Abstract
A new class of leaf stalk fibers of the palm tree were extracted and treated with a 5% NaOH solution for 1 h, 2 h, 6 h, and 12 h. The treated fibers were then characterized by tensile strength testing, chemical analysis, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and solid state NMR. The tensile strength of the fibers was improved with an alkali treatment, and the 6 h treatment resulted in the maximum fiber strength. The maximum cellulose content was present in the 6 h-treated fibers; cellulose content was reduced with a longer treatment (12 h). Similarly, SEM, FTIR, XRD, and NMR confirmed the removal of hemicelluloses from the raw fiber surface and the formation of new hydrogen bonds between the cellulose fibril chains with respect to the duration of the treatment. The 5% alkali treatment also improved the fiber density from 0.85 gm/cc (raw fiber) to 1.05 gm/cc, 1.13 gm/cc, 1.17 gm/cc, and 1.25 gm/cc after the 1 h, 2 h, 6 h, and 12 h treatments, respectively.
Download PDF
Full Article
Effect of Surface Treatment on the Physical, Chemical, and Mechanical Properties of Palm Tree Leaf Stalk Fibers
Arun Kumar Rout,a,* Jnanaranjan Kar,b Dipak Kumar Jesthi,a and Alekh Kumar Sutar b
A new class of leaf stalk fibers of the palm tree were extracted and treated with a 5% NaOH solution for 1 h, 2 h, 6 h, and 12 h. The treated fibers were then characterized by tensile strength testing, chemical analysis, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and solid state NMR. The tensile strength of the fibers was improved with an alkali treatment, and the 6 h treatment resulted in the maximum fiber strength. The maximum cellulose content was present in the 6 h-treated fibers; cellulose content was reduced with a longer treatment (12 h). Similarly, SEM, FTIR, XRD, and NMR confirmed the removal of hemicelluloses from the raw fiber surface and the formation of new hydrogen bonds between the cellulose fibril chains with respect to the duration of the treatment. The 5% alkali treatment also improved the fiber density from 0.85 gm/cc (raw fiber) to 1.05 gm/cc, 1.13 gm/cc, 1.17 gm/cc, and 1.25 gm/cc after the 1 h, 2 h, 6 h, and 12 h treatments, respectively.
Keywords: Palm fiber; Alkali treatment; Tensile strength; XRD; FTIR; SEM; NMR
Contact information: a: School of Mechanical Engineering, KIIT University, Bhubaneswar, India, 751024; b: Department of Chemistry, Ravenshaw University, Cuttack, India, 753001;
* Corresponding author: arun.rout.6314@gmail.com
INTRODUCTION
Polymer composites that are reinforced with synthetic fibers and filled with various inorganic fillers have large-scale applications in every field of engineering. However, one problem related to these composites is their disposal, as they contain toxic ingredients that are difficult to recycle. Due to this issue, there is an increasing demand for biodegradable composites (Chand and Fahim 2008), and plant-based fibers are slowly replacing the conventional synthetic fibers in various applications. In addition to being biodegradable, these new fibers are readily available, cost-effective, and have properties comparable to synthetic fibers (Peng et al. 2011; Ratna Prasad and Mohana Rao 2011). For better mechanical performance in a natural fiber-reinforced composite, the fiber must be compatible with the matrix, but their interfacial adhesion is generally poor because the fiber is hydrophilic and the matrix is hydrophobic (Westerlind and Berg 1998). Hence, the composite shows poor mechanical properties. The interfacial adhesion can be improved if the matrix, fiber, or both are modified by chemical or physical treatments. Chemically treated natural fibers have potential to be less hydrophilic and have better bonding with the matrix (Chand and Fahim 2008; Hossain et al. 2011; Merlini et al. 2011; Ramli et al. 2011).
Sodium hydroxide (NaOH) has been investigated as a pre-treatment in many different fibers and composites. In Borassus fruit fiber, 5% NaOH treatment removes impurities and improves the tensile strength (Boopathi et al. 2012; Reddy et al. 2013). Furthermore, alkali treatment completely eliminated amorphous hemicellulose from the fiber surface (Reddy et al. 2013). After treating short and randomly oriented palmyra fibers with 5% NaOH, the tensile strength of the resulting composite was greatly influenced by the time of treatment and the fiber volume fraction (Balakrishna et al. 2013). An alkali-treated agave fiber-reinforced epoxy composite has shown better tensile, compressive, flexural, and water absorption properties than the untreated composite (Mylsamy and Rajendran 2011). Alkali treatment has also been studied in kenaf fiber-reinforced epoxy composites (Fiore et al. 2015). While the impurities were removed by 6% NaOH after 48 h, a longer treatment had a detrimental effect on the fiber surface. Aziz and Ansell (2004) observed that alkalized kenaf polyester had better mechanical properties than alkalized long hemp polyester composites. Alkali pre-treatment is effective in tropical wood polymer composites (Islam et al.2012). Comparing male and female date palm leaves, AlMaadeed et al. (2013) found that alkali-treated female leaves had better tensile properties. Anbukarasi and Kalaisevam (2015) investigated the effect of fiber volume, dimension, and alkali treatment on the luffa reinforced epoxy composites. They observed that 40% of the volume fraction of the mat reinforced composite showed better tensile, compressive, and impact strength, and this composite decomposed within a temperature range of 341.40 °C to 387.10 °C. When Bachtiar et al. (2008) investigated alkali-treated sugar palm fiber reinforced composite, they observed that the tensile modulus of the treated fiber composite was much higher than that of the untreated fiber composite. Cao et al. (2006) prepared a biodegradable composite containing alkali-treated bagasse fiber. The mechanical properties of this composite were superior to those made with untreated fibers.
A variety of other treatments have been investigated. Joseph et al. (1996) examined NaOH, isocyanate, permanganate, and peroxide treatments and the tensile properties of short sisal fiber-reinforced polyethylene composites. While a low concentration of permanganate enhanced the mechanical properties, the peroxide treatment resulted in the highest level of interfacial adhesion. Coir pith has also been treated with various chemicals (Narendar and Dasan 2014) and by bleaching, vinyl grafting, and the addition of rubber (Geethamma et al. 1998; Rout et al.2001). Liu et al. (2014) studied mercerization and a silane treatment in unidirectional abaca fiber epoxy composites; the transverse thermal conductance (TTC) increased as voids in the composite decreased. Mohanty et al. (2000) investigated bleaching, dewaxing, alkali treatment, cyanoethylation, and vinyl grafting in a biodegradable jute-fabric polyester amide composite. The tensile and bending strengths of the developed composites were improved by surface modifications, with alkali treatment and cyanoethylation producing the best mechanical properties. Alkali, acetic anhydride, acrylonitrile, KMnO4, diphenylmethane disocyanate, and benzoyl chloride treatments were used on jute fiber mat reinforced PLA composites (Khan et al. 2015); the alkali-benzoylated treated composite showed higher tensile, flexural, and impact strength than the composite made from untreated fibers. Similarly, Rahman et al. (2015) prepared a photo-catalytic fiber by modifying the surface of jute fiber with a Bi2O3/TiO2 composite. High photo-catalytic activity was observed under visible light irradiation at 420 nm.
In sum, chemical modifications of various natural fibers and the physical, chemical, and mechanical properties of their respective composites have been extensively studied. However, there is no such report available on the study of leaf stalk fibers of the palm tree, which is used in villages for various day-to-day applications. Palm trees are abundant around the world and especially in India. In this study, the prime objective was to investigate the reinforcing potential of palm leaf stalk fiber in order to develop low cost composites. Fibers were extracted from the leaf stalk and chemically treated with 5% NaOH for different amounts of time. The physical, chemical, morphological, and mechanical properties of the fibers and their resulting composites were studied.
Fig. 1. Extracted raw fibers of leaf stalk
EXPERIMENTAL
Materials
Extraction of fiber
The raw leaf stalks of the palm tree were collected from villages near Bhubaneswar, India, and immersed in tap water for a week. The stalks were then thoroughly washed in tap water. Fibers were extracted from the leaf stalk surface and dried in sunlight for three days. The extracted fibers were then kept in an oven at 105 °C for 2 h to remove all moisture. Finally, the raw fibers were cut with scissors to obtain suitable dimensions of 200 mm in length, 5 mm in width, and 0.4 mm in thickness (Fig. 1).
Alkali treatment of fibers
The dry fibers were treated with a 5% NaOH solution for 1 h, 2 h, 6 h, and 12 h at room temperature and then washed with distilled water to remove excess NaOH. The fibers were then neutralized with a diluted HCl solution at room temperature. The fibers were again washed with distilled water and then dried at room temperature for 24 h.
Physical Properties
Weight loss
The weight loss of the fiber after the alkali treatment was determined using Eq. 1 (Narendar and Dasan 2014),
where W1 and W2 are weight of the fiber before and after treatment, respectively.
Surface morphology
The treated and untreated fibers were examined by a field emission scanning electron microscope (FESEM) (FEI Nova Nano SEM-450, Eindoven, Netherlands).
Fiber density
The Archimedes principle was employed to find the density of the fiber. The weighed quantity of the fiber was completely immersed in water, and the volumetric displacement was observed. The weight-to-volume ratio yielded the density of the fiber.
Chemical Properties
Cellulose content
Dry leaf stalk fiber (1 g) was immersed into a mixture of 1.72% of NaCl and 3 drops of H2SO4 in water for 1 h. The excess fluid was removed, and 4 N ammonia was added. The residue was then washed with distilled water, dried at room temperature, and weighed. The percentage of the cellulose was calculated by the ratio of the residue weight to that of the dry sample weight.
Hemicellulose content
Dry leaf stalk fiber (1 g) was immersed in 5% NaOH at room temperature for 0.5 h and then neutralized with 10 N HCl. The fiber was then dried in an oven at 105 °C for 2 h, and the difference in weight was noted as the hemicellulose content.
Lignin content
Dry leaf stalk fiber (1 g; L1) was immersed into a mixture of 12.5 mL of H2SO4 and 300 mL of water at room temperature and refluxed for 2 h. The solvents were removed, and the residue was weighed (L2). The lignin percentage was calculated using Eq. 2:
Wax content
The wax content was measured using a Soxhlet apparatus (Borosil, Mahapatra Scientific Supplier, Bhubaneswar, India). Petroleum benzene was heated to 70 °C, and one gram of the leaf stalk fiber was refluxed in the liquid for 1 h. The samples were then dried and weighed. The difference in weight between the raw and the treated fiber was determined as the wax content (Boopathi et al. 2012).
Moisture content
The moisture content of the fiber was determined as described (Narendar and Dasan 2014). A sample (1 g; N1) was heated in an oven at 105 °C for 2 h. The sample was cooled in a desiccator and weighed every hour by replacing it in the oven until it reached a constant weight (N2). The moisture content was determined using Eq. 3:
X-ray diffraction (XRD)
The XRD pattern of fibers was recorded using a Rigaku Dmax Ultima-4 diffractometer (Tokyo, Japan) with Ni-filtered Cu Kα radiation. The diffraction intensities were measured from 10 deg. to 80 deg. (2θ range) at a speed of 20°/min. The crystallinity of the fiber was calculated by using Eq. 4,
where and
are the intensities of crystalline and amorphous material, respectively.
Fourier transform infrared spectroscopy (FTIR)
The fiber samples were examined with a Thermo Nicolet IS-05 FTIR spectrophotometer (Kolkata, India), with scanning from 4000 to 500 cm-1 and a resolution of 2 cm-1.
Tensile testing
Tensile properties were determined with an Instron 3369 machine (Kolkata, India) at room temperature with a gauge length of 50 mm and a crosshead speed of 10 mm/min. The test was repeated five times for each sample, and the average value was recorded.
NMR analysis
The high resolution solid state 13C spectra were obtained through a JEOL Resonance ECX 400 spectrometer (Tokyo, Japan) equipped with a CP MAS probe operating at 400 MHz. The samples were packed into zirconia type rotors of 5 mm, and spun at 8 kHz.
RESULTS AND DISCUSSION
When palm fibers are treated with NaOH, a major modification occurs in the form of removal of hydrogen bonding in the network structure as shown in the following reaction (Reddy et al. 2013):
Alkali treatment improves the fiber surface adhesive characteristics by removing surface impurities and thereby producing a rough surface as shown in Fig. 7. Besides, alkali treatment leads to breaking down of the fiber bundles into smaller fiber components in a process referred to as fiber fibrillation. Therefore, alkali treatment reduces fiber diameter and as a result increases the aspect ratio (Joseph et al. 1996). The effect of alkali treatment on the palm fiber is discussed in measuring density, weight loss, chemical properties, tensile properties, XRD analysis, FTIR analysis, SEM and NMR study.
Fiber Density
Alkali treatment had a positive impact on fiber density (Table 1); the density increased with increasing treatment time. This result may reflect the densification of cell wall as a result of removal of non cellulosic part after prolonged alkali treatment. Similar observations have been made in Borassus fruit fibers and coir pith (Boopathi et al. 2012; Narendar and Dasan 2014).
Table 1. Density of Raw and NaOH-Treated Palm Fiber
Weight Loss
Alkali treatment noticeably reduced the fiber weight, which may have indicated the removal of wax and other non-cellulosic materials. More impurities were removed by longer treatments. After 1 h, alkali-treated fibers showed a 12% weight loss, which increased to 27%, 40%, and 56% for 2, 6, and 12 h, respectively. Similar results were observed for coir pith (Narendar and Dasan 2014).
Chemical Properties of the Fiber
The chemical composition of treated and untreated fibers is presented in Table 2. The 6 h-treated fiber showed the maximum cellulose content of 72.35%, and this value dropped to 61.26% for the 12-h treatment. Hemicellulose was more sensitive to the duration of the alkali treatment, as it was reduced from 13.02% to 0.00% after 2 h in 5% NaOH; wax contents behaved similarly (Gassan and Bledzki 1997). However, the lignin content was reduced from 4.52% (raw fiber) to 2.9% after the 12-h treatment. In sum, alkali treatment enhanced the cellulose content and removed surface impurities from the fiber, which leads to better mechanical properties. Similar observations have been made in Borassus fruit fibers (Boopathi et al. 2012; Reddy et al. 2013).
Table 2. Chemical Composition of Raw and NaOH-Treated Fiber
Tensile Properties of the Fiber
The tensile strength and modulus of the raw fiber increased with increasing length of the treatment, up to 6 h. When the treatment was extended to 12 h, the strength and modulus values were reduced, as shown in Table 3 and in Figs. 2 and 3. This may be due to the rupture of hemicellulose bonds existing between the fiber surfaces, which have made the fiber more homogenous. As a result, the load carrying capacity of the fiber was improved. In case of untreated fibers, presence of hemicelluloses in the inter-fibrillar region separates the cellulose chains from one another and due to this barrier, the molecular chains are in a state of strain. When this barrier (hemicelluloses) is removed, new hydrogen bonds are formed between the cellulose chains and the fibrils are now more capable to carry external loads. However, excess treatment of alkali leads to rupture of the fibril, which reduces the tensile strength of fiber. Similar observations have been found in Borassus fruit fiber (Reddy et al. 2013).
Table 3. Tensile Properties of Untreated and Treated Palm Fiber
Fig. 2. Tensile strength of untreated and treated palm fiber
Fig. 3. Tensile modulus of untreated and treated palm fiber
X-ray Diffraction Analysis
XRD analysis of the raw fiber and alkali-treated fibers showed two major reflections corresponding to the 2θ values of 17 deg. and 22 deg. (Fig. 4). The sharp and intense reflection at 22 deg. signified the presence of crystalline cellulose. The low angle of reflection (17 deg.) represented amorphous hemicelluloses. The crystallinity index of each sample was calculated using Eq. 4. The crystallinity index of the raw fiber was 46.37. This value increased to 57.55 after the 6-h treatment but decreased to 48.9 for the 12-h treatment. This result was attributed to the removal of amorphous hemicelluloses and the exposure of crystalline cellulose on the fiber surface. Similar observations were made in Borassus fruit fiber and coir pith (Reddy et al. 2013; Narendar and Dasan 2014).
FTIR Analysis
The FTIR of raw fiber contained an O-H stretching band at 3431 cm-1 (Fig. 5). The band at 2925 cm-1 indicated the C-H modes of methyl and methylene groups (Boopathi et al. 2012). Similarly, the peak at 1741 cm-1 indicated the presence of C=O stretching in acetyl groups of hemicelluloses. The peak at 1254 cm-1 was due to C-O stretching in hemicelluloses (Reddy et al. 2013). There was no peak at 1741cm-1 for the alkali-treated fibers, which indicated the removal of hemicelluloses, as previously noted (Reddy et al. 2013). Similarly, the peak at 1254 cm-1 was not visible, confirming the lack of C-O stretching from hemicelluloses. The band at 1037 cm-1 was gradually reduced with extended alkali treatment, which indicated the reduction of lignin content (Boopathi et al. 2012).
Fig. 4. XRD patterns of untreated palm fiber and fiber treated with 5% NaOH for 1, 2, 6, or 12 h
Fig. 5. FTIR spectra of raw and alkali-treated fiber
Surface Morphology of the Fiber
Fiber morphology was studied by SEM (Figs. 6, 7, and 8). The raw fiber surface was compact, smooth, and contained a white layer of visible impurities. In the micrograph of fiber treated for 6 h with 5% NaOH, there were many pores visible on the fiber surface. Most surface impurities including hemicellulose, wax, and lignin were removed. When the fibers were treated for a longer duration, the rough and degraded surfaces reflected the breakage or partial removal of crystalline cellulose materials (Fig. 7). Also, fiber weight loss was observed, which may signify the removal of impurities. Similar observations were made in Borassus fruit fiber (Boopathi et al. 2012; Reddy et al.2013). The rough surface of the fiber increases the wettability with the polymer, and consequently, the mechanical properties of the developed composites. However, the longer alkali treatment was detrimental to fiber strength due to the partial removal of the aligned grain structured cellulose content (Fig. 8). Therefore, the treatment should be judiciously selected for developing laminated composites reinforced with these fibers.
Fig. 6. SEM of raw palm fiber (500x magnification)
Fig. 7. SEM of palm fiber treated with 5% NaOH for 6 h (1000x magnification)
Fig. 8. SEM of palm fiber treated with 5% NaOH for 12 h (1000x magnification)
Solid State NMR Analysis
Solid state NMR spectra of raw and alkali treated palm fiber are shown in Fig. 8.
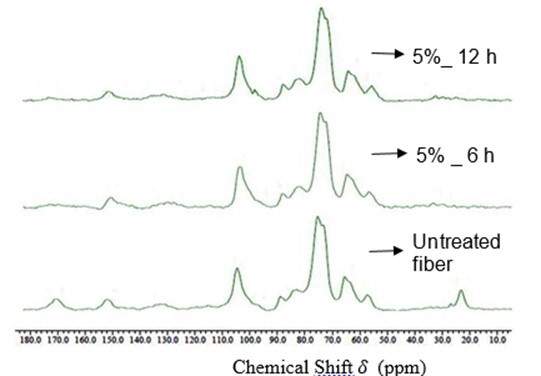
Fig. 9. CP/MAS 13 C NMR spectra of raw and alkali treated fiber
In the untreated fiber, it can be observed that the most intense signals were from cellulose carbons that appear between 60 to 110 ppm. The signals from 60 to 70 ppm are assigned to C6, from 70 to 80 ppm to C2, C3, and C5, from 80 to 90 ppm to C4, and from 97 to 108 ppm to C1. The signal of methyl and carboxylic carbons of acetyl groups attached to hemicelluloses indicated at 22 and 170 ppm respectively. The less intense signals resonated at 56 ppm, from 130 to 136 ppm and from 154 to 158 ppm were assigned to methoxyl and aromatic groups of lignin. For the alkali treated fibers, it can be seen (Fig. 9) that the signals at 22 and 170 ppm due to acetyl groups of hemicelluloses were not found, which confirmed the removal of hemicelluloses from the surface. Similarly, the signals in the lignin aromatic region, that is, from 130 to 136 ppm and from 154 to 158 ppm were gradually reduced, which indicated the partial removal of lignin content from the treated fiber surface. These observations are in agreement with the previous works (Martins et al. 2006; Wikberg and Mannu 2004; Stewart et al. 1997).
CONCLUSIONS
- Alkali treatment improved the density of the raw fiber from 0.85 gm/cc to 1.05, 1.13, 1.17, and 1.25 gm/cc after 1, 2, 6, or 12 h of treatment, respectively.
- Alkali treatment removed hemicelluloses, lignin, and wax from the fiber surface, making it rough. The removal of hemicelluloses, lignin, and other surface impurities was confirmed with SEM, FTIR, XRD and NMR analyses. The treatment led to a better fiber-matrix interface, fiber wetting characteristics, and improved mechanical properties.
- The tensile strength value of the fiber treated for 6 h with 5% NaOH was higher than other samples, which reflected the highest cellulose content in these fibers. A longer treatment (12 h) reduced the tensile strength.
- The 6 h-treated fibers showed the best mechanical properties. Hence, fibers treated for 6 h with 5% NaOH can be used as reinforcements in composites for various applications.
ACKNOWLEDGMENTS
The authors thank the All India Council of Technical Education (AICTE), New Delhi, for financial support per Research Promotion Scheme sanction No. 20/AICTE/ RIFD/RPS 42/2012-13.
REFERENCES CITED
AlMaadeed, M. A., Kahraman, R., Khanam, P. N., and Al-Maadeed, S. (2013). “Characterization of untreated and treated male and female date palm leaves,” Mater. Design 43, 526-531. DOI: 10.1016/j.matdes.2012.07.028
Anbukarasi, K., and Kalaiselvam, S. (2015). “Study of effect of fibre volume and dimension on mechanical, thermal, and water absorption behaviour of luffa reinforced epoxy composites,” Mater. Design 66, 321-330. DOI: 10.1016/j.matdes.2014.10.078
Aziz, S. H., and Ansell, M. P. (2004). “The effect of alkalization and fibre alignment on the mechanical and thermal properties of kenaf and hemp bast fibre composites: Part 1- polyester resin matrix,” Compos. Sci. Technol. 64(9), 1219-1230. DOI: 10.1016/j.compositesb.2013.07.006
Bachtiar, D., Sapuan, S. M., and Hamdan, M. M. (2008). “The effect of alkaline treatment on tensile properties of sugar palm fibre reinforced epoxy composites,” Mater. Design 29(7), 1285-1290. DOI: 10.1016/j.matdes.2007.09.006
Balakrishna, A., Rao, D. N., and Rakesh, A. S. (2013). “Characterization and modeling of process parameters on tensile strength of short and randomly oriented Borassus flabellifer (Asian palmyra) fiber reinforced composite,”Compos. Part B-Eng. 55, 479-485. DOI: 10.1016/j.compositesb.2013.07.006
Boopathi, L., Sampath, P. S., and Mylsamy, K. (2012). “Investigation of physical, chemical, and mechanical properties of raw and alkali treated Borassus fruit fiber,” Compos. Part B-Eng. 43(8), 3044-3052. DOI: 10.1016/j.compositesb.2012.05.002
Chand, N., and Fahim, M. (2008). Tribology of Natural Fiber Polymer Composites, CRC Press, Boca Raton, FL, USA.
Cao, Y., Shibata, S., and Fukumoto, I. (2006). “Mechanical properties of biodegradable composites reinforced with bagasse fibre before and after alkali treatments,” Compos. Part A-Appl. Sci. 37(3), 423-429. DOI: 10.1016/j.compositesa.2005.05.045
Fiore, V., Bella, G. D., and Valenza, A. (2015). “The effect of alkaline treatment on mechanical properties of kenaf fibers and their epoxy composites,” Compos. Part B-Eng. 68, 14-21. DOI: 10.1016/j.compositesb.2014.08.025
Gassan, J., and Bledzki, A. K. (1997). “The influence of fiber -surface treatment on the mechanical properties of jute-polypropylene composites,” Compos. Part A-Appl. Sci. 28(12), 1001-1005. DOI: 10.1016/s1359-835X(97)00042-0
Geethamma, V. G., Mathew, K. T., Lakshminarayana, R., and Thomas, S. (1998). “Composite of short coir fibers and natural rubber: Effect of chemical modification, loading, and orientation of fiber,” Polymer 39(6-7), 1483-1491. DOI: 10.1016/S0032-3861(97)00422-9
Hossain, M. K., Dewan, M. W., Hosur, M., and Jeelani, S. (2011). “Mechanical performances of surface modified jute fiber reinforced biopol nanophased green composites,” Compos. Part B-Eng. 42(6), 1701-1707. DOI: 10.1016/j.compositesb.2011.03.010
Islam, M. S., Hamdan, S., Jusoh, I., Rahman, M. R., and Ahmed, A. S. (2012). “The effect of alkali pre-treatment on mechanical and morphological properties of tropical wood polymer composites,” Mater. Design 33, 419-424. DOI: 10.1016/j.matdes.2011.04.044
Joseph, K., Thomas, S., and Pavithran, C. (1996). “Effect of chemical treatment on the tensile properties of short sisal fiber-reinforced polyethylene composites,” Polymer 37, 5139-5149. DOI: 10.1016/0032-3861(96)00144-9
Khan, G. M. A., Shaikh, H., Alam, M. S., Gafur, M. A., and Al-Zhrani, S. M. (2015). “Effect of chemical treatment on the physical properties of non-woven jute/PLA biocomposites,” BioResources 10(4), 7386-7404. DOI: 10.15376/biores.10.4.7386-7404
Liu, K., Zhang, X., Takagi, H., Yang, Z., and Wang, D. (2014). “Effect of chemical treatments on transverse thermal conductivity of unidirectional abaca fiber/epoxy composite,” Compos. Part A-Appl. Sci. 66, 227-236. DOI: 10.1016/j.compositesa.2014.07.018
Martins, M. A., Forato, L. A., Mattoso, L. H. C., and Colnago, L. A. (2006). “A solid state 13C high resolution NMR study of raw and chemically treated sisal fibers,” Carbohydrate Polymers 64, 127-133. DOI:10.1016/j.carbpol.2005.10.034
Merlini, C., Soldi, V., and Barra, G. M. O. (2011). “Influence of fiber surface treatment and length on physic-chemical properties of short random banana fiber-reinforced castor oil polyurethane composites,” Polym. Testing. 30(8), 833-840. DOI: 10.1016/j.polymertesting.2011.08.008
Mohanty, A. K., Khan, M. A., and Hinrichsen, G. (2000). “Influence of chemical surface modification on the properties of biodegradable jute fabrics-polyester amide composites,” Compos. Part A-Appl. Sci. 31(2), 143-150. DOI: 10.1016/S1359-835X(99)00057-3
Mylsamy, K., and Rajendran, I. (2011). “The mechanical properties, deformation, and thermo mechanical properties of alkali treated and untreated agave continuous fibre reinforced epoxy composites,” Mater. Design 32(5), 3076-3084. DOI: 10.1016/j.matdes.2010.12.051
Narendar, R., and Dasan, K. P. (2014). “Chemical treatments of coir pith: Morphology, chemical composition, thermal, and water retention behaviour,” Compos. Part B-Eng. 56, 770-779. DOI: 10.1016/j.compositesb.2013.09.028
Peng, X., Fan, M., Hartley, J., and Al-Zubaidy, M. (2011). “Properties of natural fiber composites made by pultrusion process,” J. Compos. Mater. 46(2), 237-246. DOI: 10.1177/0021998311410474
Ratna Prasad, A. V., and Mohana Rao, K. (2011). “Mechanical properties of natural fiber reinforced polyester composites: Jowar, sisal, and bamboo,” Mater. Design 32(8-9), 4658-4663. DOI: 10.1016/j.matdes.2011.03.015
Rahman, A., Ching, Y. C., Ching, K. Y., Awanis, N., Chakraborty, A. K., Chuah, C. H., and Liou, N. S. (2015). “Surface modification of natural fiber using Bi2O3/TiO2 composite for photocatalytic self-cleaning,” BioResources10(4), 7405-7418. DOI: 10.15376/biores.10.4.7405-7418
Ramli, R., Yonus, R. M., Beg, M. D. H., and Alam, A. K. M. M. (2011). “Effects of different coupling reagents on mechanical and interfacial properties of oil palm fiber reinforced polypropylene composites,” J. Reinf. Plast. Compos. 30(4), 301-308. DOI: 10.1177/0731684410395419
Reddy, K. O., Maheswari, C. U., Shukla, M., Song, J. I., and Rajulu, A. V. (2013). “Tensile and structural characterization of alkali treated Borassus fruit fine fibers,” Compos. Part B-Eng. 44(1), 433-438. DOI: 10.1016/j.compositesb.2012.04.075
Rout, J., Mishra, M., Tripathy, S. S., Nayak, S. K., and Mohanty, A. K. (2001). “The influence of fibre treatment on the performance of coir-polyester composites,” Compos. Sci. Technol. 61(9), 1303-1310. DOI: 10.1016/S0266-3538(01)00021-5
Stewart, D., Azzini, A., Hall, A. T. and Morrison, I. M. (1997). “Sisal fibers and their constituent non-cellulosic polymers,” Industrial Crops and Products 6(1), 27-26. DOI: 10.1016/S0926-6690(96)00172-0
Westerlind, B. S., and Berg, J. C. (1998). “Surface energy of untreated and surface- modified cellulose fibers,” JAPS36(3), 523-534. DOI: 10.1002/app.1988.070360306
Wikberg, H., and Mannu, S. L. (2004). “Characterization of thermally modified hard and soft woods by13C CP MAS NMR,” Carbohydrate Polymers 58, 461-466. DOI:10.1016/j.carbpol.2004.08.008
Article submitted: Nov. 13, 2015; Peer review completed: Jan. 9, 2016; Revised version received & accepted: March 19, 2016; Published: March 31, 2016.
DOI: 10.15376/biores.11.2.4432-4445
