Abstract
Significant developments in the area of wood modification have been achieved during the last three decades. These developments can be attributed to increased environmental concerns, the escalating demand for a high and constant quality of wood products, and the rising prices of the durable tropical timber as well as its very limited availability, as affected by illegal logging. As a consequence, a number of wood modification techniques such as chemical and impregnation modifications or heat treatments have been introduced, and some of these technologies have reached the industrial level. This review paper deals with two successful technologies, that is, wood acetylation and wood furfurylation. It briefly discusses the advantages of the new modified products and presents in short their improved properties. Published findings indicate that chemical modification of wood, to a full or partial degree, by means of acetylation or furfurylation, offers a way to transform low durability wood species to new ‘green’ wood materials having advanced qualities and properties.
Download PDF
Full Article
Chemical Modification of Wood by Acetylation or Furfurylation: A Review of the Present Scaled-up Technologies
George I. Mantanis
Significant developments in the area of wood modification have been achieved during the last three decades. These developments can be attributed to increased environmental concerns, the escalating demand for a high and constant quality of wood products, and the rising prices of the durable tropical timber as well as its very limited availability, as affected by illegal logging. As a consequence, a number of wood modification techniques such as chemical and impregnation modifications or heat treatments have been introduced, and some of these technologies have reached the industrial level. This review paper deals with two successful technologies, that is, wood acetylation and wood furfurylation. It briefly discusses the advantages of the new modified products and presents in short their improved properties. Published findings indicate that chemical modification of wood, to a full or partial degree, by means of acetylation or furfurylation, offers a way to transform low durability wood species to new ‘green’ wood materials having advanced qualities and properties.
Keywords: Wood chemistry; Chemical modification; Acetylation; Acetic anhydride; Furfuryl alcohol; Furfurylation; Accoya; Kebony
Contact information: Research Lab of Wood Science & Technology, Technological Education Institute (TEI) of Thessaly, Dept. of Wood & Furniture Design and Technology, GR-43100, Karditsa, Greece; Email: mantanis@teilar.gr
INTRODUCTION
Wood has been utilized by humans for many centuries, mainly for fuel, shelter, weapons, tools, and furniture. As a material, wood is considered to be easy-to-work, renewable, readily available, and sustainable. For the most part, it has been used without any modification (Rowell 2014, 2016). Solid timber and lumber long have been treated for decay and fire resistance, as recorded in ancient, historic sources. Nonetheless, most modern applications of wood involve little treatment, mostly limited to coatings or finishes (Rowell 2016).
Humans have learned to use wood, accepting that it changes dimensions with changing moisture content, or in contact with water (Mantanis et al. 1994). It can be degraded by a wide variety of microorganisms, it burns, and it is decomposed by ultraviolet energy (Stamm 1964; Rowell 1983). With an increased awareness of the fragility of the environment and the need for durability in wood products, new technologies have been developed to increase the service life of wood without the use of toxic chemicals (Rowell 1983, 1984; Militz 1991; Westin 1996; Hill 2006; Rowell et al. 2009; Rowell 2012; Gérardin 2016). Issues of sustainability and carbon sequestration converge in this search for new ‘green’ technologies to improve durability, stability, and performance of wood, especially in exterior applications (Rowell 2016).
In addition, the progress of regulations in Europe, North America, and elsewhere, on the use of biocide products, has led to novel developments in the field of wood modification (Gérardin 2016). This has led to an increasing attention for non-biocide treatments including chemical modification, thermal modification, or impregnation modification, in an attempt to face the forthcoming prohibition of biocide-treated products (Hill 2006; Rowell 2012). Through chemical modification of wood, low-durability species can be upgraded to new modified wood products with advanced properties, without any deleterious effects to the natural environment or to the human beings (Hill 2006; Rowell 2014).
This work reviews present technologies of chemical modification of wood, namely, acetylation and furfurylation, which currently have reached the industrial-scale level.
CHEMICAL MODIFICATION OF WOOD
Chemical modification of wood takes place when a chemical reaction of a reagent occurs with the polymeric constituents of wood (lignin, hemicelluloses, or cellulose), resulting thus in the formation of a stable covalent bond between the reagent and the cell wall polymers (Rowell 1982, 1983; Hill 2006).
In general, chemical modification of wood can be regarded as an active modification because it results in a distinct chemical change in the macromolecules of the cell wall. Currently, much is known about the modes of action of modified wood, which includes the following: i) the equilibrium moisture content is lowered in modified wood, and hence it is harder for fungi to obtain the moisture required for decay; ii) there is a physical blocking of the entrance of decay fungi to micropores of the cell walls; and/or, iii) inhibition of the action of specific enzymes (Hill 2006; Rowell et al. 2009; Rowell 2012). These three are only parts of the whole mechanism.
Up to the present, the most prominent processes that involve chemical modification of the cell wall of wood, to full or partial degree, are the acetylation and furfurylation of wood, respectively; both have been scaled up today to the industrial level. These two technologies are reviewed in the sections that follow.
Acetylation
The first recorded experiment of wood acetylation was carried out in Germany by Fuchs (1928) using the chemical agent acetic anhydride, with sulphuric acid as a catalyst. Horn (1928) also acetylated beech wood, but in order to remove hemicelluloses in a similar lignin isolation procedure. Tarkow was actually the first scientist who described the use of acetylation process in an attempt to stabilize wood from swelling in the water (Tarkow 1946; Tarkow et al. 1946). Since the ‘40s, many laboratories worldwide have performed experiments on the acetylation of wood in a variety of different ways, and by using various wood species and agricultural resources (Rowell 1983, 1984).
Early attempts to commercialize the process of wood acetylation failed in the USA (Koppers Inc. in 1961), Russia (1977), and Japan (Daiken Inc. in 1984) due to the high production costs involved (Rowell 2012). Pioneering work in scaling-up laboratory acetylation to the semi-industrial level, was successfully carried out at Stichting Hout Research (SHR, The Netherlands) by Prof. H. Militz and his coworkers during the ‘90s (Militz 1991; Beckers and Militz 1994; Beckers et al. 1994).
Wood acetylation, using primarily acetic anhydride, has been initially carried out as a liquid phase reaction (Rowell 1983, 1984). The early work was initiated using acetic anhydride catalyzed by zinc chloride or pyridine (Tarkow 1946). Through the years, many other catalysts have been evaluated, both with liquid and vapour systems. Some of the catalysts used include sodium acetate, potassium acid, urea-ammonium sulphate, magnesium persulfate, and dimethylformamide (Rowell 1983). Most acetylation reactions today are realized without the use of a catalyst (Rowell 2012; Larsson-Brelid 2013).
The reaction of acetic anhydride with wood polymers results in the esterification of the accessible hydroxyl groups in the cell wall (Rowell 1983), with the formation of a by-product, acetic acid (Fig. 1). The by-product is, for the most part, removed from the final modified material (Lankveld 2017) as the human nose is quite sensitive to the odour of acetic acid. Like untreated wood, acetylated wood is comprised only of carbon, hydrogen, and oxygen, and it contains absolutely nontoxic constituents (Hill 2006). In fact, acetylation of wood is a single-addition chemical reaction (Rowell 1984; Rowell et al. 1994) which means that one acetyl group is on one hydroxyl group without any polymerization (Fig. 1).
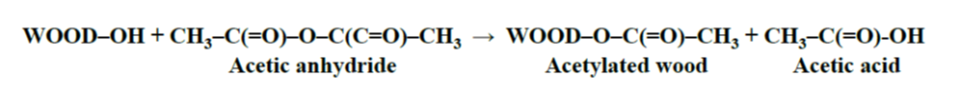
Fig. 1. The main acetylation reaction of wood with acetic anhydride (Rowell 1983)
During the acetylation process, wood material at 100 to 120 °C reacts with acetic anhydride in the absence of catalyst, and at an acetyl weight gain of 16% to 19%, approximately 90% of the lignin is esterified and 25% of the holocellulose (Rowell et al. 1994; Rowell 2012). As expected, 100% of the hydroxyl groups in the hemicelluloses, which are readily accessible, are substituted. It has been shown that lignin is the most reactive constituent of the cell wall during the acetylation reaction (Rowell et al. 1994).
Acetylated wood is presently commercialised by the company Accsys Technologies in Arnhem, The Netherlands. It is marketed under the commercial name Accoya®, utilizing mainly the species radiata pine (Pinus radiata) and alder (Alnus sp.), and technically, on the average, has a 20% acetyl weight gain. Annually, approximately 40,000 m3 acetylated timber is produced by Accsys (2017), while the company will increase its capacity to 60,000 m3 in the year 2018 (Lankveld 2017). Accoya wood is today available in many countries, and its main uses are for exterior windows and doors, decking, cladding, and civil construction mainly in outdoors, above- and in-ground contact (Lankveld 2017).
Today, the acetylation process, as applied in liquid phase, yields chemically modified timber that has considerably improved physical, mechanical, and biological material properties (Hill 2006; Jones and Hill 2007; Rowell et al. 2009; Larsson-Brelid 2013; Alexander et al. 2014; Gérardin 2016), which are presented below.
- The biological durability of wood (EN 350-2) is improved to the highest durability class (‘class 1’), which corresponds to the durable tropical species teak (Tectona grandis), ipé (Handroanthus ), and merbau (Intsia spp.). Acetylated wood exhibits considerably increased biological resistance to brown- and white-rot fungi (Larsson-Brelid et al. 2000; Papadopoulos and Hill 2002; Mohebby 2003; Mohebby and Militz 2010; Rowell 2012; Larsson-Brelid 2013; Alexander et al. 2014; Rowell 2016).
- Acetylated wood, at 20% loading, obtains a fiber saturation point below 15%; thus, the cell wall becomes highly hydrophobic (Papadopoulos and Hill 2003; Hill 2006; Rowell 2012). Consequently, swelling and shrinkage properties are reduced by 70% to 75% as compared to untreated wood (Jones and Hill 2007; Rowell 2012). The reason for that is simply because the cell wall is filled with chemically bonded acetyl groups that take up space within the cell wall (Rowell 1983; Rowell et al. 1994; Hill 2006; Jones and Hill 2007).
- Accoya wood at high acetyl loading is very resistant to subterranean and Formosan termites (Alexander et al. 2014). This field study against termites verified the high resistance of acetylated material to attack by termites.
- Acetylated pine wood with high acetyl loading (>20% acetyl weight gain) has been shown to provide excellent resistance to borer attacks even after 11 years of field exposure; e., in better order than chromium copper arsenate (CCA) impregnated pine wood (Westin et al. 2016). It is believed that acetylated wood at high loading can be confirmed as marine-borer resistant timber for long periods of time.
- An increase of 15% to 30% of hardness of the material can be reached (Rowell 2012).
- The acetylation treatment has no negative impact on the strength properties of wood (Jorissen and Luning 2010; Bongers et al. 2013; Larsson-Brelid 2013).
- Acetylated wood is marketed today as a ‘green’ product with several environmental benefits (Jones and Hill 2007; Lande et al. 2008; Van der Lugt et al. 2016). According to a recent study of Van der Lugt et al. (2016), acetylated wood has considerably lower carbon footprint than steel, concrete, and unsustainably sourced azobé. In fact, Accoya wood does have CO2 negative life-cycle-analysis over a full-life cycle.
Recently in Germany, Accoya wood has gained acceptance for use in exterior windows by the German Association of Windows and Facades (VFF).
Furfurylation
Research relating to chemical modification of wood with furfuryl alcohol (C5H6O2), which has been referred to in the literature as ‘furfurylation of wood’, was initiated by Goldstein and Stamm (Goldstein 1959; Stamm 1977). By using cyclic carboxylic anhydrides, mainly maleic anhydride, as key catalysts, furfurylated wood, having properties superior to those produced with the early developed systems, was achieved by Prof. M. Schneider (1995) in Canada. Novel research was also carried out by Dr. M. Westin and his coworkers (Westin 1996; Westin et al. 1998; Lande et al. 2004a, 2004b), at SP Sweden, which led to a new technology based on stable solutions with good impregnating capacity, as well as some promising properties such as resistance to decay.
Furfuryl alcohol is a liquid produced from agricultural wastes such as sugar cane and corn cobs. Furfurylation is accomplished by impregnating the wood with a mixture of furfuryl alcohol and catalysts, and then heating it to cause polymerization (Schneider 1995; Westin 1996). The purpose of furfurylation is to improve some physical, mechanical, and biological properties of the lignocellulosic material (e.g., resistance to biological degradation, dimensional stabilization, resistance to weathering, and hardness) by applying a proprietary furfuryl alcohol polymer, which notably is nontoxic (Lande et al. 2004b, 2008).
The polymerization of furfuryl alcohol polymer in the wood is a rather complex chemical reaction. The question of whether wood furfurylation is a true chemical modification of the cell wall remains unanswered by the scientific community. Some scientists believe that it comprises a chemical modification process, since the furfuryl alcohol polymer reacts with itself and possibly reacts with lignin in the cell walls (Nordstierma et al. 2008; Lande et al. 2008; Li et al. 2016; Gérardin 2016). Hence, the furfuryl alcohol complexes are predominantly deposited in the wood cavities, and also in the cell walls (Fig. 2). Polymerization takes place in the microscopic cell cavities, which is easily detected using several microscopic techniques (Thygesen et al. 2010). The study of Thygesen et al. (2010) also revealed that when higher amounts of catalyst were added to the impregnation liquid, a red-shift in the fluorescence from the furfurylated wood was seen, corresponding to an increased conjugation length of conjugated poly-(furfuryl alcohol) formed within the cell wall of wood.
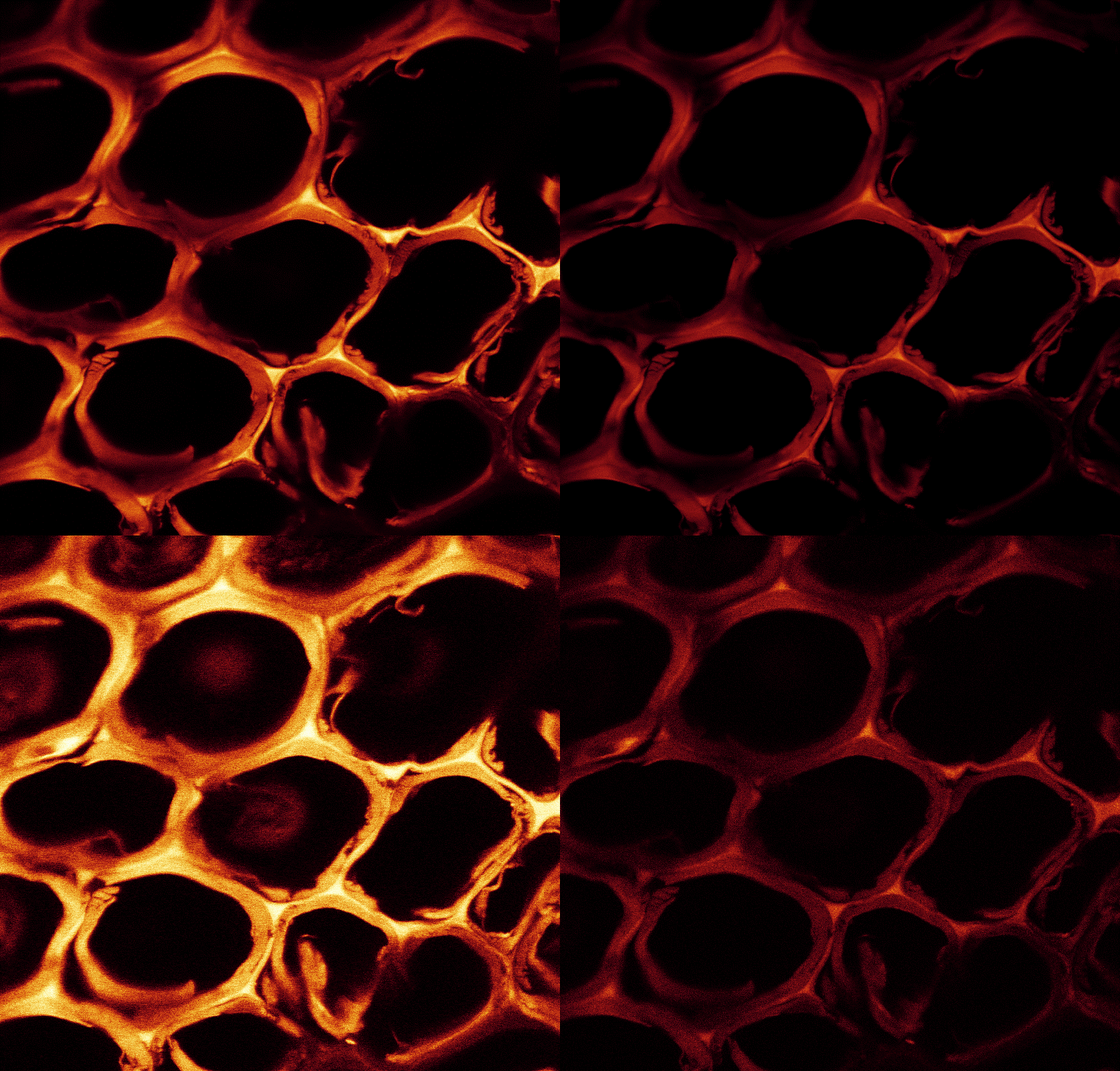
Fig. 2. Cross section of radiata pine (Pinus radiata) wood with cell walls containing furan polymer. Image through fluorescence microscopy (courtesy: L. Garbrecht Thygesen, RVAU, Copenhagen)
Recent nanoindentation studies demonstrated improvements in indentation modulus and hardness of furfurylated wood cells demonstrated. The work indicated that furfuryl alcohol indeed penetrated wood cells during the modification process (Li et al. 2016). Another perception is that furfurylation leads to permanent ‘bulking’ of cell wall, meaning that the cells are swollen in a permanent way. One possible explanation is that the furfuryl alcohol polymer inside the cell wall occupies some of the space normally filled with water molecules, when wood is under swelling in humid conditions (Lande et al. 2008).
On the contrary, other scientists insist that furfurylation of wood is an impregnation modification process in which the properties of the furfurylated material appear more like those of a polymer-filled cell wall rather than a reacted cell wall (Rowell 2012; Larsson-Brelid 2013). However, it may be possible that a branch from the polymer chain forms a connection to the wood polymers via the lignin hydroxyls (Thygesen et al. 2010; Gérardin 2016).
The technology for industrially producing furfurylated wood is presently applied by the Norwegian company Kebony AS (formerly Wood Polymer Technologies). The industrial process of wood furfurylation consists of the following production steps (Lande et al. 2004a):
- Storage and mixing of chemicals: The treating solutions are mixed in a separate mixing tank where different chemicals (furfuryl alcohol, initiators/catalysts, buffering agents, surfactants, water) are added. The mixed solution is pumped to one of the buffer tanks.
- Impregnation: The wood material (i.e. treatable softwoods or hardwoods) is vacuum pressure impregnated with the treating solution by a full-cell process with a vacuum step, a pressure step, and a short post-vacuum step.
- Reaction/curing: In-situ polymerization of the chemicals and grafting reactions with the wood polymeric components occur during this step. The curing chamber is heated with direct injection of steam, where the temperature achieved depends on the product use. The chamber is operated as a closed system during the curing period except for a ventilation period at the end. The ventilation gas is cooled, and the condensate is separated from the gas. Condensate goes back to the condensate tank for re-use.
- Drying: Final drying of the wood material in a kiln dryer is essential to minimize emissions and to obtain desirable final moisture content.
- Cleaning: The emissions during the process are managed by cleaning the ventilated gases.
By using cyclic anhydrides as catalysts, the impregnation solution is stable at room temperature. The polymerization reaction is initiated by heating. In the initial phase of polymerization, there are two competing condensation reactions (González et al. 1992). The possible cross-linking patterns of furfuryl alcohol polymer chains have been proposed by González et al. (1992), who demonstrated that the high amount of permanent wood cell-wall bulking in the furfurylated wood is evidence of the grafting reactions at early polymerization stages. In addition, it has been concluded (González et al. 1992; Lande et al. 2004b) that with the catalytic systems used for furfurylation of wood it is more likely that grafting to hemicelluloses and most of all to lignin is dominant. As a consequence, a possible grafting reaction between the furfuryl alcohol and a guaiacyl unit of lignin (the predominant unit in softwood lignins) is evidently probable. Gérardin (2016) in his recent review work reported that the furfurylation of wood as a process is based on the in-situ polymerization of furfuryl alcohol, with the main chemical reactions demonstrated in Fig. 3.
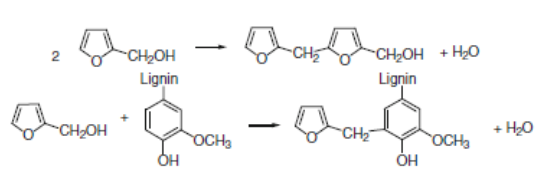
Fig. 3. Main reactions involved in the polymerization of furfuryl alcohol (Gerardin 2016)
According to the literature (Lande et al. 2004a; Lande 2008; Lande et al. 2008; Rowell 2012; Larsson-Brelid 2013; Mantanis and Lykidis 2015), the furfurylation process results in a modified wood product that has significantly improved material properties and characteristics, which are summarized below.
- The biological durability of wood is upgraded to ‘class 1’ (Gérardin 2016). Results from decay resistance test ongoing for 9 years, have shown that furfurylated wood of moderate loading, g., at 30 to 35% of weight percentage gain (WPG), has comparable biological resistance with that of pine wood treated with copper chromium arsenate (Lande et al. 2008; Larsson-Brelid 2013).
- The mechanical properties of wood, except for impact resistance, are enhanced when wood is treated with the furfuryl alcohol polymer. As a matter of fact, furfurylated wood is characterized by greater hardness, elastic and rupture moduli, as compared to untreated wood but, on the other hand, it is more brittle (Larsson-Brelid 2013).
- Furfurylated wood at a loading>35% exhibits very good dimensional stability (Lande et al. 2008), and resistance to weathering (Mantanis and Lykidis 2015). It was reported that furfurylation leads to an anti-shrink efficiency of 60%, at a WPG of approximately 35% (Lande et al. 2008).
- Furfurylated wood is resistant to marine borers (Westin et al. 2016) only when the WPG is very high (>50%). Not really satisfactory results were obtained after 16-year exposure at reasonable loadings of 30 to 35% (e., slight or moderate wood failure to marine borers was observed at these levels of WPG or lower).
- Recent studies regarding ecotoxicology of furfurylated wood and leachates from furfurylated wood have shown no significant ecotoxicity, while its combustion did not release any volatile organic compounds or polyaromatic hydrocarbons above the normal levels of wood combustion (Pilgård et al. 2010a, 2010b).
- Furfurylated wood is a ‘green’ wood product that holds an ecological label in the Scandinavian market, named ‘Swan’. Furfurylation of wood is, therefore, believed to be a safe process for the environment (Gérardin 2016).
At present, Kebony AS (Norway) produces two distinct furfurylated wood products, namely:
- ‘Kebony Clear’: a highly-loaded, dark, hard furfurylated wood currently used for flooring that simulates tropical hardwood. The wood species used for this are radiata pine (Pinus radiata), southern yellow pine (Pinus sp.), and maple (Acer sp.). Typically, the mean WPG of this product is approx. 35%.
- ‘Kebony Character’: a more lightly-loaded furfurylated wood presently used as decking, siding, roofing, and outdoor furniture mainly sold in the Scandinavian markets. This wood material is produced today from Scots pine (Pinus sylvestris) wood; it has an average WPG of 20% (Lande 2017).
In addition, Kebony wood has been recently used in the production of exterior windows. Following a series of extensive quality tests in Germany (Bollmus et al. 2012), furfurylated wood has been recommended by the German Association of Windows and Facades. Results from a project, entitled ‘Winfur’, indicated that the use of furfurylated wood in windows has not caused any problems, fulfilling the criteria for the Scandinavian P-mark and the German RAL certificates (Bollmus et al. 2012).
Today, the company Kebony AS (Norway) produces the above-listed modified products at approximately 22,000 m3 annually (as in 2017). It is expected to increase its production capacity by building a new factory in Belgium (Lande 2017).
CONCLUSIONS
This work briefly reviewed the two most successful chemical modification technologies of wood, up to this date, that is, acetylation and furfurylation. These technologies have given rise to new modified products with improved physical, mechanical, and biological properties; this can gradually change the mistaken perspective of customers about wood being a fragile, instable, and easily degraded construction material. As presented, both technologies, achieving a full or partial degree of chemical modification of the cell wall of wood, have a high potential and possess a ‘green’ character having many ecological benefits. For each of them, as disclosed in the literature, a very large number of quality tests has been carried out worldwide, demonstrating the technical advantages and enhanced durability of the chemically modified timber products.
It is noted, though, that the two products of chemically-modified wood differ in several structural facets. Furfurylated wood has, in general, a higher level of hardness, is more rigid, has an excellent tropical-timber-like appearance and texture, and it is better suited for applications like decking. A point to notice is that furfurylated wood products always have a dark colour. On the other hand, acetylated wood, besides its high biological durability, has a superior dimensional stability (i.e., distinctly higher than that of furfurylated wood), and it can be used not only in joinery products (windows, doors), but also in several structural applications. Nevertheless, the technical remarks mentioned above are not precise for all the cases, since the modified products may differ depending upon the final end-use, or project.
Evidently, as of today, acetylation and furfurylation exhibit a high potential to pave the way for innovative marine-borer resistant wood products. Nonetheless, the commercially available Accoya and Kebony modified timber products currently are not marketed or sold as marine-borer resistant wood. This product type of high requirements can be achieved in practice, only if high percentage gains are applied. In conclusion, both modification technologies (acetylation and furfurylation) yield non-toxic, environmentally sound, alternative materials to tropical hardwoods. However, there are certainly a number of technical differences between the two modified materials that set the two apart. Last but not least, as both products are being graded as in durability ‘class 1’ (EN 350-2), they can offer durable and sustainable alternatives for wooden or mixed outdoor structures that can have noteworthy lifetime duration of 30 years, or even more.
ACKNOWLEDMENTS
This review paper acknowledges four respected wood scientists, namely, Roger M. Rowell (Univ. of Wisconsin-Madison), Holger Militz (Univ. of Goettingen), Marc H. Schneider (Univ. of New Brunswick), and Mats Westin (SP Sweden), for their long-standing, diligent work and pioneering scientific contributions on the acetylation and furfurylation of wood. The author highly acknowledges the COST Action FP1407. This review work received no funding, and the author has absolutely no conflict of interest.
REFERENCES CITED
Alexander, J., Hague, J., Bongers, F., Imamura, Y., and Roberts, M. (2014). “The resistance of Accoya® and Tricoya® to attack by wood-destroying fungi and termites,” in: Proceedings of the 45th Annual Meeting of the International Research Group (IRG) on Wood Protection, 11-15 May 2014, St. George, Utah, USA, Document IRG/WP 14/40658, Section 4, pp. 1-10.
Beckers, E. P. J., and Militz, H. (1994). “Acetylation of solid wood. Initial trials on lab and semi- industrial scale,” in: Proc. of Second Pacific Rim Bio-Based Composites Symposium, Vancouver, Canada, pp. 125-135.
Beckers, E. P. J., Militz, H., and Stevens, M. (1994). “Resistance of acetylated wood to basiomycetes, soft rot and blue stain,” in: Proceedings of the 25th Annual Meeting of the International Research Group (IRG) on Wood Protection, Bali, Indonesia, Document IRG/WP 94/40021.
Bollmus, S., Treu, A., Westin, M., Brynildsen, P., and Militz, H. (2012). “Use of furfurylated wood for the production of windows – Results from the WinFur project,” in: Proceedings of the 6th European Conference on Wood Modification (ECWM), Ljubljana, Slovenia, 16-18 Sept. 2012, pp. 99-107.
Bongers, F., Alexander, J., Marcroft, J., Crawford, D., and Hairstans, R. (2013). “Structural design with Accoya wood,” International Wood Products Journal 4(3), 172-176. DOI: 10.1179/2042645313Y.0000000041
EN 350-2 (1994). “Durability of wood and wood-based products. Natural durability of solid wood. Part 2: Guide to natural durability and treatability of selected wood species of importance in Europe,” European Committee for Standardization (CEN), Brussels, Belgium, pp. 1-36.
Fuchs, W. (1928). “Genuine lignin. I. Acetylation of pine wood,” Berichte 61B, 948-951.
Gérardin, P. (2016). “New alternatives for wood preservation based on thermal and chemical modification of wood – A review,” Annals of Forest Science 73, 559-570. DOI: 10.1007/s13595-015-0531-4
Goldstein, I. (1959). “Impregnating solutions and method of impregnation therewith,” Patent US No. 2,909,450, USA.
González, R., Martinez, R., and Ortiz, P. (1992). “Polymerizationof furfuryl alcohol with trifluoroacetic acid: The influence of experimental conditions,” Makromol. Chem. 193, 1-9. DOI: 10.1002/macp.1992.021930101
Hill, C. A. S. (2006). Wood Modification – Chemical, Thermal and Other Processes, Wiley Series in Renewable Resources, Ed. J. Wiley and Sons, Chichester, United Kingdom, pp. 260.
Horn, O. (1928). “Acetylation of beech wood,” Berichte 61B, 2542-2545.
Jones, D., and Hill, C. A. S. (2007). “Wood modification – A brief overview of the technology,” in: Proceedings of the 5th COST E34 International Workshop, M. Sernek (ed.), Bled, Slovenia, 6-7 Sept. 2007, pp. 1-9.
Jorissen, A., and Luning, E. (2010). “Wood modification in relation to bridge design in the Netherlands,” in: Proceedings of the 11th World Conference on Timber Engineering (WCTE 2010), Trentino, Italy, June 2010, A. Ceccottiand J.-W.van der Kuilen (eds.), pp. 1-9.
Lande, S., Westin, M., and Schneider, M. (2004a). “Properties of furfurylated wood,” Scandinavian Journal of Forest Research 19(5), 22-30. DOI: 10.1080/0282758041001915
Lande, S., Eikenes, M., and Westin, M. (2004b). “Chemistry and ecotoxicology of furfurylated wood,” Scandinavian Journal of Forest Research 19(5), 14-21. DOI: 10.1080/02827580410017816
Lande, S. (2008). “Furfurylation of wood – Wood modification by the use of furfuryl alcohol,” Ph.D. Thesis. Norwegian University of Life Science, Ås, Norway.
Lande, S., Eikenes, M., Westin, M., and Schneider, M. (2008). “Furfurylation of wood: Chemistry, properties and commercialization,” in: Development of Commercial Wood Preservatives, ACS Symposium Series No. 982, pp. 337-355. DOI: 10.1021/bk-2008-0982.ch020
Lande, S. (2017). Kebony AS, Norway (Personal communication).
Lankveld, C. (2017). Accsys Technologies, Arnhem, The Netherlands (Personal communication).
Larsson-Brelid, P. (2013). “Benchmarking and state-of-the-art report for modified wood,” SP Report No. 54, Swedish Institute of Wood Technology SP, Stockholm, Sweden, pp. 1-31.
Larsson-Brelid, P., Simonson, R., Bergman,Ö., and Nilsson, T. (2000). “Resistance of acetylated wood to biological degradation,” Holz als Roh Werkstoff 58(5), 331-337. DOI: 10.1007/s001070050439
Li, W., Ren, D., Zhang, X., Wang, H., and Yu, Y. (2016). “The furfurylation of wood: A nanomechanical study of modified wood cells,” BioResources 11(2), 3614-3625. DOI: 10.15376/biores.11.2.3614-3625
Mantanis, G., Young, R. A., and Rowell, R. M. (1994). “Swelling of wood. Part 1. Swelling in water,” Wood Science and Technology 28, 119-134. DOI: 10.1007/bf00192691
Mantanis, G., and Lykidis, C. (2015). “Evaluation of weathering of furfurylated wood decks after a 3-year outdoor exposure in Greece,” DrvnaIndustrja 66(2), 115-122. DOI: 10.5552/drind.2015.1425
Militz, H. (1991). “The improvement of dimensional stability and durability of wood through treatment with non-catalysed acetic acid anhydride,” Holz als Roh- und Werkstoff 49(4), 147-152. DOI: 10.1007/BF02607895
Mohebby, B. (2003). “Biological attack of acetylated wood,” Ph.D. thesis, Institute of Wood Biology and Wood Technology, University of Goettingen, Goettingen, Germany.
Mohebby, B., and Militz, H. (2010). “Microbial attack of acetylated wood in field soil trials,” International Biodeterioration& Biodegradation 64, 41-50. DOI: 10.1016/j.ibiod.2009.10.005
Nordstierma, L., Lande, S., Westin, M., Karlsson, O., and Furo, I. (2008). “Towards novel wood-based materials: Chemical bonds between lignin-like model molecules and poly (furfuryl alcohol) studied by NMR,” Holzforschung, 62, 709-713.
Papadopoulos, A. N., and Hill, C. A. S. (2002). “The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophor aputeana,” Holz als Roh Werkstoff 60, 329-332. DOI: 10.1007/s00107-002-0327-8
Papadopoulos, A. N., and Hill, C. A. S. (2003). “The sorption of water vapour by anhydride modified softwood,” Wood Science and Technology 37(3-4), 221-231. DOI: 10.1007/s00226-003-0192-6
Pilgård, A., De Vetter, L., Van Acker. J., and Westin, M. (2010a). “Toxic hazard of leachates from furfurylated wood: Comparisons between two different aquatic organisms,” Environmental Toxicology Chemistry 29, 1067-1071. DOI: 10.1002/etc.132
Pilgård, A., Treu, A., van Zeeland, A. N., Gosselink, R. J., and Westin, M. (2010b). “Toxic hazard and chemical analysis of leachates from furfurylated wood,” Environmental Toxicology Chemistry 29, 1918-1924. DOI: 10.1002/etc.244
Rowell, R. M. (1982). “Distribution of reacted chemicals in southern pine modified with acetic anhydride,” Wood Science 15(2), 172-182.
Rowell, R. M. (1983). “Chemical modification of wood: A review,” Commonwealth Forestry Bureau, Oxford, England, No. 6, pp. 363-382.
Rowell, R. M. (1984). “The chemistry of solid wood,” in: Advances in Chemistry Series No. 207, American Chemical Society, Washington DC, USA, pp. 540. DOI: 10.1021/ba-1984-0207
Rowell, R. M., Simonson, R., Hess, S., Plackett, D. V., Cronshaw, D., and Dunningham, E. (1994). “Acetyl distribution in acetylated whole wood and reactivity of isolated wood cell wall components to acetic anhydride,” Wood and Fiber Science 26(1), 11-18.
Rowell, R. M., Ibach, R. E., McSweeny, J., and Nilsson, T. (2009). “Understanding decay resistance, dimensional stability and strength changes in heat treated and acetylated wood,” Wood Material Science and Engineering 1-2, 14-22. DOI: 10.1080/17480270903261339
Rowell, R. M. (2012). Handbook of Wood Chemistry and Wood Composites, 2nd Ed., CRC Press, Taylor and Francis Group, Boca Raton, Florida, USA, pp. 703. DOI: 10.1201/b12487
Rowell, R. M. (2014). “Acetylation of wood – A review,” Int. J. Lignocellulosic Products 1(1), 1-27.
Rowell, R. M. (2016). “Dimensional stability and fungal durability of acetylated wood,” Drewno 59(197), 139-150.
Schneider, M. H. (1995). “New cell wall and cell lumen wood polymer composites,” Wood Science and Technology 29, 121-127.
Stamm, A. J. (1964).Wood and Cellulose Science, Roland Press, New York, USA.
Stamm, A. J. (1977). “Dimensional stabilization of wood with furfuryl alcohol,” in: Wood Technology: Chemical Aspects, Goldstein (ed.), ACS Symposium Series, Volume 43, American Chemical Society, Washington, USA, pp. 141-149. DOI: 10.1021/bk-1977-0043.ch009
Tarkow, H. (1946). “A new approach to the acetylation of wood,” USDA Forest Service, Forest Products Laboratory, Madison, Wisconsin, USA, pp. 9.
Tarkow, H., Stamm, A. J., and Erickson, E. (1946). “Acetylated wood,” Report no. 1593, USDA Forest Service, Forest Products Laboratory, Madison, Wisconsin, USA, pp. 29.
Thygesen, L. G., Barsberg, S., and Venås, T. M. (2010). “The fluorescence characteristics of furfurylated wood studied by fluorescence spectroscopy and confocal laser scanning microscopy,” Wood Science and Technology 44(1), 51-65. DOI: 10.1007/s00226-009-0255-4
Westin, M. (1996). “Development and evaluation of new alternative wood preservation treatments,” Final report to The Swedish Council for Forestry and Agri. Res. (SJFR), (In Swedish with English summary), pp. 1-25.
Westin, M., Nilsson, T., and Hadi, Y. S. (1998). “Field performance of furfuryl alcohol treated wood,” in: Proceedings of the 4th Pacific Rim Bio-Based Composites Symposium, Bogor, Indonesia. pp. 305-331.
Westin, M., Larsson-Brelid, P. L., Nilsson, T., Rapp, A., Dickerson, J. P., Lande, S., and Cragg, S. (2016). “Marine borer resistance of acetylated and furfurylated wood – Results from up to 16 years of field exposure,” in: Proceedings of the 47th Annual Meeting of the International Research Group (IRG) on Wood Protection, Lisbon, Portugal, 15-19 May 2016, IRG/WP 16-40756, Section 4, pp. 1-9.
Van der Lugt, P., Bongers, F., and Vogtländer, J. (2016). “Environmental impact of constructions made of acetylated wood,” in: Proceedings of the World Conference on Timber Engineering (WCTE 2016), 22-25 Aug. 2016, Vienna, Austria, pp. 1-6.
Article submitted: March 1, 2017; Peer review completed: March 27, 2017; Revised version received: April 9, 2017; Accepted: April 10, 2017: Published: April 18, 2017.
DOI: 10.15376/biores.12.2.Mantanis
