Abstract
Cork is one of the most important forest products in Portugal. The cork processing industry is highly resource-efficient, and the only residue is cork powder, which is too small for agglomerate production. This work studied the usage of cork powder for the production of added-value products via polyol liquefaction. Liquefactions were performed in a reactor using a mixture of polyethylene glycol (PEG 400) and glycerol as solvents, which were catalyzed by the addition of sulphuric acid. Several cork-to-solvent ratios, reaction temperatures, and reaction times were tested. Polyurethane foams were prepared by combining polyol mixtures with a catalyst, surfactant, blowing agent, and polymeric isocyanate. Mechanical tests of the produced foams were conducted, and compressive modulus of elasticity and compressive stress at 10% deformation were determined. The results show that the best conditions for obtaining high liquefaction yields are as follows: 160 °C for 1 h; glycerol-to-PEG 400 ratio of 1:9; cork-to-solvent ratio of 1:6; and 3% H2SO4 catalyst addition. The Fourier Transform Infrared (FTIR) spectra indicated that the lignocellulosic fractions of the cork were more selectively dissolved during acidified polyol liquefaction than the suberin. With liquefied cork powder using these optimized conditions, it is possible to produce polyurethane foams with desired properties.
Download PDF
Full Article
Cork Liquefaction for Polyurethane Foam Production
Bruno Esteves,a,b,* Yuliya Dulyanska,a Catarina Costa,a José Vicente,a Idalina Domingos,a Helena Pereira,b Luis Teixeira de Lemos,a and Luísa Cruz-Lopes a
Cork is one of the most important forest products in Portugal. The cork processing industry is highly resource-efficient, and the only residue is cork powder, which is too small for agglomerate production. This work studied the usage of cork powder for the production of added-value products via polyol liquefaction. Liquefactions were performed in a reactor using a mixture of polyethylene glycol (PEG 400) and glycerol as solvents, which were catalyzed by the addition of sulphuric acid. Several cork-to-solvent ratios, reaction temperatures, and reaction times were tested. Polyurethane foams were prepared by combining polyol mixtures with a catalyst, surfactant, blowing agent, and polymeric isocyanate. Mechanical tests of the produced foams were conducted, and compressive modulus of elasticity and compressive stress at 10% deformation were determined. The results show that the best conditions for obtaining high liquefaction yields are as follows: 160 °C for 1 h; glycerol-to-PEG 400 ratio of 1:9; cork-to-solvent ratio of 1:6; and 3% H2SO4 catalyst addition. The Fourier Transform Infrared (FTIR) spectra indicated that the lignocellulosic fractions of the cork were more selectively dissolved during acidified polyol liquefaction than the suberin. With liquefied cork powder using these optimized conditions, it is possible to produce polyurethane foams with desired properties.
Keywords: Cork; Liquefaction; Polyurethane foams; Valorization
Contact information: a: Centre of Studies in Education, Technologies and Health, School of Technology of Viseu, Department of Wood Engineering, Superior School of Technology of Viseu, Polytechnic Institute of Viseu, Portugal; b: Forest Research Centre, School of Agriculture, University of Lisbon, Portugal;
* Corresponding author: bruno@estgv.ipv.pt
INTRODUCTION
Cork is a non-wood forest product of high ecological and economic importance. It is obtained by sustainable harvesting of cork oak forests and provides the raw material for a strong industrial chain. Cork is a cellular material with a unique chemical composition, which differs from wood and other lignocellulosic materials. Suberin, a polyester macromolecule composed of long-chain fatty acids and alcohols condensed with glycerol, is a major structural component of cork, in association with lignin and with low amounts cellulose and hemicelluloses (Pereira 2007, 2015).
Industrial processing of raw cork produces 20% to 30% residue, primarily in the form of cork powder, which has a small particle size and has low valorisation. This powder mainly is burned for energy production (Sousa et al. 2006). Therefore, the chemical conversion of this waste cork powder into a high-value product is an attractive option to increase the overall cork chain value and to increase cork oak sustainability.
Biomass liquefaction at low temperatures and pressures may be one of the best options to obtain chemicals from these materials in the future. Under these conditions, biomass liquefaction with polyhydric alcohols, such as glycerol, ethylene glycol, or polyethylene glycol (PEG) (Kurimoto and Tamura 1999; Yamada and Ono 2001; Niu et al. 2011; Martins et al. 2013) affords large molecules that can be subsequently polymerized to produce adhesives, plastics, or other polymers (Alma et al. 1998; Zhang et al. 2007; Lin et al. 2014). The choice of biomass liquefying solvent, as well as the catalyst (e.g., acid, base or other cyclic carbonates), will influence the composition of molecules extracted (Xie and Chen 2005). There are mainly two reactions occurring in lignocellulosic liquefaction by polyalcohol: decomposition and polycondensation reactions (Niu et al. 2011). Cellulose decomposition in polyalcohol liquefaction was studied by Yamada and Ono (2001) who concluded that EG-glucosides are produced initially but these compounds are afterwards further degraded, leading to levulinates. According to Niu et al. (2011), cellulose is degraded through the cleavage of β–O–4 bonds due to the dehydration reaction with hydrogen ions from an acid catalyst. Lignin degradation has been studied using phenol as solvent (Lin et al. 1997): guaiacol and phenolated derivatives are produced, resulting from the cleavage of β–O–4 linkages. In wood, the behaviour of lignin and polysaccharides cannot be studied separately, as shown by Kobayashi et al. (2004), who found that wood lignin was converted to N,N-dimethylformamide (DMF)-soluble compounds in the early stage, followed by cellulose dissolution into DMF and later by condensation reactions between the depolymerized and degraded compounds from cellulose and lignin, resulting into DMF-insoluble compounds. Polycondensation reactions only occur between polysaccharides and lignin derivatives and not by themselves (Kobayashi et al. 2004). A higher amount of low weight polyalcohols such as glycerol has been shown to significantly reduce polycondensation reactions. In accordance with D’Souza et al. (2015), this is due to the highly polar hydroxyl groups (primary) with short chains that create a highly protic solvent that improves conversion.
Wood liquefaction using phenol results in products with properties similar to those of conventional phenolic resins (Zhang et al. 2005; Kunaver et al. 2010); these liquefied products have been used as adhesives, as well as raw materials for manufacturing molding compounds and adhesives, such as resole and Novolak phenolic resins (Alma et al. 1996; Maldas et al. 1997). When using polyhydric alcohols as liquefaction agents, other resins can be produced, such as polyurethanes (Lee and Lin 2008).
Biomass contains high amounts of hydroxyl functional groups, so liquefied biomass with polyhydric alcohol solvents can be used directly as polyols for polyurethane production. Polyurethanes (PUs) are widely used as flexible foams in furniture, as rigid foams for insulation in walls and roofs, and as thermoplastic polyurethanes in footwear, coatings and adhesives (Pan 2011). Currently, the PU industry depends on fossil fuels for the raw materials of its main monomer units (Desroches et al. 2012).
The chemical composition of cork, with its high levels of lipophilic extractives and suberin (Pereira 2013), has great potential as the starting raw materials for polyurethane production, which has been investigated in previous studies using depolymerized suberin (Cordeiro et al. 1998). Liquefaction of cork is a recent research subject, and most of the work is based on the cork dust from the cork stoppers industry. Yona et al. (2014) examined the liquefaction of cork dust using glycerol and PEG 400 solvent mixtures under acidic and alkaline conditions; the authors achieved a liquid fraction yield of 43% to 50% with sulfuric acid as catalyst and 61% to 85% with a sodium hydroxide catalyst. Soares et al. (2014) studied ecopolyol production from industrial cork powder and concluded that the minimum residue content (29 wt.%) was obtained at 150 °C, 4 wt.% of sulfuric acid, and 60 min.
Mateus et al. (2015) compared the conventional heating of cork in the presence of p-toluenesulfonic acid with heating by ultrasounds and found that the constant rate for the ultrasound-induced process was 4.5 times higher than the conventional method.
A recent study by dos Santos et al. (2016), who liquefied cork dust with a mixture of 2 ethyl-hexanol, DEG, and p-toluene sulfonic acid, demonstrated that the aqueous extract of the liquefied cork could be used to bond lignocellulosic surfaces with low solid suspension with less than 6.5% of components from fossil sources. The potential use of the organic extract from liquefied cork was studied by Mateus et al. (2016), who concluded that this extract presented a higher heating value (HHV) than that of the liquefied cork or even cork dust, and therefore it could be used as a feedstock in steam furnaces.
The optimization of cork liquefaction should therefore be examined in relation to catalyst, reaction temperature, and reaction time to produce polyurethane foams. Thus, the objective of this paper is to report results regarding the liquefaction of cork powders of different particle sizes using various glycerol/PEG mixtures as solvents under acidic conditions; various solvent compositions, solvent-to-cork ratios, reaction temperatures, and reaction times were tested. The liquefaction reaction was monitored using FTIR analysis of the solid phase and liquefied material. The liquefied cork was processed into polyurethane foams, which were characterized in relation to structure and compressive behaviour.
EXPERIMENTAL
Sample Preparation and Characterization
Raw cork planks from Quercus suber were obtained in a local producer and dried at room temperature. The planks were milled using a Retsch Cross Beater Mill SK 100 (Retsch, Haan, Germany); the resulting powder was sieved into four fractions: greater than 40 mesh; 40 to 60 mesh; 60 to 80 mesh; and lower than 80 mesh. Only the fractions 40 to 60, 60 to 80, and lower than 80 mesh were used for the liquefaction tests. Since the lower than 80 mesh size was the one used in most of the liquefaction tests, this was the fraction that was characterized in terms of extractives, suberin, lignin, and holocellulose.
The samples were dried in an oven until complete dryness. The samples were Soxhlet extracted with 250 mL solvent during 6 h, 16 h, and 16 h with dichloromethane, ethanol, and water, respectively. The extracts were concentrated in a rotary evaporator under reduced pressure and then transferred to a pre-weighed petri dish. Dichloromethane and ethanol extracts were air dried and placed in an oven at 100 ºC for 1 h. Water extracts were dried directly in the oven. The percentage of extractives obtained with each solvent was determined gravimetrically in relation to initial dry mass according to TAPPI 204 Mac-88.
Suberin was determined in 1.5 g of extractive-free cork powder that was refluxed with 100 mL of a 3 % methanolic solution of NaOCH3 for 3 h. The sample was filtrated in a pre-weighed G2 crucible and washed with methanol. The percentage of suberin was determined as the mass loss of the residue after drying.
Insoluble lignin was determined by the Klason method in suberin-free samples by weighing 350 mg of sample to a 50 mL beaker and adding 3 mL of iced sulphuric acid at 72%. The sample was placed in a thermostatic bath at 30 ºC for 1 h with periodically mixing with a small glass rod. The second hydrolysis was done in an autoclave at 120 ºC during 1 h after diluting the sample with 84 mL of distilled water and placing it in a flask. Insoluble lignin was filtered in a G4 crucible washed with hot water to remove the excess of acid. Holocellulose was determined by difference.
Liquefaction Reaction
Cork liquefaction was performed with a polyol solvent mixture of glycerol and polyethylene glycol (PEG 400) utilizing H2SO4 as the catalyst. The tested cork-to-solvent ratios were 1:3, 1:5, and 1:6, and the glycerol-to-PEG ratios were 9:1, 1:1, and 1:9. The catalyst was added at 3% of the solvent mass.
The liquefaction process was conducted in a 600-mL Parr 5100 low pressure reactor that was indirectly heated with an oil jacket (Parr Instrument Co., Moline, IL). The dried cork powder (10 g), solvent, and catalyst were mixed to obtain a homogenous mixture prior to being placed into the reactor. The mixture was transferred to the reactor when the reactor reached the desired temperature, and the reactor was then sealed. The reactor’s stirrer was set at 70 rpm. The liquefaction reaction temperatures tested (i.e., temperature of the oil in the reactor jacket) were 150, 160, and 170 °C. The reaction time ranged between 30 and 120 min.
After the required reaction time had passed, the products of the liquefaction were removed from the reactor and were mixed with approximately 100 mL of a 1,4-dioxane-water solution (4:1 v/v). The mixture was filtered by pumping it to a Büchner funnel containing filter paper to separate the solid residue from the liquefied cork fraction. The filtered solids were oven-dried and weighed; afterwards, the liquefaction yield was determined in accordance to Eq. 1:
![]() (1)
(1)
Polyurethane Foam Production
Prior to foam production, the prepared polyols were neutralized with a 1 N KOH solution. Polyurethane foams were prepared in accordance with the method described by Gama et al. (2015). Liquefied cork (4 g) was mixed with 4% (w/w) catalyst (Polycat 34), 4% (w/w) surfactant (Tegostab® B8404), and 0.25% (w/w) of water as blowing agent. Afterwards 8 g of polymeric isocyanate (Voranate M229 MDI-methylenediphenyl diisocyanate) were added, and the resulting mixture was vigorously shaken in a propylene beaker with an IKA Ost Basic mixer with rotating blades at 700 rpm until the foam started to grow. The resulting foam was left to stabilize for 24 h at room temperature. The foams were prepared with cork liquefied by a mixture of Glycerol:PEG 9:1 at 160 ºC. PUF01 used a 1:3 cork-to-solvent ratio (2 h), PUF02 used a 1:5 cork-to-solvent ratio (2 h), PUF03 used a 1:6 cork-to-solvent ratio (2 h), and PUF04 used a 1:6 cork-to-solvent ratio (1 h).
Polyurethane Foam Mechanical Testing
Compression tests of the produced polyurethane foams (PUFs) were done in accordance with the ISO 844 (2014) standard using a Servosis ME-405/5 universal testing machine (Servosis, Madrid, Spain) at a compression rate of 5 mm/min. Cubic samples (18 mm x18 mm x18 mm) were cut from the various PUFs; the samples were weighed and their densities were calculated. Five replicates were made for each foam. The stress-strain curves were recorded, and the compressive modulus of elasticity and the stress at 10% deformation were determined.
Scanning Electronic Microscopy (SEM)
The cellular structures of the PUFs were observed using a scanning electron microscope, which was coupled to an energy dispersion spectrometer (SEM-EDS). Small samples (5 mm x 5 mm x 5 mm) were cut from each PUF and fixed in a sample holder with a double-sided tape. The sample was covered with carbon and analyzed with a Hitachi S4100 SEM coupled with an EDS system (RonTech AG, Felsberg, Switzerland). The morphologies of the samples were observed in relation to cell topology and size.
Fourier Transform Infrared spectroscopy (FTIR)
The cork and the solid residue samples were ball-milled and were dried overnight at 100 °C. Afterwards, approximately 2 mg of the sample was mixed with KBr in the proportion of 1:100 (w/w) and subsequently pressed at 8 tons of pressure for 3 min. The FTIR spectra of samples were recorded using a Mattson 7000 FTIR spectrophotometer (Mattson Instruments, Inc., Madison, WI) operating at 64 scans/min with a resolution of 4.0 cm-1 over the 4000 to 400 cm-1 range.
The liquefied material was dried in an oven at 100 ºC for one week in order to assure that water was completely removed. FTIR-ATR spectra were taken in a Perkin Elmer UATR Spectrum Two with 72 scans/min with a resolution of 4.0 cm-1 over the 4000 to 400 cm-1 range. After performing the background, the liquefied material was placed in the sample holder and pressed against the crystal. The average of three spectra was used.
RESULTS AND DISCUSSION
Chemical Characterization
Table 1 presents the chemical characterization of the cork sample used in this work. The extractive percentage (14.1) was comparable to the average percentage reported by Pereira (2013). There was a similar percentage of water and ethanol extracts, higher than dichloromethane. The suberin content was smaller than the average (42.8%) reported by Pereira (2013). Nevertheless, it was clearly in the interval reported (23.1 to 54.2%). On the other side, lignin (31.4%) was higher than the average (22%) but also inside the interval (17.1 to 36.4%). The lower amount of suberin might result from the fraction used in the tests (< 80 mesh) since this has been observed for Pseudotsuga menziesii bark (Ferreira et al. 2015)
Table 1. Average Polyurethane Foam Density and Mechanical Properties (with ± St. Dev.)
Cork Liquefaction
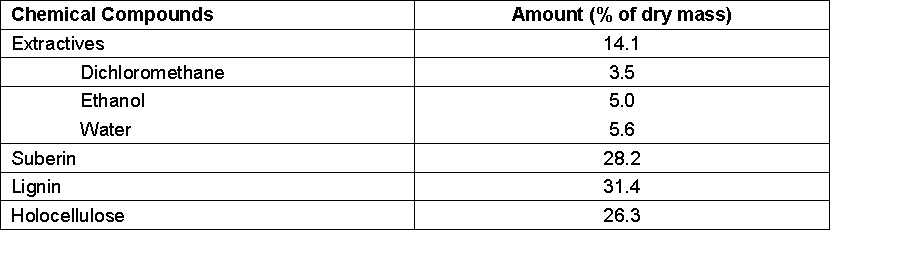
Figure 1 summarizes the yield results obtained for cork liquefaction with respect to solvent composition (i.e., glycerol-to-PEG ratio), reaction temperature, reaction time, and solvent-to-cork ratio. The maximum liquefaction yield attained was 75%. This value showed that liquefaction of all the cork components could be achieved partially under the test conditions examined. Cork contains on average 14.1 extractives, 28.2% suberin, 31.4% lignin, and 26.3% polysaccharides (Table 1). Similar results with a 71% liquefaction yield were achieved at 150 °C for 60 min using 4 wt.% sulfuric acid by Soares et al. (2014). It is noteworthy that a previous attempt to liquefy cork by Yona et al. (2014) using glycerol/PEG with an acid catalyst was only able to obtain a 45% liquefaction yield.
The liquefaction yield increased from 40% at 150 °C to 75% at 160 °C, whereas a further temperature increase to 170 °C did not increase the liquefaction yield. The results showed that at temperatures higher than 160 ºC both suberin and the remaining lignocellulosic components were liquefied to some extent, since the suberin amount in cork is higher than 25%.
Slightly different results were presented by Soares et al. (2014), who obtained higher liquefaction yield at lower reaction temperatures; the authors attributed this observation to the condensation of liquefied intermediates at the higher temperatures. In this work, the effects of polycondensation were not obvious, since there is no significant decrease in the liquefaction yield for higher liquefaction times as seem before with other materials (Martins et al. 2013)
This does not mean that there is no recondensation, because for higher liquefaction times there might exist an equilibrium between the higher liquefaction of cork components and the recondensation of the liquefied material. Soares et al. (2014) suggested that condensation reactions occurred at the higher reaction temperatures with the small percentage of glycerol (10%) used in their study. Kurimoto and Tamura (1999) proposed that re-condensation only happens when both the cellulose and the lignin are liquefied; however, they also proposed that this reaction can be inhibited by the addition of low-molecular weight glycols, such as glycerol. The use of glycerol limits the re-condensation reactions and at the same time lowers the cost of the process since glycerol is a less expensive chemical than other polyalcohols. The possible use of crude glycerol in cork liquefaction could further decrease the cost of the process. This concept was applied successfully by Hu et al. (2012) in the liquefaction of soybean straw.
Liquefaction yields increased as the reaction time was increased, which approached a maximum at approximately 60 min. Additional reaction time did not appreciably increase the liquefaction yield and would cause the process to be more expensive. Variations of the liquefaction yields with a range of cork-to-solvent ratios showed that yields increased as the amount of solvent increased to a moderate extent; a 1:3 ratio afforded a liquefaction yield of 60%, which increased to 75% at a 1:6 ratio. The composition of the liquefaction solvent showed that it had an appreciable impact on the liquefaction yields that were obtained.
A decrease in the PEG proportion in the solvent mixture (i.e., an increase in the glycerol proportion) led to a decrease in the liquefaction yield. For example, a 9:1 glycerol-to-PEG solvent composition only afforded 30% liquefaction of the cork. Nevertheless, a satisfactory liquefaction yield of approximately 60% was obtained when using a 1:1 glycerol-to-PEG solvent mixture.
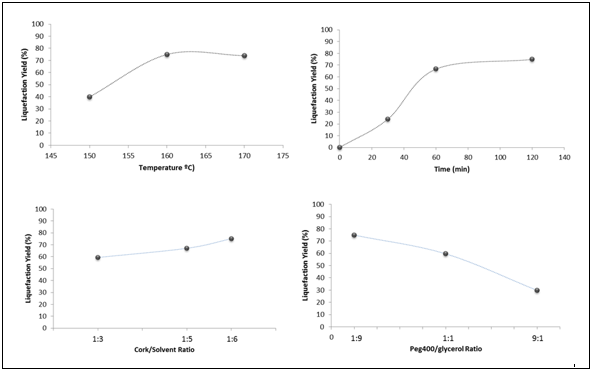
Fig. 1. Variation of liquefaction yield (%) with reaction temperature (top left), time (top right), cork-to-solvent ratio (bottom left), and PEG 400-to-glycerol ratio (bottom right). The general conditions used were 160 °C with 60 min reaction time, cork/solvent ratio of 1:6 and PEG/Glycerol ratio 1:9.
Figure 2 illustrates how cork powder particle size affects liquefaction yield. There were no noticeable differences observed between the 60 to 80 mesh powder and the lower than 80 mesh fraction; however, the larger particle sizes (i.e., higher than 60 mesh) were liquefied only to a small extent (50 to 60%). Therefore, particles of approximately 0.177 to 0.250 mm (60 to 80 mesh) should be sufficiently small to ensure good liquefaction yields.
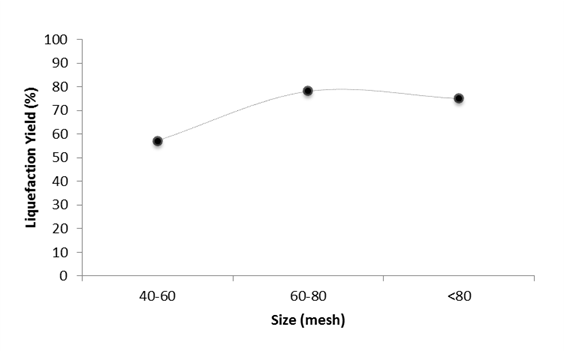
Fig. 2. Influence of cork powder particle size on liquefaction yield
Figure 3 compares the FTIR spectra of the original cork, the liquefied material, and the solid residue from post-liquefaction treatment (for a sample with a 67% liquefaction yield). Several differences were observed in the spectra that may provide insights into the liquefaction process.
Fig. 3. FTIR spectra for the original cork material for liquefied and solid residue
Overall, significant differences were observed between the spectra of cork and both the liquefied material and the solid residue, thereby showing that the structure of the main cork polymers were changed by the liquefaction process. There is a large reduction of the absorption peak at 1610 cm-1, which is assigned to the elongation of the aromatic C=C units, as well as the absorption peak at 1510 cm-1, which is characteristic of lignin structures in both the solid residue and the liquefied material. The peaks around 2930 and 1740 cm-1 are indicative of suberin, corresponding to C-H stretching and to the C=O band, respectively, of suberin (Graça and Pereira 2000; Lopes et al. 2000). The peak located at 1265 cm-1 is assigned to the epoxy ring, which is also characteristic of suberin (Graça and Pereira 2000). Comparatively, these suberin-related peaks increased in the post-treatment solids while in the liquefied material a clear decrease is observed for the peak around 1735 cm-1. In relation to the peak at 2930 cm-1 the differences are difficult to see because of the appearance of a high intensity peak at around 2870 cm-1. This peak is usually assigned to C-H stretching of the methyl group while methylene absorbs at lower and methine at higher wavenumbers (Esteves et al. 2013). This might also explain the high intensity of the peak around 1260 cm-1 that is also characteristic of the methyl group. The higher intensity of these peaks in the liquefied material suggests the existence of smaller molecules which was to be expected.
There was an increase in the absorption peak around 1040 cm-1, which is characteristic of C-O vibrations in polysaccharides and tended to shift to approximately 1090 cm-1. This increase was observed in the solid residue and also in the liquefied material spectra.
Comparison of the cork and the post-liquefaction solids showed that the solid residues were enriched in suberin, which indicated that the liquefaction process selectively dissolved the lignocellulose components, particularly the lignin, while leaving the suberin intact for the most part. This observation is attributed to suberin’s resistance to acid degradation; on the other hand, suberin is susceptible to alkaline degradation and dissolution via saponification. Yona et al. (2014) reported that they were only able to liquefy 47% of the cork when using an acid catalyst. Nevertheless, the present results indicated that a liquefaction yield of approximately 70% can be obtained despite the acid resistance of suberin. Liquefaction of suberin may impart different properties than those usually attained for other liquefied lignocellulosic materials, which may or may not be an important benefit.
Polyurethane Foams
One of the main products that can be produced with liquefied lignocellulosic materials is polyurethane foams. Several attempts have been made to use liquefied wood (Alma and Shiraishi 1998), sugar cane bagasse (Hakim et al. 2011), and wheat straw (Chen and Lu 2009), as well as corn bran, stover, and stalks (Lee et al. 2000; Wang et al. 2008; Yan et al. 2008), to produce polyurethane foams. Recently, liquefied cork has been used for the production of polyurethane foams, although the investigations did not report the liquefaction yields nor the amount of suberin that had been dissolved (Gama et al. 2015).
Figure 4 shows the surface of the PUFs derived from liquefied cork from SEM analysis. In all the cases, the PUFs exhibited a typical cellular structure. These PUFs had closed cells with a polyhedral structure, which differ from the open cellular structure typically observed for flexible polyurethane foams.
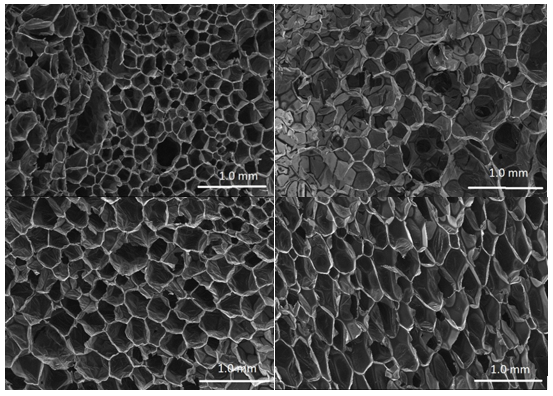
Fig. 4. Micrographs at 30x magnification for: a (top left) PUF01 using a 1:3 cork-to-solvent ratio (2 h); b (top right) PUF02 using a 1:5 cork-to-solvent ratio (2 h); c (bottom left) PUF03 using a 1:6 cork-to-solvent ratio (2 h); and d (bottom right) PUF04 using a 1:6 cork-to-solvent ratio (1 h)
There was a variation in the cell dimensions within each type of foam to a certain extent. Some PUFs were nearly homogeneous, while others were heterogeneous. For example, the ranges of cell diameters were between 0.13 and 0.66 mm in PUF01, between 0.26 and 0.92 mm in PUF02, between 0.26 and 0.53 mm in PUF03, and between 0.13 and 0.39 mm in PUF04 (Fig. 4). Hakim et al. (2011) disclosed a relationship between cell uniformity and pore size of PUFs using polyols made from liquefy sugar cane bagasse. The authors observed that PUFs prepared using polyols containing more than 20% of polyol produced from sugar cane have a very heterogeneous surface, with irregular pore size and shape. In the present study, the use of 100% bio-based polyol for PUF production afforded foams that were dominated by smaller pore size and greater homogeneity.
The FTIR-ATR spectra of the liquefied cork and of the foams produced are presented in Fig. 5. There were large differences between the spectra mainly due to the addition of isocyanate in the polyurethane foams. There were some differences between foams, although they all exhibited peaks at similar wavenumbers. The band between 3200 and 3500 cm-1, which was due to stretching of O-H groups in liquefied cork within the foam, also included a contribution from symmetric and asymmetric stretching of the N-H from the urethane and urea groups (Gama et al. 2015), and that is probably the reason why the band for PUFs was at lower wavenumbers (3300 cm-1) than for liquefied cork (3450 cm-1). The bands at 2900 to 2800 cm-1 were composed by the overlapping of the asymmetric stretch vibrations and symmetric stretch vibrations of -CH2– and -CH3. The C=O linkage exhibits strong absorptions between 1750 and 1700 cm-1, and the precise wavenumber depends of the functional group structural location. The band at 1220 cm−1 associated with C-O stretching vibrations was stronger in the foams than in liquefied cork. The small peak around 2270 cm−1, observed in PUF02 was due to residual NCO groups (Gama et al. 2015).
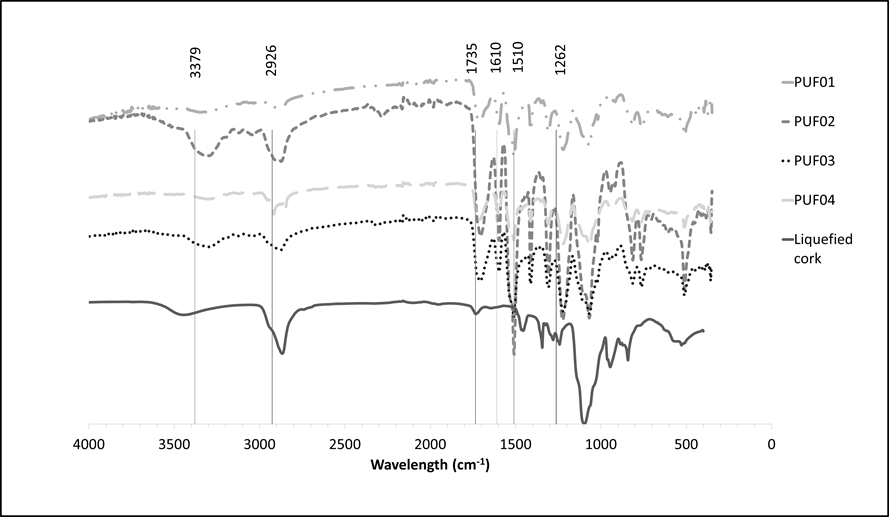
Fig. 5. FTIR-ATR spectra of original cork and polyurethane foams produced
Figure 6 presents the stress-strain compression curves for one PUF sample; all PUFs showed similar compressive behaviour. This compression behaviour is typical of cellular solids (Gibson and Ashby 1999). Cork itself is a closed-cell foam with a specific gravity of approximately 0.15 (Ashby and Medalist 1983) that contains small cells (Pereira et al. 1987). The stress-strain curves of PUFs of this study were similar to stress-strain curves reported by other PUF investigators (Gibson et al. 1981; Oliveira et al. 2014), although with higher stress values.
The compressive stress was initially linear to compressive strain until approximately 10% strain. This corresponds to the elastic zone where deformations are not permanent. Gibson and Ashby (1999) indicated that this initial linear region primarily arises from the bending of cell struts. In foams with closed cells, in this initial region, there is the stretching of the membranes due to changes in fluid pressure inside the cells. After this initial linear region of the stress-strain curve, the stress value plateaus as the strain increases; this corresponds to where the cell walls of the foam are deformed. This plateau is one of the advantages of foams because the foam is able to absorb kinetic energy while limiting the stress transmitted to relatively low levels, which is important for crash protection (e.g., in helmets). These foams differ from those of solid materials, such as metals, which generally do not have an extended stress-strain plateau under compression (Ridha 2007). This stress-strain plateau is different between foam types. In elastomeric foams there is elastic buckling, in elastic-plastic foams the formation of plastic hinges and in elastic-brittle foams, brittle crushing (Gibson and Ashby 1999). The stress-strain curves shown in Fig. 6 are consistent with elastomeric foams. The final densification zone begins at about 50% to 70% strain where the cell walls are crushed.
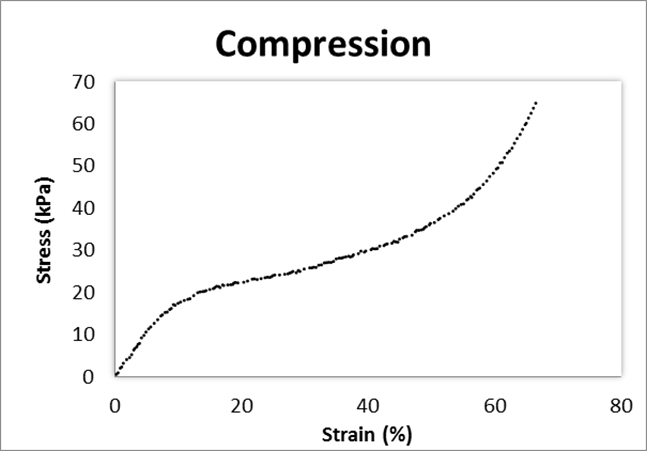
Fig. 6. Example of the compression curve for the PUF sample with 1:3 cork-to-solvent ratio
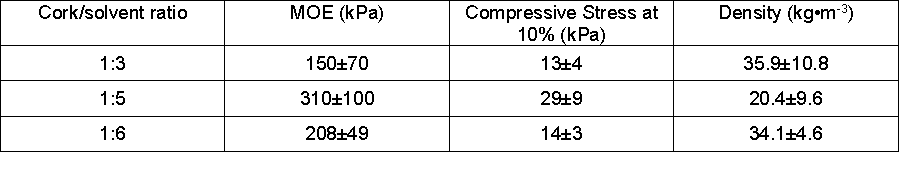
Table 2 presents the modulus of elasticity (MOE) and the stress at 10% deformation for foams produced with various cork-to-solvent ratios (1:3, 1:5, and 1:6). The mean densities ranged from 20 to 36 kg•m-3. Slightly higher values were found for polyurethane foams produced from liquefied corn bran, which had foam densities in the range of 35 to 45 kg•m-3 (Lee et al. 2000). Higher densities were achieved by Gama et al. (2015) with foams prepared with liquefied cork with densities between 57.4 to 70.7 kg•m-3.
The compression modulus of elasticity (MOE) for the three tested foams ranged from 150 to 310 kPa; stress at 10% deformation ranged from 13 to 29 kPa. There was a large between-sample variations of the foams, as is shown by the standard deviation of the mean values. No trends were observed for the variation regarding the cork-to-solvent ratio or with PUF density. The results obtained are within the range of values reported by Gama et al. (2015) for PUFs obtained from acid liquefied cork (i.e., MOE ranged from 183 to 475 kPa, and stress at 10% deformation ranged from 7.7 to 34.6 kPa). Higher values were reported by Lee et al. (2000) for liquefied corn bran PUFs with compressive strength at 10% strain of 76 kPa and compressive MOE of 1140 kPa. However, all of the foams in this study have compressive strengths that were lower than commercial foams, which have compressive strength at 10% strain of about 100 kPa (Guo et al. 2000).
Table 2. Average Polyurethane Foam Density and Mechanical Properties (with ± St. Dev.)
CONCLUSIONS
- The results of this study showed that it is possible to liquefy a large proportion of cork residuals under mild conditions with an acid catalysis, which can be used to produce polyurethanes foams (PUFs).
- The best conditions to obtain high liquefaction yields from cork residuals were: 160 °C for 1 h; glycerol-to-PEG 400 solvent ratio of 1:9; cork-to-solvent ratio of 1:6; and 3% H2SO4 catalyst charge.
- The FTIR spectra indicated that the lignocellulosic fraction of cork was dissolved preferentially during the liquefaction reaction versus the suberin.
- The SEM micrographs of different PUFs showed that the cellular structure depends on the characteristics of the polyol used. Overall, it is possible to obtain PUFs with acceptable quality, although more studies are needed to achieve foam properties similar to those that are commercially available.
ACKNOWLEDGMENTS
Funding from the Portuguese Foundation for Science and Technology (FCT) for the Center for Studies in Education, Technologies and Health (CIandDETS) is acknowledged, as well as for the Forest Research Centre (UID/AGR(0239/2013).
REFERENCES CITED
Alma, M., and Shiraishi, N. (1998). “Preparation of polyurethane-like foams from NaOH-catalyzed liquefied wood,” Eur. J. Wood Prod. 56(4), 245-246. DOI: 10.1007/s001070050311
Alma, M. H., Yoshioka, M., Yao, Y., and Shiraishi, N. (1996). “The preparation and flow properties of HC1 catalyzed phenolated wood and its blends with commercial novolak resin,” Holzforschung 50(1), 85-90. DOI: 10.1515/hfsg.1996.50.1.85
Alma, M. H., Yoshioka, M., Yao, Y., and Shiraishi, N. (1998). “Preparation of sulfuric acid-catalyzed phenolated wood resin,” Wood Sci. Technol. 32(4), 297-308. DOI: 10.1007/BF00702897
Ashby, M. F., and Medalist, R. M. (1983). “The mechanical properties of cellular solids,” Metall. Trans. A 14(9), 1755-1769. DOI: 10.1007/BF02645546
Chen, F., and Lu, Z. (2009). “Liquefaction of wheat straw and preparation of rigid polyurethane foam from the liquefaction products,” J. Appl. Polym. Sci. 111(1), 508-516. DOI: 10.1002/app.29107
Cordeiro, N., Belgacem, M. N., Silvestre, A. J. D., Pascoal Neto, C., and Gandini, A. (1998). “Cork suberin as a new source of chemicals,” Int. J. Biol. Macromol. 22(2), 71-80. DOI: 10.1016/S0141-8130(97)00090-1
Desroches, M., Escouvois, M., Auvergne, R., Caillol, S., and Boutevin, B. (2012). “From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products,” Polym. Rev. 52(1), 38-79. DOI: 10.1080/15583724.2011.640443
dos Santos, R. G., Carvalho, R., Silva, E. R., Bordado, J. C., Cardoso, A. C., do Rosário Costa, M., and Mateus, M. M. (2016). “Natural polymeric water-based adhesive from cork liquefaction,” Industrial Crops and Products 84, 314-319. DOI: 10.1016/j.indcrop.2016.02.020
D’Souza, J., Wong, S. Z., Camargo, R., and Yan, N. (2015). “Solvolytic liquefaction of bark: understanding the role of polyhydric alcohols and organic solvents on polyol characteristics,” ACS Sustainable Chemistry & Engineering 4(3), 851-861. DOI: 10.1021/acssuschemeng.5b00908
Esteves, B., Velez Marques, A., Domingos, I., and Pereira, H. (2013). “Chemical changes of heat treated pine and eucalypt wood monitored by FTIR,” Maderas Cienc. Tecnol. 15, 245-258. DOI: 10.4067/S0718-221X2013005000020
Ferreira, J. P. A., Miranda, I., Gominho, J., and Pereira, H. (2015). “Selective fractioning of Pseudotsuga menziesii bark and chemical characterization in view of an integrated valorization,” Industrial Crops and Products 74, 998-1007. DOI: 10.1016/j.indcrop.2015.05.065
Gama, N. V., Soares, B., Freire, C. S., Silva, R., Brandão, I., Neto, C. P., Barros-Timmons, A., and Ferreira, A. (2015). “Rigid polyurethane foams derived from cork liquefied at atmospheric pressure,” Polym. Int. 64(2), 250-257. DOI: 10.1002/pi.4783
Gibson, L. J., and Ashby, M. F. (1999). Cellular Solids: Structure and Properties, 2nd Ed., Cambridge University Press, Cambridge, UK.
Gibson, L. J., Easterling, K. E., and Ashby, M. F. (1981). “The structure and mechanics of cork,” Proc. Roy. Soc. Lond. A 77(1769), 99-117.
Graça, J., and Pereira, H. (2000). “Methanolysis of bark suberins: Analysis of glycerol and acid monomers,” Phytochem. Anal. 11(1), 45-51. DOI: 10.1002/(SICI)1099-1565(200001/02)11:1<45::AID-PCA481>3.0.CO;2-8
Guo, A., Javni, I., and Petrovic, Z. (2000). “Rigid polyurethane foams based on soybean oil,” J. Appl. Polym. Sci. 77(2), 467-473. DOI: 10.1002/(SICI)1097-4628(20000711)77:2<467::AID-APP25>3.3.CO;2-6
Hakim, A. A., Nassar, M., Emam, A., and Sultan, M. (2011). “Preparation and characterization of rigid polyurethane foam prepared from sugar-cane bagasse polyol,” Mater. Chem. Phys. 129(1), 301-307. DOI: 10.1016/j.matchemphys.2011.04.008
Hu, S., Wan, C., and Li, Y. (2012). “Production and characterization of biopolyols and polyurethane foams from crude glycerol based liquefaction of soybean straw,” Bioresour. Technol. 103(1), 227-233. DOI: 10.1016/j.biortech.2011.09.125
ISO 844 (2014). “Rigid cellular plastics — Determination of compression properties,” International Organization for Standardization, Geneva, Switzerland.
Kobayashi, M., Asano, T., Kajiyama, M., and Tomita, B. (2004). “Analysis on residue formation during wood liquefaction with polyhydric alcohol,” Journal of Wood Science, 50(5), 407–414. DOI: 10.1007/s10086-003-0596-9
Kunaver, M., Jasiukaityte, E., Cük, N., and Guthrie, J. T. (2010). “Liquefaction of wood, synthesis and characterization of liquefied wood polyester derivatives,” J. Appl. Polym. Sci. 115(3), 1265-1271. DOI: 10.1002/app.31277
Kurimoto, Y., and Tamura, Y. (1999). “Species effects on wood-liquefaction in polyhydric alcohols,” Holzforschung 53(6), 617-622. DOI: 10.1515/hf.1999.102
Lee, W.-J., and Lin, M.-S. (2008). “Preparation and application of polyurethane adhesives made from polyhydric alcohol liquefied Taiwan acacia and China fir,” J. Appl. Polym. Sci. 109(1), 23-31. DOI: 10.1002/app.28007
Lee, S.-H., Yoshioka, M., and Shiraishi, N. (2000). “Liquefaction of corn bran (CB) in the presence of alcohols and preparation of polyurethane foam from its liquefied polyol,” J. Appl. Polym. Sci. 78(2), 319-325. DOI: 10.1002/1097-4628(20001010)78:2<319::AID-APP120>3.0.CO;2-Z
Lin, L., Yao, Y., Yoshioka, M., and Shiraishi, N. (1997). “Liquefaction mechanism of lignin in the presence of phenol at elevated temperature without catalysts. Studies on ß-O-4 lignin model compound. I. Structural characterization of the reaction products,” Holzforschung 51(4), 316-324.
Lin, R., Sun, J., Yue, C., Wang, X., Tu, D., and Gao, Z. (2014). “Study on preparation and properties of phenol-formaldehyde-Chinese fir liquefaction copolymer resin,” Maderas-Cienc. Tecnol. 16(2), 159-174. DOI: 10.4067/s0718-221×2014005000013
Lopes, M. H., Gil, A. M., Silvestre, A. J. D., and Neto, C. P. (2000). “Composition of suberin extracted upon gradual alkaline methanolysis of Quercus suber L. cork,” J. Agr. Food Chem. 48(2), 383-391. DOI: 10.1021/jf9909398
Maldas, D., Shiraishi, N., and Harada, Y. (1997). “Phenolic resol resin adhesives prepared from alkali-catalyzed liquefied phenolated wood and used to bond hardwood,” J. Adhes. Sci. Technol. 11(3), 305-316. DOI: 10.1163/156856197X00714
Martins, J., Lopes, L. C., and Esteves, B. (2013). “Otimização da liquefação da madeira de Pinus pinaster com poliálcoois,” Silva Lusit. 21(Special Issue June 2013), 177-183 (in Portuguese).
Mateus, M. M., Acero, N. F., Bordado, J. C., and dos Santos, R. G. (2015). “Sonication as a foremost tool to improve cork liquefaction,” Industrial Crops and Products 74, 9-13.
Mateus, M. M., Bordado, J. C., and dos Santos, R. G. (2016). “Potential biofuel from liquefied cork – Higher heating value comparison,” Fuel, 174, 114-117.
Niu, M., Zhao, G., and Alma, M. (2011). “Polycondensation reaction and its mechanism during lignocellulosic liquefaction by an acid catalyst: A review,” For. Stud. China 13(1), 71-79. DOI: 10.1007/s11632-011-0109-7
Oliveira, V., Rosa, M. E., and Pereira, H. (2014). “Variability of the compression properties of cork.” Wood Sci. Technol. 48(5), 937-948. DOI: 10.1007/s00226-014-0651-2
Pan, H. (2011). “Synthesis of polymers from organic solvent liquefied biomass: A review,” Renew. Sustain. Energ. Rev. 15(7), 3454-3463. DOI: 10.1016/j.rser.2011.05.002
Pereira, H. (2007). Cork: Biology, Production and Uses, Elsevier, London, UK.
Pereira, H. (2013). “Variability of the chemical composition of cork,” BioResources 8(2), 2246-2256. DOI: 10.15376/biores.8.2.2246-2256
Pereira, H. (2015). “The rationale behind cork properties: A review of structure and chemistry,” BioResources 10(3), 6207-6229. DOI: 10.15376/biores.10.3.6207-6229
Pereira, H., Rosa, M. E., and Fortes, M. A. (1987). “The cellular structure of cork from Quercus suber L.,” IAWA J. 8(3), 213-218. DOI: 10.1163/22941932-90001048
Ridha, M. (2007). Mechanical and Failure Properties of Rigid Polyurethane Foam under Tension, Ph.D. dissertation, National University of Singapore, Singapore.
Soares, B., Gama, N., Freire, C., Barros-Timmons, A., Brandão, I., Silva, R., Pascoal Neto, C., and Ferreira, A. (2014). “Ecopolyol production from industrial cork powder via acid liquefaction using polyhydric alcohols,” ACS Sust. Chem. Eng. 2(4), 846-854. DOI: 10.1021/sc400488c
Sousa, A. F., Pinto, P. C., Silvestre, A. J., and Pascoal Neto, C. (2006). “Triterpenic and other lipophilic components from industrial cork byproducts,” J. Agr. Food Chem. 54(18), 6888-6893. DOI: 10.1021/jf060987+
Wang, T., Zhang, L., Li, D., Yin, J., Wu, S., and Mao, Z. (2008). “Mechanical properties of polyurethane foams prepared from liquefied corn stover with PAPI,” Bioresour. Technol. 99(7), 2265-2268. DOI: 10.1016/j.biortech.2007.05.003
Xie, T., and Chen, F. (2005). “Fast liquefaction of bagasse in ethylene carbonate and preparation of epoxy resin from the liquefied product,” J. Appl. Polym. Sci. 98(5), 1961-1968. DOI: 10.1002/app.22370
Yamada, T., and Ono, H. (2001). “Characterization of the products resulting from ethylene glycol liquefaction of cellulose,” J. Wood Sci. 47(6), 458-464. DOI: 10.1007/BF00767898
Yan, Y., Pang, H., Yang, X., Zhang, R., and Liao, B. (2008). “Preparation and characterization of water‐blown polyurethane foams from liquefied cornstalk polyol,” J. Appl. Polym. Sci. 110(2), 1099-1111. DOI: 10.1002/app.28692
Yona, A. M. C., Budija, F., Kričej, B., Kutnar, A., Pavlič, M., Pori, P., and Petrič, M. (2014). “Production of biomaterials from cork: Liquefaction in polyhydric alcohols at moderate temperatures,” Ind. Crop. Prod. 54, 296-301. DOI: 10.1016/j.indcrop.2014.01.027
Zhang, Q., Zhao, G., and Jie, S. (2005). “Liquefaction and product identification of main chemical compositions of wood in phenol,” For. Stud. China 7, 31-37. DOI: 10.1007/s11632-005-0018-8
Zhang, T., Zhou, Y., Liu, D., and Petrus, L. (2007). “Qualitative analysis of products formed during the acid catalyzed liquefaction of bagasse in ethylene glycol,” Bioresour. Technol. 98(7), 1454-1459. DOI: 10.1016/j.biortech.2006.03.029
Article submitted: August 30, 2016; Peer review completed: October 15, 2016; Revised version received and accepted: December 7, 2016; Published: February 10, 2017.
DOI: 10.15376/biores.12.2.2339-2353
