Abstract
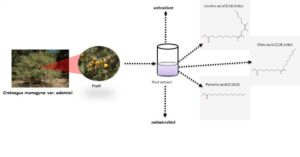
Fruit of the Crataegus species (Rosaceae), known as the “Hawthorn” wild plant, has important benefits for human health. The purpose of this study was to determine the chemical properties of one of the local endemic taxa, Crataegus monogyna var. odemisii in west Turkey. Based on the chemical analysis results, antioxidant and antimicrobial activities of fruit extracts, the fruit lipid contents, total phenol, and total flavonoid contents were studied. Five different methods were used to measure antioxidant capacity, namely the CUPRAC, β-Carotene-linoleic acid, DPPH•, ABTS•+, and CUPRAC analyses were used for the measurement of antioxidant capacity. The results showed that the highest antimicrobial activity was against C. albicans, and it was more effective against Gr+ bacteria than Gr- bacteria. The qualitative, as well as quantitative, analyses of lipid contents of the fruits of C. monogyna var. odemisii were made by gas chromatographic analysis. Folin-Ciocalteu reagent was used for determining the total phenolic content, and aluminum chloride was used for determining the flavonoid content. Results revealed that there was a statistically significant relationship between the antioxidant activities and the phenolic and flavonoid contents in the extracts of Hawthorn fruits.
Download PDF
Full Article
Chemical Properties, Antioxidant, and Antimicrobial Activities of Fruit extracts of Crataegus monogyna var. odemisii
Sevgin Özderin *
Fruit of the Crataegus species (Rosaceae), known as the “Hawthorn” wild plant, has important benefits for human health. The purpose of this study was to determine the chemical properties of one of the local endemic taxa, Crataegus monogyna var. odemisii in west Turkey. Based on the chemical analysis results, antioxidant and antimicrobial activities of fruit extracts, the fruit lipid contents, total phenol, and total flavonoid contents were studied. Five different methods were used to measure antioxidant capacity, namely the CUPRAC, β-Carotene-linoleic acid, DPPH•, ABTS•+, and CUPRAC analyses were used for the measurement of antioxidant capacity. The results showed that the highest antimicrobial activity was against C. albicans, and it was more effective against Gr+ bacteria than Gr- bacteria. The qualitative, as well as quantitative, analyses of lipid contents of the fruits of C. monogyna var. odemisii were made by gas chromatographic analysis. Folin-Ciocalteu reagent was used for determining the total phenolic content, and aluminum chloride was used for determining the flavonoid content. Results revealed that there was a statistically significant relationship between the antioxidant activities and the phenolic and flavonoid contents in the extracts of Hawthorn fruits.
DOI: 10.15376/biores.19.1.1542-1557
Keywords: Phenolics; Flavonoids; Antioxidant activity; Turkey
Contact information: Department of Forest, Muğla Sıtkı Koçman University, 480800 Muğla, Turkey;
* Corresponding author: sevginozderin@mu.edu.tr
GRAPHICAL ABSTRACT
INTRODUCTION
Located in the temperate zone, Turkey has a rich biodiversity, despite its sensitive ecosystems because of its geographical location and topographical structure (Güner et al. 2012). Ecologically sensitive areas are usually defined based on the level of reaction and/or adaptability of an area to environmental changes produced by internal and external variables (Mingwu et al. 2010; Liang and Li 2012). There are several types and combinations of the following ecosystems found in Turkey: wetland, coastal, marine, mountain, forest, steppe, and agricultural (Küçük and Ertürk 2013; Şimşek 2014). These diverse ecosystems and habitats contribute to the wide range of species that exist in Turkey (MOE 2001; Kiziroglu et al. 2006).
Plants have been used throughout history for food, beverage preparation, medical purposes, etc. The use of medicinal plants is being increasingly studied currently as a significant factor for improving people’s overall health and their positive effects on health. These properties are primarily explained in terms of the secondary metabolites formed from plants, and their various biological activities (Kaur and Kapoor 2008).
The Rosaceae family, one of the largest flowering plant families spreading worldwide, is represented by up to 240 species (Dönmez 2014; Dönmez and Özderin 2019; eFloras 2023). Although hawthorn (Crataegus spp.) is widely spread in Northern Europe, temperate regions of Asia, Africa, and North America, Turkey is an important gene center for the Crataegus genus (Dönmez 2014). The genus Crataegus spp. has many species in Turkey, and there are ongoing botanical studies on Crataegus species grown in Turkey (Dönmez 2004; Dönmez and Özderin 2019). Some features of the genus are polymorphic, such as leaf and nucleus morphologies, number of kernels, and fruit color. Polymorphism and hybridization are the reasons why there are so many synonyms in the Crataegus species (Dönmez 2004). In Turkey, the genus Crataegus comprises 28 taxa (Güner et al. 2012), corresponding to 25 species (Dönmez 2014), and 10 of which are endemic (Güner et al. 2012). Hawthorn (Crataegus L.) is a woody species belonging to the genus that sheds its leaves in winter, is rarely semi-evergreen and, is usually in the form of 2 to 5 m bushes with multiple branches or trunks, reaching up to 10 m. They usually have thorns, and the fruits of most of the species start to ripen from the beginning of autumn to mid-autumn (Davis 1972; Seçmen et al. 1989). The leaves may be simple or lobed, and the fruits may be yellow, red, purple, or black (Dönmez 2004). Dark reddish fruit is characterized by a greenish calyx. Another distinctive feature is the presence of the indumentum, the leaf shape, and the denticulate leaves (Dönmez 2004).
Taxa belonging to the genus Crataegus (Rosaceae), known as “hawthorn”, is one of the most common plants traditionally used in the treatment of different diseases, and as bio-nutrients, in addition to its use as a fruit tree and for landscape purposes. Various parts (leaves, flowers, shoots, fruits, and roots) of the hawthorn (Crataegus) species have been used for many years. Hawthorn species’ dried leaves, flowers, and fruits can be prepared into a tea and utilized for the treatment of a variety of conditions, including hemorrhoids, memory loss, attention deficit, eye bleeding, oral malodor, kidney diseases, high blood pressure, cancer, arteriosclerosis, liver pains, coughing, throat inflammation, and heart palpitations (Baytop 1994; Edwards et al. 2012; Ku et al. 2015). In Greece and China, leaves and fruits of these species are consumed fresh or cooked, and composted, marmalade, milk desserts, confectioneries, and sugar bars are used to make vinegar, fruit juice, and other soft drinks (Miandji 2010). Although it is not common in Turkey, it is available in the market as fresh fruit, vinegar, marmalade, and leaf-flower tea, and people also consume it as tea (Altundağ and Öztürk 2011).
As a plant species that has been used for medicinal purposes and traditional medicine for many years, hawthorn is also widely used as a food and beverage. The hawthorn species, which are common and traditionally used in folk medicine, contain flavonoids, vitamins, saponin, essential oils, organic acids, and procyanidins in their flowers and fruits, and therefore have high antioxidant, antimicrobial, antifungal, anti-inflammatory, and antiviral activities (Ozyurek et al. 2012; Çalışkan et al. 2012; Garcia-Mateos et al. 2013; Özderin et al. 2016).
There is a growing interest in medicinal plants and their phenolic content due to health benefits when applied for therapeutic purposes. Antioxidant activity is the most known bioactivity of phenolic compounds, which helps to prevent or reduce diseases associated with oxidative stress (Hiransai et al. 2016). According to numerous scientific studies, phenolic compounds have a variety of bioactivities. Moreover, further research is needed to understand their main mechanism in the organisms, to improve their sustainable bioavailability, increase extraction methods, diversify the areas of application, and ensure the establishment of stable procedures (Albuquerque et al. 2021). Many studies indicate great interest in flavonoids, one of the most prominent groups of phenolic metabolites (Su et al. 2021). It is generally known that during the extraction procedure, solvents with high polarities, such as ethyl acetate and methanol, easily dissolve phenolic compounds of the plant, and hence it can be stated that phenolic components are extracted easily (Alara et al. 2021). Selecting the appropriate solvent system also affects the amount of phenolic components extracted and the rate of extraction (Xu and Chang 2007).
This study aims to determine for the first time the chemical content, total phenolic content, total flavonoid content, as well as the antioxidant and antimicrobial activities of fruit samples of Crataegus monogyna var. odemisii, to determine the effect of hexane, ethyl acetate, and ethanol solvents on the extraction performance.
EXPERIMENTAL
Plant Material
Fruit samples of Crataegus monogyna var. odemisii Dönmez & Özderin were collected from İzmir, Ödemiş-Bozdag, Turkey, during the fruit ripening period (October) in 2022. The samples from the plant and its fruits were dried using the herbarium techniques, their authenticity was confirmed by Prof. Dr. Ali A. Dönmez, and a voucher specimen was kept at the Herbarium of the Hacettepe University (HUB), Turkey. The samples were kept in the above-mentioned herbarium. Before the analysis, the harvested samples were first allowed to ripen at room temperature for a week.
Methods
Preparation of extracts and determination of the lipid compositions of fruit pulp and fruit seed extracts
Crataegus monogyna var. odemisii plant and fruit samples were packaged and transferred to the laboratory the same day of harvest without exposure to sunlight. The fruit parts were dried for about 2 weeks under shade. The dried fruit parts (pulp and seed) were ground using a mechanical grinder to obtain its fine powder before the extraction. Briefly, the powdered hawthorn samples were extracted separately with solvents of increasing polarity. First individual 10 g of hawthorn was taken, and 40 mL of hexane and ethyl acetate was added to it. It was extracted with an orbital shaker for 2 weeks at room temperature. Then, extraction was continued again for 10 days at 25 °C. After the extraction, ethyl acetate, and hexane were evaporated in a rotary evaporator to obtain raw extracts. Next, the hexane and ethyl acetate extracts were mixed with methanol and various organic solvents were used to perform further extraction. After removing the solvents, raw extracts of each solvent were obtained.
GC-MS analysis
Approximately 100 mg of the hexane extract was mixed with 10 mL of hexane in a 20 mL test tube. Following the vortexing for 5 min, 100 µL of solution in 2 N methanol (KOH solution) was added. The tube was closed and vortexed for 1 min. After centrifuging for 10 min at 4000 rpm, Nagel Chromaphil Xtra was used to filter the upper phase through a PTFE – 20/25 0.20 µm filter, then 2.0 µL solution was added into the Agilent 7890A GC-5975C MS device (Figs. 1 and 2). An ion trap MS spectrometer and a DB-1 fused silica non-polar capillary column (30 m × 0.25 mm I.D., film thickness 0.25 µm) were used for the GC–MS analyses. The carrier gas was helium with 1.4 mL/min flow rate. The injector and MS transfer line temperatures were 220 and 290 °C, respectively. The ion source temperature was at 200 °C. The injection volume was 0.2 µL, with a split ratio of 20:1. EI–MS measurements were taken at 70 eV ionization energy. The mass range was from m/z 28 to 650 amu. Scan time was 0.5 s with 0.1 s interscan delays. The initial oven temperature was held at 100 °C for 5 min, then increased up to 238 °C with 3 °C/min increments and kept at this temperature for 9 min. Identification of components of the hexane extract was based on GC retention indices and computer matching with the Wiley, NIST-2008 and TRLIB libraries, as well as by comparison of the fragmentation patterns of the mass spectra which reported in the literature (Adams 2007).
Fig. 1. The GC-MS chromatogram of the fruit extracts of C. monogyna var. odemisii; (Abundance/retention time)
Fig. 2. The GC-MS chromatogram of the reference compounds (Abundance/retention time)
Determination of Total Phenolic and Flavonoid Contents
Folin-Ciocalteu reagent was used in determining the total phenolic content (TPC) of the extracts based on the above-mentioned method with small modifications (Chandra et al. 2014). Of the test sample, 0.2 mL was added to 0.6 mL of distilled water, together with 0.2 mL of Folin-Ciocalteu reagent (1:1). The mixture was stirred for 5 min. Then, 1 mL of saturated Na2CO3 solution (8% w/v in H2O) was added, and distilled water was added to obtain the final volume of 3 mL. The reaction mixture of each sample was incubated in complete darkness for 30 min. Next, the blue-colored mixture was centrifuged. Then its absorbance at 765 nm was measured. Pyrocatechol was used to obtain a standard curve, and the phenolic content was calculated as pyrocatechol equivalents PE/g of dried plant material. Each experiment was repeated three times. The AlCl3 colorimetric method was used to determine the total flavonoid content (TFC) of the extracts as described in a previous study (Chandra et al. 2014) with small modifications. The calibration curve was established using the quercetin standard. As a stock solution, 5.0 mg of quercetin was dissolved in 1.0 mL methanol, and pure methanol was added to obtain different serial dilutions. Then, 0.6 mL of 2% AlCl3 was added and mixed into 0.6 mL diluted quercetin standard solutions or extracts, and the solution was incubated at room temperature for 1 h. The absorbance at 420 nm of each mixture was then found against a blank. The calibration curve was used for determining the total flavonoid content in test samples in mg quercetin equivalent (QE)/g of dried plant. Each experiment was repeated three times.
Antioxidant activity analysis
Five different methods used were as follows: ABTS+ (2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) radical cation, DPPH (2,2-diphenyl-1-picryl hydrazylhydrazyl) radical scavenging assay, CUPRAC (cupric reducing antioxidant capacity), β-carotene linoleic acid assay, and metal chelation. These methods were used for measuring the antioxidant potential of the extracts. Lipid peroxidation inhibition was evaluated using a β-carotene-linoleic acid assay as described in another research article (Tel-Cayan and Duru 2019). Radical scavenging potentials were measured with the help of DPPH and ABTS+, using spectrophotometry as previously explained (Tel et al. 2012). CUPRAC was determined using a method described in another study (Apak et al. 2004). In the above-mentioned assays, atocopherol and BHA (butylated hydroxyanisole) were used as antioxidant standards to compare the activities of the extracts. In the metal chelation assay, EDTA molecule was used as the reference compound. In this method, it was compared with the value of EDTA to determine the antioxidant activity of the extract (Decker and Welch 1990).
Determination of antimicrobial effects
Antimicrobial activities of pigments extracted from Streptomyces spp. were determined by the minimum inhibitory concentration (MIC) method (CLSI 2006). The antimicrobial activity of the pigments was studied using standard test bacteria Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Candida albicans ATCC 10239, and Bacillus subtilis ATCC 6633.
The broth microdilution method was used to determine the MIC values of the newly obtained pigments against the test microorganisms (CLSI 2006). The MIC tests were conducted with Mueller Hinton Broth (MHB) using microplates (Nunc F96 Microwell plates, Nunclon Δ, Denmark) for polystyrene culture media kept in a sterilized condition. The suspensions at 5 × 105 CFU/mL concentrations were prepared for all tested microorganisms. In plaques, the medium was placed on the first row, and the bacteria + medium mixture was placed on the second row. For the other rows, 0.200 μL samples of extracts and bacteria mixed with the medium were added to the wells to obtain the final extract concentrations (0.312, 0.625, 1.25, 2.5, 5, and 10 mg/mL). Bacillus subtilis and S. aureus were incubated at 37 ± 0.1 °C, E. coli and P. aeruginosa were incubated at 30 ± 0.1 °C for 24 h, and C. albicans was incubated at 30 ± .0.1 °C for 24 to 48 h, and the final, turbidity-free clear well was selected for the MIC value. The measurements were repeated three times in the study.
RESULTS AND DISCUSSION
Lipid Compositions
In this study, gas chromatography coupled with mass selective detector (GC/MSD) systems were used to qualitatively and quantitatively analyze the lipid contents in fruit seeds of C. monogyna var. odemisii. Using reference standard substances with certified lipid contents, retention times were defined using the NIST 2008 and Wiley 2008 libraries, and percentage amounts were calculated. The results of the obtained analysis are shown in Table 1. Looking at the lipid content in fruit kernel ethyl acetate extract of C. monogyna, 21.1% were 9-octadecenal (z), 20.4% were ɣ-sitosterol, and 20.3% were β-monolinolein components, whereas C. monogyna fruit parts in hexane extract had lipid contents of 49.3% linoleic acid, 30.6% oleic acid, and 10.5% palmitic as major components, as shown in Table 2.
There has been great interest in medicinal aromatic plants, which play an important role, particularly in human nutrition. They have been used in the treatment of diseases since ancient times. Wild fruits, well adapted to local climatic conditions, contain a high content of phytochemicals (Calışkan et al. 2012; Özyürek et al. 2012; Özderin et al. 2016; Özderin 2022). As a result of different extraction methods, the chemical components of hawthorn species have been determined in various studies. Hawthorn leaves, flowers, and fruits contain various chemical components, such as flavonoids (0.1% to 1% in fruits, 1% to 2% in flowers or leaves), triterpene acids (0.5% to 1.4% in fruits), oligomeric proanthocyanidins (OPCs, 1% to 3% in flowering leaves of fruits), organic acids (2% to 6%), sterols, and trace amounts of cardioactive amines. Of these components, flavonoids and OPCs constitute the two main groups of bioactive components (Bechkri et al. 2017). In the seed oil of C. monogyna Jacq., 10 components were identified. The major components of C. monogyna seed oil were linoleic acid (44.2%), and oleic acid (28.3%). According to the results, the seed oils of C. azarolus L. contain tetradecamethyl-cycloheptasiloxane (39.4%), mandelic acid, 3,4-dihydroxytetramethylsilyl (19.2%), dodecamethylcyclohexasiloxane (17.1%), and decamethylcyclopentasiloxane (10.6%). In the evaluation of the chemical content in fruit kernel ethyl acetate extract of C. monogyna var. odemisii, 21.1% were 9-octadecenal(z), 20.4% were ɣ-sitosterol, 20.3% were β-monolinolein components, and the hexane extract of the C. monogyna fruit parts had 49.3% linoleic acid, 30.6% oleic acid, 10.4% palmitic acid as major components, which were similar to the study by Bechkri et al. (2017), who found linoleic acid (44.2%), oleic acid (28.3%), and palmitic acid (6.56%) in C. monogyna fruit parts samples.
Table 1. Lipid Composition of Methyl Esters in the Fruit Seeds of C. monogyna var. odemisii fruit parts (Ethyl Acetate Extracts)
Table 2. Lipid Composition of Methyl Esters in the Fruit of C. monogyna var. odemisii (Hexane Extracts)
Total Phenolic and Total Flavonoid Contents
Phenolic and flavonoid compounds are among the important classes of bioactive components in plants, which are known to have antioxidant activities (Bahorun et al. 2003). Flavonoids are a class of polyphenolic compounds found almost everywhere in plants. Because of their myriad pharmacological activities, ranging from antioxidant capacity to protective activity against diseases, such as cancer, diabetes, inflammation, and chronic diseases, flavonoids are by far the most studied secondary plant metabolite classes (Habtemariam and Varghese 2014). The total phenolic and total flavonoid contents in the fruit parts of C. monogyna var. odemisii extracted with three different solvents (hexane, ethyl acetate, and ethanol) are presented in Table 3. According to the results of the total phenolic-flavonoid analysis, the extract with the highest phenolic and flavonoid contents was ethanol extract (204.4 ± 0.86 mg PEs/g and 77.83 ± 0.84 mg QEs/g).
The phenolic compounds in plants are mostly associated with bioactivity resulting from the synergistic or additive effect of different classes of compounds found in plant extract (Ramful et al. 2011). Various studies have reported that the total phenolic content in different extracts from C. monogyna flowers and leaves ranges from 108.6 to 343.5 mg/g GAE/g (Öztürk and Tuncel 2011). The total phenolic and flavonoid contents of C. azarolus leaf extracts were 38.2 to 396.0 mg GAE/g and 2.12 to 32.6 mg QE/g, respectively (Amel et al. 2014). The amount of TPC of hawthorn fruits extracts ranged from 21.2 to 69.1 mg GAE/g (Alirezalu et al. 2020).
Table 3. Total Phenolic and Total Flavonoid Contents of the Extracts
Given the many pharmacological effects of phenolic compounds, the antioxidant effects of phenolic compounds have been demonstrated in several experimental studies. Different results have been obtained in studies where the total amount of phenolic substances contained in the fruit of different hawthorn species was examined. For example, Ercisli et al. (2015) noted that the TPC of 18 different hawthorn varieties extracted with acetone:water:acetic acid (70:20.5:0.5) mixture was in the range of 660 to 3460 mg GAE 100 g-1 of fresh weight. Similarly, Çalışkan et al. (2012) determined that the TPC in methanol extraction of 15 different hawthorn varieties varied in the range of 26.6 to 57.1 mg GAE g-1 dry weight, while Mraihi et al. (2013) determined that the phenolic substance content in extractions with methanol:water (80:20) mixture in C. monogyna and C. azarolus pulps were 122.3 and 60.9 mg GAE 100 g-1 dry weight.
Tahirovic and Basic (2014) used water, 50% methanol and 50% ethanol, 80% methanol, 80% ethanol, pure ethanol, and pure methanol for extractions, and determined the highest TPC in C. monogyna species as 4.60 mg GAE g-1 fresh fruit using 80% methanol, and noted that phenolic substance amounts were 2.02, 4.18, and 3.01 mg GAE g-1 fresh weight, using water, 50% methanol, and pure methanol, respectively. In another study, it was recorded that the TPC of C. oxyacantha hawthorn fruit extracts in ethanol, ethanol:water, methanol, methanol:water, and water extractions were 2.12, 19.32, 18.21, 30.63, and 24.89 mg gallic acid equivalent g-1, respectively (Kostic et al. 2012).
In another study, it was determined that phenolic compounds from C. orientalis fruit were better extracted in methanol:water mixture and that antioxidant activity was higher in this extract (Çoklar and Akbulut 2016). Many research studies examined the compound and antioxidant capacity for several Crataegus species parts, including leaves, fruits, and flowers (Bernatoniene et al. 2008; Bouzid et al. 2011). Some experimental studies determined the fruit antioxidant activities of Crataegus species of different origins (Bahorun et al. 2003; Pliszka et al. 2016; Cosmulescu et al. 2017). According to the results of these studies, antioxidant activity was mainly associated with phenolic procyanidinine and flavonoid contents (Ljubuncic et al. 2005, and to a lesser extent total phenols (Bahorun et al. 2003). Another study found that the highest concentration of flavonoids in ethyl acetate extracts of C. monogyna leaves and fruits were 122.98 ± 0.21 and 58.81 ± 0.12 mg RU/g dry weight, respectively, while the lowest concentration of flavonoids in chloroform extracts of leaves and fruits were 36.01 ± 0.16 and 21.11 ± 0.11 mg RU/g dry weight, respectively, and the amount of flavonoid content extracted was following this order: ethyl acetate > ethanol > chloroform. In the same study, they determined that the phenolic content of C. monogyna Jacq varied in the range of 38.05 ± 0.18 to 152.87 ± 0.11 mg GAE/g dry weight in fruit samples, and in the range of 83.36 ± 0.21 to 365.11 ± 0.32 mg GAE/g dry weight in the leaf samples (Dekic et al. 2020). In the Eastern region of Algeria, polyphenol and flavonoid values in samples of C. monogyna fruit were determined as 43.9 mg EAG/mg and 5.41 μg EQ/mg, respectively (Bouzi et al. 2011). According to the results of the total phenolic and flavonoid analysis in C. monogyna var. odemisii fruit samples, it was determined in this study that ethanol extract led to the highest phenolic and flavonoid component contents (204.4 ± 0.86 mg PEs/g and 77.83 ± 0.84 mg QEs/g). When compared the studies with other hawthorn species in the literature, it is seen that C. monogyna var. odemisii fruit samples have higher phenolic and flavonoid contents.
Antioxidant Activity Results
All tested extracts of C. monogyna var. odemisii fruit samples had strong antioxidant activity. Of these extracts, ethanol extract, in particular, showed a higher activity than BHA and α-tocopherol, which are used as standard antioxidants in DPPH, ABTS, and CUPRAC tests. Ethanol extract showed antioxidant activities with 1C50 values of 18.31 ± 0.45 μg/mL in the DPPH test, 9.25 ± 0.36 μg/mL in the ABTS test, 15.74 ± 0.55 μg/mL in the CUPRAC test, and 52.80 ± 0.90 μg/mL in metal chelating assay. In the same tests, ethyl acetate extract showed antioxidant activity with IC50 values of 18.48 ± 0.34 μg/Ml, in the DPPH test, 13.51 ± 0.70 μg/mL in the ABTS test, 22.30 ± 0.80 μg/mL in the CUPRAC test, and 81.20 ± 0.75 μg/mL in metal chelating assay, and hexane extract in showed antioxidant activity of 125.5 ± 1.37 μg/mL in the DPPH test, 107.5 ± 1.20 μg/mL in the ABTS test, 121.1 ± 1.18 μg/mL in the CUPRAC test. Table 4 shows the results of the antioxidant activity of the samples. According to these results, it is believed that ethanol extract can be used as a potential antioxidant.
Table 4. Antioxidant Activity of the Extracts Using β-Carotene-linoleic acid, ABTS•+, DPPH•, CUPRAC, and Metal Chelating Assays a
a Values represent the means ± SEM of three parallel sample measurements (p < 0.05)
b NT: not tested
cA0.50 values represent the means ± S.E. of three parallel measurements (p < 0.05)
Antioxidant activities of Crataegus species (C. oxyacantha, C. orientalis, C. aronia syn. azarolus, C. monogyna, C. meyeri, and C. pontica) have been reported. An ethanol extract of C. orientalis leaves has been shown to have good activity according to the DPPH radical scavenging experiment (62.9% at 10 mg/mL ± 3.40%), and β-carotene bleaching experiment (42.4% at 1 mg/mL) (Bor et al. 2012). The antioxidant activities of methanol extracts from flowers and leaves of 14 Crataegus spp. in different regions of Turkey were examined using different experiments. And CUPRAC (cupric reducing antioxidant capacity), ABTS/persulfate, FRAP (Ferric Reducing Antioxidant Power), and Folin experiments showed that the most active was the C. pentagyna leaf extract, compared to the Crataegus extracts (TEACCUPRAC = 0.378 ± 0.004, TEACFRAP = 0.132 ± 0.001, TEAC Folin = 0.752 ± 0.201 mmol TR g-1, TEACABTS = 0.423 ± 0.112). It was stated that the antioxidant activities of C. pentagyna flowers and leaves reduce copper and iron ions, Folin reagent and ABTS radical cation. In the same study, they determined the total antioxidant capacities (TAC values) values of the leaves and flowers of different Crataegus species obtained from different regions as Folin > CUPRAC > ABTS/TEAC > FRAP. It was found that C. monogyna samples showed a high antioxidant activity, which was attributed to flavonoids and procyanidin contents (Özyürek et al. 2012). Çalışkan et al. (2012) determined that the antioxidant activity results of fruit extracts obtained from different hawthorn taxa ranged from 21.4% to 33.2%. In CUPRAC analysis, determined that leaves of Hawthorn (C. monogyna) have the highest activity with a value of 4.30±0.98 µmol Trolox equivalents/g sample (Ceylan et al. 2019). The antioxidant activities of hawthorn fruit extract from a study of three antioxidant study techniques showed that ECE had statistically reliably stronger activity in free radical scavenging. Using different procedures to get different indices of the antioxidant activity of the same product allows one to assume that distinct chemical components are involved in the inactivation of free radicals in DPPH·, ABTS·+, or TNI (Bernatoniene et al. 2008). According to numerous studies, the total phenolic and flavonoid content of many plant species varied depending on the polarity of the solvents used for the extraction (Zhou and Yu 2004; Mohsen and Ammar 2009). However, it has been stated in different studies that even some small morphological differences that Crataegus species can lead to different chemical content and antioxidant capacity (Özyürek et al. 2012).
Antimicrobial Effects
According to the result of the minimum inhibitory activity (MIC) test of C. monogyna var. odemisii, the highest antimicrobial activity against on C. albicans was achieved by 0.625 mg/mL of ethanol extract, 1.25 mg/mL of ethyl acetate extract, and 2.5 mg/mL of hexane extract. MIC values ranging from 0.625 to >10 mg mL was observed in our test in microorganisms (Table 5). Low MIC values are the desired result. Because it is known that the lower this value is, the lower the concentration of it is effective against the relevant microorganisms. According to the results, the extracts contain higher antimicrobial activity against Gr+ bacteria than Gr- bacteria. Especially, hexane extracts with a concentration of 1.25 mg/mL on B. subtilis and S. aureus had an inhibitory effect.
Consistent with the results obtained in the study, other studies have identified the activity of leaf ethanol extract of C. monogyna against common pathogens of S. aureus, E. coli, and P. aeruginosa. They found that the MIC of ethanol extract of C. monogyna leaves was in the range of 0.512 to 1.024 mg/mL against S. aureus but was inactive against E. coli and P. aeruginosa (Belabdelli et al. 2022). They determined that the MIC of C. elbursensis fruit flesh ranges between 5 to 10 mg/mL and minimum bactericidal concentration (MBC) values vary between 5 and 20 mg/mL, while the bacteriostatic activity of C. elbursensis seed extracts of fruit kernel varies between 10 and 40 mg/mL (Salmanian et al. 2014). They found that the highest antibacterial activity in methanol extracts of C. monogyna leaves had a 12-mm inhibition zone and a MIC value of 0.625 mg/mL against S. aureus. Methanol extracts of C. monogyna leaves had activity against Enterobacter aerogenes (10-mm inhibition zone, 1.25 mg/mL MIC value), E. coli (10-mm inhibition zone, 1.25 mg/mL MIC value), and methanol and water extracts of leaves had activity against E. coli and S. aureus (1.25 and 0.625 mg/mL MIC values, respectively) (Yigit et al. 2014). Although the solvents in the present study were not the same, the findings were similar. In another study, C. tanacetifolia (Lams) Press and C. bornmuelleri Zabel samples had varied, widely intermittent antimicrobial activity against certain human and plant pathogenic microbes and food toxic substances (Yigit et al. 2014). In another study, regarding the evaluation of the antibacterial activity of ethanol extracts of C. monogyna leaves, the MIC against S. aureus was in the range of 0.512 to 1.024 mg/mL and it was inactive against E. coli and P. aeruginosa and MIC values ranging from 0.625 to >10 mg mL were observed to test microorganisms (Güven et al. 2006). Upon comparison of the results from this study with the literature, the author’s results were consistent with the previous studies (Güven et al. 2006; Belabdelli et al. 2022).
In this study, the phenolic content of the extracts of C. monogyna var. odemisii fruit samples were determined for the first time and it was found that the strong antioxidant and antimicrobial activities were associated with a high content of chemical components, phenolic, and flavonoid compounds.
Table 5. The MIC Test Results of C. monogyna var. odemisii Fruit Extracts
CONCLUSIONS
- In this study, it was found for the first time the fruit extracts of C. monogyna var. odemisii contain phytochemicals that are important for health. The findings showed that the fruit extracts of C. monogyna var. odemisii contained noticeable levels of lipid components, flavonoids, and phenols. Based on its chemical composition it can be utilized in medicine and food industry, pharmaceutical applications as a natural source of antioxidants, antimicrobials, and functional food/additives production
- Consequently, the consideration of the morphological differences and ecological preferences of Crataegus monogyna var. odemisii should be assigned to ‘sensitive variety’, and there is no information about the phytochemicals of C. monogyna var. odemisii in litarature which restricts the range of its uses. The data obtained at the end of this study will hopefully contribute to the literature and thus increase the usage areas of hawthorn species, nothing is known about the phytochemicals of C. monogyna var. odemisii, which restricts the range of its uses. The data obtained at the end of this study will hopefully contribute to the literature and thus increase the usage areas of hawthorn species.
REFERENCES CITED
Adams, R. P. (2007). Identification of Essential Oil Components by Gas Chromatography/ Mass Spectrometry, 4th Ed., Allured Publishing Corporation, Carol Stream, Illinois.
Alara, O. R., Abdurahman, N. H., and Ukaegbu, C. I. (2021). “Extraction of phenolic compounds: A review,” Current Research in Food Science 4(2021), 200-214. DOI: 10.1016/j.crfs.2021.03.011
Albuquerque, B. R., Heleno, S. A., Oliveira, M. B. P., Barros, L., and Ferreira, I. C. (2021). “Phenolic compounds: Current industrial applications, limitations and future challenges,” Food & Function 12(1), 14-29. DOI: 10.1039/D0FO02324H
Alirezalu, A., Ahmadi, N., Salehi, P., Sonboli, A., Alirezalu, K., Mousavi Khaneghah, A., Barba, F. J., Munekata, P. E. S., and Lorenzo, J. M. (2020). “Physicochemical characterization, antioxidant activity, and phenolic compounds of hawthorn (Crataegus spp.) fruits species for potential use in food applications,” Foods 9(4), 436.
Altundag, E., and Ozturk, M. (2011). “Ethnomedicinal studies on the plant resources of east Anatolia,” Procedia-Social and Behavioral Sciences 19, 756-777. DOI: 10.1016/j.sbspro.2011.05.195
Amel, B. K., Seddik, A., Shtaywy, D., Saliha, A. Z., Mussa, B., Assia, D., Saliha, B., Abderahmane, and Smain, A. (2014). “Phytochemical analysis, antioxidant activity and hypotensive effect of Algerian azarole (Crataegus azarolus L.) leaves extracts,” Research Journal of Pharmaceutical, Biological and Chemical Sciences 5(2), 286-305.
Apak, R., Güçlü, K., Özyürek, M., and Karademir, S. E. (2004). “Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method,” Journal of Agricultural and Food Chemistry 52(26), 7970-7981. DOI: 10.1021/jf048741x
Bahorun, T., Aumjaud, E., Ramphul, H., Rycha, M., Luximon‐Ramma, A., Trotin, F., and Aruoma, O. I. (2003). “Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts,” Food/Nahrung 47(3), 191-198. DOI: 10.1002/food.200390045
Baytop, T. (1994). Dictionary of Turkish Plant Names (Turkish Linguistic Council Publication No. 578). Turkish Linguistic Council, Ankara (in Turkish).
Bechkri, S., Berrehal, D., Semra, Z., Bachari, K., Kabouche, A., and Kabouche, Z. (2017). “Composition and biological activities of seeds oils of two Crataegus species growing in Algeria,” Journal of Materials and Environmental Sciences 8(5), 1526-1530. DOI: 10.1016/j.scienta.2023.112007
Belabdelli, F., Bekhti, N., Piras, A., Benhafsa, F. M., Ilham, M., Adil, S., and Anes, L. (2022). “Chemical composition, antioxidant and antibacterial activity of Crataegus monogyna leaves’ extracts,” Natural Product Research 36(12), 3234-3239. DOI: 10.1080/14786419.2021.1958215
Bernatonienė, J., Masteikova, R., Majienė, D., Savickas, A., Kėvelaitis, E., Bernatonienė, R., Pečiūra, R., Dvoráčková K., Civinskienė G., Lekas R., et al. (2008). “Free radical-scavenging activities of Crataegus monogyna extracts,” Medicina 44(9), article 706. DOI: 10.3390/medicina44090091
Bor, Z., Arslan, R., Bektaş, N., Pirildar, S., and Dönmez, A. A. (2012). “Antinociceptive, antiinflammatory, and antioxidant activities of the ethanol extract of Crataegus orientalis leaves,” Turkish Journal of Medical Sciences 42(2), 315-324. DOI: 10.3906/sag-1011-1304
Bouzid, W., Yahia, M., Abdeddaim, M., Aberkane, M. C., and Ayachi, A. (2011). “Évaluation de l’activité antioxydante et antimicrobienne des extraits de l’aubépine monogyne [Evaluation of the antioxidant and antimicrobial activity of monogyne Hawthorn extracts],” Lebanese Science Journal 12(1), 59-69.
Çalişkan, O., Gündüz, K., Serçe, S., Toplu, C., Kamiloğlu, Ö., Şengül, M., and Ercişli, S. (2012). “Phytochemical characterization of several hawthorn (Crataegus spp.) species sampled from the Eastern Mediterranean region of Turkey,” Pharmacognosy Magazine 8(29), 16-21. DOI: 10.4103/0973-1296.93305
Ceylan, Ş., Yarar, R., Camadan, Y., Saral, Ö., and Özşen, Ö. (2019). “Determination of antioxidant and antimicrobial activities of some medicinal plants grown in Şırnak region of Turkey,” Erzincan University Journal of Science and Technology 12(2), 628-638. DOI: 10.18185/erzifbed.461319
Chandra, S., Khan, S., Avula, B., Lata, H., Yang, M. H., Elsohly, M. A., and Khan, I. A., (2014). “Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study,” Evidence Based Complementary and Alternative Medicine 2014, article ID 253875. DOI: 10.1155/2014/253875
Clinical and Laboratory Standards Institute (CLSI) (2006). Methods for Dilution Microbial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 7th Edition, M7-A7, CLSI, Wayne, PA, USA.
Çoklar, H., and Akbulut, M. (2016). “Effect of different solvents on extraction of phenolic compounds and antioxidant activity of hawthorn (Crataegus orientalis) fruit,” Derim 33(2), 237-248. DOI: 10.16882/derim.2016.267908
Cosmulescu, S., Trandafir, I., and Nour, V. (2017). “Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties,” International Journal of Food Properties 20(12), 3124-3134. DOI: 10.1080/10942912.2016.1274906
Davis, P. H. (1972). Flora of Turkey and the East Aegean Islands, Edinburg University Press, Edinburg, Scotland.
Decker, E. A., and Welch, B. (1990). “Role of ferritin as a lipid oxidation catalyst in muscle food,” Journal of Agricultural and food Chemistry 38(3), 674-677. DOI: 10.1021/jf00093a019
Dekić, V., Ristić, N., Dekić, B., and Ristić, M. (2020). “Phenolic and flavonoid content and antioxidant evaluation of hawthorn (Crataegus monogyna Jacq.) fruits and leaves extracts,” Bulletin of Natural Sciences Research 10(1), 20-25. DOI: 10.5937/univtho10-25574
Dönmez, A. A. (2004). “The genus Crataegus L. (Rosaceae) with special reference to hybridization and biodiversity in Turkey,” Turkish Journal of Botany 28(1), 29-37.
Dönmez, A. A. (2014). “Nomenclatural, taxonomic and biogeographic novelties in the Turkish Crataegus L. (Rosaceae-Maleae) taxa,” Adansonia 36(2), 245-253. DOI: 10.5252/a2014n2a7
Dönmez, A. A., and Özderin, S. (2019). “Additional contributions to taxonomy, nomenclature and biogeography of the Turkish Crataegus (Rosaceae) taxa,” PhytoKeys 122(1), article ID 33002. DOI: 10.3897/phytokeys.122.33002
Edwards, J. E., Brown, P. N., Talent, N., Dickinson, T. A., and Shipley, P. R. (2012). “A review of the chemistry of the genus Crataegus,” Phytochemistry 79, 5-26. DOI: 10.1016/j.phytochem.2012.04.006
eFloras. org. (2023). (http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=108272), Accessed 28 Oct 2023.
Ercisli, S., Yanar, M., Sengul, M., Yildiz, H., Topdas, E. F., Taskin, T., and Yilmaz, K. U. (2015). “Physico-chemical and biological activity of hawthorn (Crataegus spp. L.) fruits in Turkey,” Acta Scientiarum Polonorum-Hortorum Cultus 14(1), 83-93. DOI: 10.1007/s10341-022-00676-9
García-Mateos, R., Ibarra-Estrada, E., and Nieto-Angel, R. (2013). “Antioxidant compounds in hawthorn fruits (Crataegus spp.) of Mexico,” Revista Mexicana de Biodiversidad 84(4) 1298-1304. DOI: 10.7550/rmb.35675
Güner, S., Aslan, T., Ekim, M., Vural, M., and Babaç, M. T. (2012). Plant List of Turkey: Vascular Plants, Nezahat Gökyiǧit Botanical Garden Press, ANG Foundation, İstanbul, Turkey.
Güven, K., Yücel, E., and Cetintaş, F. (2006). “Antimicrobial activities of fruits of Crataegus and Pyrus species,” Pharmaceutical Biology 44(2), 79-83. DOI: 10.1080/13880200600591253
Habtemariam, S., and K. Varghese, G. (2014). “The antidiabetic therapeutic potential of dietary polyphenols,” Current Pharmaceutical Biotechnology 15(4), 391-400. DOI: 10.2174/1389201015666140617104643
Hiransai, P., Tangpong, J., Kumbuar, C., Hoonheang, N., Rodpech, O., Sangsuk, P., Kajklangdon U., and Waraphorn I. (2016). “Anti-nitric oxide production, anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia,” Asian Pacific Journal of Tropical Biomedicine 6(11), 950-956. DOI: 10.1016/j.apjtb.2016.02.002
Kaur, C., and Kapoor, H. C. (2001). “Antioxidants in fruits and vegetables–the millennium’s health,” International Journal of Food Science & Technology 36(7), 703-725.
Kiziroglu, I. Adızel, Ö., and Bahadır, M. (2006). “Nature reserves and biodiversity in Turkey and related policy measures along with joining the EU,” Fresenius Environmental Bulletin 15(9b), 1156-1160.
Kostić, D. A., Velicković, J. M., Mitić, S. S., Mitić, M. N., and Randelović, S. S. (2012). “Phenolic content, and antioxidant and antimicrobial activities of Crataegus oxyacantha L. (Rosaceae) fruit extract from Southeast Serbia,” Tropical Journal of Pharmaceutical Research 11(1), 117-124. DOI: 10.4314/tjpr.v11i1.15
Ku, S. K., Zhou, W., Lee, W., Han, M. S., Na, M., and Bae, J. S. (2015). “Anti-inflammatory effects of hyperoside in human endothelial cells and in mice,” Inflammation 38(2), 784-799. DOI: 10.1007/s10753-014-9989-8
Küçük, M., and Ertürk, E. (2013). “Biodiversity and protected areas in Turkey,” Sains Malaysiana 42(10), 1455-1460.
Liang, C., and Li, X. (2012). “The ecological sensitivity evaluation in Yellow River delta national natural reserve,” CLEAN Soil, Air, Water 40(10), 1197-1207. DOI: 10.1002/clen.201200051
Ljubuncic, P., Portnaya, I., Cogan, U., Azaizeh, H., and Bomzon, A. (2005). “Antioxidant activity of Crataegus aronia aqueous extract used in traditional Arab medicine in Israel,” Journal of Ethnopharmacology 101(1-3), 153-161. DOI: 10.1016/j.jep.2005.04.024
Miandji, M. A. (2010). Atlas of Medicinal Plants, Bilgi Publishing House, Ankara, Turkey.
Mingwu, Z., Haijiang, J., Desuo, C. and Chunbo, J. (2010). “The comparative study on the ecological sensitivity analysis in Huixian Karst wetland, China,” Procedia Environmental Sciences 2, 386-398. DOI: 10.1016/j.proenv.2010.10.043
Mohsen, S. M., and Ammar, A. S. (2009). “Total phenolic contents and antioxidant activity of corn tassel extracts,” Food Chemistry 112(3), 595-598. DOI: 10.1016/j.foodchem.2008.06.014
MOE (2001). The National Strategy and Action Plan for Biodiversity in Turkey, Ministry of Environment, Ankara, p. 37.
Mraihi, F., Journi, M., Chérif, J. K., Sokmen, M., Sokmen, A., and Trabelsi-Ayadi, M. (2013). “Phenolic contents and antioxidant potential of Crataegus fruits grown in Tunisia as determined by DPPH, FRAP, and β-carotene/linoleic acid assay,” Journal of Chemistry 2013, article ID 378264. DOI: 10.1155/2013/378264
Özderin, S. 2022. “Determination of some chemical properties of wild pear (Pyrus spinosa Forsk.),” BioResources 17(1), 1659-1669. DOI: 10.15376/biores.17.1.1659-1669
Özderin, S., Fakir, H., and Dönmez, E. (2016). “Chemical properties of hawthorn (Crataegus L. spp.) taxa naturally distributed in western Anatolia part of Turkey,” Šumarski List 140(7-8), 369-375. DOI: 10.31298/sl.140.7-8.5
Öztürk, N., and Tunçel, M. (2011). “Assessment of phenolic acid content and in vitro antiradical characteristics of hawthorn,” Journal of Medicinal Food 14(6), 664-669. DOI: 10.1089/jmf.2010.0063
Özyurek, M., Bener, M., Guclu, K., Dönmez, A. A., Selcuk, S., Pirildar, S., Mericli, A. H., and Apak, R. (2012). “Evaluation of antioxidant activity of Crataegus species collected from different regions of Turkey,” Records of Natural Products 6(3) 263-277. DOI: 10.1515/pjfns-2017-0010
Pliszka, B., Huszcza-Ciołkowska, G., and Wierzbicka, E. (2016). “Effects of solvents and extraction methods on the content and antiradical activity of polyphenols from fruits Actinidia arguta, Crataegus monogyna, Gaultheria procumbens, and Schisandra chinensis,” Acta Scientiarum Polonorum Technologia Alimentaria 15(1), 57-63. DOI: 10.17306/J.AFS.2016.1.6
Ramful, D., Aumjaud, B., Neergheen, V. S., Soobrattee, M. A., Googoolye, K., Aruoma, O. I., and Bahorun, T. (2011). “Polyphenolic content and antioxidant activity of Eugenia pollicina leaf extract in vitro and in model emulsion systems,” Food Research International 44(5), 1190-1196. DOI: 10.1016/J.FOODRES.2010.09.024
Salmanian, S. S. M. A., Sadeghi Mahoonak, A. R., Alami, M., and Ghorbani, M. (2014). “Phenolic content, antiradical, antioxidant, and antibacterial properties of hawthorn (Crataegus elbursensis) seed and pulp extract,” Journal of Agricultural Science and Technology 16(2), 343-354.
Seçmen, Ö., Gemici, Y., Leblebici, Y., Görk, G., and Bekat, L. (1989). Systematics of Seed Plants, Ege University Faculty of Science Publicatio, İzmir (In Turkish).
Şimşek, E. (2014). “Protection of biodiversity in Turkey,” Public and Private International Law Bulletin 34(1), 73-92.
Su, H. B., Lei, C., Seung, J. J., Hyeung-Rak, K., Take, J. N., and Sang, G. L. (2021). “The comparison of total phenolics, total antioxidant, and anti-tyrosinase activities of Korean sargassum species,” Journal of Food Quality 2021, article ID 6640789. DOI: 10.1155/2021/6640789
Tahirović, A., and Bašić, N. (2014). “Phenolic content and antioxidant activity of Crataegus monogyna L. fruit extracts,” Radovi Šumarskog Fakulteta Univerziteta u Sarajevu 44(2), 29-40. DOI: 10.54652/rsf.2014.v44.i2.99
Tel, G., Apaydın, M., Duru, M. E., and Öztürk, M. (2012). “Antioxidant and cholinesterase inhibition activities of three Tricholoma species with total phenolic and flavonoid contents: The edible mushrooms from Anatolia,” Food Analytical Methods 5(3), 495-504. DOI: 10.1007/s12161-011-9275-4
Tel-Çayan, G., and Duru, M. E. (2019). “Chemical characterization and antioxidant activity of Eryngium pseudothoriifolium and E. thorifolium essential oils,” Journal of Research in Pharmacy 23(6), 1106-1114. DOI: 10.35333/jrp.2019.75
Xu, B. J., and Chang, S. K. C. (2007). “A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents’ Journal of Food Science 72(2), 159-166. DOI: 10.1111/j.1750-3841.2006.00260.x
Zhou, K., and Yu, L. (2004). “Effects of extraction solvent on wheat bran antioxidant activity estimation,” LWT-Food Science and Technology 37(7), 717-721. DOI: 10.1016/j.lwt.2004.02.008
Article submitted: November 4, 2023; Peer review completed: January 3, 2024; Revised version received and accepted: January 16, 2024; Published: January 19, 2024.
DOI: 10.15376/biores.19.1.1542-1557
