Abstract
Rosewood furniture and handicrafts are appreciated by Chinese people on account of their rich aroma and pleasing feel. The unique characteristics of rosewood are attributed to the presence of certain organic compounds in its gum canal and parenchyma cells. However, modern wood drying is different from traditional technology with respect to protecting those valuable organic compounds in wood. In this study, to investigate the valuable organic compounds in Dalbergia bariensis, and the effect of drying treatments on their preservation rates, wood extracts, untreated and treated with conventional drying (CD), vacuum drying (VD), and vacuum freeze drying (VFD), were analyzed by gas chromatography- mass spectrometry (GC-MS). The results indicated that there were some compounds with obvious pharmaceutical functions in Dalbergia bariensis, which can be used to improve the furniture function in health care. Also, the preservation of these compounds was affected by drying treatment; VFD drying preserved the maximum amount of organic compounds in wood.
Download PDF
Full Article
Effects of Different Drying Treatments on Preservation of Organic Compounds in Dalbergia bariensis Wood
Lin Yang,a Tao Jiang,b,c Honghai Liu,a and Kaifu Li b,*
Rosewood furniture and handicrafts are appreciated by Chinese people on account of their rich aroma and pleasing feel. The unique characteristics of rosewood are attributed to the presence of certain organic compounds in its gum canal and parenchyma cells. However, modern wood drying is different from traditional technology with respect to protecting those valuable organic compounds in wood. In this study, to investigate the valuable organic compounds in Dalbergia bariensis, and the effect of drying treatments on their preservation rates, wood extracts, untreated and treated with conventional drying (CD), vacuum drying (VD), and vacuum freeze drying (VFD), were analyzed by gas chromatography- mass spectrometry (GC-MS). The results indicated that there were some compounds with obvious pharmaceutical functions in Dalbergia bariensis, which can be used to improve the furniture function in health care. Also, the preservation of these compounds was affected by drying treatment; VFD drying preserved the maximum amount of organic compounds in wood.
Keywords: Dalbergia bariensis; Organic compounds; Extract; Effect of drying; Aroma of wood
Contact information: a: College of Furniture and Industrial Design, Nanjing Forestry University, No.159 Longpan Road, Nanjing, Jiangsu 210037, China; b: College of Forestry, South China Agricultural University, No.483 Wushan Road, Tianhe District, GuangZhou 510642, China; c: CEPREI Laboratory, Guangzhou 510075 , China; *Corresponding author: kfli@scau.edu.cn
INTRODUCTION
Dalbergia is a large genus in the pea family, Fabaceae, subfamily Faboideae. The genus has a wide distribution, native to the tropical regions of central South America, Africa, Madagascar, and southern Asia. Many species of Dalbergia are important timber trees, valued for their decorative and often fragrant wood, which is rich in aromatic oils. The most famous of these species are used for rosewood furniture. Previous reports have shown that some crude extracts and pure compounds from the heartwood of the genus have various bioactivities (Ito et al. 1994; Chuankhayan et al. 2007; Umehara et al.2009; Innocent et al. 2010).
Dalbergia odorifera T. Chen is the most representative type of fragrant rosewood tree that grows in Asia. The dried heartwood is an important traditional medicine in Asia, named “Jiangxiang” in Chinese. Dissipating blood stasis, regulating the flow of qi, and relieving pain are its main actions in traditional Chinese medicines (Liu et al. 2005). Modern pharmacological studies have shown that Jiangxiang possesses various biological activities, such as anti-inflammatory (Lee et al. 2009), antiplatelet (Tao and Wang 2009), anticoagulant, antitumor, anti-hyperlipidemic, and vasodilative effects (Zhao et al. 2000), as well as stimulating the activity of tyrosinase (Wu and Wang 2003). In addition to the medical functions of Dalbergia species, recent studies (Edmone 2006; Khyasudeen and Abu Bakar 2006; Wang 2006) have focused on enhancing the added value of rosewood furniture. However, Dalbergia odorifera T. Chen is rarely used for rosewood furniture because of the fear of exhaustion of resources (Xu 2013). Dalbergia bariensis, a valuable reddish hardwood with good thermal diffusivity and permeability, is used widely in the rosewood furniture industry.
People like Dalbergia bariensis not only because of its color, density, watermark, and wood grain, but also because of the scent released from the wood, which has a refreshing function, and the pleasing feel. Consumers are increasingly interested in the comfortable feeling coming from aromatic smells and the sense of touch in daily life. All the healthy characteristics of Dalbergia bariensis are related to the extracts from its gum canal and parenchyma cells. In general, wood extracts contains various types of organic compounds, and the most common compounds include polyphenols, terpenes, lipids, flavonoids, lignans, and water-soluble carbohydrates (Zhao et al.2002). However, there have not been any related studies on extracts from Dalbergia bariensis in China. In addition, the valuable organic compounds existing in wood may be destroyed because of the high dying temperature and the long drying process of modern conventional kiln (CK) drying (Esteves et al. 2011), resulting in decreased healthy functions of rosewood.
Therefore, to investigate the constituents and to identify the medically valuable organic compounds in Dalbergia bariensis, the wood was extracted with various solvents and the extracts were analyzed with gas chromatography-mass spectrometry (GC-MS) in this study. The effects of drying on organic compounds extracted by conventional drying (CD), vacuum drying (VD), and vacuum freeze drying (VFD) of the untreated and treated samples with the above three drying methods were also investigated.
EXPERIMENTAL
Materials
The raw material was Dalbergia bariensis heartwood, identified and provided by Zhongshan Dongcheng Furniture Co., Ltd (China). The initial moisture content of the material was approximately 40%.
Methods
Wood drying
The Dalbergia bariensis heartwood was sawed into three end-matched samples with dimensions of 30 mm (T) × 30 mm (R) × 100 mm (L). These samples were dried to an absolutely dry state by CD, VD, and VFD (Table 1). The absolutely dry state means the mass difference was within 0.02 g between the last two weight measurement intervals in 2 h (Cai et al. 2005).
Table 1. Conditions for the Various Drying Methods
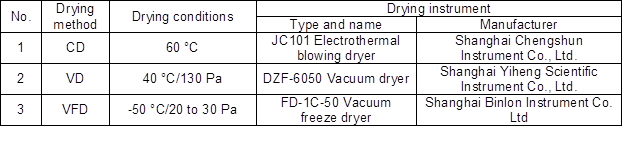
Extraction
The untreated Dalbergia bariensis heartwood and the three absolutely dry samples were riven, ground, and sieved (40-mesh) as quickly as possible. Then, 2 g of each sample was wrapped in quantitative filter paper and placed in a Soxhlet extractor with 90 mL of benzene-ethanol (1:2, v/v) solvent. The benzene and ethanol were both analytical reagents. The benzene-ethanol solvent has a wide range of polarity, so almost all kinds of component in wood tissue can be dissolved, and it is not easy to volatilize (Zhao et al. 2002; Enma et al. 2014). The extraction step was performed for 6 h, and each extraction cycle took approximately 10 min with adjustable temperature. After obtaining the extract, the three wood residues of absolutely dry samples were baked to constant weight to calculate their extract yields,
(1)
where C is the extract yield; M1 is the mass of absolutely dry sample before extraction; and M2 is the mass of wood residues after extraction.
GC-MS analysis
An Agilent (USA) 6890N+5795C GC-MS and Agilent DB-5MS capillary quartz column (30 m × 0.25 mm × 0.25 μm) were used to analyze the extracts in this study. Each extract was concentrated to approximately 1mL using a rotary evaporator (Shanghai YaRong Biochemical Instrument Co., Ltd) and then transferred to a 5 mL volumetric flask, diluted with benzene-ethanol solvent to the volume mark, and filtered with a microporous membrane of 0.45 μm. Then, 1 μL of the final extracted sample was injected into the GC-MS using a split ratio of 20:1 and inlet temperature of 290 °C. Helium was used as a carrier gas at a constant flow rate of 1.4 mL/min. The oven temperature program was initially set to 80 °C for the first 5 min, rose to 120 °C at a rate of 20 °C/min, then rose to 250 °C at a rate of 10 °C/min, and finally to 300 °C at a rate of 5 °C/min, where it was held for 5 min. The ionization mode of MS was electron ionization (EI), electron energy was 70eV, ion source temperature was 200 °C, quadropole temperature was 150 °C, and mass scan range was 30 to 500 amu. Identification of compounds was based on comparison of their spectra and relative abundance with NIST08 libraries (http://www.nist.gov/srd/). Those compounds with more than 80% matching degree same as previous reports (Zhao et al. 2014) were qualitative in this study.
RESULTS AND DISCUSSION
Compounds Extracted from Dalbergia bariensis
The GC-MS successfully separated most compounds in the extract of raw Dalbergia bariensis heartwood, as shown by the presence of 95 peaks and their relative quantitative contents in the total ion chromatograms (Fig. 1).
There were a total of 42 compounds out of the 95 candidate compounds that had been identified and assigned chemical names (Table 2). These identified compounds primarily included aromatic compounds, aldehydes, ketones, and esters, and their peak areas occupied 75.571% of the total peak area. The main components were 6H-benzofuro[3,2-c][1]benzopyran-3-ol, 6a, 11a-dihydro-9-methoxy-, (6aR-cis)- (20.762%); 6a, 12a-dihydro-6H-(1,3)dioxolo(5,6)benzofuro(3,2-c)chromen-3-ol (15.052%); estroxide (10.314%); 4H-1-benzopyran-4-one, 7-hydroxy-3-(4-methoxyphenyl)-(9.642%); pseudobaptigenin (6.437%); methyl3-(1-formyl-3,4-methylenedioxy)benzoate (3.413%); Liquiritigenin (2.911%); benzofuran, 2,3-dihydro-(2.084%), and pyrimido[1,2-a]indole, 4-isopropyl-5-methyl-2-phenyl-(1.48%).
Fig. 1.Total ion chromatograms of the extracts of Dalbergia bariensis
Among these compounds, phenol and 4-methyl-(p-cresol) are used as disinfectants, resorcinol can sterilize, so it is often added in cosmetics, drug paste, and ointment for treating skin disease. Benzene and 1,2,3-trimethoxy-5-(2-propenyl)(Elemicin) have hypnotic and antiseptic effects. β-Endesmol is one of the main effective components of traditional Chinese medicine. Rhizoma atractylodishas a certain effect on protecting liver cells, treatment of diabetes, and gastrointestinal disorders, and is also a drug in treatment of nervous system diseases (Yoshinobu et al. 1983; Nakai et al. 2003; Jeon et al.2007; Yang et al. 2011).
Table 2. Identified Compounds inDalbergia bariensis
2-Propenal, 3-(4-hydroxy-3-methoxyphenyl)-(Conifer aldehyde) are used as antifungal and anti-swelling agents and as the biosynthesis inhibitor for prostaglandin, 9, 12-Octadecadienoic acid (Z,Z)- which has the effects of decreasing the blood lipid level and blood pressure and softening the human blood vessels. It also facilitates microcirculation, which can prevent or reduce the incidence of cardiovascular disease and is especially useful for the prevention of high blood pressure, high cholesterol, angina, coronary heart disease (CHD), and atherosclerosis. Meanwhile, 9,12-octadecadienoic acid (Z,Z)(linoleic acid) is also regarded as a scavenger receptor and helps prevent cardiovascular disease and atherosclerosis (Rodrigues 2010). 9,12,15-octadecatrienoic acid, (Z,Z,Z)-(linolenic acid) has the effect of reducing blood fat and blood pressure (Lorente et al. 2012). Pinocembrin can resist Staphylococcus aureus infection and inflammation (Fu et al. 2013; Duan et al. 2006). 6H-Benzofuro[3,2-c] (Ito et al. 2003) benzopyran-3-ol, 6a,11a-dihydro-9-methoxy-, (6aR-cis)-(medicarpin), flavonoids with high biological activity (Li et al.2001; Martinez et al. 2011) have been detected in licorice and other herbal products. 4H-1-benzopyran-4-one and 7-hydroxy-3-(4-methoxyphenyl), which have anti-cancer effects, are beneficial for prevention of colon, breast, and prostate cancers and improvement of menopausal symptoms and hot flashes, cyclic breast pain or tenderness (mastalgia).
Liquiritigenin has the effects of anti-cancer, anti-ulcer, anti-bacteria, and anti-virus (Maggiolini et al. 2002; Kanno et al. 2005; Renugadevi 2010; Fu et al. 2013). At the same time, 4′-methoxy-5,7-dihydroxy isoflavone; 2-butenedioic acid (Z)-, diethyl ester; 4-methyl-2,5-dimethoxybenzaldehyde, and other components are often used as pharmaceutical intermediates. From these compounds we can see Dalbergia bariensis has high medical value and health care potential to human.
Extract Yields of Absolutely Dry Samples
Modern conventional kiln (CK) processing is used for wood drying in China and throughout the world. There have been many studies on drying technology, drying schedules, and drying properties of rosewood (Li et al. 2001; Cai and Sun 2013; Torelli et al.1995a,b); however, few studies have been carried out on the effect of wood extracts of drying methods. In this study, wood extracts were studied and analyzed. The extract yields of the absolutely dry samples were calculated using Eq. 1 and compared among the three drying methods, as shown in Table 3. The extract yield from the absolutely dry sample by the VFD method was the maximum (24.471%). This result demonstrates that the VFD method outperformed the CD and VD methods with respect to preserving the organic compounds existing in Dalbergia bariensis. In the VFD process, the sample was frozen quickly at -18 to 30 °Cat first, so the internal moisture was fixed in its original position and became uniform with tiny ice formation, which was sublimated directly into water vapor under vacuum conditions. Eventually, moisture was removed, and the sample became dry. The drying process occurred at low temperature and vacuum conditions so that thermal reaction and oxidation can be avoided (Maggiolini et al. 2002; Sadoth et al. 2012).
Table 3. Extract Yields of the Absolutely Dry Samples Dried by Various Methods
Comparison of the Three Extracts from Absolutely Dry Samples
To further verify the above experimental results concerning the extract yields of absolutely dry samples, the three extracts were analyzed by GC-MS. Although 25 compounds out of 50 peaks had been identified in the extract sample treated by VFD, only 25 and 50 compounds out of 25 and 61 peaks had been identified in extract samples treated by CD and VD, respectively.
The identified components in the three types of absolutely dry samples were all fewer than that in the raw material, which has 25 unique components. Specifically, only 10 kinds of compounds with obvious health care effects were detected after drying compared with un-dried materials, in which 13 kinds were detected. Benzene, 1,2,3-trimethoxy-5-(2-propenyl)-; 2-propenal,3-(4-hydroxy-3-methoxyphenyl)-; 9,12-octadeca-dienoic acid (Z,Z)-; and 9,12,15-octadecatrienoic acid, (Z,Z,Z)- were not be detected in heartwood material after drying. Those peaks area corresponding to the compounds were compared and are shown in Table 4.
Table 4. Comparison of Peak Area Corresponding to Each Compound
ND: not detected
Under set operating conditions, the mass of analyzed components or their concentrations in carrier gas is proportional to the corresponding peak areas. Therefore, it is feasible to use peak area to represent the relative amount of components (Yoshinobu 1983; Xu et al. 2010). For the VFD sample, the total peak area was larger than those of either the CD sample or the VD sample. Except for 1-hexanol,2-ethyl-; benzofuran, 2,3-dihydro-; phenol,3-methoxy-; 4-methyl-2,5-dimethoxybenzaldehyde; 1,2-benzene-dicarboxylic acid, mono(2-ethylhexyl) ester, and 7-acetyl-4,6-dihydroxy-2′,5-dimethyl-3-methylenespiro(benzofuran-2(3H),1′-(2)cyclopenten)-4′-one, every peak area in the VFD sample was also larger than the corresponding peak areas of the CD sample or VD sample.
Fig. 2. Comparison of pharmaceutical ingredients peak area
a: Phenol, 4-methyl-; b: 2-Butenedioic acid(Z)-,diethyl ester; c: Resorcinol; d: 4-Methyl-2,5-dimethoxybenzaldehyde; e: β-Eudesmol; f: Pinocembrin; g: 6H-Benzofuro[3,2-c][1]benzopyran-3-ol, 6a,11a-dihydro-9-methoxy-, (6aR-cis)-; h: 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-methoxyphenyl)-; i: 4′-Methoxy-5,7-dihydroxy isoflavone; j: Liquiritigenin
The peak areas of 10 kinds of pharmaceutical ingredients from samples treated by the three drying methods were plotted and compared (Fig. 2). In general, the sample treated by VFD had a higher mass for each pharmaceutical ingredient than the other samples, excepting 4-methyl-2,5-dimethoxybenzaldehyde. Clearly, the sample treated by VFD had a larger mass of phenol, 4-methyl; β-eudesmol; 6H-benzofuro[3,2-c][1] benzopyran-3-ol, 6a,11a-dihydro-9-methoxy-, (6aR-cis)-;4H-1-benzopyran-4-one, 7-hydroxy-3-(4-methoxy-phenyl)-; and 4′-methoxy-5,7-dihydroxy isoflavone in comparison to the samples treated by CD or VD. Thus, VFD can be regarded as the most favorable method for preserving the components and their mass in Dalbergia bariensis, compared with the other two drying methods.
For the VD sample, the total peak areas were the smallest. This result is consistent with the conclusion from calculating the extract yields.
CONCLUSIONS
- The extracts of Dalbergia bariensis heartwood were analyzed by GC-MS. A total of 42 compounds were identified in the extracts.
- Several compounds have obvious medical efficacy, e.g., anti-bacteria, anti-inflammation, antioxidation, scavenging free radicals, reducing blood lipid, anti-cancer, and physiological benefits, such as phenol, 4-methyl-,2-butenedioic acid(Z)-,diethyl ester, and resorcinol etc. Therefore, Dalbergia bariensis also has high value and potential to be used for rosewood furniture products as a novel health care function.
- VFD method has better performance over CD and VD methods with respect to preserving the physiologically valuable ingredients and total mass of organic compounds in Dalbergia bariensis.
ACKNOWLEDGMENTS
This study was financially supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the second batch of Special University-Industry Cooperation Research Funds in 2013 (grant no. 2013C2FC0038), the Startup Foundation of Scientific Research, Nanjing Forestry University (GXL026), National Natural Science Foundation of China (Grant No. 31570558 and 31500479), and the Science and Technology Innovation Project of Guangdong Yihua Timber Co., Ltd (Grant No.YH-NL-2014002A).
REFERENCES CITED
Cai, Y. C., Chen, G. Y., Ai, M. Y., and Sun, H. F. (2005). “Discussion on improving measurement accuracy of wood moisture content by oven drying method,” Journal of Beijing Forestry University 27(S1), 64-67.DOI:10.13332/j.1000-1522.2005.s1.015
Chuankhayan, P., Rimlumduan, T., Svasti, J., and Cairns, J. R. (2007). “Hydrolysis of soybean isoflavonoid glycosides by Dalbergia beta-glucosidases,” Journal of Agricultural and Food Chemistry 55(6), 2407-2412.DOI: 10.1021/jf062885p
Conde, E., Fang, W. W., Hemming, J., Willför, S., Domínguez, H., and Parajó, J. C. (2014). “Recovery of bioactive compounds from Pinus pinaster wood by consecutive extraction stages,” Wood Science and Technology 48(2), 311-323.DOI: 10.1007/s00226-013-0604-1
Dong, H. J., and Sun, Y. (2013). “Study on drying schedule for scented rosewood and rosewood board,” Furniture 34(2), 30-33.
Duan, Y. B., Qi, Y., Ji, Z., Fang, G., and Cheng, Y. H., and Wu, S. (2006). “Synthesis and antimicrobial activity of pinocembrin and its derivatives,” Chinese Journal of Medicinal Chemistry 16(6), 342-346.
Edmone, R. (2006). “Volatile organic compounds and formaldehyde in nature, wood and wood based panels,”Holz als Roh-und Werkstoff 64(2), 144-149.DOI: 10.1007/s00107-005-0061-0
Esteves, B., Videira, R., and Pereira, H. (2011). “Chemistry and ecotoxicity of heat-treated pine wood extractives,” Wood Science and Technology 45(4), 661-676.DOI: 10.1007/s00226-010-0356-0
Fu, Y., Chen, J., Li, Y.J., Zheng, Y. F., and Li, P. (2013). “Antioxidant and anti-inflammatory activities of six flavonoids separated from liquorice,” Food Chemistry 141(2), 1063-1071.DOI: 10.1016/j.foodchem.2013.03.089
Innocent, E., Magadula, J. J., Kihampa, C., and Heydenreich, M. (2010). “Bioactive isoflavones from Dalbergia vacciniifolia(Fabaceae),” Natural Product Communications 5(6), 903-906.
Ito, C., Itoigawa, M., Kanematsu, T., Ruangrungsi, N., Higashihara, H., Tokuda, H., Nishino, H., and Furukawa, H. (2003). “New cinnamylphenols from Dalbergia species with cancer chemopreventive activity,” Journal of Natural Products 66(12), 1574-1577. DOI:10.1021/np0302450
Jeon, S., Kim, H. K., Kim, H. J., Do, G. M., Jeong, T. S, Park, Y. B., and Choi, M. S. (2007). “Hypocholesterolemic and antioxidative effects of naringenin and its two metabolites in high-cholesterol fed rats,” Translational Research 149(1), 15-21.DOI: 10.1016/j.trsl.2006.08.001
Kanno, S., Tomizawa, A., Hiura, T., Osanai, Y., Shouji, A., Ujibe, M., Ohtake, T., Kimura, K., and Ishikawa, M. (2005). “Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice,” Biological and Pharmaceutical Bulletin 28(3), 527-530.DOI: 10.1248/bpb.28.527
Khyasudeen, S. F., and Abu Bakar, M. (2006). “Aromatherapy: It’s effect on brain signal, math computation, blood pressure and heart rate,” 3rd Kuala Lumpur International Conference on Biomedical Engineering, Kuala Lumpur, Malaysia, pp.447-450.
Lee S. H., Kim, J. Y., Seo, G. S., Kim, Y.C., and Sohn, D. H. (2009). “Isoliquiritigenin, from Dalbergia odorifera, up-regulates anti-inflammatory heme oxygenase-1 expression in RAW264.7 macrophages,” Inflammation Research 58(5), 257-262.DOI: 10.1007/s00011-008-8183-6
Li, W. D., Kan Y. M., Hibg, M., and Zhu, Q. (2001). “The inhibitory effects of homopterocarpin and medicarpin on human’s liver cancer cells in vitro,” Journal of Shenyang Pharmaceutical University 18(3), 211-212.DOI:10.14066/j.cnki.cn21-1349/r.2001.03.016
Li, Y. Z., Ma, Z. F., Liu, Z. J., and Zhang, H. (2001). “Drying characters of five tropical wood species,” China Forest Products Industry 28(6), 10-12.
Liu, R. X., Wang, Q., Guo, H. Z., Lia, L., Bi, S. K., and Guo, D. A. (2005). “Simultaneous determination of 10 major flavonoids in Dalbergia odorifera by high performance liquid chromatography,” Journal of Pharmaceutical and Biomedical Analysis 39(3-4), 469-476. DOI: 10.1016/j.jpba.2005.04.007
Lorente, C. S., Costa, A.G., Navas, C. S., Zabala, M., Martinez, J. A., and Moreno, A. M. J. (2012). “Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence,” Journal of Physiology Biochemistry 69(3), 633-651. DOI: 10.1007/s13105-013-0265-4
Maggiolini, M., Statti, G., Vivacqua, A., Gabriele. S., Rago, V., Loizzo, M., Menichini, F., and Amdò, S. (2002). “Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells,” Journal of Steroid Biochemistry and Molecular Biology 82(4-5), 315-322.DOI: 10.1016/S0960-0760(02)00230-3
Martinez-Sotres, C., Lopez-Albarran, P., Cruz-de-Leon, J., Garcia-Moreno, T., Rutiaga-Quinones, J.G., Vazquez-Marrufo, G., Tamariz-Mascarua, J., and Herrera-Bucio, R. (2012). “Medicarpin, an antifungal compound identified in hexane extract of Dalbergia congestiflora Pittier heartwood,” International Biodeterioration and Biodegradation69, 38-40.DOI: 10.1016/j.ibiod.2011.11.016
Nakai, Y., Kido, T., Hashimoto, K., Kase, Y., Sakakibara, I., Higuchi, M., and Sasaki, H. (2003). “Effect of the rhizomes of Atractyledes lancea and its constituents on the delay of gastric emptying,” Journal of Ethnopharmacology 84(1), 51-55.DOI: 10.1016/S0378-8741(02)00260-X
Renugadevi, J., and Prabu, S. M. (2010). “Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin,” Experimental Toxicologic Pathology 62(2), 171-181.DOI: 10.1016/j.etp.2009.03.010
Rodrigues, H.G., Vinolo, M. A. R., Magdalon, J., Fujiwara, H., Cavalcanti, D. M. H., Farsky, S. H. P., Calder, P. C., Hatanaka, E., and Curi, R. (2010).“Dietary free oleic and linoleic acid enhances neutrophil function and modulates the inflammatory response in rats,” Lipids45 (9), 809-819.DOI: 10.1007/s11745-010-3461-9
Sadoth, S. T., Wahbi, J., Francoise, M., and Jean-Rodolphe, P. (2012). “Colour alteration and chemistry changes in oak wood (Quercus pedunculata Ehrh) during plain vacuum drying,” Wood Science and Technology 46(1-3), 177-191.DOI: 10.1007/s00226-010-0381-z
Tao, Y., and Wang, Y. (2009). “Bioactive sesquiterpenes isolated from the essential oil of Dalbergia odorifera T. Chen,” Fitoterapia 81(5), 393-396.DOI: 10.1016/j.fitote.2009.11.012
Torelli, N., and Gorisek, Z.(1995a).“Mexican tropical hardwoods: Stepwise shrinkage and traverse shrinkage anisotropy,”Holz als Roh-und Werkstoff 53(3), 155-157.DOI: 10.1007/s001070050107
Torelli, N., and Gorisek, Z. (1995b). “Mexican tropical hardwoods: Seasoning characteristics and recommended drying schedules,”Holz als Roh-und Werkstoff 53(5), 355-356.DOI:10.1007/s001070050107
Umehara, K., Nemoto, K., Matsushita, A.,Terada, E., Monthakantirat, O., De-Eknamkul, W., Miyase, T., Warashina, T., Degawa, M., and Noguchi, H. (2009).“Flavonoids from the heartwood of the Thai medicinal plant Dalbergia parviflora and their effects on estrogenic-responsive human breast cancer cells,” Journal of Natural Products72(12), 2163-2168. DOI:10.1021/np900676y
Wang, K. (2006). “The health care effect and harm of wood on human (1) The health care effect of wood on human,” China Forest Products Industry 33(1), 65-67.
Wu, K. K., and Wang, F. (2003).“Study of kinetics for activation of Chinese medicinal plant Dalbergia odorifera T. Chen to tyrosine,” China Surfactant Detergent & Cosmetics 33(3), 204-206.DOI: 10.13218/j.cnki.csdc.2003.03.019
Xu, M. Q. (2013). “Our redwood furniture industry needs new way to go,” Furniture & Interior Design 11, 11-12.
Xu, J. H., Wen, S. W., and Wu, D.P. (2010). “Influence of temperature on pythoncidere of Lavandula angustifolia Mill flower,”Acta Ecologica Sinica 30(3), 645-651.
Yang, J., Li, Q., Zhou, X.D., Kolosov, V. P, and Perelman, J. M. (2011). “Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-kappa B activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells,” Molecular and Cellular Biochemistry 351(1-2), 29-40.DOI: 10.1007/s11010-010-0708-y
Yoshinobu, K., Masahiro, T., and Hiroshi H. (1983). “Antihepatotoxic principles of atractylodes rhizomes,” Journal of Natural Products 46(5), 651-654.DOI:10.1021/np50029a010
Zhao, Q., Guo, J., and Zhang, Y. (2000). “Chemical and pharmacological research progress of Chinese drug ‘‘JiangXiang’’ (Lignum Dalbergia odorifera),”Journal of Chinese Pharmaceutical Science 9(1), 1-5.
Zhao, R. J., Li, J., Fang, G. Z., and Li, S.J. (2002). “The effects of wood extraction on mammal growth,” China Wood Industry 16(3), 19-21.
Zhao, X., Zhao X. H., Zu Y. G., Z. Q., Wu Y. (2014).“The Analysis of Volatile Composition from the Leaves and Culm of Neosinocalamus affinis in Muchuan of Sichuan Province,” Botanical Research 3(6), 249-256. DOI: 10.12677/BR.2014.36031
Article submitted: April 29, 2015; Peer review completed: July 24, 2015; Revised version received: August 25, 2015; Accepted: August 26, 2015; Published: September 2, 2015.
DOI: 10.15376/biores.10.4.7092-7104
