Abstract
The spill of crude oil products into the environment has a negative impact on the ecosystem. Sorption materials are utilized as the means of their elimination. The sorption capacity of selected organic and inorganic natural sorbents, such as needles (Larix decidua, Abies alba, and Pinus sylvestris), sawdust from logging (Fagus sylvatica, Picea abies), leaf residues (Fagus sylvatica), moss (Ceratodon purpureus), soil, and synthetic sorbents Absodan Plus, expanded perlite, Eco-dry plus, and Reo Amos were all tested according to the standard ASTM F726 (2012). The natural sorbents were tested at various moisture contents (wet, air-dry, and dry) ranging from 0 to 82%. The pollutant used in the experiment was the low-viscosity engine oil 10W 40. The best sorption capacity among the wet sorbents was achieved with larch needles (11.1 g/g). Moss exhibited the best sorption capacity (25.2 g/g) among the air-dry sorbents. Regarding air-dry sorbents, larch needles, spruce sawdust, and beech sawdust showed the best results. When further dried, their sorption capacity decreased. Soil was the least efficient natural sorbent with a sorption capacity that ranged from 0.45 to 3.82 g/g. The best sorption capacity of 11.5 g/g among the synthetic sorbents was in Reo Amos. The sorption capacity of natural and synthetic substances was comparable.
Download PDF
Full Article
Comparison of Natural and Synthetic Sorbents’ Efficiency at Oil Spill Removal
Miroslav Mojžiš,a,* Tatiana Bubeníková,b Martin Zachar,a Danica Kačíková,a and Jaroslava Štefková c
The spill of crude oil products into the environment has a negative impact on the ecosystem. Sorption materials are utilized as the means of their elimination. The sorption capacity of selected organic and inorganic natural sorbents, such as needles (Larix decidua, Abies alba, and Pinus sylvestris), sawdust from logging (Fagus sylvatica, Picea abies), leaf residues (Fagus sylvatica), moss (Ceratodon purpureus), soil, and synthetic sorbents Absodan Plus, expanded perlite, Eco-dry plus, and Reo Amos were all tested according to the standard ASTM F726 (2012). The natural sorbents were tested at various moisture contents (wet, air-dry, and dry) ranging from 0 to 82%. The pollutant used in the experiment was the low-viscosity engine oil 10W 40. The best sorption capacity among the wet sorbents was achieved with larch needles (11.1 g/g). Moss exhibited the best sorption capacity (25.2 g/g) among the air-dry sorbents. Regarding air-dry sorbents, larch needles, spruce sawdust, and beech sawdust showed the best results. When further dried, their sorption capacity decreased. Soil was the least efficient natural sorbent with a sorption capacity that ranged from 0.45 to 3.82 g/g. The best sorption capacity of 11.5 g/g among the synthetic sorbents was in Reo Amos. The sorption capacity of natural and synthetic substances was comparable.
Keywords: Natural sorbents; Synthetic sorbents; Oil spills; Sorption capacity
Contact information: a: Department of Fire Protection, Faculty of Wood Sciences and Technology, Technical University in Zvolen, T. G. Masaryka 24, 960 01 Zvolen, Slovakia; b: Department of Chemistry and Chemical Technologies, Faculty of Wood Sciences and Technology, Technical University in Zvolen, T. G. Masaryka 24, 960 01 Zvolen, Slovakia; c: The Institute of Foreign Languages, Technical University in Zvolen, T. G. Masaryka 24, 960 01 Zvolen, Slovakia; *Corresponding author: xmojzism1@is.tuzvo.sk
INTRODUCTION
Despite the constantly growing utilization of biomass and other alternative energy sources and substituents, petroleum or crude oil products are still being used to a considerable extent. The most common crude oil products include oils, lubricants, fuels, such as diesel fuel or petrol, Vaseline, engine oils, and gearbox oils. The advantageous properties behind their usage are their ability to decrease friction, corrosion protection, heat transfer under mechanical friction, and use as fuels in combustion engines and spark-ignition engines. There are situations, including transportation, processing, pumping, careless handling and using or even an accident, when a spill or spilling in the environment can occur. When there is a spill from a transportation device or from vehicles, these substances spread across the solid surface or across the surface of liquids such as flowing or still waters. Another disadvantage of almost all kinds of oils, lubricants, and fuels, is their flammability, and in the case of an accident, their negative impact on the environment. Crude oil and crude oil products are classified as hazardous substances. For example, car petrol is classified by Regulation EC No.1272/2008 as flammable liquid substances (Flam. Liq. 1), skin irritating (Skin Irrit. 2), and toxic inhalation (Asp. Tox. 1). It can cause cancer (Carc. 1B) and genetic damage (Muta. 1B) and is suspected of reproduction toxicity (Repr. 2). Oil spills also present a considerably negative impact on the environment; moreover, they are toxic to water organisms (Aquatic Chronic 2). Therefore, it is crucial in the case of a spill to eliminate their spreading and to decontaminate the affected area, soil, or water surface. There are various sorption materials used as prevention and also intervention measures.
Synthetic sorbents were not originally intended as sorption materials, but rather as waste material or a side product of industrial processing. Ash and pet dust are examples of products not originally intended as sorbents for spilled oil (Sun et al. 2013). Their ability to take up oil-based substances is seen as a side effect that led to the beginning of their use. The examples of synthetic sorbents include ash, other rock-based substances, like perlite, clay, and limestone (Rajaković-Ognjanović et al. 2008; Cheng et al. 2011; Bi et al. 2013; Kończewicz et al. 2013; Song et al. 2014; Wang et al. 2014; Bandura et al. 2015), plastics– polypropylene and polyurethane (Choi et al. 2011; Lee et al. 2013; Ge et al. 2014; Saleem et al. 2015; Zhang et al. 2017), carbon (Dong et al. 2012; Yoon et al. 2014), cellulose (Feng et al. 2015), and others. Synthetic sorbents are intentionally manufactured to serve as sorption materials to remove carbon and chemical spills. They might be further treated, for example by hydrophobization, which makes them hydrophobic and oleophilic. These types of sorbents have been processed as high-quality sorption materials.
For a spill into the environment, various natural sorption materials are used, which can be found naturally in the surroundings of the spill or the incident. and can be used instantly or at least until other sorbents are available. Another advantageous property of natural sorbents is their biological degradability. Some types of the sorption materials can affect the sorbed substance by various biological reactions and may degrade the substance being sorbed (Gertler et al. 2009; Lin et al. 2014; Olalekan et al. 2014). The use of inorganic and organic natural sorbents to remove oil products has been investigated (Singh et al. 2014; Wong et al. 2016). Natural sorbents include wood sawdust (Annunciado et al. 2005; Očkajová et al. 2016; Očkajová et al. 2018; Meng et al. 2019), cellulose-based material (Hubbe et al. 2013), natural clay (Zadaka-Amir et al. 2013), leaves (Sidik et al. 2012), rice straw (Hoang et al. 2018a; Hoang et al. 2018b) peat (Ribeiro et al. 2003), cotton (Carmody et al. 2007) and various natural fibres (Moriwakia et al. 2009; Abdelwahab 2014; Idris et al. 2014).
There are some sorption materials, either natural or synthetic, that can be used repeatedly (Karakutuk and Okay 2010; Korhonen et al. 2011; Bi et al. 2012; Hashim et al. 2012; Sun et al. 2013; Gu et al. 2014; Pham and Dickerson 2014; Qiu et al. 2015).
Sorbents generally can be described as insoluble substances or compound substances most often of solid state intended to capture spilled substances in a liquid or gaseous state. The sorption capacity depends on the kind of oil product, the size of the surface area where the oil product can be sorbed, and the type of surface (Fingas 2016). The surface size of sorption materials and increasing their sorption capacity depends on the amount of inner cracks, pores, and capillaries, as shown in Fig. 1.
A typical feature of sorption materials is their ability to sorb a certain amount of liquid in relation to their own weight (Ankowski et al. 2011). The sorbents appear mostly in the form of textile or a loose material. The loose sorption materials can further be divided into synthetic and natural. Currently, a majority of commercially available sorption materials are synthetic materials and they are of a hydrophobic nature, which makes them a perfect sorption material for various oil-based substances (Wang et al. 2013).
Fig. 1. Adsorption mechanism (redrawn from Vivek 2012)
When there is a spill of the hazardous material into the environment, it is important to stop the spill, limit its spread, and consequently facilitate its removal and disposal. A suitable way to decontaminate such a polluted environment is the utilization of various sorption materials that are often used at emergency services interventions.
The aim of the paper is to compare the sorption capacity of organic and inorganic natural sorption materials for used low-viscosity engine oil 10W 40 according to ASTM F726 (2012). The natural sorbents were chosen from an area that is prone to the spill of oil products because of the presence of heavy traffic roads and frequent occurrence of car accidents. This paper focuses on the spill of low-viscosity oil only on solid surface found within the forest environments. It does not discuss the spill of low-viscosity oil onto water surfaces. The natural sorbents were tested at three different moisture contents (wet, air-dry, and dry) as the moisture content in nature changes depending on vegetation season and climatic conditions. Synthetic sorbents were chosen based on their frequent use by emergency services. The 10W 40 low-viscosity oil is a component in both combustion and spark-ignition engines.
EXPERIMENTAL
Materials
The samples of various loose materials taken for further testing were taken directly from the natural environment in the original condition while maintaining their shape and form. The samples were taken from mixed forests nearby the town of Zvolen (Slovakia) at 300 m above the sea level in the spring time of the year 2018. The samples from the surface included fallen needles, leaves (from the previous autumn), moss (from the prior year), surface soil (maximum 2 cm depth), and sawdust recovered immediately after felling the trees. The samples of natural sorbents taken in such fashion were tested at three different moisture contents ranging from 0 to 82%.
Fig. 2. Air-dry samples of natural sorbents: a) Larch needles, b) Fir needles, c) Pine needles, d) Beech sawdust, e) Spruce sawdust, f) Leaf residues, g) Moss, and h) Soil
Larch needles (Larix decidua), at the collection of samples, originally had a moisture content of 45.5% and the average needle size was 1 × 1 × 35 mm3. Fir needles (Abies alba) originally had a moisture content of 27.2% and the needle size was 1 × 2 × 30 mm3. Pine needles’ (Pinus sylvestris) moisture content was 26.8% and the needles were 1 × 1 × 75 mm in size. Beech sawdust (Fagus sylvatica) had a moisture content at sampling of 33.9% and the particle size was 0.5 × 4 × 4 mm3. Spruce sawdust’s (Picea abies) moisture content was 46.2% and the size of particles was 0.5 × 2 × 4 mm3. The leaf residues’ (Fagus sylvatica) moisture content was 34.2% and the particle size was 0.3 × 70 × 40 mm3. Moss’ (Ceratodon purpureus) moisture content at sampling was 81.9% and the particle size was 3 × 3 × 50 mm3. Lastly, the soil´s moisture content was 26.4% and the particle size was from 0.1 mm up to 4 mm.
The first test was conducted immediately after sampling; the samples were marked as wet and their moisture content was determined. The second test was performed when the samples had been air-dried (marked as air-dry). They had been dried at the room temperature until the stabile state. The third test was performed with the samples dried to the absolute moisture content of 0 to 2% at the 103° to 105 °C temperature range (marked as dry samples). The moisture content was determined via a gravimetric method by universally applicable lab heating/drying oven with air circulation (UNB 200; Memmert Ltd., Schwabach, Germany). Table 1 states the mentioned tested sorbents and their average moisture content at each measurement.
The synthetic sorption materials commonly used during interventions were chosen for comparison. The synthetic sorbents were Absodan Plus, expanded perlite, Eco-dry Plus, and Reo Amos. The synthetic sorbents were in the loose form in an untreated condition as shown in Fig. 3. Absodan Plus (Imerys Industrial Minerals Denmark A/S, Fur, Denmark) is a mineral-based loose sorbent, based on Danish Moler clay 4 with the particle size of 1.5 to 3 mm. It is suitable to sorb liquids from a hard surface. Absodan Plus is a versatile sorbent for almost all types of liquids such as oils, acids, alkaline substances, oil products, water, and organic solvents.
Fig. 3. Samples of synthetic sorbents: a) Absodan Plus, b) Expanded perlit, c) Eco-dry Plus, and d) Reo Amos
Expanded perlite (LBK Perlite Ltd., Lehôtka pod Brehmi, Slovakia) is an expanded amorphous aluminous silicate that is of a volcanic rock origin in a grain form with particles 0.5 to 2.5 mm in size. It has excellent thermal insulation and sound insulation properties. It is non-flammable and antimicrobial resistant and mold resistant. It is most often used to clean garages, pump stations, floors of production facilities, and other facilities where a spill of oil products could occur. Eco-dry Plus (REO AMOS SLOVAKIA, Ltd., Bratislava, Slovakia) is a non-dust, non-flammable, and health safe, rock-based crumb with 0.5 to 2.5 mm sized particles. It is suitable for removing or treating oil products, cutting and cooling emulsions, and aqueous solutions that it can sorb rapidly. It is a chemically stable substance, excluding hydrofluoric acid and 50% sodium hydroxide. Reo Amos (REO AMOS SLOVAKIA, Ltd., Bratislava, Slovakia) is a slightly dusty mixture of loose sorbents, it is granulated, chemically stable, and treated by a hydrophobic treatment. The particle size is 0.5 to 5 mm and it is designed to remove oil products from various rugged surfaces and still waters.
Sorption properties of the selected sorbents were tested for sorption of an oil product- the used engine oil 10W 40 (Total Slovakia, Ltd., Bratislava, Slovakia), which is commonly used in combustion and spark-ignition engines. The engine oil was chosen because it spills most often during car accidents at road collisions.
Methods
The moisture content of the natural sorbents at the individual tests was determined gravimetrically by drying in a kiln at the temperature of 103° to 105 °C to a constant weight (Kačík and Solár 1999).
The sorption capacity was stated by the standard testing method of sorption properties of adsorbents ASTM F726 (2012). The mentioned testing method refers to the laboratory testing of sorbents’ properties to remove oil substances and other floating, non-soluble liquids that do not form emulsions. The method was applied to test type II (loose) sorbents. The tested sorption materials were conditioned at 23 ± 4 °C and a relative humidity of 70% ± 20% for 24 h before the actual testing as per ASTM F726 (2012). The natural sorbents were not conditioned for the wet and dry moisture content tests so that sorbents maintained their moisture content for testing. The samples of the sorption materials were tested according to the testing method for short-time sorption of oil substances in laboratory conditions. A total of 5.00 g of the sorbent was weighed for each test. The crystallizing dish (23-cm diameter) was filled with the test liquid at least up to 2.5 cm height, which equals to 1 L of the sorbate (the low-viscosity engine oil 10W 40) that was refilled to the original amount after every test. To achieve higher accuracy, the empty testing sample holder was left sunk for 1 min in the engine oil and then left to drop off for 15 min. This procedure enabled the authors to achieve more precise results as the differences in the oil absorbed in the mesh of the holder were eliminated. Every measurement was repeated three times as stated in the standard. The achieved results were calculated according to Eqs. 1 and 2 and the average sorption capacity was calculated. The used sorbents and oils were ecologically disposed.
The sorbed crude oil substance in the sorbent m1 (g) was calculated according to Eq. 1,
m1 = m2 – m3 – m4 (1)
where m1 is the weight of the sorbate (g), m2 is the weight of the wet test sample holder, a crystallizing dish and wet sorbent (g), m3 is the weight of the wet sample holder and the dish (g), and m4 is the weight of the dry sorbent (g).
The sorption capacity a1 (g/g) was calculated using Eq. 2,
a1 = m1 / m4 (2)
where a1 is the sorption capacity (g/g), m1 is the weight of the sorbate (g), and m4 is the weight of the dry sorbent (g).
The results were evaluated in the STATISTICA program (Statsoft, Inc., version 7.0, Prague, Czech Republic). The average values of sorption capacities of the selected sorbents and the values of the moisture content were evaluated and interpreted by an analysis of variance and Duncan´s test where the significance level is 5%.
RESULTS AND DISCUSSION
The research was based on the technical standard ASTM F726 (2012) focusing on the examination and comparison of the sorption capacity of several kinds of sorbents to remove engine oil spills. Natural sorbents were tested at three different moisture contents (wet, air-dry, and dry) that are stated in Table 1. The given figures demonstrate that natural sorbents occur in a wide range of moisture contents. This is due to the different ability of the natural materials to sorb water vapors from the air.
Table 1. Tested Natural Sorbents and Their Moisture Contents (%) at the Individual Tests
The achieved values of the sorption capacities of the tested sorbents at different moisture contents and their statistical assessment is stated in Table 2.
The sorption capacity values for the low-viscosity engine oil 10W 40 differed widely and are given in Table 2 for the various moisture contents (0 to 82%, Table 1).
Table 2 shows the achieved average values of sorption capacity at various moisture contents including the statistical parameters (standard error, confidence interval, standard deviation, and relative standard deviation).
Table 2. Sorption Capacity of Natural Sorbents at Various Moisture Contents and Relevant Statistical Data
For the wet test, when the moisture content of the natural sorbents was the highest, 26% to 82%, the best sorption capacity was achieved by larch needles with the value of 11.1 g/g. The lowest sorption capacity was achieved by soil with the value of 0.81 g/g. Table 2 shows that the increased moisture content (26.45%) of this natural sorbent negatively influenced its sorption capacity.
For the air-dry test of the natural sorbents when their moisture content ranged from 7 to 13% (except soil whose moisture content was 2%), the best sorption capacity was achieved by moss. Decreasing its moisture content from the original 81.9% to 12.3% caused a considerable increase of sorption capacity up to 25.2% (g/g). The change in moisture content positively influenced the sorption capacity of all natural sorbents, except fir needles and pine needles. The soil sorbent, which was minimally efficient during the wet test, increased its sorption capacity greatly, however, it remained minimally efficient compared to the other natural sorbents.
For the dry test, the decreased moisture content of natural sorbents led to an increase in the sorption capacity of moss and leaf residue samples. The most efficient was moss with 28.4 g/g, and the least efficient was soil, which was demonstrated not only in this test but in all tests at all moisture contents.
Despite the various moisture contents, the most influencing factor is the overall surface area. The sorption capacity for all tested samples was influenced most by the nominal surface area which is shown in detail in Figs. 2 and 3.
Figure 4 presents the variance of the sorption capacities of individual natural sorbents at three different moisture contents (wet, air-dry, and dry).
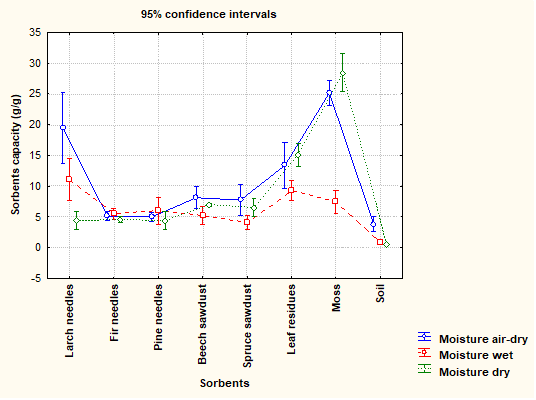
Fig. 4. Sorption capacity at different moisture contents
Comparing all three tests, it can be stated that the decreasing moisture content for the natural sorbent samples led to a slight decrease of sorption capacity for fir needles and pine needles; however, it implied an increase of sorption capacity for moss and leaf residues. For the other natural sorbents, the highest sorption capacity was found at the air-dry moisture content.
Tables 3, 4, and 5 state the Duncan test values for the chosen natural sorbents at different moisture contents. The results implied statistically significant differences at wet moisture content tests for larch needles, leaf residues, and soil, which state statistically significant differences in sorption capacity compared to all sorbents. The other tested sorbents stated non-significant statistical difference in some cases.
The results given in Table 4 show that a statistically significant difference at air-dry moisture content tests was achieved by larch needles, leaf residue, and moss, which show statistically significant differences compared to all other sorbents. Other tested sorbents achieved non-significant statistical differences in some cases.
Table 3. Results of Duncan’s Test for Wet Moisture Content Tests (P-values)
Table 4. Table Results of Duncan’s Test for Air-dry Moisture Content Tests (P-values)
Table 5 presents the statistically significant difference for dry moisture content tests achieved by leaf residue, moss, and soil, which achieved statistically significant differences compared with all other sorbents. Other tested sorbents showed non-significant statistical differences in some cases.
Table 6 presents the maximum sorption capacity of the selected sorption materials of synthetic nature that were conditioned in laboratory conditions for 24 h before tests according to ASTM F726 (2012). The selected synthetic sorbents are commonly used in the interventions of emergency forces. As Table 3 states, the highest sorption capacity was found for Reo Amos with the sorption capacity of 9.24 g/g and the lowest for Absodan Plus with the sorption capacity of 1.06 g/g. An even lower sorption capacity of Absodan Plus for oils (0.5 to 0.6 g/g) is stated by Bandura et al. (2015), who tested sorption capacity of sorbents of the zeolite base. The sorption capacity was 3.33 g/g for expanded perlite, which is probably the most frequently used sorption material of emergency forces interventions. Similar results for expanded perlite (2 to 4 g/g) are also stated by Teas et al. (2001).
Table 5. Results of Duncan’s Test for Dry Moisture Content Tests (P-values)
Table 6. The Sorption Capacity of the Selected Synthetic Sorbents
The comparison of natural and synthetic sorbents revealed that their sorption capacity is comparable; some natural sorbents displayed higher sorption capacity than synthetic sorbents, which is confirmed by other authors as well. For instance, Wong et al. (2016) present the overview of the sorption capacities of various natural materials and fibers and state that the application of natural sorbents based on plant fibers shows a great potential. Likon et al. (2013) compared the sorption capacity of various natural fibers and reported the outstanding sorption capacity of lignocellulose fiber made of Populus nigra ‘Italica’, whose sorption capacity for oil ranged depending on their density from 182 to 211 g/g.
Certainly, sorption capacity is not the only property of sorption materials that is considered during the selection of a proper sorbent. The other important properties include chemical resistance, non-flammability, cohesion, and the ability of mechanical loading of sorbents after sorption of a hazardous substance. Despite everything, the popularity of green materials (lignocellulose fibers, cellulose, modified cellulose, etc.) as sorption materials is constantly growing. They hold several advantages over synthetic sorbents. They are biodegradable, environmentally friendly, and do not cause secondary pollution (Chen et al. 2015). The further treatment of the used sorption materials is discussed in the Waste Oil Directive 75/439/EEC.
CONCLUSIONS
- Regarding synthetic sorbents, the highest sorption capacity was achieved by Reo Amos at 9.24 g/g. The sorption capacity of synthetic and natural sorbents was comparable. The choice of suitable sorption material depends on a variety of factors, for instance, the kind of sorbed substance, chemical resistance of the sorbent, non-flammability, degradability, coherence, and mechanical loading, in addition to the price and availability.
- When testing natural sorbents of the highest moisture content 26 to 82% (wet), the highest sorption capacity was demonstrated by larch needles at 11.1 g/g and the least efficient sorption capacity was shown by soil at 0.81 g/g. The increased moisture content of the natural materials negatively influences the sorption capacity, except the natural sorbents fir needles and pine needles.
- During the air-dry tests of natural sorbents of the 2 to 13% moisture content, the highest sorption capacity was achieved by moss at 25.2 g/g. The samples of larch needles, spruce sawdust, and beech sawdust achieved the best results in this test; when they were further dried, their sorption capacity decreased.
- When the moisture content further decreased (dry), the sorption capacity of moss and leaf residues increased. Moss was considered the most efficient sorbent of all tested sorbents. Its sorption capacity at this test was 28.4 g/g. The soil sample was the least efficient sorbent at all three moisture contents.
- It can be stated that the parameter of moisture content of the tested natural sorbents is a statistically significant parameter towards its sorption capacity. The differences in the sorption capacity of eight tested natural sorbents were computed by Duncan‘s test. The test showed which values were statistically significant and they are shown in Tables 3, 4, and 5.
- Subjected to an immediate use of the sorbent, during the spill of crude oil products in the environment, various natural sorption materials that can be found naturally in the surroundings of the spill can be used until another sorbent material arrives.
ACKNOWLEDGMENTS
The research was supported by the KEGA agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic, No. 030UMB-4/2017.
REFERENCES CITED
ASTM F726-12 (2012). “Standard test method for sorbent performance of adsorbents,” ASTM International, West Conshohocken, PA, USA.
European Commission (EC) Regulation 1272/2008 (2008). “European commission (EC) Regulation 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006,” European Union, Brussels, Belgium.
European Commission (EC) Waste Oil Directive 75/439/EEC (1998). “Resolution on the communication from the Commission to the European Parliament and Council concerning the application of Directives 75/439/EEC,” European Union, Brussels, Belgium.
Abdelwahab, O. (2014). “Assessment of raw Luffa as a natural hollow oleophilic fibrous sorbent for oil spill cleanup,” Alexandria Engineering Journal 53(1), 213-218. DOI: 10.1016/j.aej.2013.11.001
Annunciado, T. R., Sydenstricker, T. H. D., and Amico, S. C. (2005). “Experimental investigation of various vegetable fibers as sorbent materials for oil spills,” Marine Pollution Bulletin 50(11), 1340-1346. DOI: 10.1016/j.marpolbul.2005.04.043
Ankowski, A., Poterek, M., and Lazaj, R. (2011). “The use of sorbents in actual conditions for elimination of oil derivative spillages,” Požární Ochrana 20, 5-9.
Bandura, L., Franus, M., Józefaciuk, G., and Franus, W. (2015). “Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup,” Fuel 147, 100-107. DOI: 10.1016/j.fuel.2015.01.067
Bi, H., Xie, X., Yin, K., Zhou, Y., Wan, S., He, L., and Ruoff, R. S. (2012). “Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents,” Advanced Functional Materials 22(21), 4421-4425. DOI: 10.1002/adfm.201200888
Bi, H., Yin, Z., Cao, X., Xie, X., Tan, C., Huang, X., and Lu, X. (2013). “Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents,” Advanced Materials 25(41), 5916-5921. DOI: 10.1002/adma.201302435
Carmody, O., Frost, R., Xi, Y., and Kokot, S. (2007). “Adsorption of hydrocarbons on organo-clays—implications for oil spill remediation,” Journal of Colloid and Interface Science 305(1), 17-24. DOI: 10.1016/j.jcis.2006.09.032
Chen, S., Zhang, X., Zhu, H., and Cao, X. (2015). “Optimization synthesis conditions and characterization of oil biosorbent: Sugarcane bagasse cellulose-graft-polystearylmethacrylate copolymer,” BioResources 10(1), 1357-1365. DOI: 10.15376/biores.10.1.1357-1365
Cheng, M., Gao, Y., Guo, X., Shi, Z., Chen, J. F., and Shi, F. (2011). “A functionally integrated device for effective and facile oil spill cleanup,” Langmuir 27(12), 7371-7375. DOI: 10.1021/la201168j
Choi, S. J., Kwon, T. H., Im, H., Moon, D. I., Baek, D. J., Seol, M. L., and Choi, Y. K. (2011). “A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water,” ACS Applied Materials & Interfaces 3(12), 4552-4556. DOI: 10.1021/am201352w
Dong, X., Chen, J., Ma, Y., Wang, J., Chan-Park, M. B., Liu, X., and Chen, P. (2012). “Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water,” Chemical Communications 48(86), 10660-10662. DOI: 10.1039/C2CC35844A
Feng, J., Nguyen, S. T., Fan, Z., and Duong, H. M. (2015). “Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels,” Chemical Engineering Journal 270, 168-175. DOI: 10.1016/j.cej.2015.02.034
Fingas, M. (2016). Oil Spill Science and Technology, Gulf Professional Publishing, Edmonton, Canada.
Ge, J., Ye, Y. D., Yao, H. B., Zhu, X., Wang, X., Wu, L., and Yu, S. H. (2014). “Pumping through porous hydrophobic/oleophilic materials: An alternative technology for oil spill remediation,” Angewandte Chemie 53(14), 3612-3616. DOI: 10.1002/anie.201310151
Gertler, C., Gerdts, G., Timmis, K. N., and Golyshin, P. N. (2009). “Microbial consortia in mesocosm bioremediation trial using oil sorbents, slow-release fertilizer and bioaugmentation,” FEMS Microbiology Ecology 69(2), 288-300. DOI: 10.1111/j.1574-6941.2009.00693.x
Gu, J., Jiang, W., Wang, F., Chen, M., Mao, J., and Xie, T. (2014). “Facile removal of oils from water surfaces through highly hydrophobic and magnetic polymer nanocomposites,” Applied Surface Science 301, 492-499. DOI: 10.1016/j.apsusc.2014.02.112
Hashim, D. P., Narayanan, N. T., Romo-Herrera, J. M., Cullen, D. A., Hahm, M. G., Lezzi, P., and Roy, A. K. (2012). “Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions,” Scientific Reports 2, Article Number 363. DOI: 10.1038/srep00363
Hoang, A. T., Bui, X. L., and Pham, X. D. (2018a). “A novel investigation of oil and heavy metal adsorption capacity from as-fabricated adsorbent based on agricultural by-product and porous polymer,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 40(8), 929-939. DOI: 10.1080/15567036.2018.1466008
Hoang, A. T., and Pham, X. D. (2018b). “An investigation of remediation and recovery of oil spill and toxic heavy metal from maritime pollution by a new absorbent material,” Journal of Marine Engineering & Technology, 1-11. DOI: 10.1080/20464177.2018.1544401
Hubbe, M. A., Rojas, O. J., Fingas, M., and Gupta, B. S. (2013). “Cellulosic substrates for removal of pollutants from aqueous systems: A Review. 3. Spilled oil and emulsified organic liquids,” BioResources, 8(2), 3038-3097. DOI: 10.15376/biores.8.2.3038-3097
Idris, J., Eyu, G. D., Mansor, A. M., Ahmad, Z., and Chukwuekezie, C. S. (2014). “A preliminary study of biodegradable waste as sorbent material for oil-spill cleanup,” The Scientific World Journal 2014, Article ID 638687. DOI: 10.1155/2014/638687
Kačík, F., and Solár, R. (1999). Analytická Chémia Dreva [Wood Analytical Chemistry], Technická Univerzita vo Zvolene, Zvolen, Slovakia.
Karakutuk, I., and Okay, O. (2010). “Macroporous rubber gels as reusable sorbents for the removal of oil from surface waters,” Reactive and Functional Polymers 70(9), 585-595. DOI: 10.1016/j.reactfunctpolym.2010.05.015
Korhonen, J. T., Kettunen, M., Ras, R. H., and Ikkala, O. (2011). “Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents,” ACS Applied Materials & Interfaces 3(6), 1813-1816. DOI: 10.1021/am200475b
Kończewicz, W., Grabowska, O., Lachowicz, D., and Otremba, Z. (2013). “Study on oil sorbents effectiveness,” Journal of KONES 20(1), 135-138.
Lee, M. W., An, S., Latthe, S. S., Lee, C., Hong, S., and Yoon, S. S. (2013). “Electrospun polystyrene nanofiber membrane with superhydrophobicity and superoleophilicity for selective separation of water and low viscous oil,” ACS Applied Materials & Interfaces 5(21), 10597-10604. DOI: 10.1021/am404156k
Lin, M., Liu, Y., Chen, W., Wang, H., and Hu, X. (2014). “Use of bacteria-immobilized cotton fibers to absorb and degrade crude oil,” International Biodeterioration & Biodegradation 88, 8-12. DOI: 10.1016/j.ibiod.2013.11.015
Likon, M., Remškar, M., Ducman, V., and Švegl, F. (2013). “Populus seed fibers as a natural source for production of oil super absorbents,” Journal of Environmental Management 114, 158-167. DOI: 10.1016/j.jenvman.2012.03.047
Meng, X., Wan, Y., Feng, K., Kong, H., and Liu, T. (2019). “Preparation and characteristics of three sorbents from wood chips screening reject (WCSR) modified by nitric acid, phosphoric acid, or sodium hydroxide,” BioResources 14(1), 2216-2228. DOI: 10.15376/biores.14.1.2216-2228
Moriwaki, H., Kitajima, S., Kurashima, M., Hagiwara, A., Haraguchi, K., Shirai, K., and Kiguchi, K. (2009). “Utilization of silkworm cocoon waste as a sorbent for the removal of oil from water,” Journal of Hazardous Materials 165(1-3), 266-270. DOI: 10.1016/j.jhazmat.2008.09.116
Očkajová, A., Kučerka, M., Krišťák, L., and Igaz, R. (2018). “Granulometric analysis of sanding dust from selected wood species,” BioResources 13(4), 7481-7495. DOI: 10.15376/biores.13.4.7481-7495
Očkajová, A., Kučerka, M., Krišťák, L., Ružiak, I., and Gaff, M. (2016). “Efficiency of sanding belts for beech and oak sanding,” BioResources 11(2), 5242-5254. DOI: 10.15376/biores.11.2.5242-5254
Olalekan, A. P., Dada, A. O., and Adesina, O. A. (2014). “Silica aerogel as a viable absorbent for oil spill remediation,” Journal of Encapsulation and Adsorption Sciences 4(04), 122-131. DOI: 10.4236/jeas.2014.44013
Pham, V. H., and Dickerson, J. H. (2014). “Superhydrophobic silanized melamine sponges as high efficiency oil absorbent materials,” ACS Applied Materials & Interfaces 6(16), 14181-14188. DOI: 10.1021/am503503m
Qiu, S., Jiang, B., Zheng, X., Zheng, J., Zhu, C., and Wu, M. (2015). “Hydrophobic and fire-resistant carbon monolith from melamine sponge: A recyclable sorbent for oil–water separation,” Carbon 84, 551-559. DOI: 10.1016/j.carbon.2014.12.055
Rajaković-Ognjanović, V., Aleksić, G., and Rajaković, L. (2008). “Governing factors for motor oil removal from water with different sorption materials,” Journal of Hazardous Materials 154(1-3), 558-563. DOI: 10.1016/j.jhazmat.2007.10.066
Ribeiro, T. H., Rubio, J., and Smith, R. W. (2003). “A dried hydrophobic aquaphyte as an oil filter for oil/water emulsions,” Spill Science & Technology Bulletin 8(5-6), 483-489. DOI: 10.1016/S1353-2561(03)00130-0
Saleem, J., Bazargan, A., Barford, J., and McKay, G. (2015). “Application of strong porous polymer sheets for superior oil spill recovery,” Chemical Engineering & Technology 38(3), 482-488. DOI: 10.1002/ceat.201400068
Sidik, S. M., Jalil, A. A., Triwahyono, S., Adam, S. H., Satar, M. A. H., and Hameed, B. H. (2012). “Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal,” Chemical Engineering Journal 203, 9-18. DOI: 10.1016/j.cej.2012.06.132
Singh, V., Jinka, S., Hake, K., Parameswaran, S., Kendall, R. J., and Ramkumar, S. (2014). “Novel natural sorbent for oil spill cleanup,” Industrial & Engineering Chemistry Research 53(30), 11954-11961. DOI: 10.1021/ie5019436
Song, J., Huang, S., Lu, Y., Bu, X., Mates, J. E., Ghosh, A., and Megaridis, C. M. (2014). “Self-driven one-step oil removal from oil spill on water via selective-wettability steel mesh,” ACS Applied Materials & Interfaces 6(22), 19858-19865. DOI: 10.1021/am505254j
Sun, H., Li, A., Zhu, Z., Liang, W., Zhao, X., La, P., and Deng, W. (2013). “Superhydrophobic activated carbon‐coated sponges for separation and absorption,” ChemSusChem 6(6), 1057-1062. DOI: 10.1002/cssc.201200979
Teas, C., Kalligeros, S., Zanikos, F., Stournas, S., Lois, E., and Anastopoulos, G. (2001). “Investigation of the effectiveness of absorbent materials in oil spills clean up,” Desalination 140(3), 259-264. DOI: 10.1016/S0011-9164(01)00375-7
Vivek, K. (2012). “Adsorption mechanism,” Slideshare, (http://www.slideshare.net/VivekKumar36/adsorption-regenerationvivek-kumarneeri), Accessed 23 Aug 2012.
Wang, F., Lei, S., Xue, M., Ou, J., Li, C., and Li, W. (2014). “Superhydrophobic and superoleophilic miniature device for the collection of oils from water surfaces,” The Journal of Physical Chemistry C 118(12), 6344-6351. DOI: 10.1021/jp500359v
Wang, X., Li, D., Li, W., Peng, J., Xia, H., Zhang, L., and Chen, G. (2013). “Optimization of mesoporous activated carbon from coconut shells by chemical activation with phosphoric acid,” BioResources 8(4), 6184-6195. DOI: 10.15376/biores.8.4.6184-6195
Wong, C., McGowan, T., Bajwa, S. G., and Bajwa, D. S. (2016). “Impact of fiber treatment on the oil absorption characteristics of plant fibers,” BioResources 11(3), 6452-6463. DOI: 10.15376/biores.11.3.6452-6463
Yoon, H., Na, S. H., Choi, J. Y., Latthe, S. S., Swihart, M. T., Al-Deyab, S. S., and Yoon, S. S. (2014). “Gravity-driven hybrid membrane for oleophobic–superhydrophilic oil–water separation and water purification by graphene,” Langmuir 30(39), 11761-11769. DOI: 10.1021/la5031526
Zadaka-Amir, D., Bleiman, N., and Mishael, Y. G. (2013). “Sepiolite as an effective natural porous adsorbent for surface oil-spill,” Microporous and Mesoporous Materials 169, 153-159. DOI: 10.1016/j.micromeso.2012.11.002
Zhang, X., Zhi, D., Zhu, W., Sathasivam, S., and Parkin, I. P. (2017). “Facile fabrication of durable superhydrophobic SiO2/polyurethane composite sponge for continuous separation of oil from water,” RSC Advances 7(19), 11362-11366. DOI: 10.1039/C7RA00020K
Article submitted: June 6, 2019; Peer review completed: August 16, 2019; Revised version received and accepted: September 11, 2019; Published: September 18, 2019.
DOI: 10.15376/biores.14.4.8738-8752
