Abstract
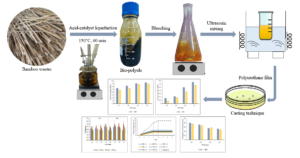
Malaysian bamboo residues were subjected to a liquefaction process. Bleaching of the liquefied product was carried out to reduce its coloration. Polyurethane (PU) films were then manufactured as a coating material by reacting the -OH groups in the bamboo material with isocyanate (-NCO). The study’s objective was to investigate the effects of bleaching on the properties of resulting polyurethane films and to elucidate its behavior as affected by the NCO/OH ratios. Bamboo residues, in powder form, were liquefied with polyethylene glycol (PEG 400) and glycerol (Gly) as reactive co-solvent, and sulphuric acid as catalyst. The obtained liquefied bamboo was then bleached with hydrogen peroxide. Bleached and unbleached liquefied bamboo were used to produce PU film by reacting isocyanate at NCO/OH ratios of 1.6, 1.8, 2.0, and 2.2. The results revealed that the NCO/OH ratios improved the mechanical performance of the PU films. Bleaching treatment slightly reduced the thermal stability and mechanical strength of the PU films. However, bleached PU films displayed lower water absorption and lower biodegradation than unbleached PU films. Nevertheless, the performance of the bleached PU films was still acceptable, indicating that the bleaching treatment using hydrogen peroxide is feasible to obtain semi-transparent film.
Download PDF
Full Article
Effects of Bleaching Treatment on the Properties of Bio-polyurethane Films from Liquefied Bamboo
Redzuan Mohammad Suffian James,a Paridah Md Tahir,a,b,* Norwahyuni Mohd Yusof,a Syeed SaifulAzry Osman Al-Edrus,a Zurina Zainal Abidin,c Ismawati Palle,d Mohd Khairun Anwar Uyup,e and Seng Hua Lee f,g,*
Malaysian bamboo residues were subjected to a liquefaction process. Bleaching of the liquefied product was carried out to reduce its coloration. Polyurethane (PU) films were then manufactured as a coating material by reacting the -OH groups in the bamboo material with isocyanate (-NCO). The study’s objective was to investigate the effects of bleaching on the properties of resulting polyurethane films and to elucidate its behavior as affected by the NCO/OH ratios. Bamboo residues, in powder form, were liquefied with polyethylene glycol (PEG 400) and glycerol (Gly) as reactive co-solvent, and sulphuric acid as catalyst. The obtained liquefied bamboo was then bleached with hydrogen peroxide. Bleached and unbleached liquefied bamboo were used to produce PU film by reacting isocyanate at NCO/OH ratios of 1.6, 1.8, 2.0, and 2.2. The results revealed that the NCO/OH ratios improved the mechanical performance of the PU films. Bleaching treatment slightly reduced the thermal stability and mechanical strength of the PU films. However, bleached PU films displayed lower water absorption and lower biodegradation than unbleached PU films. Nevertheless, the performance of the bleached PU films was still acceptable, indicating that the bleaching treatment using hydrogen peroxide is feasible to obtain semi-transparent film.
DOI: 10.15376/biores.19.2.2562-2574
Keywords: Bio-polyurethane; Liquefied bamboo; Bamboo; Coating
Contact information: a: Institute of Tropical Forestry and Forest Products, Universiti Putra Malaysia (UPM), 43400 UPM Serdang, Selangor, Malaysia; b: Faculty of Forestry and Environment, UPM, 43400 UPM Serdang, Selangor, Malaysia; c: Department of Chemical and Environmental Engineering, Faculty of Engineering, UPM, 43400 UPM Serdang, Selangor, Malaysia; d: Faculty of Tropical Forestry, Universiti Malaysia Sabah, Jalan UMS, 88400 Kota Kinabalu, Sabah, Malaysia; e: Forest Products Division, Forest Research Institute Malaysia (FRIM), 52109 Kepong, Selangor, Malaysia; f: Department of Wood Industry, Faculty of Applied Sciences, Universiti Teknologi MARA (UiTM) Cawangan Pahang Kampus Jengka, 26400 Bandar Tun Razak, Pahang, Malaysia; g: Institute for Infrastructure Engineering and Sustainable Management (IIESM), Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia;
* Corresponding authors: parida@upm.edu.my; leesenghua@uitm.edu.my
GRAPHICAL ABSTRACT
INTRODUCTION
Polyurethane (PU) is a polymeric material that is typically derived from petroleum by reacting isocyanate with two or more isocyanate groups with a suitable polyol having two or more hydroxyl groups (Saha et al. 2020). Many efforts have recently been made to develop low-cost raw materials for the PU industry (Kaur et al. 2022). Liquefaction of renewable biomass resources is one of the effective and widely accepted approaches for lowering biodiesel production costs, directly converting whole biomass into liquids, and achieving high effective utilization and development of biomass waste (Beims et al. 2020). This approach relies on the utilization of liquefied wood products as polyols to prepare polyurethane (PU) resin. The outcome of this method is PU films with enhanced mechanical properties (Lee and Chao 2018).
Liquefied wood and other lignocellulosic materials have been reported to be employed in the production of PU films. Kurimoto et al. (2001) reported using liquefied sugi wood to make PU films. Lee et al. (2015) developed PU films using liquefied sugi wood and ethylene glycol (EG)-based polyester polyols. It was discovered that films made with a combination of liquefied sugi wood and EG-based polyester polyols had better mechanical properties than EG-based polyester polyols alone. The PU films are suitable for coating applications. Based on Hrastnik et al. (2014), liquefied contaminated wood could be reused for surface coating instead of being burnt or land filled. Although films derived from liquefied wood exhibit promising potential as coating materials, it is noteworthy that they typically possess a black or dark brown coloration. Therefore, in order to make the PU films lighter in color and more aesthetically appealing, bleaching treatment is necessary. Cheumani-Yona et al. (2015) bleached liquefied black poplar wood with hydrogen peroxide and made PU films out of it. While the bleaching treatment did successfully turn the color of the films from dark brown to yellowish, the authors reported that the bleaching treatment did not affect the properties of the films produced.
Other lignocellulosic materials could potentially be liquefied and used for PU films production, for example, bamboo. In Malaysia, bamboo is abundantly available and is used for the manufacturing of various bamboo-based products. The residues of the bamboo during such activities can serve as a promising liquefaction feedstock. To the best of our knowledge, there is currently no research available on the effects of bleaching treatment on the performance of PU films derived from liquefied bamboo. Hence, the primary objective of this study was to investigate the impact of bleaching liquefied bamboo on the properties of the resultant polyurethane (PU) films. The study aimed to elucidate the influence of NCO/OH ratios on various aspects, including chemical, physico-mechanical, thermal properties, and the degradation of bio-PU films under indoor soil burial conditions.
EXPERIMENTAL
Materials Preparation
Bamboo residues were purchased from a local bamboo processing factory in Sungai Senam, Sik, Kedah, Malaysia. The bamboo residues were ground and sieved to obtain 60-mesh sizes. The polymeric methylene diphenyl diisocyanate (pMDI) was supplied by Sigma Aldrich. The ethyl acetate and acetone used were reagent grade and used as received.
Liquefaction of Bamboo
The reaction was carried out in a 500-mL round bottom flask using a mixture of polyethylene glycol (PEG 400) and glycerol (Gly) at a 9:1 mass ratio as the reactive co-solvent, and sulphuric acid as the catalyst. The amount of bamboo used was 15 wt% of co-solvent and the catalyst-to-solvent mass ratio was 3%. PEG 400, glycerol, and bamboo sawdust were charged into the round bottom flask and stirred using a mechanical stirrer and heated at 150 °C for 60 min. The liquefied mixture was cooled down and diluted to ten times its weight with methanol to stop further reaction. The insoluble bamboo residue in the solution was filtered under vacuum through a porcelain Buchner funnel with Whatman™ filter paper grade No. 4 (25 µm) and dried in a vacuum at 80 °C for 24 h. The water content of the liquefied wood was approximately 2%, as measured using a moisture analyzer (Shidmazu-MOU63u model).
Bleaching of Liquefied Bamboo
Liquefied bamboo (30 g) was diluted in a 500-mL conical flask with a mixture of 100 mL of 1,4-dioxane and water (4:1 v/v) before 1.5 g of solid potassium carbonate was added. At room temperature, hydrogen peroxide was added dropwise and stirred continuously at 1000 rpm. The color of polyol gradually changes over time. Following the completion of the designated reaction time, a 40% aqueous solution of sodium hydroxide (NaOH) was employed to adjust the pH of the reaction to approximately 8.5. After the treatment period, they were removed by filtering under vacuum via a Buchner funnel (porcelain) using Whatman™ filter paper No. 4. The solvent in the filtrate was evaporated at 80 °C under reduced pressure. The discolored liquefied polyol sample was redissolved in a fresh solution of 1,4-dioxane and water (4:1 v/v), and the pH was adjusted to 4 to 4.5 using a solution of sulphuric acid (5 M) to allow additional salt precipitation, and again filtered. The solvent was then removed in a vacuum at 80 °C for 24 h until reached 2% of water content.
Preparation of Polyurethane Film
Unbleached liquefied bamboo (ULB) and bleached liquefied bamboo (BLB) with a hydroxyl number of 342.83 mg.KOH/g and 214.09 mg.KOH/g, respectively, were dosed with an appropriate amount of pMDI (isocyanate content of 7.43 mmol-1) and ethyl acetate, as shown in Table 1. The dosing was given by the NCO/OH ratio. The NCO/OH ratio was calculated by the Eq. 1 following Kurimoto et al. (2000). In this study, four NCO/OH ratios of 1.6, 1.8, 2.0, and 2.2 were utilized to generate bio-PU films. These films were assigned the designations UPU1.6, UPU1.8, UPU2.0, and UPU2.2 for the unbleached polyurethane, and BPU1.6, BPU1.8, BPU2.0, and BPU2.2 for the bleached polyurethane, respectively.
NCO/OH ratio = (MpMDI.WpMDI) / (Mpolyol +Wpolyol +Wwater ×2/18 ×1000) (1)
where MpMDI represents the content of the -NCO group in pMDI (7.43 mmol/g), WpMDI is the weight of pMDI, Mpolyol is the content of the -OH group in ULB or BLB (hydroxyl number/56.1 the unit is mmol/g), Wpolyol is the weight of ULB or BLB, Wwater is the water included in ULB or BLB, and 2/18 × 1000 is 2 g water/18 × 1000 (while 18 is the molecular weight of H2O).
Table 1. Ingredients for PU Film Formation
The ULB and BLB samples were diluted with twice the amount of distilled acetone using ultrasonics for 60 s before vigorously mixing with pre-determined pMDI and 10% EA for another 20 s. The polymerized mixtures were then poured into a Petri dish for solution-casting to obtain 0.25-mm thick films. The bio-PU films obtained were gradually dried at room temperature overnight before being dried in an oven at 80 °C for 8 h in an oven.
Film Characterization
The unbleached and bleached polyol film samples were analyzed by Fourier transform infrared (FTIR) spectroscopy in the wavelength range of 4000 to 400 cm-1. Samples were cast onto a KBr disc for the IR measurement.
Thermogravimetric analysis was performed using the Perkin Elmer apparatus (Pyris 1 TGA) to understand the thermal behavior of the obtained bio-based polyurethane. A measurement was taken using 5 mg sample at a temperature range of 30 to 650 °C, with a ramp heating rate of 20 °C/min. Results were recorded based on the weight loss of 5%, 50%, as well as the maximum degradation rate and ash residue at 600 °C.
The viscoelastic behavior was investigated with a tensile mode from room temperature to 120 °C at a heating rate of 2 °C min-1 at a frequency of 1.0 Hz at dimension of 30 × 7 × 2 mm. This test was completed using a DMA Q 800 analyzer (TA Instruments).
The mechanical properties of the prepared films were determined in terms of tensile strength (measured in MPa) and elongation at break (measured in %) according to ASTM D882 (2018). This was done using a universal material testing machine (Cometech -500N: Model QC50881) at room temperature, with a relative humidity of 56 ± 5%. Film samples were cut into rectangular shapes with length and width of 75 mm × 10 mm, respectively, and conditioned for 48 h before testing. The initial grid separation was set to 50 mm, and the crosshead speed was 5 mm-1 (Shamsuri and Daik 2013). Young’s modulus, tensile strength, and the elongation at break were calculated using data from stress and strain measurements. The data obtained was reported as the mean of at least five replicates of each sample.
The hydrophilicity of the polyurethanes was quantified by measuring the amount of water that each films absorbed (Aung et al. 2014). Approximately 5 g of film samples were weighed to the nearest 0.0001 g and dried in a drying oven (100 °C ± 2 °C; 24 h) to obtain initial dry matter weight of the films. The dried film was immersed in 50 mL of distilled water and gently agitated (20 °C ± 2 °C; 24 h). After 7 days, the films were taken out of the water and dried (100 °C ± 2 °C; 24 h) to determine the weight of the dry matter.
The degradation test was done following Deshmukh et al. (2021). Five 10 mm × 10 mm samples were cut, weighed, and buried in a 50-mL capacity tube containing 50 g of moist gardening soil. Inside the tube, the soil height was 100 mm, and the test film samples were placed at a depth of 50 mm. Every alternate day, 3 mL of water was dropped over the open surface of the soil to maintain humidity. The test was carried out for 30, 60, and 90 days. After degradation period, samples were taken out from soil, rinsed with water to remove adhered soil, and dried to constant weight in the oven at 40 °C. The weight of the specimens before and after soil burial tests were recorded.
RESULTS AND DISCUSSION
FTIR Spectra of Bio-PU Film as Affected by NCO/OH Ratios
The chemical structures of the bio-PU film using various ratios of NCO/OH were analyzed through FTIR spectroscopy, as shown in Fig. 1.
Fig. 1. FTIR Spectra of unbleached polyurethane (UPU) and bleached polyurethane (BPU) at different NCO/OH ratio (1.6, 1.8, 2.0 & 2.2)
The presence of peaks associated with the urethane linkage can clearly be observed in the IR-bands located at 3460, 3310, and 3290 cm-1. The absorption band at 3460 cm-1 could be assigned to hydroxyl groups in the cured films. Certain hydroxyl groups in the films may have remained unreacted, possibly due to steric hindrances in the liquefied bamboo compounds. The absence of transmittance at 2270 cm-1, which is attributed to the (NCO) group in pMDI, was observed in the spectrum of the PU film. This observation suggests that the NCO group had undergone complete reaction with the polyol, as reported by Lee et al. (2015). The isocyanate group in the PU film was completely consumed through the production of either urethane or urea linkage (Kurimoto et al. 2000). The bands observed in 1720, 1702, 1597, and 1222 cm-1 indicate the presence of urethane linkages, which were formed through the co-polymerization of bamboo polyol and pMDI. The band at 1702 cm-1 is the stretching vibration of hydrogen-bonded carbonyl groups between N-H and C=O groups of urethane linkages, while 1720 cm-1 is assigned to “free” carbonyl groups. The two bands at 1597 cm-1 and 1222 cm-1 are attributed to the stretching of N-H and C-N bonds, respectively (Palle et al. 2023).
Thermo-Gravimetric Analysis
The effects of the NCO/OH ratio on the TGA and DTG curves of bio-PU films, both unbleached and bleached, are shown in Figs. 2 and 3, respectively. The summarized data are presented in Table 2. The derivative of weight-curves reveals the presence of five thermal degradation curves, which are located at approximately 110, 280, 340, 390, and 470 °C for the UPU sample. Similarly, for the BPU sample, the curves are apparent at 110, 220, 350, 410, and 480 °C. The initial mass loss, occurring at temperatures ranging from 70 to 160 °C, is attributed to the dehydration of the solvent and the trapped moisture within the film. A higher temperature was required to achieve a 10% weight loss at higher NCO/OH ratios for both unbleached and bleached bio-PU film. The degradation processes occurred in a temperature range of approximately 250 to 270 °C, which corresponds to the urethane linkages or the post-curing process. The decomposition step, derived from urea, occurred in the third stage at approximately 350 °C. The fourth degradation stage involved a rapid weight loss taking place at approximately 380 to 450 °C, corresponding to polyol. The high degradation ca. > 450 °C, may potentially be associated with the isocyanate linkages.
Fig. 2. TGA and DTG of unbleached Bio-PU (UPU) films
Fig. 3. TGA and DTG of unbleached Bio-PU (BPU) films
Table 2. Thermal Degradation Stability of Bio-PU Films
Generally, bleached bio-PU had higher thermal stability compared to that of unbleached bio-PU, as shown by its higher degradation temperatures. The finding was in line with study reported by Beg and Pickering (2008), where they reported that the bleached fiber has higher Tmax compared to that of unbleached fiber, probably due to the reduction of lignin and hemicellulose content after bleaching treatment. Meanwhile, excess isocyanate in the bio-PU films may result in the formation of higher amount of isocyanate linkages. The isocyanate group is the most thermal stable structure in the polyurethane system (Kurimoto et al. 2001), and therefore the thermal stability increased with increasing NCO/OH ratio.
Dynamic Mechanical Analysis
Figures 4 and 5 shows the storage modulus, loss modulus, and tan ẟ curves of UPU and BPU films prepared with different NCO/OH ratios, respectively. Both UPU2.2 and BPU2.0 films had higher storage modulus than others throughout the temperature range. At high temperatures, the phase transition for all UPU and BPU films showed no phase, indicating that they had lower thermal activity (Lee and Chao 2018). The tan ẟ curves of the UPU and BPU films showed a peak at the glass transition (Tg) different temperature. Noticeably, the glass transition (Tg) of the PU films became broadened and shifted to a higher temperature as the -NCO/-OH ratio increased, indicating the decrement of the polymer’s chain flexibility and becoming more rigid and more non-uniform (Lee and Chao 2018; Palle et al. 2023).
Fig. 4. (a) Storage modulus, (b) loss modulus, and (c) tan ẟ of unbleached bio-PU (UPU) films
Fig. 5. (a) Storage modulus, (b) loss modulus, and (c) tan ẟ of bleached bio-PU (BPU) films
Mechanical Properties of Bio-PU Films
A high NCO/OH ratio generally increases the cross-linking density of the PU polymer, thereby improving the mechanical properties of the final products (Das et al. 2015). Table 3 lists the mechanical properties of bleached and unbleached bio-PU films at different NCO/OH ratios. Generally, the UPU film had higher tensile strength and less elongation at breaking point compared with the BPU films, indicating a higher Young’s modulus and more brittle behavior. The findings suggested that, though not significant, the bleaching treatment did affect the mechanical properties of the bio-PU films. Typically, the reduced strength observed in bleached PU may be attributed to the presence of salt residues resulting from sodium hydroxide solution (during bleaching process), which can impact the characteristics of cured bio-PU films using liquid polyol as a base (Cheumani Yona et al. 2015). However, the reduction in strength after bleaching treatment can be compensated by increasing the NCO/OH ratio. Based on Table 3, the Young’s modulus and tensile strength values increased, while the elongation at break decreased with the addition of NCO for UPU and BPU films. These effects could be attributed to an increase in crosslink densities of PU films, because a higher crosslink density results in a more rigid material.
Table 3. Mechanical Properties of the Bio-PU Films
Note: E = Young’s modulus
σb = Tensile strength
εb = Elongation at break
Water Absorption of Bio-PU Films
The water absorption of bio-PU films was shown to have lower values compared to the jatropha oil-based PU adhesive, as depicted in Fig. 6. The PU film made from liquefied bamboo gradually absorbed water until the third day, after which the increase became minimal. In the case of the PU film based on jatropha oil, it took four days for the film to achieve stability, with only a slight increase observed until the conclusion of the testing period. This behavior indicates that polyurethane made from liquefied bamboo could exclude water more effectively, thus making a relatively good moisture resistant coating system. The water absorption pattern observed in both UPU and BPU, across various NCO ratios, displayed a consistent trend from day 1 to day 7. Notably, BPU2.2 exhibited the lowest water absorption at 6.2%, while UPU1.6 had the highest water absorption at 12.4%, representing a 50% higher absorption. Generally, bleached bio-PU films (BPU) had lower water absorption than non-bleached bio-PU films, probably due to the reducing hemicellulose content after bleaching (Beg and Pickering 2008). Meanwhile, the water absorption of the films decreased as the NCO/OH ratio increased.
Fig. 6. Water absorption of bio-PU films at different time points
Soil Burial Degradation of Bio-PU Films
In Fig. 7, the weight gain of UPU and BPU films is depicted after 30, 60, and 90 days of soil burial tests.
Fig. 7. Weights of UPU and BPU of bio-PU films during soil burial test
An initial increase from 0 days to 30 days was observed. The weight increased around 0.79% to 1.57% and 1.16% to 2.36% for UPU and BPU, respectively, over a period of 30 days. The increase in weight is likely related to the absorption of water in the early stage of the test, which demonstrates a similar trend to the water solubility test. As the time progressed, the UPU and BPU started to lose weight. The weights for UPU and BPU after 60- and 90-days exposure decreased approximately 0.6% to 8.06% and 0.59% to 1.4%, respectively. It was observed that the bleached PU films degraded at a less rapid rate than that of unbleached film. This phenomenon may be attributed to the use of hydrogen peroxide as a bleaching agent, which potentially possesses antimicrobial properties capable of either destroying or inhibiting the growth of microorganisms (Juven and Pierson 1996). On the other hand, it could also be attributed to the lower water absorption of BPU films, as mentioned earlier. UPU is more hydrophilic than BPU and therefore it absorbs higher amount of water during the soil burial test which increases its susceptibility to microbial attack. According to Zuliani et al. (2022), increasing water absorption can increase the surface area for microbial attack, which can then promote the hydrolysis of ester groups on the PU networks, thereby increase the rate of biodegradation of the UPU films with higher hydrophilicity. The NCO/OH ratio of the PU films also plays an important role in the biodegradation rate of the PU films. The higher the NCO/OH ratio, the lower the degradation rate. A study by Abd Hilmi et al. (2023) reported a similar finding, where the PU made from Eucalyptus pellita wood polyol at the lowest NCO/OH ratio of 1.8 experienced the highest degradation rate of 14.0%.
CONCLUSIONS
- Bio-polyurethane (bio-PU) films with lighter color were successfully prepared from liquefied bamboo residues bleaching with hydrogen peroxide.
- The bleaching treatment employing hydrogen peroxide had minimal impact on the properties of the bio-polyurethane (PU) films. There was a slight decrease in the mechanical properties and thermal stability of the bio-PU films after the bleaching treatment. However, the subtle nature of these changes suggests that the use of hydrogen peroxide as a bleaching agent is acceptable in achieving PU films with a lighter color.
- Bleached bio-PU films had lower water absorption compared to unbleached bio-PU films. Consequently, the biodegradation rate of the bleached bio-PU films was also lower than that of unbleached films. The findings suggested that it could be beneficial in applications where water resistance and durability are of concern.
- Elevating the NCO/OH ratio demonstrated an augmentation in the mechanical strength and overall performance of the bio-polyurethane (PU) films. This adjustment could function as a compensatory measure when seeking specific desired properties in the films.
ACKNOWLEDGEMENTS
This study was supported by the Higher Education Center of Excellence (HICoE) Phase 2 grant (Project title: “Mill production of laminated bamboo board from buluh madu (Gigantochloa albociliata) for structural applications,” vote number: 5210010 project code: 800-3/8/HICoEF2/2023/5210010) provided by the Malaysian Ministry of Higher Education (MOHE). This study was also supported by the Malaysian Timber Industry Board (MTIB) under project vote numbers: 6300258 and 6300313.
REFERENCES CITED
Abd Hilmi, N. H., Lodin, V., Jesuet, M. S. G., Salim, S., Lee, S. H., Hori, N., Takemura, A. and Palle, I. (2023). “Biodegradability properties of polyurethane film made from Eucalyptus pellita wood polyol,” Borneo International Journal of Biotechnology 3, 147-158. DOI: 10.51200/bijb.v3i.4720
ASTM D882-18 (2018). “Standard test method for tensile properties of thin plastic sheeting,” ASTM International, West Conshohocken, PA, USA.
Aung, M. M., Yaakob, Z., Kamarudin, S., and Abdullah, L. C. (2014). “Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive,” Industrial Crops and Products 60, 177-185. DOI: 10.1016/j.indcrop.2014.05.038
Beg, M. D. G., and Pickering, K.L. (2008). “Accelerated weathering of unbleached and bleached Kraft wood fibre reinforced polypropylene composites,” Polymer Degradation and Stability 93(10), 1939-1946. DOI: 10.1016/j.polymdegradstab.2008.06.012
Beims, R. F., Hu, Y., Shui, H., and Xu, C. (2020). “Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Products applications and challenges to develop large-scale operations,” Biomass and Bioenergy 135, article ID 105510. DOI: 10.1016/j.biombioe.2020.105510
Cheumani-Yona, A. M., Budija, F., Hrastnik, D., Kutnar, A., Pavlič, M., Pori, P., Tavzes, Č., and Petrič, M. (2015). “Preparation of two-component polyurethane coatings from bleached liquefied wood,” BioResources 10(2), 3347-3363. DOI: 10.15376/biores.10.2.3347-3363
Deshmukh, A. R., Aloui, H., Khomlaem, C., Negi, A., Yun, J.-H., Kim, H.-S., and Kim, B. S. (2021). “Biodegradable films based on chitosan and defatted Chlorella biomass: Functional and physical characterization,” Food Chemistry 337, article ID 127777. DOI: 10.1016/j.foodchem.2020.127777
Hrastnik, D., Humar, M., Kričej, B., Pavlič, M., Pori, P., Cheumani Yona, A.M., and Petrič, M. (2014). “Polyurethane coatings from liquefied wood containing remains of a copper‐, chromium‐, and boron‐based wood preservative,” Journal of Applied Polymer Science 131(19), article ID 40865. DOI: 10.1002/app.40865
Juven, B. J., and Pierson, M. D. (1996). “Antibacterial effects of hydrogen peroxide and methods for its detection and quantitation,” Journal of Food Protection 59(11), 1233-1241. DOI: 10.4315/0362-028X-59.11.1233
Kaur, R., Singh, P., Tanwar, S., Varshney, G., and Yadav, S. (2022). “Assessment of bio-based polyurethanes: Perspective on applications and bio-degradation,” Macromolecules 2(3), 284-314. DOI: 10.3390/macromol2030019
Kurimoto, Y., Takeda, M., Doi, S., Tamura, Y., and Ono, H. (2001). “Network structures and thermal properties of polyurethane films prepared from liquefied wood,” Bioresource Technology 77(1), 33-40. DOI: 10.1016/s0960-8524(00)00136-x
Kurimoto, Y., Takeda, M., Koizumi, A., Yamauchi, S., Doi, S., and Tamura, Y. (2000). “Mechanical properties of polyurethane films prepared from liquefied wood with polymeric MDI,” Bioresource Technology 74(2), 151-157. DOI: 10.1016/s0960-8524(00)00009-2
Lee, W. J., Kuo, E. S., Chao, C. Y., and Kao, Y. P. (2015). “Properties of polyurethane (PUR) films prepared from liquefied wood (LW) and ethylene glycol (EG),” Holzforschung, 69(5), 547-554. DOI: 10.1515/hf-2014-0142
Lee, W. J., and Chao, C. Y. (2018). “Effect of containing polyhydric alcohol liquefied wood on the properties of thermoplastic polyurethane resins,” 76(6), 1745-1752. DOI: 10.1007/s00107-018-1338-4
Palle, I., Lodin, V., Mohd Yunus, A. A., Lee, S. H., Md Tahir, P., Hori, N., Antov, P., and Takemura, A. (2023). “Effects of NCO/OH ratios on bio-based polyurethane film properties made from Acacia mangium liquefied wood,” Polymers 15(5), article ID 1154. DOI: 10.3390/polym15051154
Saha, P., Aloui, H., Yun, J.-H., Kim, H.-C., and Kim, B. S. (2020). “Development of a novel composite film based on polyurethane and defatted Chlorella biomass: Physical and functional characterization,” Journal of Applied Polymer Science 138(14), article ID 50152. DOI: 10.1002/app.50152
Shamsuri, A., and Daik, R. (2013). “Utilization of an ionic liquid/urea mixture as a physical coupling agent for agarose/talc composite films,” Materials 6(2), 682-698. DOI: 10.3390/ma6020682
Zuliani, A., Rapisarda, M., Chelazzi, D., Baglioni, P., and Rizzarelli, P. (2022). “Synthesis, characterization, and soil burial degradation of biobased polyurethanes,” Polymers 14(22), article ID 4948. DOI: 10.3390/polym14224948
Article submitted: December 18, 2023; Peer review completed: January 27, 2023; Revised version received and accepted: February 24, 2024; Published: March 4, 2024.
DOI: 10.15376/biores.19.2.2562-2574
