Abstract
Arbuscular mycorrhizae (AM) fungi can increase the biomass of host plants that are used as biofuel feedstock. However, little is known about the effects of AM fungi during aqueous ammonia pretreatment of biomass to remove lignin or on the enzymatic hydrolysis of cellulose. The analysis of mycorrhizal colonization (Rhizophagus irregularis or Glomus versiforme) on stems of black locust (Robinia pseudoacacia) plants in their first and second year of growth revealed that the presence of AM fungi and the growth time significantly influenced the lignin and cellulose content of untreated black locust but had no effect on the content of stems pretreated with aqueous ammonia. The presence of AM fungi and/or the growth time also affected the black locust stem structure and the chemical structure of lignin. Hydrolysis of mycorrhizal and non-mycorrhizal biomass produced similar glucose yields (except for the second-year R. irregularis biomass, which produced significantly less glucose than the other treatments). The results suggest that mycorrhizal black locust biomass is a suitable substrate for biofuel production.
Download PDF
Full Article
Delignification and Hydrolyzation of Mycorrhizal Black Locust Biomass Pretreated with Aqueous Ammonia
Xiaoqin Zhu,a,b Ming Tang,a,* and Hui Chen a
Arbuscular mycorrhizal (AM) fungi can increase the biomass of host plants that are used as biofuel feedstock. However, little is known about the effects of AM fungi during aqueous ammonia pretreatment of biomass to remove lignin or on the enzymatic hydrolysis of cellulose. The analysis of mycorrhizal colonization (Rhizophagus irregularis or Glomus versiforme) on stems of black locust (Robinia pseudoacacia) plants in their first and second year of growth revealed that the presence of AM fungi and the growth time significantly influenced the lignin and cellulose content of untreated black locust but had no effect on the content of stems pretreated with aqueous ammonia. The presence of AM fungi and/or the growth time also affected the black locust stem structure and the chemical structure of lignin. Hydrolysis of mycorrhizal and non-mycorrhizal biomass produced similar glucose yields (except for the second-year R. irregularis biomass, which produced significantly less glucose than the other treatments). The results suggest that mycorrhizal black locust biomass is a suitable substrate for biofuel production.
Keywords: Arbuscular mycorrhizal fungi; Black locust; Lignin content; Cellulose content; Enzymatic hydrolysis; Glucose yield
Contact information: a: State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources (South China Agricultural University), Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou 510642, China; b: Key Laboratory of Plant-Microbe Interactions, Shangqiu Normal University, Shangqiu 476000, China;
* Corresponding author: tangmingyl@163.com
INTRODUCTION
Black locust (Robinia pseudoacacia L.) is a hardy, productive, fast-growing leguminous tree that can be grown in temperate regions worldwide and has the potential to be utilized as a biofuel feedstock (Gasol et al. 2010). Black locust is an important short-rotation forestry crop with many combustion properties similar to other fuels used in power plants (Monedero et al. 2017). Many lectures reported that black locust have higher biomass than those of poplar (Populus) and willow (Salix spp.) (Bongarten et al. 1992; Gruenewald et al. 2007; Grünewald et al. 2009). Black locust as a biofuel feedstock can be directly used as firewood or converted to gas (Geyer and Walawender 1994), oil (Balat 2010) or ethanol (González-García et al. 2011; 2012).
Black locust has high glucan/cellulose content, which is a desirable characteristic for cellulosic ethanol production (Torget et al. 1991). To optimize the sugar yields of 1-year-old black locust, Garlock et al. (2012) investigated the use of an ammonia fiber expansion pretreatment and the enzymatic conversion of black locust to soluble sugars. It was reported that the glucan/cellulose content of black locust wood was 37.15% and that the acid-insoluble lignin content was 21.19%.
The cellulose and lignin contents of plant cell walls significantly influence the enzymatic hydrolysis of plant biomass to soluble sugars. Cellulose can be hydrolyzed into sugars and then fermented to produce bioethanol. Lignin has a negative effect on the biofuel production process. Increasing the cellulose content or reducing the lignin content or delignification of a biofuel can cause an enhancement in enzymatic hydrolysis efficiency and sugar yield (Alvira et al. 2010). Aqueous ammonia pretreatment is an efficient delignification methodology, which selectively extracts lignin from lignocellulosic biomass at low temperature, and then improves enzyme accessibility to cellulose for enzymatic hydrolysis (Sousa et al. 2016).
Genetically modified “energy trees” or genetic mutations to reduce lignin content induce complex responses in plants, and the benefits of such genetic manipulations are still under debate (Zeng et al. 2014). It is well known that soil organisms can influence plant lignin content and that modifications to the lignin biosynthetic pathway can influence soil organisms (Bennett et al. 2015). Arbuscular mycorrhizal (AM) fungi are important soil organisms that can form a symbiotic association with more than 80% of plants, enhancing the plant’s growth and stress resistance (Smith and Read 2008). Liu et al. (2014) investigated the effects of AM fungi on the biomass and bioenergy production of poplar: AM fungi significantly enhanced the biomass and affected the lignin content of poplar. Zhu et al. (2014; 2017) found that AM fungi enhanced the biomass and calorific value of black locust. However, to the authors’ knowledge, little is known about whether AM fungi can influence the cellulose and lignin content of black locust, and whether the pretreatment and hydrolysis steps of the biofuel production process are affected by using biomass that has been colonized by AM fungi.
The general objectives of this study were to determine whether the AM fungal species Rhizophagus irregularis and Glomus versiforme have any effect on the cellulose and lignin content of black locust, and whether the aqueous ammonia pretreatment and enzymatic hydrolysis processes are affected when feedstocks colonized with R. irregularis or G. versiforme are used.
EXPERIMENTAL
Materials
Planting, harvest, and sample preparation
The plants used in this study were grown on a culture substrate of soil and sand that had been sieved through a 2-mm sieve, mixed (sand/soil, 1:1 v/v), and then autoclaved at 121 °C for 2 h. Each pot (top diameter, 17 cm; depth, 16 cm) was filled with 1.5 kg of culture substrate. Three black locust seeds were sown in each pot. Seedlings were thinned to one seedling per pot after 10 d of germination. The seedlings were inoculated with either AM inocula (R. irregularis or G. versiforme) or without AM (non-mycorrhizal, NM). The R. irregularis and G. versiforme inocula used in this research were provided by the Bank of Glomales in China. The inocula comprised spores (approximately 50 spores/g), hyphae, infected root fragments, and sand. For mycorrhizal treatment, 20 g of the inoculum (either R. irregularis or G. versiforme) was applied to the germinated seeds. For the NM treatment, 20 g of the sterilized inoculum with 10 mL of mycorrhizal fungal-free filtrate meshing (through 1-μm nylon mesh) from the AM fungi inocula was applied next to the germinated seeds. Each treatment included five replicates, and the pots were randomly arranged in the greenhouse. The seedlings were planted in June 2011 and harvested in either October 2011 (year 1) or October 2012 (year 2). The seedlings were cultured under natural light at a temperature ranging between 12 °C and 35 °C and a humidity ranging between 40% and 85% from June to October 2011 or from June 2011 to October 2012. Each pot was supplemented with 100 mL of Hoagland nutrient solution every week and 200 mL of water every three days. The black locust stems and branches were harvested during year one or year two, when they were 5 or 17 months old, respectively. They were dried at 60 °C for 48 h and then milled (approximately 0.25 mm).
Methods
Cellulose and lignin content analysis
The cellulose and lignin content were determined using the National Renewable Energy Laboratory (NREL) standard protocols (Sluiter et al. 2010). The samples after Soxhlet extraction were hydrolyzed with 72% sulfuric acid (Luoyang Chemical Reagent Factory, China) for 1 h and then autoclaved after being diluted to 4% sulfuric acid for 1 h at 121 °C. The autoclaved samples were filtered, and the dried residue was weighed to determine the Klason lignin content (Jia et al. 2013).
Aqueous ammonia pretreatment
First, Soxhlet extraction was conducted with 95% ethanol (Luoyang Chemical Reagent Factory, China) for 10 h to remove the soluble content (e.g., phenols and flavonoids) of the samples. After Soxhlet extraction, the milled samples (about 4 g) were pretreated at 70 °C for 48 h in a screw-capped 100-mL bottle with a 21% (w/w) aqueous ammonia solution (Luoyang Chemical Reagent Factory, China) and a solid-to-liquid ratio of 1:10. The solid was recovered using a filtration cloth and washed with distilled water until the pH of the filtrate was between 6 and 7. Then, the solid was air-dried and stored at –18 °C (Jia et al. 2013).
Enzymatic hydrolysis and glucose content analysis
Enzymatic hydrolysis experiments were conducted using a 5-mL working volume with a loading of 2.0% dry solids after aqueous ammonia pretreatment (w/v) in screw-capped 10-mL tubes. The pH of each sample was adjusted to 5 using a 50-mM sodium citrate buffer. The reaction mixtures were incubated at 50 °C on an orbital shaker at 200 rpm.
The commercial enzyme preparations Celluclast 1.5 L® (cellulase, Novo Nordisk A/S, Bagsværd, Denmark) and Novozyme 188® (β-glucosidase, Novo Nordisk A/S, Bagsværd, Denmark) were used for the hydrolysis experiments. The filter paper activity (FPA) of the cellulase was 74.7 filter paper units per milliliter (FPU/mL), according to the standardized IUPAC filter paper assay (Ghose 1987). The β-glucosidase activity was 8451 nkat/mL (~380 IU/mL), which was measured according to the method described by Bailey and Linko (1990). A loading of 15 FPU/g of dry matter of cellulase was used, which was supplemented with 500 nkat/g of dry matter of β-glucosidase and 0.55 mg/mL of xylanase. The enzymatic hydrolysis was stopped at 48 h by boiling the samples for 10 min. After cooling, the samples were centrifuged at 10,000 × g for 10 min, and the glucose content of the supernatants was analyzed using a biological sensing analyzer (SBA-40C, Shanghai, China).
Scanning electron microscopy (SEM) analysis
Physical changes in the samples and materials after aqueous ammonia pretreatment or hydrolysis were observed by SEM, operated at an accelerating voltage of 10 kV. The samples were air-dried and mounted on aluminum sample stubs. The mounted samples were then placed in a sputter-coating chamber and coated with a thin layer of gold by high-vacuum evaporation magnetron sputtering (208HR, Cressington, England) before examining the samples using a scanning electron microscope (JSM–6360LV, JEOL, Japan).
Fourier transform infrared spectroscopy (FTIR) analysis
The samples used in the FTIR spectra analysis were pressed into a disc with KBr and analyzed on a Vetex70 (Bruker Corp., Germany). The background spectrum of the diamond window without the sample was subtracted from that of each sample spectrum. In total, 16 scans were collected from each measurement in a wavelength range of 4000 to 400 cm-1.
Statistical analyses
The data of the cellulose and lignin contents, glucose yield (the data for Table 1, Table 2, and Fig. 1) were subjected to a one-way analysis of variance (ANOVA). The means were compared by performing a Duncan’s test at p < 0.05 using SPSS 16.0 (SPSS, Chicago, IL).
RESULTS AND DISCUSSION
Effects of AM Fungi on the Cellulose and Lignin Content of Black Locust
Before the aqueous ammonia pretreatment, the cellulose and lignin contents of the year-one R. irregularis-colonized black locust plants were 19.9% and 13.4% greater, respectively, than those of the non-mycorrhizal plants (p < 0.05), and the lignin content of the year-two black locust was 11.6% greater than that of the non-mycorrhizal plants (Table 1). The lignin contents of the year-one and year-two G. versiforme-colonized black locust plants were 12.4 % and 7.9% greater, respectively, than those of the non-mycorrhizal plants, but G. versiforme colonization did not significantly (p > 0.05) affect the cellulose content. AM fungi are known to absorb nutrition and exchange the carbohydrate from the host, which influence the photosynthesis efficiency (Miransari 2011; Sheng et al. 2011). In the previous studies, the authors found that AM fungi R. irregularis and G. versiforme enhanced photosynthesis ability of black locust, and increased the carbon content and bioenergy (Zhu et al. 2014, 2017). Liu et al. (2014) reported that AM fungi improved the poplar photosynthesis, cellulose content, and lignin content. AM fungi increase lignin synthesis to enhance the host’s resistance to disease or adverse environments (Zhang et al. 2018). In this study, R. irregularis and G. versiforme had different effects on the lignin and the cellulose content of black locust.
After aqueous ammonia pretreatment, the lignin content of the black locust biomass was significantly lower than that of the untreated biomass (Table 1). The lignin content of year-two black locust colonized by R. irregularis or G. versiforme was approximately 40.0% lower than that of the untreated mycorrhizal black locust, while that of year-one non-mycorrhizal black locust was 25.0% lower than that of the untreated non-mycorrhizal black locust. However, after the aqueous ammonia pretreatment, the lignin content of all the black locust biomass was not significantly (p > 0.05) different, regardless of the treatment.
The cellulose content of the aqueous ammonia-treated year-two black locust biomass that had received the non-mycorrhizal, R. irregularis, or G. versiforme treatments was significantly (p < 0.05) higher, being 32.5%, 19.5%, and 15.0%, respectively, than that of the untreated biomass. After the aqueous ammonia pretreatment, the cellulose content of the year-one biomass that had received the non-mycorrhizal, R. irregularis, or G. versiforme treatments also appeared significantly (p < 0.05) greater (29.8%, 28.2%, and 28.1%, respectively) than that of the untreated biomass. Lignin and cellulose affect the enzymatic hydrolysis of cellulose and hemicellulose in the biofuel production process (Lin et al. 2014). The bark of younger trees, which has a high lignin content, comprises 4% to 17% of the stem's biomass (Tharakan et al. 2003; Mészáros et al. 2004; Serapiglia et al. 2009). Lignin causes non-productive adsorption of cellulase and inhibits cellulose hydrolysis. Ammonia pretreatment removes some of the lignin and alters its structure, which enhances the relative cellulose and hemicellulose content and the surface of cellulose fibers (Jung et al. 2011; Kim et al. 2011). Table 1. Impact of AM Fungi on the Cellulose and Lignin Contents of Black Locust (mean ± SD)

Different letters within each column indicate that the values are significantly different (p < 0.05).
Effects of AM Fungi and Growth Year on Lignin and Cellulose Content
AM fungi affect the lignin and cellulose content of the host. Liu et al. (2014) reported that different AM fungi species exhibits various functions on the component contents of poplar. AM fungi could influence the expression of lignin synthesis-related genes in cotton, mediating lignin synthesis (Zhang et al. 2018). In the present study, AM fungi and growth year significantly affected the cellulose content of black locust before and after aqueous ammonia pretreatment. Only AM fungi impacted lignin content before aqueous ammonia pretreatment. Growth year had no effect on lignin content (Table 2).
Table 2. The Significance of AM Fungi, Growth Time, and Aqueous Ammonia Pretreatment on Cellulose, Lignin Content, and Glucose Yield
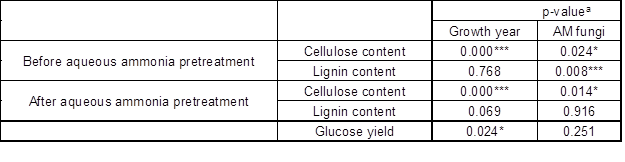
a: *, p = 0.05; **, p = 0.01; and ***, p = 0.001.
Glucose Yield
The highest glucose yields were obtained from year-one non-mycorrhizal black locust, with a conversion rate of 69.7% (Fig. 1). The glucose yield obtained from year-two black locust plants that had formed a mycorrhizal association with R. irregularis was only 56.1%. Glucose yields of approximately 65.0%, which indicate no significant (p > 0.05) difference between the highest glucose yield, were obtained from the plants that received R. irregularis or G. versiforme and the other treatments. Simply reducing the lignin content may not increase the glucose yield (Meng and Ragauskas 2014). Garlock et al. (2012) studied the effect of ammonia fiber expansion pretreatment between 120 and 180 °C and enzymatic hydrolysis of black locust. The studies obtained results showing glucose yields of more than 70% at 180 °C, with high enzyme loading. In this experiment, the glucose yields obtained were almost as high as those reported by Garlock et al. (2012), suggesting that aqueous ammonia pretreatment at 70 °C was a suitable temperature for the pretreatment of black locust biomass in the biofuel production process. Black locust has antibacterial compounds, such as phenols and flavonoids, which reduce the enzymatic activity in the hydrolysis process (Burner et al. 2008). In this study, the soluble content (e.g., phenols and flavonoids) of the samples was removed by Soxhlet extraction to enhance enzymatic activity.
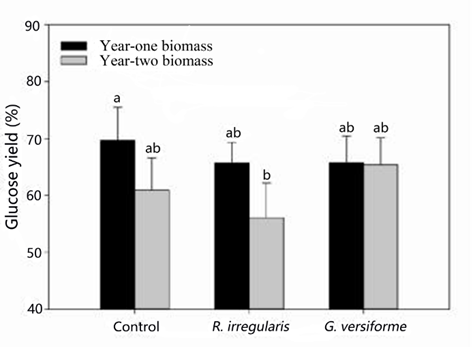
Fig. 1. The glucose yields obtained from black locust biomass that received either a non-mycorrhizal treatment (control) or mycorrhizal treatment
In addition to the lignin content, many other factors, such as the sample’s cellulose and hemicellulose content, influence enzymatic hydrolysis (Gourlay et al. 2013; Bals et al. 2014). The degradation of hemicellulose by xylanase promotes cellulose hydrolysis (Hu and Arantes 2011). Qing and Wyman (2011) enhanced the hydrolysis of corn stover by adding xylanase and β-xylosidase, obtaining a glucose yield that was 27% greater than that obtained without the addition of xylanase and β-xylosidase. In the present study, the addition of xylanase did not enhance glucose yield, possibly because the hemicellulose content was so low that it had no adverse impact on hydrolysis.
SEM Analysis
Changes in morphology of the feedstock after pretreatment were observed by SEM. The stem fibers of black locust plants that had formed a mycorrhizal association with R. irregularis (Fig. 2b) were smoother and more compacted and ordered than those of the non-mycorrizal and G. versiforme biomass (Fig. 2a, c), which suggested that AM fungi influenced the structure of black locust.
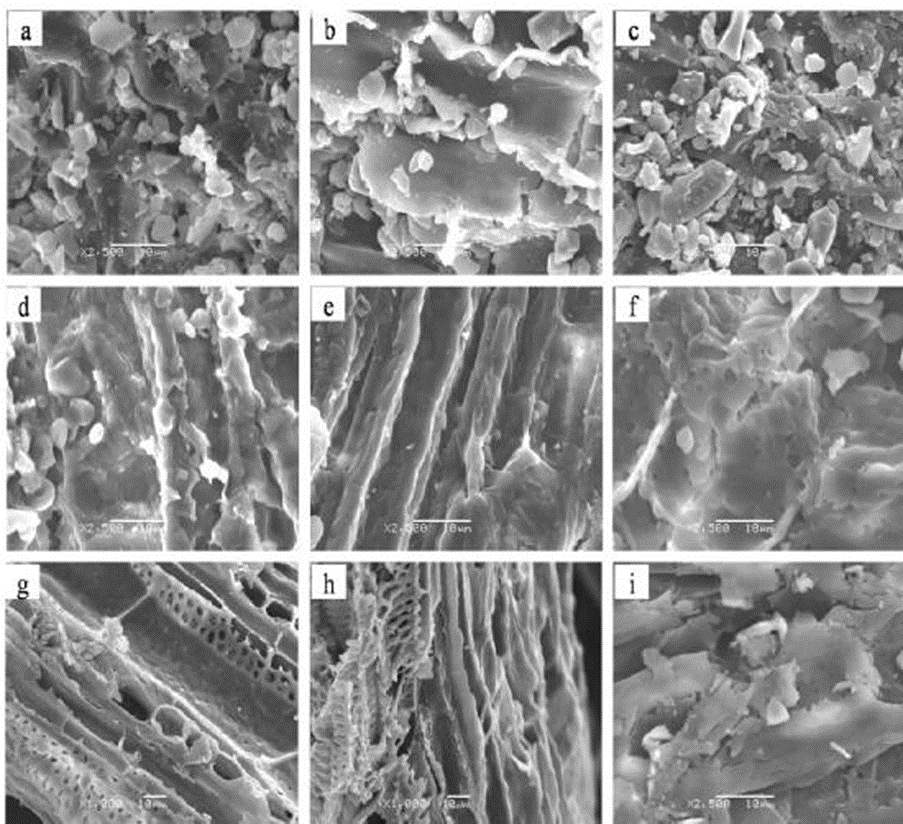
Fig. 2. SEM images of the stems of year-one black locust seedlings: a, b, and c (a ×2500; b ×2500, c ×1000) are images of untreated non-mycorrhizal, Rhizophagus irregularis, and Glomus versiforme black locust, respectively; d, e, and f (d ×2500; e ×2500, f ×2500) are images of non-mycorrhizal, R. irregularis, and G. versiforme black locust after aqueous ammonia pretreatment; g, h, and i (g ×1000; h ×1000, i ×2500) are images of non-mycorrhizal, R. irregularis, and G. versiforme black locust after enzymatic hydrolysis
After the aqueous ammonia pretreatment, the surface of the fibers appeared rough, with more micropores (Fig. 2d–f), especially fibers of black locust plants that had formed an association with G. versiforme (Fig. 2f). After enzymatic hydrolysis, its fiber structure broke down because of the loss of cellulose and hemicelluloses (Fig. 2g–i). This destruction by delignification during aqueous ammonia pretreatment, which seemed to increase enzyme accessibility and enzymatic hydrolysis (Cha et al. 2014). Delignification after the aqueous ammonia pretreatment, the stems of black locust colonized by R. irregularis or G. versiforme had nearly the same lignin content as the non-mycorrhizal stems for the enzymatic hydrolysis.
FTIR Analysis
The FTIR spectra (Fig. 3) revealed changes in the chemical structure of the black locust by showing changes in the characteristic adsorption peaks of the chemical functional groups. Lignin is an aromatic polymer with four characteristic peaks at 1600, 1580, 1500, and 1450 cm–1. The alkyl C=O of the lignin side chain linking with an aromatic group has a peak at 1640 cm–1 and absorption bands at 1610 and 1516 cm–1 (Zhao et al. 2008; Gao et al. 2012). Guaiacal rings and syringyl rings are also important functional groups of lignin, with characteristic peaks at 1218, 1268 (C-O of guaiacal ring), and 1315 cm–1 (C-O of syringyl ring) (Hsu et al. 2010). The different intensity bands for all the lignin peaks demonstrated that R. irregularis and G. versiforme had an important influence on the lignin chemical structure.
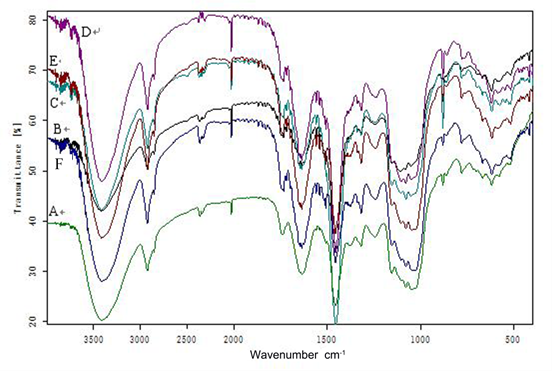
Fig. 3. Infrared spectroscopic analysis of black locust: A, B, and C are FTIR analyses of year-one non-mycorrhizal, Rhizophagus irregularis, or Glomus versiforme black locust, respectively; D, E, and F are FTIR analyses of year-two non-mycorrhizal, R. irregularis, and G. versiforme black locust, respectively
CONCLUSIONS
The presence of AM fungi enhanced the lignin and cellulose content of black locust; however, after aqueous ammonia pretreatment, AM fungi had no effect on the lignin and cellulose content.
AM fungi affected the structure of black locust stems and the chemical structure of lignin. Apart from the year-two R. irregularis treatment, the glucose yield (%) obtained from mycorrhizal black locust biomass was not significantly different from that obtained from non-mycorrhizal biomass.
Mycorrhizal black locust biomass was shown to be a suitable substrate for biofuel production.
ACKNOWLEDGEMENTS
We acknowledge the financial support of the National Key Research and Development Program of China (2018YFD0600203-3), the National Natural Science Foundation of China (41671268), and the Shaanxi Science and Technology Innovation Project Plan (2016KTCL02-07).
REFERENCES CITED
Alvira, P., Tomás-Pejó, E., Ballesteros, M., and Negro, M. J. (2010). “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review,” Bioresour. Technol. 101(13), 4851-4861. DOI: 10.1016/j.biortech.2009.11.093
Bailey, M. J., and Linko, M. (1990). “Production of β-galactosidase by Aspergillus oryzae in submerged bioreactor cultivation,” J. Biotechnol. 16(1), 57-66. DOI: 10.1351/pac198759020257
Balat, M. (2010). “Bio-oil production from pyrolysis of black locust (Robinia pseudoacacia) wood,” Energ. Explor. Exploit. 28(3), 173-186. DOI: 10.1260/0144-5987.28.3.173
Bals, B. D., Gunawan, C., Moore, J., Teymouri, F., and Dale, B. E. (2014). “Enzymatic hydrolysis of pelletized AFEX™-treated corn stover at high solid loadings,” Biotechnol. Bioeng. 111(2), 264-271. DOI: 10.1002/bit.25022
Bennett, A. E., Grussu, D., Kam, J., Caul, S., and Halpin, C. (2015). “Plant lignin content altered by soil microbial community,” New Phytol. 206(1), 166-174. DOI: 10.1111/nph.13171
Bongarten, B. C., Huber, D. A., and Apsley, D. K. (1992). “Environmental and genetic influences on short rotation biomass production of black locust (Robinia pseudoacacia L.) in the Georgia Piedmont,” Forest Ecol. Manag. 55(1-4), 315-331. DOI: 10.1016/0378-1127(92)90108-L
Burner, D., Carrier, D., Belesky, D., Pote, D., Ares, A., and Clausen, E. (2008). “Yield components and nutritive value of Robinia pseudoacacia and Albizia julibrissin in Arkansas, USA,” Agroforest. Syst. 72(1), 51-62. DOI: 10.1007/s10457-007-9098-x
Cha, Y. L., Yang, J., Ahn, J. W., Moon, Y. H., Yoon, Y. M., Yu, G. D., An J. H., and Choi I. H. (2014). “The optimized CO2-added ammonia explosion pretreatment for bioethanol production from rice straw,” Bioprocess Biosyst. Eng. 37(9), 1907-1915. DOI: 10.1007/s00449-014-1165-x
Gao, A. H., Bule, M. V., Laskar, D. D., and Chen, S. (2012). “Structural and thermal characterization of wheat straw pretreated with aqueous ammonia soaking,” J. Agr. Food Chem. 60(35), 8632-8639. DOI: 10.1021/jf301244m
Garlock, R. J., Wong, Y. S., Balan, V., and Dale, B. E. (2012). “AFEX pretreatment and enzymatic conversion of black locust (Robinia pseudoacacia L.) to soluble sugars,” Bioenerg. Res. 5(2), 306-318. DOI: 10.1007/s12155-011-9134-6
Gasol, C. M., Brun, F., Mosso, A., Rieradevall, J., and Gabarrell, X. (2010). “Economic assessment and comparison of acacia energy crop with annual traditional crops in Southern Europe,” Energ. Policy 38(1), 592-597. DOI: 10.1016/j.enpol.2009.10.011
Geyer, W. A., and Walawender, W. P. (1994). “Biomass properties and gasification behavior of young black locust,” Wood Fiber Sci. 26(3), 354-359
Ghose, T. K. (1987). “Measurement of cellulase activities,” Pure Appl. Chem. 59(2), 257-268
González-García, S., Gasol, C. M., Moreira, M. T., Gabarrell, X., Rieradevall, P. J., and Feijoo, G. (2011). “Environmental assessment of Acacia (Robinia pseudoacacia L.)-based ethanol as potential transport fuel,” Int. J. Life Cycle Ass. 16(5), 465-477. DOI: 10.1007/s11367-011-0272-z
González-García, S., Moreira, M. T., Feijoo, G., and Murphy, R. J. (2012). “Comparative life cycle assessment of ethanol production from fast-growing wood crops (black locust, eucalyptus and poplar),” Biomass Bioenerg. 39(1), 378-388. DOI: 10.1016/j.biombioe.2012.01.028
Gourlay, K., Hu, J., Arantes, V., Andberg, M., Saloheimo, M., Penttilä, M., and Saddler, J. (2013). “Swollenin aids in the amorphogenesis step during the enzymatic hydrolysis of pretreated biomass,” Bioresour. Technol. 142(4), 498-503. DOI: 10.1016/j.biortech.2013.05.053
Gruenewald, H., Brandt, B. K. V., Schneider, B. U., Bens, O., Kendzia, G., and Hüttl, R. F. (2007). “Agroforestry systems for the production of woody biomass for energy transformation purposes,” Ecol. Eng. 29(4), 319-328. DOI: 10.1016/j.ecoleng.2006.09.012
Grünewald, H., Böhm, C., Quinkenstein, A., Grundmann, P., Eberts, J., and von Wühlisch, G. (2009). “Robinia pseudoacacia L.: A lesser known tree species for biomass production,” Bioenerg. Res. 2(3), 123-133. DOI: 10.1007/s12155-009-9038-x
Hsu, T. C., Guo, G. L., Chen, W. H. and Hwang, W. S. (2010). “Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis,” Bioresource Technol. 101(13), 4907-4913. DOI: 10.1016/j.biortech.2009.10.009
Hu, J., Arantes, V., and Saddler, J. N. (2011). “The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: Is it an additive or synergistic effect?” Biotech. Biofuels 4(1), 1-14. DOI: 10.1186/1754-6834-4-36
Jia, L., Sun, Z., Ge, X., Xin, D., and Zhang, J. (2013). “Comparison of the delignifiability and hydrolysability of wheat straw and corn stover in aqueous ammonia pretreatment,” BioResources 8(3), 4505-4517. DOI: 10.15376/biores.8.3.4505-4517
Jung, Y. H., Kim, I. J., Han, J. I., Choi, I. G., and Kim, K. H. (2011). “Aqueous ammonia pretreatment of oil palm empty fruit bunches for ethanol production,” Bioresour. Technol. 102(20), 9806-9809. DOI: 10.1016/j.biortech.2011.07.050
Kim, J. W., Kim, K. S., Lee, J. S., Park, S. M., Cho, H. Y., Park, J. C., and Kim, J. S. (2011). “Two-stage pretreatment of rice straw using aqueous ammonia and dilute acid,” Bioresour. Technol. 102(19), 8992-8999. DOI: 10.1016/j.biortech.2011.06.068
Lin, L., Yan, R., Jiang, W., Shen, F., Zhang, X., Zhang, Y., Deng, S., and Li, Z. (2014). “Enhanced enzymatic hydrolysis of palm pressed fiber based on the three main components: Cellulose, hemicellulose, and lignin,” Appl. Biochem. Biotech. 73(2), 409-420. DOI: 10.1007/s12010-014-0848-8
Liu, T., Wang, C. Y., Chen, H., Fang, F. R., Zhu, X. Q., and Tang, M. (2014). “Effects of arbuscular mycorrhizal colonization on the biomass and bioenergy production of Populus × canadensis ‘Neva’ in sterilized and unsterilized soil,” Acta Physio. Plant. 36(4), 871-880. DOI: 10.1007/s11738-013-1465-9
Meng, X., and Ragauskas, A. J. (2014). “Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates,” Curr. Opin. Biotech. 27(6), 150-158. DOI: 10.1016/j.copbio.2014.01.014
Mészáros, E., Jakab, E., Várhegyi, G., Szepesváry, P., and Marosvölgyi, B. (2004). “Comparative study of the thermal behavior of wood and bark of young shoots obtained from an energy plantation,” J. Anal. Appl. Pyrol. 72(2), 317-328. DOI: 10.1016/j.jaap.2004.07.009
Miransari, M. (2011). “Arbuscular mycorrhizal fungi and nitrogen uptake,” Arch. Microbiol. 193(2), 77-81. DOI: 10.1007/s00203-010-0657-6
Monedero, E., Hernández, J. J., and Collado, R. (2017). “Combustion-related properties of poplar, willow and black locust to be used as fuels in power plants,” Energies 10(7), 997. DOI: 10.3390/en10070997
Qing, Q., and Wyman, C. E. (2011). “Supplementation with xylanase and β-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover,” Biotechnol. Biofuels 4(1), 18. DOI: 10.1186/1754-6834-4-18
Serapiglia, M., Cameron, K., Stipanovic, A., and Smart, L. (2009). “Analysis of biomass composition using high-resolution thermogravimetric analysis and percent bark content for the selection of shrub willow bioenergy crop varieties,” Bioenerg. Res. 2(1-2), 1-9. DOI: 10.1007/s12155-008-9028-4
Sheng, M., Tang, M., Zhang, F. F., and Huang, Y. H. (2011). “Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress,” Mycorrhiza 21(5), 423-430. DOI: 10.1007/s00572-010-0353-z
Sluiter, J. B., Ruiz, R. O., Scarlata, C. J., Sluiter, A. D., and Templeton, D. W. (2010). “Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods,” J. Agr. Food Chem. 58(16), 9043-9053. DOI: 10.1021/jf1008023
Smith, S. E., and Read, D. J. (2008). “Arbuscular mycorrhizas,” in: S. E. Smith and D. J. Read, Mycorrhizal Symbiosis, 3rd Ed., Academic Press, New York, pp. 31-134.
Sousa, L. C., Jin, M., Chundawat, S. P. S, Bokade, V., Tang, X., Azarpira, A., et al. (2016). “Next-generation ammonia pretreatment enhances cellulosic biofuel production,” Energy Environ. Sci. 9(4), 1215-1223. DOI: 10.1039/c5ee03051j
Tharakan, P. J., Volk, T. A., Abrahamson, L. P., and White, E. H. (2003). “Energy feedstock characteristics of willow and hybrid poplar clones at harvest age,” Biomass Bioenerg. 25(6), 571-580. DOI: 10.1016/S0961-9534(03)00054-0
Torget, R., Walter, P., Himmel, M., and Grohmann, K. (1991). “Dilute-acid pretreatment of corn residues and short-rotation woody crops,” Appl. Biochem. Biotechnol. 28(1), 75-86. DOI: 10.1007/BF02922590
Zeng, Y., Zhao, S., Yang, S., and Ding, S. Y. (2014). “Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels,” Curr. Opin. Biotechnol. 27(6), 38-45. DOI: 10.1016/j.copbio.2013.09.008
Zhang, Q., Gao, X., Ren, Y., Ding, X., Qiu, J., Li, N., Zeng, F., and Chu. Z. (2018). “Improvement of Verticillium wilt resistance by applying arbuscular mycorrhizal fungi to a cotton variety with high symbiotic efficiency under field conditions,” Int. J. Mol. Sci. 19(1), 241. DOI: 10.3390/ijms19010241
Zhao, X., Wu, R., and Liu, D. (2011). “Production of pulp, ethanol and lignin from sugarcane bagasse by alkali-peracetic acid delignification,” Biomass Bioenerg. 35(7), 2874-2882. DOI: 10.1016/j.biombioe.2011.03.033
Zhu, X. Q., Wang, C. Y., Chen, H., and Tang, M. (2014). “Effects of arbuscular mycorrhizal fungi on photosynthesis and energy content of black locust seedlings,” Photosynthetica 52(2), 247-252. DOI: 10.1007/s11099-014-0031-z
Zhu, X. Q., Tang, M., and Zhang, H. Q. (2017). “Arbuscular mycorrhizal fungi enhanced the growth, photosynthesis, and calorific value of black locust under salt stress,” Photosynthetica 55(2), 378-385. DOI: 10.1007/s11099-017-0662-y
Article submitted: April 9, 2018; Peer review completed: July 7, 2018; Revised version received and accepted: November 30, 2018; Published: December 12, 2018.
DOI: 10.15376/biores.14.1.965-976
