, Iran),” Orient. J. Chem. 27(4), 1345-1350.
Sijacic-Nikolic, M., Vilotic, D., Veselinovic, M., Mitrovic, S., and Jokanovic, D. (2010). “Bald cypress (Taxodium distichum (L.) Rich.) in the protected area Veliko ratnoostrvo,” Bull. Fac. Forestry 103, 173-184. DOI: 10.2298/GSF1103173S
Starks, C. M., Norman, V. L., Williams, R. B., Goering, M. G., Rice, S. M., O’Neil-Johnson, M., and Eldridge, G. R. (2014). “Antibacterial activity of Taxodium ascendens diterpenes against methicillin-resistant Staphylococcus aureus,” Nat. Prod. Communic. 9(8), 1129-1130. DOI: 10.1177/1934578X1400900817
Su, Z., Yuan, W., Wang, P., and Li, S. (2013). “Ethnobotany, phytochemistry, and biological activities of Taxodium Rich,” Pharm. Crop. 4, 1-14. DOI: 10.2174/2210290601304010001
Yin, J., Li, X., Huang, F. F., Lu, M. H., Yang, J., and Zhu, L. Y. (2019). “Chemical composition, antioxidant and anticancer activity of the essential oil from Myrica rubra leaves,” in: IOP Conf. Series: Earth Environ. Sci. 346, Article ID 012085. DOI: 10.1088/1755-1315/346/1/012085
Article submitted: October 11, 2023; Peer review completed: November 18, 2023; Revised version received: November 22, 2023; Accepted: November 28, 2023; Published: December 7, 2023.
DOI: 10.15376/biores.19.1.751-765
APPENDIX
Fig. A1. The GC/MS chromatogram of the essential oil of T. distichum winter cone scale (December 2022)
Fig. A2. The GC/MS chromatogram of the essential oil of T. distichum winter seed (December 2022)
Fig. A3. The GC/MS chromatogram of the essential oil of T. distichum winter thin branch along with leaf (December 2022)
