Abstract
A variety of water-retaining hydrogels possessing macroporous interiors resembling a honeycomb framework were developed by free radical polymerization of acrylamide (AM), starch, and TEMPO-oxidized nanofibrillated cellulose (TONFC) (Poly (AM-co-TONFC/Starch) (PATS) hydrogels). With the help of ultrasound, nano precipitated calcium carbonate (NPCC) was gelatinized in the preformed NPCC suspension in a monomer solution that forms a hydrogel. When NPCC was dispersed in water, its surface was positively charged, and NPCC could be used as a condensation center to form hydrogen bonds with AM, TONFC, and starch, shortening the distance between AM, TONFC, and starch, increasing the pore size, and thinning the pore wall. The uniform dispersion of NPCC in hydrogels could also promote a more uniform degree of cross-linking in hydrogels. When the hydrogels was extruded and deformed by external forces, the stress of hydrogels was more uniform, so that they could withstand more forces without collapsing. When 4% NPCC was added (relative to starch and TONFC), the pore size of PATS2 hydrogel was uniformly distributed in the range of 10 to 20 μm. The maximum tensile strength of PATS2 hydrogel reached 84.8 KPa, and the elongation at break was 399%. The water absorption reached 172.3, and 65% water content was maintained after 720 h.
Download PDF
Full Article
Effect of Nano Precipitated Calcium Carbonate on the Properties of Hydrogels Prepared with Acrylamide, Starch, and TEMPO-oxidized Nanocellulose
Tianjiao He, Guolin Tong,* Penghui Li, Chen Miao, Xin Zhang, and Xuewen Xu
A variety of water-retaining hydrogels possessing macroporous interiors resembling a honeycomb framework were developed by free radical polymerization of acrylamide (AM), starch, and TEMPO-oxidized nanofibrillated cellulose (TONFC) (Poly (AM-co-TONFC/Starch) (PATS) hydrogels). With the help of ultrasound, nano precipitated calcium carbonate (NPCC) was gelatinized in the preformed NPCC suspension in a monomer solution that forms a hydrogel. When NPCC was dispersed in water, its surface was positively charged, and NPCC could be used as a condensation center to form hydrogen bonds with AM, TONFC, and starch, shortening the distance between AM, TONFC, and starch, increasing the pore size, and thinning the pore wall. The uniform dispersion of NPCC in hydrogels could also promote a more uniform degree of cross-linking in hydrogels. When the hydrogels was extruded and deformed by external forces, the stress of hydrogels was more uniform, so that they could withstand more forces without collapsing. When 4% NPCC was added (relative to starch and TONFC), the pore size of PATS2 hydrogel was uniformly distributed in the range of 10 to 20 μm. The maximum tensile strength of PATS2 hydrogel reached 84.8 KPa, and the elongation at break was 399%. The water absorption reached 172.3, and 65% water content was maintained after 720 h.
DOI: 10.15376/biores.17.3.5079-5094
Keywords: Water-retaining hydrogel; Nano precipitated calcium carbonate; TONFC; Cassava starch
Contact information: College of Light Industry and Food Engineering, Nanjing Forestry University, Longpan Road 159, Nanjing 210037, China; *Corresponding author: gtong@njfu.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
Nano-composite hydrogels can be prepared by combining inorganic and organic nanoparticles with hydrogels, which can improve the strength and other related properties of hydrogels. A wide range of nanoparticles, such as carbon-based, polymeric, ceramic, and metallic nanomaterials can be integrated within the hydrogel networks to obtain nanocomposites with superior properties and tailored functionality. Nanocomposite hydrogels can be engineered to possess superior physical, chemical, electrical, and biological properties (Gaharwar et al. 2014). The nanoparticles in hydrogels are often in the form of physical entanglement or hydrogen bonding cross-linking, either by encapsulation or by hydrogen bonding to the hydrogel (Goenka et al. 2014).
The structural combination of polymer hydrogel networks and nanoparticles can provide superior properties for composite materials in a variety of applications, including catalysis, electronics, biosensing, drug delivery, nanomedicine, and agricultural horticulture. Such blending may result in synergistic performance enhancement of the components, such as mechanical strength of the hydrogel and simultaneous reduction of nanoparticle aggregation (Thoniyot et al. 2015). The excellent properties such as rigidity, small size effect, and thermal stability of nanomaterials can impart favorable characteristics to the hydrogels; thus, the mechanical properties and swelling properties of hydrogels could be increased (Xia et al. 2010). For example, hydrogel composites made from silica nanoparticles and modified polyethylene glycol showed significant increases in mechanical strength and bioactivity, compared to hydrogels without nanoparticles (Skelton et al. 2013; Liu et al. 2014). When gold nanoparticles are introduced into a poly-N-isopropylamide gel system, the mechanical properties and thermal response change (Marcelo et al. 2014). Chitosan hydrogel containing copper nanoparticles has been applied to plants to accelerate the growth rate of tomato fruit and improve the quality of fruit (Antonio et al. 2016).
Nanocellulose has the advantages of large specific surface area, high strength, good hydrophilicity, and high surface activity, which can be effectively applied in hydrogels and provide excellent performance for hydrogels (Wang et al. 2012). When 10% CNF was added to a polyacrylamide/acrylic acid hydrogel network, the mechanical strength was approximately 13 times that of P(AM/AA) hydrogel, and the elastic modulus increased nearly 10 times (Okumura and Ito 2001). A biodegradable hydrogel microsphere has been prepared by using porous collagen and cellulose nanofibres, which contain basic fibroblast growth factor for long-term release and thus promotes angiogenesis (Li et al. 2014). Graft polymerization of TEMPO-oxidised nanofibres and N-isopropylacrylamide results in a composite hydrogel with both thermal and pH-responsive properties, which could be applied for drug release (Masruchin et al. 2018). The addition of nanocellulose thus can improve the mechanical strength and enhance the biodegradability of the hydrogel.
The preparation of nanocellulose is complex and expensive, so its wide application in daily life is limited. As an active precursor of hydrogel preparation, starch with low price and easy preparation has attracted much attention due to its rich hydroxyl group and good crosslinking performance (Sun et al. 2012; Lima-Tenorio et al. 2015; Banerjee et al. 2019). Because of its poor mechanical properties, it has not been fully explored in material science (Dragan 2014). Starch granules are an active ingredient that can transform polyacrylamide/alginate hydrogel matrices into tissue-like materials with a variety of relevant functions (Fang et al. 2020). At the same time, starch is a suitable choice of potential self-healing enhancers. Cassava starch has been introduced into polyacrylamide (PAA) and 2-propyl amide-2-methyl-1-propane sulfonic acid (AMPS) to prepare self-healing double-network hydrogels with self-healing properties (Shang et al. 2021).
Starch and nanocellulose were used in this work to prepare hydrogels, and NPCC nanoparticles were introduced into the hydrogels to take advantage of their small size effect, surface effect, and hydrophilicity. Using ultrasound, NPCC was combined with AM, TONFC, and starch as a hydrogel network by blend polymerization (Thoniyot et al. 2015), so that NPCC was evenly dispersed in the hydrogel network. Three groups of hydrogels were prepared: poly-(AM-co-TONFC) (PAT), poly-(AM-co-Starch) (PAS), and poly-(AM-co-TONFC/Starch) (PATS) hydrogels. The properties of these three hydrogels were investigated to explore the effect of NPCC. The microstructure, thermal properties, mechanical properties, rheological properties, water absorption, and water retention of hydrogels were investigated in this work. The optimum proportion of NPCC was explored to prepare NPCC-hydrogel composites with certain mechanical strength and excellent water retention. The investigated hydrogels can be used in agriculture and horticulture as a water slow-release materials, which can be used to reduce the acidity of soil.
EXPERIMENTAL
Materials
Nano precipitated calcium carbonate (NPCC) was purchased from Jiangsu Xianfeng Nanomaterials Technology Co Ltd. Acrylamide was purchased from Shanghai Lingfeng Chemical Reagent Co Ltd. N-N’ methylene bisacrylamide was purchased from Sinopharm Chemical Reagent Co Ltd. Ammonium persulfate was purchased from Nanjing Chemical Reagent Co., Ltd. Cassava starch was from Yuanye Biotechnology Co. TEMPO oxidation of nanocellulose was performed in the lab, with a carboxyl content of 1.851 mmol/g.
Methods
Synthesis of hydrogels
Free radical polymerization was performed on cassava starch (ST), TEMPO-oxidized nanocellulose (TONFC), a 1:1 mixture of cassava starch and TONFC (TONFC/ST), and acrylamide (AM). The amount of crosslinking agent and initiating additive was not changed; only the amount of NPCC was changed. Three hydrogels with different monomer ratios (PAS hydrogels, PAT hydrogels, and PATS hydrogels) were obtained. Briefly, 1 g of starch was gelatinized in water. A suspension of well-dispersed NPCC (0%, 2%, 4%, 6%, 8%, and 10% relative to the starch mass) was added, and then 4 g of acrylamide, 0.06 g of N, N’ methylene bisacrylamide, and 0.03 g of ammonium persulphate were added to the mixture. Distilled water was added to 100 g. The mixture was stirred for 5 min, and then ultrasonic treatment was conducted with the probe ultrasonic cell crusher of Fangxu Technology, Shanghai. The probe of ultrasonic cell crusher was placed in the mixture and dispersed by ultrasound effect for 5 min, so that the components were evenly mixed. At this time, the hydrogel had not yet formed, the probe was removed, and the container was paced with the mixture into another, larger container with water. Then the probe was placed in the water, across the water, the mixture was subjected to ultrasound across the container, without damaging the shape of the hydrogel. The crude hydrogel products could be obtained by continuous ultrasound for 15 min. The resulting crude hydrogel product was immersed in distilled water for three consecutive days to remove unreacted water-soluble components, with water changes every hour on the first day and twice daily on the second two days. The hydrogels were placed in air for 24 h to equilibrate to constant weight and placed in a prepared ethanol solution (80% v/v) for 6 h to obtain the final hydrogel product. PAT hydrogels and PATS hydrogels were synthesized similarly. The water temperature was monitored during the ultrasonic process to prevent excessive gelatinization of cassava starch due to high temperature. The sonication power was 85 W. Taking PATS hydrogels as an example, the NPCC additions were varied, and the reformed hydrogels were named PATS0, PATS2, PATS4, PATS6, PATS8, and PATS10, where the numbers indicated the mass percentage of NPCC in TONFC and Starch.
Morphology of NPCC
NPCC was placed in an oven at 105 °C for 6 h to dry. A JSM-7600f field-emission electron microscope (Japan Electronics Co., Ltd., Tokyo, Japan) was used to characterize the morphology and particle size distribution of NPCC. The voltage was set at 1 to 3 kV, and the working distance was 5 to 8 mm.
Morphology of hydrogels
The hydrogel samples were cut to a suitable size, frozen in liquid nitrogen, and freeze-dried using an SCIE-NTZ-12N freeze-dryer (Xinzhi Bio, Ningbo, China) for 24 h. The freeze-dried samples were stored in a vacuum oven. Samples were glued to the metal sample stage by double-sided tape to ensure a flat shooting surface, gold sprayed, and then observed under a Quanta 200 environmental scanning electron microscope (FEI, Hillsboro, OR, USA).
Elemental analysis (EDS)
Hydrogels were freeze-dried to obtain dry gels. Elemental analysis was carried out using a 2400 II Energy Dispersive Spectrometer (FEI, Hillsboro, OR, USA) to observe the distribution of calcium elements in the gel skeleton.
Thermogravimetric analysis (TG)
The dry gels were tested and recorded at different temperatures using a TGA209 F1 thermogravimetric analyzer (NETZSCH, Selb, Germany). The heating conditions were: 25 to 600 °C in an N2 environment, with a temperature rise rate of 10 °C/min and a gas flow rate of 20 mL/min.
Rheological properties
The hydrogels were prepared into cylindrical shapes of 10 mm diameter and 5 mm thickness. The rheological properties of the hydrogels were tested using a 20 mm diameter vertebral plate rotor using a MARS60 micro infrared coupled rheometer (Thermo Fisher, Waltham, MA, USA). The test was performed at 25 °C with a strain scan of the sample, selected shear stress of 5 Pa in the linear viscoelastic region, and an oscillation frequency f of 0.1 to 5 Hz, followed by an oscillation frequency scan to obtain the viscoelastic change curve.
Tensile properties
The hydrogel samples were prepared into rectangular shapes of 60 mm in length, 10 mm in width, and 1 mm in thickness. Tensile strength and elongation at break were obtained by using an SLBL 500N electronic universal testing machine (Shimadzu, Osaka, Japan) at a speed of 20 mm/min until the hydrogel was pulled apart. Five sets of parallel experiments were carried out for each sample.
Water retention properties
Cylindrical hydrogel samples of 20 mm in length and 20 mm in diameter were freeze-dried, and the initial mass of the dried hydrogel was recorded as Wd, after which the dried gel was immersed in water at a temperature of 25 ℃. After each predetermined time, the gel was removed from the water and its weight was recorded. The weight of the gel was recorded as Wt after it reached equilibrium weight. The water floating on the surface of the gel was dried with filter paper before weighing. The water absorption, WU, was used as a measure of the swelling properties of the hydrogel as shown in Eq. 1.
(1)
The hydrogels were subjected to a dehydration test in a constant environment. The samples were immersed in a mixture of glycerol and water (1:1) for two h, wiped dry of the surface solution, and with the weight recorded as W0. They were then placed at a temperature of 25 ℃ and a humidity of 50 and their mass, recorded as W, was recorded after each predetermined time.
The water content WR was used as a measure of the hydrogel’s ability to lock in water as shown in Eq. 2.
RESULTS AND DISCUSSION
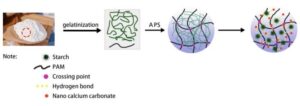
Figure 1 shows the synthesis of PAS hydrogels. During the gelatinization process, the starch absorbs water and expands, and the internal crystallization area changes from a close arrangement to a loose state. The starch particles are gradually destroyed, and the molecular chain is completely extended. In the presence of ammonium persulfate (APS), starch was oxidized and generated free radicals, which were polymerized with acrylamide (AM). In the presence of N,N’ methylene diacrylamide (MBA), the hydrogel system with a three-dimensional network structure was formed by chemical cross-linking with a covalent bond. Under the action of ultrasound, NPCC could be evenly dispersed in the hydrogel system, and due to its high hydrophilicity, it was easy to form hydrogen bonds with water, hydroxyl, and carboxyl groups. The synthesis of PAT hydrogels and PATS hydrogels also used the same mechanism, but the crosslinking strength was different due to the different monomer feeding ratios.
Fig. 1. Synthesis of PAS hydrogels
Morphology of Hydrogels
Porosity is a distinguishing feature of hydrogels. Porosity, pore size, and pore interconnectivity play an important role in the direct function of biomaterial scaffolds (Zhao et al. 2015). The porosity and pore size of hydrogels can be adjusted by varying different parameters, such as temperature, polymer or monomer concentration, cross-linking, freezing rate, organic solvent, or salt addition (Dinu et al. 2007; Savina et al. 2016). This study showed that the pore size of the hydrogels was altered by the addition of NPCC.
Hydrogels were freeze-dried to obtain dry gels, which were used to observe their internal microstructure. Figures 2a and 2b showed the cross-sections of PAT0 and PAT10, respectively. Due to its high specific surface area and aspect ratio, TONFC could be used as a chemical crosslinking point during hydrogel formation to improve the crosslinking density with polymer molecules. The pore wall of PAT0 hydrogel without NPCC was thick, the pore size was about 5 to 30 μm, the pore shape was irregular, the pore distribution was uneven, some parts had high porosity, and some parts had no pores. However, the PAT10 hydrogel presented a relatively uniform honeycomb structure with a thin pore wall and a relatively regular cavity shape.
Figures 3a and 3b showed cross-sections of PAS0 and PAS10, respectively. The pore size of PAS0 hydrogels ranged from 10 to 80 μm, with larger pores in some places and relatively non-porous in others, and an overall lower porosity, along with thicker pore walls. The pore size of the PAS10 hydrogel was 30 to 50 μm, and the pores were extremely well distributed and honeycomb-like in structure, with substantially thinner pore walls compared to the PAS0 hydrogel, thus presumably leading to a reduction in hydrogel strength.
Fig. 2. SEM micrographs of PAT hydrogels; (a) cross-section of PAT0 at 120X magnification; (b) cross-section of PAT10 at 120X magnification
Fig. 3. SEM micrographs of PAS hydrogels; (a) cross-section of PAS0 at 120X magnification; (b) cross-section of PAS10 at 120X magnification
In this group of hydrogels, the effect of different NPCC additions on the microscopic morphology of PAST hydrogels was explored. As shown in Fig. 4, the pore sizes of PATS hydrogels were between those of PAT and PAS hydrogels. The internal pore sizes of PATS0 hydrogel had a wide range of pore size distribution and became significantly larger with the addition of NPCC. The pore size of PATS2 hydrogel was 5 to 20 μm with a relatively higher uniformity of pore distribution. The pore size of PAST4 hydrogel was 20 to 30 μm and the pore size was relatively uniform. The pore size of PAST6 hydrogel reached 40 to 50 μm. When the NPCC addition reached 10%, the pore size of PAST10 hydrogel reached 60 μm. The pore wall became extremely thin, but the uniformity of pore distribution was further improved. As the pores become larger, the pore interoperability within the hydrogel could be more clearly seen.
Comparing Figs. 2, 3, and 4, the addition of NPCC affected the microscopic morphology of all three hydrogels. The pore size of the PAT hydrogels was smaller than that of the PAS hydrogels due to the higher chemical cross-linking between TONFC and acrylamide molecular chains.
Fig. 4. SEM micrographs of PATS hydrogels; (a) cross-section of PATS0 at 120X magnification; (b) cross-section of PATS2 at 120X magnification; (c) cross-section of PATS4 at 120X magnification; (d) cross-section of PATS6 at 120X magnification; (e) cross-section of PATS8 at 120X magnification; (f) cross-section of PATS10 at 120X magnification.
Fiber Morphology
As inorganic nanoparticles, calcium carbonate had no active group on its surface, so it could not form chemical crosslinking with polymer materials. However, it had a great influence on hydrogel morphology. It was speculated that the atoms on the surface of CaCO3 particles were easy to coordinate with ions in water due to their unsaturated chemical bonds, and the surface Ca atoms existed in the form of Ca-OH. Therefore, NPCC was easy to form hydrogen bonds with starch, TONFC, and acrylamide molecular chains. NPCC became a condensation center and brought the molecular chains closer together.
To test this conjecture, the distribution of NPCC in the hydrogel system need to be further explored to understand the link between the variation of NPCC content and pore wall thickness. Therefore, in this study, an energy dispersive spectrometer from FEI, USA, was used to perform dot scanning of the dry gels to observe the distribution characteristics of calcium elements in the gel skeleton. Due to the three-dimensional porous structure of the hydrogel, it was not possible to perform a large area scan to determine the elemental distribution, so multiple small areas of one of the cavity walls of the gel were selected for framing under a high magnification electron microscope, and a small area of punctured elemental analysis was performed to observe the difference in NPCC content in the thinner and thicker areas of the pore walls. Multiple cavity walls were selected in different areas for spot elemental analysis to obtain the distribution pattern of NPCC.
Figure 5 shows the electron micrograph of a PAT2 hydrogel. A region was boxed for one of the cavity walls and a range of 10 regions was selected, where Spectrum 1, 4, 7, and 10 were cross-linking points between the pores and the pore walls were thicker compared to the other regions. Table 1 shows the elemental Ca content of the selected 10 regions.
Fig. 5. ESEM micrographs of PAT2 hydrogel
Table 1. Elemental Analysis of PAT2 Hydrogel
Combined with Fig. 5 and Table 1, the 1, 4, 7, and 10 areas had thicker pore walls and larger areas. The same areas also had lower elemental calcium content, all less than 0.3%. Area 1 had the largest area and the thickest pore walls, and the lowest elemental calcium content, only 0.21%. In contrast, the calcium content of the thinner pore wall areas was relatively higher, all above 0.35%.
The elemental analysis of different areas of the hydrogel with different pore walls was repeatedly punctured, all showing a similar pattern to the above. In the hydrogel skeleton, the thinner the pore wall, the more NPCC was distributed in the region, while the pore cross-linking point and the region with thicker pore walls had less NPCC. This study demonstrated that the presence of NPCC as a cohesive center in the hydrogel system could reduce the distance between the molecular chains of starch, TONFC, and acrylamide, thus making the pore walls of the gel thinner. With the increase of NPCC, the pore walls became thinner and the pore size became larger, while the ultrasound effect made the NPCC uniformly distributed in the hydrogel, to make the pore distribution more uniform.
Thermal Stability
The thermal stability of hydrogels depends to a large extent on the matrix structure and how the polymer is molded; it is more influential than the thermal stability of the polymer itself (Liu and Cui 2011). Because the amount of monomer, crosslinker, and initiator was not changed and only the amount of NPCC was changed, the TG curves of the six hydrogel samples in this group were obtained by thermogravimetric analysis using PATS hydrogels as an example. The TG curves of PATS hydrogels are shown in Fig. 6.
Fig. 6. TG curves of PATS hydrogels
The starting decomposition temperature of PATS hydrogels was in the vicinity of 280 ℃, and the maximum weight loss rate temperature was all-around 380 ℃. Due to the little addition of NPCC, the starting decomposition temperature and the maximum always rate temperature of the hydrogels were not significantly affected. This result indicates that the connection between the molecular chains of NPCC, starch, and TONFC was a physical interaction without the formation of covalent bonds, and therefore it had almost no effect on the thermal decomposition temperature of the hydrogels.
Rheological Properties
The viscoelastic change curves of the three groups of hydrogels are shown in Fig. 7. As the oscillation frequency increased, the energy storage modulus (G’) of the hydrogels prepared with different NPCC additions was greater than the energy dissipation modulus (G”), indicating that the hydrogel system was a gel network with dominant elastic behavior.
Fig. 7. Viscoelastic curves of PAT hydrogels, PAS hydrogels, and PATS hydrogels
Among the three groups of hydrogels, the elastic modulus of PAT hydrogel was the highest, followed by PATS hydrogel, and PAS hydrogel was the lowest. With the increase of NPCC addition, the elastic modulus of all three groups of hydrogels showed a trend of first increasing and then decreasing, and the elastic modulus reached the highest when the addition of NPCC reached 4% and began to decrease when it was higher than 4%. However, the addition of NPCC increased the elastic modulus of PAS hydrogel the most and had relatively less effect on PAT and PATS hydrogels. When 4% NPCC was added, the elastic modulus of PAS2 hydrogel increased by 103.0% compared to PAS0, while PAT2 and PATS2 only increased by 34.2% and 35.8%, respectively. The modulus of elasticity of PAS0 hydrogel was 136.7 Pa, PAT0 hydrogel was 464 Pa, and PATS0 hydrogel was 352 Pa. It could be seen that the modulus of elasticity was greatly improved when the nanocellulose replaced part of the starch. The modulus of elasticity of PATS2 hydrogel was 477 Pa, which was slightly higher than that of PAT0 hydrogel, indicating that the addition of this part of NPCC could compensate for the difference in starch and TONFC on the elastic modulus of hydrogel.
Tensile Properties
The mechanical strength of macroporous materials depends mainly on the composition and structure of the hydrogel (Sood et al. 2016). The degree of swelling, the composition of the copolymer monomer, the polymerization conditions, and the crosslinking density are important parameters that influence the mechanical properties of the hydrogel (Argun et al. 2014). The structural combination of NPCC and hydrogels could improve the mechanical strength of hydrogels. The tensile properties of hydrogels could reflect their toughness, so the tensile properties of composite hydrogels obtained from three groups of PAT, PAS, and PATS hydrogels, prepared at different NPCC additions were explored (Fig. 8).
Fig. 8. Tensile stress-strain curves of different hydrogels; (a) PAT hydrogels; (b) PAS hydrogels; (c) PATS hydrogels
When 4% NPCC was added, the tensile strength of hydrogel was the highest, while the elongation at break decreased with the increase of NPCC. The results showed that the proper addition of NPCC could improve the homogeneity of crosslinking density of hydrogel so that the hydrogel could disperse the stress more evenly when it was stretched by an external force, thus effectively improving the tensile properties of the hydrogel. Excessive addition of NPCC led to an increase in brittleness and a decrease in the toughness of hydrogel, which led to a decrease of tensile strength and elongation at break.
The tensile strength of the three groups of hydrogels was highest for PAT hydrogels, followed by PATS hydrogels, and lowest for PAS hydrogels. However, the trend of elongation at break was the opposite. The addition of NPCC improved the tensile strength of PAS hydrogels to a greater extent and had relatively less effect on PAT hydrogels. 74.7 kPa was the maximum tensile strength of PAT0 hydrogels and 270% elongation at break, while 24.8 kPa was the maximum tensile strength of PAS0 hydrogels and 648% elongation at break. The maximum tensile strength of PATS0 hydrogel was 55.1 kPa when half of the starch was replaced by TONFC, which was a great improvement compared to PAS. When 4% NPCC was added, the tensile strength of PAST4 reached 84.8 kPa and the elongation at break reached 399%, which was better than PAT0 hydrogel.
Water Retention Properties
When the porous material comes into contact with solvent molecules (usual water), the polymer molecular chains swell to a full solvents state and the cross-linked structure exerts a force in the opposite direction, pulling the polymer molecular chains back until the swelling and contraction forces cancel each other out and swelling equilibrium is achieved. During the swelling process, the network pores are rapidly filled with solvent, while the polymeric regions of the hydrogel absorb solvent molecules from the environment, the amount of absorption depending on the affinity of the solvent molecules for the polymer chains (Bilici et al. 2010).
Fig. 9. (a) The water uptake of PAT, PAS and PATS hydrogels; (b) The water retention of PAS0, PAT0, PATS0, PATS2 hydrogels
As shown in Fig. 9a, the water uptake of PAS hydrogels was higher than that of PAT hydrogels, while the water uptake of PATS hydrogel was between the two. With the increase of NPCC, the water absorption showed a trend of rapid increase at first and then a slow decline. From 0% to 4%, the water absorption increased rapidly, and when the addition amount further increased, the cross-linking mechanism of hydrogel was interfered with. The water absorption gradually showed a gentle downward trend. Because with the addition of NPCC, the pore size became larger, the pore distribution was more uniform, the porosity increased, and the water content increased, while when the NPCC was too much, the pore size became too large, and the pore wall became thinner, which led to the toughness of the hydrogels decreased, resulting in a decrease in the water absorption properties of the hydrogels.
The water uptake ratio of PAT0 hydrogel was 97.7, and that of PAS0 hydrogel was 111.3, and when a part of starch replaced nanocellulose, the water uptake of PAT0 hydrogel was between the two, adding 4% NPCC, the water absorption of PATS2 hydrogel increased to 172.34.
As shown in Fig. 9b, the water retention of PAS hydrogel was much lower than that of PAT hydrogel. The crosslinking strength between TONFC and acrylamide was higher than that of starch, so even though the water absorption of PAS hydrogel was higher, its water loss rate was faster. In contrast, PAT hydrogels had lower water absorption and slower water loss rates. While the PATS hydrogel combined TONFC and ST, under the addition of NPCC, the prepared PATS2 hydrogel combined the advantages of both, with both high water absorption and high water retention.
Under the constant environment of 25 ℃ and 50 humidity, four groups of hydrogels, PAS0, PAT0, PATS0, and PATS2, were selected to verify the influence of NPCC on the water retention of hydrogels. As shown in Figure 9b, the water retention of PAS0 hydrogel was much lower than that of PAT0 hydrogel. The cross-linking strength between TONFC and acrylamide was higher than that of starch, so PAS hydrogels lost water faster even though they absorbed more water. PAT hydrogels, by contrast, absorbed less water and lost it more slowly. When the time reached 240 h, the water retention rate of PAS0 hydrogel was only 37.2%, and that of PAT0 hydrogel was 56.0%. However, the PATS hydrogel combined with TONFC and ST combined the advantages of the two hydrogels under the addition of 4% NPCC. After 720 h, the PATS4 hydrogel could still reach 65.5% water retention, with both high water absorption capacity and high water retention.
- The moderate addition of nano precipitated calcium carbonate (NPCC) improved the uniformity of hydrogel crosslinking, so that hydrogel could disperse stress more evenly when subjected to external forces, so its mechanical properties were improved. However, when the amount of NPCC was too much, the pore size was too large, the pore wall was too thin, the brittleness of hydrogel was increased, and its mechanical properties declined rapidly. When the NPCC content was 4% (relative to the mass percentage of starch and TEMPO-oxidized nanofibrillated cellulose (TONFC), the hydrogels had the best mechanical properties.
- The moderate addition of NPCC increased the pore size, porosity, and cross-linking uniformity of hydrogels, thus improving the water absorption properties of hydrogels. In addition, due to the differences in crosslinking strength between starch, TONFC, and acrylamide (AM), poly-AM-co-starch (PAS) hydrogels showed a trend of high water absorption but poor water retention, while poly-AM-TONFC (PAT) hydrogels showed a slightly lower water absorption but good water retention. Therefore, when the content of NPCC was 4% (relative to the mass percentage of starch and TONFC), PATS hydrogels combined with starch and TONFC had a high water absorption and water retention.
- Starch had the advantage of low cost, PAS hydrogel had higher water absorption but lower mechanical strength; The preparation of TONFC was relatively more complicated and expensive, but the mechanical properties of PAS hydrogels were better. PATS hydrogel combining starch and TONFC, after adding 4%NPCC, the PATS4 hydrogel obtained, not only surpassed PAT0 hydrogel in mechanical properties and water retention but also surpassed PAS0 hydrogel in water absorption and elongation at break. Especially compared with PAT0 hydrogel, the addition of starch reduced the cost, while the addition of a small amount of NPCC also improved the performance. PATS4 hydrogel, with good mechanical properties, excellent water retention, and low cost, could be widely used in agriculture and horticulture as a sustained-release material.
- NPCC in composite hydrogels is mostly hydrogen bond bonding and physical entanglements, without forming covalent bonds. The authors guess that, as a coagulation center, NPCC could shorten the distance between starch, TONFC, and acrylamide molecular chains, thus thinning the pore wall and enlarging the pore size of the hydrogel. Therefore the pore distribution was more uniform.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and National Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07402004).
REFERENCES CITED
Antonio, J. M., Hortensia, O. O., Fabian, P. L., Gregorio, C. P., and Adalberto, B. M. (2016). “Cu nanoparticles absorbed on chitosan hydrogels positively alter morphological, production and quality characteristics of tomato,” Journal of Applied Botany and Food Quality 89, 183-189. DOI: 10.5073/JABFQ.2016.089.023
Argun, A., Can, V., Altun, U., and Okay, O. (2014). “Nonionic double and triple network hydrogels of high mechanical strength,” Macromolecules 47, 6430-6440. DOI: 10.1021/ma5014176
Banerjee, S. L., Swift, T., Hoskins, R., Rimmer, S., and Singha, N. K. (2019). “A muscle mimetic polyelectrolyte-nanoclay organic-inorganic hybrid hydrogel: Its self- healing, shape-memory and actuation properties,” Journal of Materials Chemistry B 7 (9), 1475-1493. DOI: 10.1039/C8TB02852D
Bilici, C., Karayel, S., Demir, T. T., and Okay, O. O. (2010). “Self-oscillating pH-responsive cryogels as possible candidates of soft materials for generating mechanical energy,” Journal of Applied Polymer Science 118, 2981-2988. DOI: 10.1002/app.32693
Dinu, M. V., Ozmen, M. M., Dragan, E. S., and Okay, O. (2007). “Freezing as a path to build macroporous structures: Superfast responsive polyacrylamide hydrogels,” Polymer 48, 195-204. DOI: 10.1016/j.polymer.2006.11.022
Dragan, E. S. (2014). “Design and applications of interpenetrating polymer network hydrogels. A review,” Chemical Engineering Journal 243, 572-590. DOI: 10.1016/j.cej.2014.01.065
Fang, Y., Han, E., Zhang, X. X., Jiang, Y., and Tian, B. (2020). “Dynamic and programmable cellular-scale granules enable tissue-like materials,” Matter 2(4), 948-964. DOI: 10.1016/j.matt.2020.01.008
Gaharwar, A. K., Peppas, N. A., and Khademhosseini, A. (2014). “Nanocomposite hydrogels for biomedical applications,” Biotechnology and Bioengineering 111(3). DOI: 10.1002/bit.25160
Goenka, S., Sant, V., and Sant, S. (2014). “Graphene-based nanomaterials for drug delivery and tissue engineering,” Journal of Control Release 173(1), 75-88. DOI: 10.1016/j.jconrel.2013.10.017
Li, W., Lan, Y., Guo, R., Zhang, Y., Xue, W., and Zhang, Y. (2014). “In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor,” Journal of Biomaterials Applications 29(6), 882-893. DOI: 10.1177/0885328214547091
Lima-Tenorio, M. K., Tenorio-Neto, E. T., Guilherme, M. R., Garcia, F. P., Nakamura, C. V., Pineda, E. A. G., and Rubira, A. F. (2015). “Water transport properties through starch-based hydrogel nanocomposites responding to both pH and a remote magnetic field,” Chemical Engineering Journal 259, 620-629. DOI: 10.1016/j.cej.2014.08.045
Liu, Y., and Cui, Y. (2011). “Preparation and properties of temperature-sensitive soy protein/poly (N-isopropylacrylamide) interpenetrating polymer network hydrogels,” Polymer International 60, 1117-1122. DOI: 10.1002/pi.3050
Liu, Y., Meng, H., Konst, S., Sarmiento, R., Rajachar, R., and Lee, B. P. (2014). “Injectable dopamine-modified poly(ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity,” ACS Applied Materials & Interfaces 6(19), 16982-16992. DOI: 10.1021/am504566v
Marcelo, G., López-González, M., Mendicuti, F., Tarazona, M. P., and Valiente, M. (2014). “Poly(N-isopropylacrylamide)/gold hybrid hydrogels prepared by catechol redox chemistry. Characterization and smart tunable catalytic activity,” Macromolecules 47(17), 6028-6036. DOI: 10.1021/ma501214k
Masruchin, N., Park, B. D., and Causin, V. (2018). “Dual-responsive composite hydrogels based on TEMPO-oxidized cellulose nanofibril and poly (N-isopropylacrylamide) for model drug release,” Cellulose 25(1), 485-502. DOI: 10.1007/s10570-017-1585-2
Okumura, Y., and Ito, K. (2001). “The polyrotaxane gel: A topological gel by figure-of-eight cross-links,” Advanced Materials 13(7), 485-487. DOI: 10.1002/1521-4095(200104)13:7<485::AID-ADMA485>3.0.CO;2-T
Savina, I. N., Ingavle, G. C., Cundy, A. B., and Mikhalovsky, S. V. (2016). “A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications,” Scientific Reports 6, 21154-21162. DOI: 10.1038/srep21154
Shang, X. Q., Wang, Q. L., Li, J., Zhang, G., Zhang, J., Liu, P., and Wang, L. (2021). “Double-network hydrogels with superior self-healing properties using starch reinforcing strategy,” Carbohydrate Polymers (257), article no. 117626. DOI: 10.1016/j.carbpol.2021.117626
Skelton, S., Bostwick, M., O”Connor, K., Konst, S., Casey, S., and Lee B P. (2013). “Biomimetic adhesive containing nanocomposite hydrogel with enhanced materials properties,” Soft Matter 9(14), 3825. DOI: 10.1039/c3sm27352k
Sood, N., Bhardwaj, A., Mehta, S., and Mehta, A. (2016). “Stimuli-responsive hydrogels in drug delivery and tissue engineering,” Drug Delivery 23, 748-770. DOI: 10.3109/10717544.2014.940091
Sun, J. Y., Zhao, X. H., Illeperuma, W. R. K., Chaudhuri, O., Oh, K. H., Mooney, D. J., Vlassak, J. J., and Suo, Z. (2012). “Highly stretchable and tough hydrogels,” Nature 489(7414), 133-136. DOI: 10.1038/nature11409
Thoniyot, P., Tan, M. J., and Karim, A. A., Young, D. J., and Loh, X. J. (2015). “Nanoparticle-hydrogel composites: Concept, design, and applications of these promising, multi-functional materials,” Advance Science 2, 1-13. DOI: 10.1002/advs.201400010
Thoniyot, P., Tan, M. J., Karim, A. A., Young, D. J., and Loh, X. J. (2015). “Nanoparticle-hydrogel composites: Concept, design, and applications of these promising, multi-functional materials,” Advance Science 2, 1-13. DOI: 10.1002/advs.201400010
Wang, H., Gurau, G., and Rogers, R. D. (2012). “Ionic liquid processing of cellulose,” Chemical Society Reviews 41(4), 1519-1537. DOI: 10.1039/c2cs15311d
Xia, Y., Yang, P., Sun, Y., Wu, Y., Mayers, B., Gates, B., Yin, Y., Kim, F., and Yan, H. (2010). “One-dimensional nanostructures: Synthesis, characterization, and applications,” Advanced Materials 15(5), 353-389. DOI: 10.1002/adma.200390087
Zhao, F., Yao, D., Guo, R., Deng, L., Dong, A., and Zhang, J. (2015). “Composites of polymer hydrogels and nano-particulate systems for biomedical and pharmaceutical applications,” Nanomaterials 5, 2054-2130. DOI: 10.3390/nano5042054
Article submitted: June 22, 2022; Peer review completed: July 11, 2022; Revised version received: July 10, 2022; Accepted: July 12, 2022; Published: July 14, 2022.
DOI: 10.15376/biores.17.3.5079-5094
