Abstract
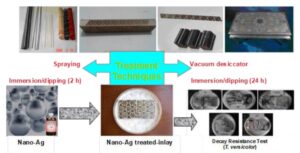
Four types of Shiraz inlay (Khatam) samples were impregnated with nano-silver solution via spraying (S), a vacuum desiccator (VD), and immersion/dipping (I/D) for 2 h and 24 h techniques. The decay resistance and dimensional stability were evaluated after each treatment. The impregnated specimens were exposed to white-rot fungus for 10 weeks by the agar-plate method, and the weight loss was determined to assess decay. The dimensional stability of samples after 2 and 24 h soaking in water were measured. According to the results, the highest weight loss (13.1%) was observed in the Shesh-Pareh Khatam (SPK)-untreated samples and the lowest weight loss (1.5%) occurred in the Ostokhani Khatam (OK)-VD-treated samples. The highest volumetric shrinkage (39.0%) was observed in the Haft Shamseh Alam-Zar Khatam (HSAZK)-untreated samples after 24 h soaking, and the lowest volumetric shrinkage (3.8%) was noted in the OK-S-treated samples after 2 h soaking. The highest volumetric swelling (64.1%) was observed in the HSAZK-untreated samples after 24 h soaking, and the lowest volumetric swelling (4%) was found in the OK-S-treated samples after 2 h soaking in water. Generally, the highest anti-shrinkage-efficiency (111.0%) and anti-swelling-efficiency (145.4%) after 24 h soaking in water were observed in the OK-S-treated and SPK-VD-treated samples, respectively.
Download PDF
Full Article
Effects of Various Treatment Methods on Decay Resistance and Dimensional Stability of Nano-silver-treated Persian/Shiraz Inlay (Khatam)
Mohammad-Hossein Salahfard, and Seyyed Khalil Hosseinihashemi*
Four types of Shiraz inlay (Khatam) samples were impregnated with nano-silver solution via spraying (S), a vacuum desiccator (VD), and immersion/dipping (I/D) for 2 h and 24 h techniques. The decay resistance and dimensional stability were evaluated after each treatment. The impregnated specimens were exposed to white-rot fungus for 10 weeks by the agar-plate method, and the weight loss was determined to assess decay. The dimensional stability of samples after 2 and 24 h soaking in water were measured. According to the results, the highest weight loss (13.1%) was observed in the Shesh-Pareh Khatam (SPK)-untreated samples and the lowest weight loss (1.5%) occurred in the Ostokhani Khatam (OK)-VD-treated samples. The highest volumetric shrinkage (39.0%) was observed in the Haft Shamseh Alam-Zar Khatam (HSAZK)-untreated samples after 24 h soaking, and the lowest volumetric shrinkage (3.8%) was noted in the OK-S-treated samples after 2 h soaking. The highest volumetric swelling (64.1%) was observed in the HSAZK-untreated samples after 24 h soaking, and the lowest volumetric swelling (4%) was found in the OK-S-treated samples after 2 h soaking in water. Generally, the highest anti-shrinkage-efficiency (111.0%) and anti-swelling-efficiency (145.4%) after 24 h soaking in water were observed in the OK-S-treated and SPK-VD-treated samples, respectively.
DOI: 10.15376/biores.17.3.5268-5284
Keywords: Nano-treated Shiraz inlay; Impregnation methods; Decay resistance; Dimensional stability; Anti-shrinkage efficiency; Anti-swelling efficiency
Contact information: Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University, Karaj, Iran; *Corresponding author: hashemi@kiau.ac.ir
GRAPHICAL ABSTRACT

INTRODUCTION
Khatam is an ancient Persian technique of inlaying. It involves decorating the surface of wood with delicate pieces of metal, bone, and wood precisely cut in geometrical figures and shapes, adhesive, and polish material (Rozi Talab and Jalali 2003; Shojanoori 2014). Common materials used in the construction of inlaid articles are gold, silver, bronze, brass, aluminum, and twisted wire (Safra 2010; Siavoshan 2019).
This craft consists in the production of incrustation patterns (generally star-shaped), with thin sticks of wood (ebony, teak, ziziphus, orange, rose, walnut, maple, or sycamore), brass (for golden parts), and camel bones (white parts). Ivory, gold, or silver can also be used for collection objects. These sticks are made into triangular beams, assembled, and glued in a strict order to create a geometrical motif such as a six-branch star included in a hexagon. Many objects can be decorated in this fashion, including jewelry boxes, tables, chessboards, pipes, desks, beds, frames, or musical instruments. In some historical mosques, it can also be found in places of pilgrimage, royal buildings, and various items that have been inlaid. These specimens can be observed in the cities of Mahshad, Qom, Shiraz, Isfahan, and Rey in Iran. It is suggested that inlay work for the first time appeared in Shiraz and the best model of this art is the inlay table that received the gold medal in the Bruxesl world exhibition in the year 1958 (Mahdavi 2022). An example of handicraft (Shiraz inlaid table) is illustrated in Fig. 1.
Fig. 1. An example of a handicraft (Shiraz inlaid table, a) that has been submitted and registered worldwide (UNESCO 2012) by the author (Mohammad-Hossein Salahfard) and received certificate (b)
Wood is a natural organic material that has been used for many centuries for the construction of buildings, bridges, and a variety of other structures (Harte 2009). This natural polymeric material is composed of three main components of cellulose, lignin, and hemicellulose. The presence of hydrophilic hydroxyl groups of wood components attracts water molecules through hydrogen bonding from the surrounding environment, resulting in the wood swelling and creates dimensional instability (Islam et al. 2012).
Wood has valuable properties and a unique structure, despite some disadvantages, such as poor dimensional stability and high moisture absorption, which results in rapid deterioration by rotting fungi (Kumar 1994; Galpperin et al. 1995; Devi et al. 2003). Unlike some other types of artifacts, such as natural or man-made stone, the wooden ones are much more exposed to degradation (Fierascu et al. 2020).
Conservation of wooden artifacts represents a common goal for researchers involved in cultural heritage studies globally (Fierascu et al. 2020). The cited authors applied six potential immersion treatments for experiments with wooden models consisting of wood spatulas with standard dimensions (150×18×1.6 mm3).
Nanotechnology applications have potential for improving materials (Moya et al. 2014). Silver nanoparticles have been considered for various applications (Mahapatra et al. 2013) that have improved wood protection (Liu et al. 2002a,b,c), behavior against fire (Taghiyari 2012), physical properties (Taghiyari and Bibalan 2013), etc. According to Dorau et al. (2004), wood decay fungi have shown susceptibility to silver nanoparticles because the silver ions, in solution, inhibit the activity of their cellulosic enzymes (Highley 1975).
Velmurugan et al. (2009) found that mycelial growth decreased with nanosilver concentration. The highest mycelial growth occurred at a concentration of 1.0 ppm, and the lowest growth was found at a concentration of 100 ppm. Liu et al. (2001, 2002a,b,c) demonstrated decreased wood decay with different stabilizing agents and with addition of various fungicides to silver nanoparticle solution.
The inlaid objects are sensitive to heat and humidity, and if they are not of high quality, when adjacent to such elements they will peel off the surface of the object. The most important dangers are due to various climatic conditions occurring in different places that may destroy this work of art, such as deformation (change of dimensions) of wood because of the sorption and desorption of moisture, swelling, and shrinkage, or the rot caused by wood-deterioration fungi.
No study has yet discussed the impregnation of inlaid works for increasing their durability against decay resistance and their dimensional stability against water sorption and desorption. In this study, the decay resistance, shrinkage and swelling behavior, anti-shrinkage, and anti-swelling efficiencies as the dimensional stabilities of several types of inlay (Shesh-Pareh Khatam (SPK), Ostokhani Khatam (OK), Haft Shamseh Alam-Zar Khatam (HSAZK), and Resorcinol Shesh-Pareh Khatam (RSPK)) treated by different impregnation methods (spraying (S), vacuum desiccator (VD), immersion/dipping 2 h (I/D 2 h), and immersion/dipping 24 h (I/D 24 h)) were investigated and compared.
EXPERIMENTAL
Inlaying Materials
Different types of wood, such as areca, ebony, jujube, elm, logwood, orange, betel nut, boxwood, alder, aspen, beech, walnut, sycamore, maple, and some colored wood, are used for inlay. Different types of metal, such as gold or silver wires, copper, brass, and aluminum, are also used for inlay. Camel leg and arm bones, horse bone, artificial bone, and elephant tusk also are used in the work. In this study, maple (Acer insigne), walnut (Juglans regia), and sycamore (Platanus orientalis) as lining and triangular wood materials, brass as metal, and camel bone were used (Table 1).
Table 1. Approximate Percentages of Persian/Shiraz Inlay Ingredients and their Density (g/cm3) in Parentheses
Adhesives including wood glue, resorcinol glue (Mitreapel Co., Aydin, Turkey), and fish glue (Kremer Pigmente GmbH & Co. KG., Aichstetten, Germany) were employed in this study. Adhesive 801 (Samad Co., Mashhad, Iran) is a standard wood adhesive based on polyvinyl acetate (PVA) that has a strong adhesion and medium water resistance. It gives a transparent and colorless adhesive film after drying. In order to waterproof the RSPK, resorcinol glue was used instead of wood glue (PVA) (Table 1).
The fish glue in granules of 250 g was used for gluing the lining wood and was also placed between the lining wood and triangular wood. The adhesive is thermoplastic and exposed to indirect heat in a bain-marie bathroom (Table 1). The sides of each frame were covered and glued with a layer of lining (shell) wood with thickness of 0.5 mm from maple wood (density of 0.63 to 0.75 g/cm3). The tools and equipment required for inlay making are: various types of graters, clamps for grinding triangles, compass and conveyor, ruler, set square, hammer, pincher, drill, circular saw, etc.
Manufacturing Process
The steps involved in inlay making are: (1) triangulation of stick; (2) warp of inlay; (3) cut of inlay; (4) gluing of inlay; and (5) lubrication. In the first stage, the wood and bone tapes with different colors are cut into dimensions of 30 cm length and 1.0 to 2.5 mm diameter or thickness (Figs. 2a, 2b, and 2c) and filed in such a way that a clean and triangular cross-section is obtained, traditionally called hexagon star “Shesh” (Fig. 2d).
Fig. 2. Grinding triangles inside a triangular mold (a); wood with color and triangular section (b); triangular wood bundle (c); “Pareh” and “Shesh” (d); view of the cross-section of an Ostokhani “Shesh” (e); triangular wood and brass wire, “Pareh”, “Shesh”, and “Luz” of inlay (f); Qameh (g); 6 cm cut “Luz”, and “Barg” of inlay (h); view of cross-section of an “Abri” inlay “Luz” (i); a sample of Haft Shamseh Alam-Zar inlay (j)
These tapes in addition to the brass tape, which has become similar to wood and bone pieces through wiredrawing, are put side-by-side in the form of a colorful and regular layout using a layer of glue. The prepared hexagon stars are fastened with a thread so that while drying, they may be stuck together very tightly “Pareh” (Fig. 2d). Figure 2e show the cross-section of an Ostokhani “Shesh”.
“Pareh” consists of a triangular wire in the middle and around it from three triangular sticks. Six “Pareh” are glued together and the “Shesh” is formed, 25 “Shesh” and 50 “Pareh” are glued together and the inlay “Luz” is formed (Fig. 2f).
This action continues until a complete flower with a height of 60 cm is obtained. Having prepared several flowers, they are put side-by-side between two boards and the spaces between them are filled with glue or wood paste. Then they are made into a monolithic piece using a press and called a rod of inlaid work, “Qameh” (Fig. 2g). Then the craftsman cuts layers with 3 mm thickness from the surface of “Qameh” using a coping saw and then it is called plate “Lengeh” or “Barg” (Fig. 2h). Figures 2i and 2j show the cross-section of an “Abri” inlay “Luz” and a sample of Haft Shamseh Alam-Zar inlay, respectively. Once the understructure is prepared via carpentry, the inlaid works are stuck-onto the background of the work through special glues and by heating based on their design, color, shape, and dimensions. After priming, rubbing, and restoring the surface of the work, the inlaid work is prepared and finally it is covered by a layer of polyester (https://isfahaninfo.com/inlaid-working/).
Preparation of Inlay Samples
In this study, two sides of “Barg” were glued by means of two layers of maple lining wood pieces with thickness of 0.5 mm. The final thickness of “Barg” was 4 mm. Then, layers with 1.7 mm thickness were cut from the surface of “Barg” using a coping saw with 0.5 mm saw thickness and the remaining 0.1 mm is the way of the saw.
Inlay samples with dimensions of 1.7 × 30 × 30 mm3 were prepared from “Barg” of HSAZK that contains bones. Inlay samples with dimension of 1.7 × 25 × 30 mm3 were prepared from “Barg” of OK that contains bones, SPK, and RSPK that was made with resorcinol adhesive.
Samples were oven-dried for 24 h at 103 ± 2 °C to determine the dry weight before treatment. Samples for each treatment were placed into treatment solutions. Each value for dimensional stability and each value for decay resistance recorded were the mean of five and four replicates, respectively.
Inlay Test Specimens Impregnation
The Inlay test specimens were treated (T) with solution of nanosilver (N-Ag) (Nanosav Co., Tehran, Iran) at a concentration of 100 ppm containing 70-nm particles.
All treatments used in the present study are summarized in Table 2.
Table 2. Nanosilver Treatment of Shiraz Inlay
Particles with diameters less than 100 nm are required to have the surface area necessary to allow a continuous release of silver ions (Velmurugan et al. 2009; Moya et al. 2014).
To reach uniform absorption and distribution of the solution, 56 test specimens (40 specimens for dimensional stability + 16 specimens for decay resistance) were continuously sprayed with treatment solution for 5 min. A set of another 56 specimens were submerged in the treatment solution applying 0.67 bar (9.8 psi) vacuum in a vacuum desiccator for 20 min according to modified method of Hosseini Hashemi and Jahan Latibari (2011). Another 56 specimens were immersed and treated in the treatment solution for 2 h. In addition, 56 specimens were immersed in the treatment solution for 24 h according to modified method of Hosseini Hashemi et al. (2010).
Specimens were then wiped to remove the excess solution from the surface and weighed to determine the amount of treating solution absorbed. In this study, 40 untreated (UT) specimens were undecayed (UD), and 16 untreated specimens were decayed (D).
Dimensional Stability Measurements
Dimensional stability data were determined by measuring the values of radial, tangential, longitudinal, and volumetric shrinkage (BV) and volumetric swelling (VS), anti-shrinkage efficiency (AShE), and anti-swelling efficiency (ASwE) of four types of inlay using the ASTM D143 (2007) standard test method.
Inlay treated and untreated samples with dimensions of 1.7 × 30 × 30 mm3 and 1.7 × 25 × 30 mm3 according to Table 2 were used for BV, SV, AShE, and ASwE tests. The inlay treated and untreated samples were dipped in distilled water for 2 or 24 h. Wet dimensions were measured to determine the swelling after each soaking time. Next, the samples were oven-dried, and their dry dimensions were again determined. The BV-2 h, SV-2 h, BV-24 h, SV-24 h, AShE-24 h, and ASwE-24 h in percentages were calculated based on the following equations,
BV-2 h (%) = [(VS2 h-VO)/VS 2 h × 100 (1)
SV-2 h (%) = [(VS2 h-VO)/VO × 100 (2)
BV-24 h (%) = [(VS24 h-VO)/VS 24 h × 100 (3)
SV-24 h (%) = [(VS24 h-VO)/VO × 100 (4)
AShE-24 h (%) = (BVc 24 h-BVt 24 h)/BVt 24 h × 100 (5)
ASwE-24 h (%) = (SVc 24 h-SVt 24 h)/SVt 24 h × 100 (6)
where VS is the saturated volume (cm3) of treated and untreated inlay specimens after each soaking time (h), and VO is the oven-dry volume (cm3) of treated and untreated inlay specimens. AShE (%) is the anti-shrinkage efficiency, BVc (%) is the volumetric shrinkage of untreated inlay after each soaking time, and BVt (%) is the volumetric shrinkage of treated inlay after each soaking time. ASwE (%) is the anti-swelling efficiency, SVc (%) is the volumetric swelling of untreated inlay after each soaking time (h), and SVt (%) is the volumetric swelling of treated inlay after each soaking time.
Decay Test
The decay test was conducted in accordance with BS 838 (1961), after exposure to Trametes versicolor for 10 weeks via the Petri plate’s method. Prior to inoculation, malt extract agar (MEA, 48 g/L) was used as an agent for the fungus culture. The agar medium was sterilized in an autoclave at a temperature of 120 °C and pressure of 1.2 atm for 20 min. Then, every Petri plate was inoculated by a small plug of T. versicolor and the Petri plates were kept at 23 °C for one week until the culture medium was fully covered by the fungus. Next, oven-dried weight of inlay specimens (of dimensions 1.7 × 30 × 30 mm3 and 1.7 × 25 × 30 mm3) for each treatment in an oven at 103 ± 2 °C for 24 h was determined. Subsequently, the sterilized samples (according to the previous method) were placed on the top of two small glass legs in Petri plate to avoid contact of specimens with fungal mycelia (Fig. 3).
Fig. 3. Shiraz inlay decay test specimens inoculated with white-rot fungus (T. versicolor) (a: two samples of SPK and b: two samples of HSAZK)
In total, 64 treated samples of four inlay types with N-Ag solution and 16 untreated samples of four inlay types were prepared (Table 2).
Both treated and untreated inlay samples were transferred to an incubator for 10 weeks and were incubated at 23 °C and 70% relative humidity until the samples were colonized by the T. versicolor. After the incubation time had elapsed, the samples were removed from the incubator and fungal mycelia were removed from the surface of the specimens. The inlay decayed samples were then placed in an oven at 103 ± 2 °C for 24 h to reach constant decayed weight (W2, g). Weight loss (WL, %) was calculated as a percentage of the initial sample weight (W1, g) using the following equation:
WL (%) = [(W1–W2)/W1] × 100 (7)
Statistical Analysis
An analysis of variance was conducted using the IBM SPSS statistics for Windows, Version 24.0, software package (SPSS Inc., IBM Corp., Armonk, NY, USA) (p< 0.05) to evaluate the effect of the different treatment methods on decay resistances and dimensional stabilities of the four inlay types. Significant difference among the average values of the inlay specimens were determined using Duncan’s multiple range test (DMRT).
RESULTS AND DISCUSSION
Weight Loss
Statistically, results in Fig. 4 showed that treatment method and inlay type had a significant effect on the WL of inlay as affected by T. versicolor. Figure 4a shows that the lowest WL values of inlay were observed via spraying (1.48%) in the treated-OK. The next-lowest results, respectively, were for the treated-RSPK, and treated-SPK by immersion/dipping 2 h and vacuum desiccator methods with WL values of 1.53%, and 1.56% (Figs. 4c and 4b).
Among the untreated inlay samples, the highest WL values were observed for UT-SPK (13.1%), followed by UT-HSAZK (4.2%), UT-RSPK (4.1%), and UT-OK (3.0%). It was observed that in the UT-OK with increasing camel bones (80%), the WL values were lower than those found with un-impregnated inlay.
The natural durability of wood was classified according to BS EN 350-2 (1994) standard of preservation of timber in the following five ratings: very durable, approximate life in ground contact (ALGC) > 25 years; durable, ALGC 15 to 25 years; moderately durable, ALGC 10 to 15 years; slightly durable, ALGC 5 to 10 years; and non-durable, ALGC > 5 years. According to the classification of natural durability, the sycamore wood is placed in the class of 5 or non-durable (ALGC > 5 years) (Crossman and Simm 2004).
Fig. 4. Change in WL of N-Ag-treated and untreated inlay samples after 10 weeks exposure to white-rot fungus (T. versicolor). * The same letters in each column indicated that there is no significant difference (p<0.05) between the inlay type and treatment method groups.
The high weight loss of UT-SPK is related further to the high percentage of sycamore wood (80%) used in the inlay and the low durability of sycamore wood against white-rot fungus. Conversely, the low weight loss of UT-OK is related further to the high percentage of camel bones (75%) and low percentage of sycamore wood (10%) used in the inlay.
Although the UT-RSPK has a high percentage of sycamore wood (80%), it has a low weight loss, which was attributed to the use of water-resistant resorcinol adhesive. Additionally, the low weight loss of UT-HSAZK was further related to the moderate percentage of camel bones (45%) and brass wire (30%) used in the inlay.
Figure 5 shows the growth of T. versicolor mycelium on the surface of untreated and nano-Ag-treated inlay samples after 10 weeks.
Fig. 5. Mycelium growth of T. versicolor fungus on treated-inlay specimens with Nano-Ag solution and decayed (S: spraying; VD: vacuum desiccator; I (2 h): immersion/dipping (2 h); I (24 h): immersion/dipping (24 h)) and the untreated decayed-inlay specimens (UT)
Moya et al. (2014) also showed that the mass loss of some commercial Costa Rica wood species after treatment by pressure and silver nanoparticles solution with a concentration of 50 ppm was less than 5% and the woods were classified as highly resistant or class A. Meanwhile, untreated wood presented losses greater than 20% with white-rot fungus.
Some studies have shown that Firmicutes and Bacteroidetes are the dominating phyla related to degradation of bones together with Proteobacteria and Actinobacteria (Damann and Jans 2017), although species of the genus Aspergillus are also known to contain collagenase-producing fungi (Yakovleva et al. 2006; Wanderly et al. 2017), which could be responsible for degradation of the collagen in the bone material. However, little research has been published on the genetics of bone-degrading fungi. The most abundant fungus genus found in the ethylenediaminetetraacetic acid (EDTA) method were either yeast (Cyberlindnera) (Kurtzman and Robnett 2013), pathogenic (Sarocladium) (Giraldo et al. 2015), or mushrooms (Clitocybe and Schizophyllum).
In addition to general environmental influences, micro-organisms play an important role in destruction of interred skeletal remains. Fungi are known to penetrate actively through hard tissues, but they also decompose dead bone by extensive excretion of secondary metabolites that partially leach the bone tissue. This leads to alterations in quality and quantity of both the organic and the inorganic bone matrix. Micro-organisms can also impregnate dead bone with persistent fluorophores and chromophores. These impregnations are often similar to various post-mortem or intra-vitam alterations, and can lead to misinterpretation (Piepenbrink 1986).
Dimensional Stability
Volumetric shrinkage of treated and untreated-inlay after 2 h soaking in distilled water
Table 3 presents the volumetric shrinkage (BV, %) in the different treated and untreated inlay samples after 2 h.
Table 3. Mean Values and Standard Deviations of BV (%) of Nano-Ag-treated and Untreated Samples after 2 h Soaking in Distilled Water
A different trend was found in BV, where the lowest BV was in the N-Ag-treated-OK and N-Ag-treated-RSPK samples using the spraying treatment, with values of 3.85% and 4.07% after 2 h, respectively.
The highest BV, with respect to the control, was in the HSAZK-treated specimens by spraying, vacuum desiccator, and immersion 2 h with values of 29.40%, 25.64%, and 23.42%, respectively. The highest and lowest BV in the untreated samples was for UT-OK and UT-RSPK with values of 31.09% and 13.48% after 2 h, respectively. In general, in both treated inlay samples with different methods and untreated inlay samples, the lowest BV was found in the RSPK samples and the highest was found in the HSAZK samples.
Volumetric shrinkage of treated and untreated-inlay after 24 h soaking in distilled water
Table 4 presents the volumetric shrinkage (BV, %) in various treatment conditions and that of untreated-inlay samples after 24 h.
A different trend was found for BV. The lowest BV was in the N-Ag-treated-RSPK samples via the spraying treatment method, with values of 10.63% after 24 h soaking in distilled water. The highest BV with respect to the control after 24 h soaking in water was found in the HSAZK-treated samples by spraying, vacuum desiccator, immersion 2 h, and immersion 24 h, with values of 38.97%, 31.11%, 28.21%, and 27.65%, respectively. The highest and lowest BV in the untreated samples was for UT-HSAZK and UT-RSPK with values of 35.87% and 17.39% after 24 h soaking in distilled water, respectively. In general, in both treated inlay samples with different methods and untreated inlay samples, the lowest BV was in the RSPK samples and the highest was found in the HSAZK samples. Totally, the best treatment method in the treated samples with the least volumetric shrinkage after 2 h and 24 h soaking in water was VD for SPK, spraying for RSPK and OK, and I/D (24 h) for HSAZK.
Table 4. Mean Values and Standard Deviations of BV (%) of Nano-Ag-treated and Untreated Samples after 24 h Soaking in Distilled Water
Volumetric swelling of treated and untreated-inlay after 2 h soaking in distilled water
Table 5 presents the volumetric swelling (SV, %) in the different treated and untreated inlay samples after 2 h soaking.
There was a different trend for SV. The lowest SV was in the N-Ag-treated-OK and N-Ag-treated-RSPK samples using the spraying treatment, with values of 4.0% and 4.25% after 2 h, respectively. The highest SV with respect to the control was in the HSAZK-treated by spraying, vacuum desiccator, and immersion 2 h with values of 42.14%, 32.67%, and 30.93%, respectively. The highest and lowest SV in the untreated samples were for the UT-SPK and UT-RSPK with values of 43.16% and 15.59% after 2 h, respectively.
Table 5. Mean Values and Standard Deviation of SV (%) of Nano-Ag-treated and Untreated Samples after 2 h Soaking in Distilled Water
Generally, in both treated-inlay samples with different methods and untreated-inlay samples, the lowest SV was in the RSPK samples and the highest was in the HSAZK samples. The best treatment method in the treated samples with the least volumetric swelling after 2 h soaking in distilled water was I/D (2 h) for SPK, spraying for RSPK and OK, and I/D (24 h) for HSAZK.
Volumetric swelling of treated and untreated-inlay after 24 h soaking in distilled water
Table 6 presents the volumetric swelling (SV, %) in the different treated and untreated inlay samples after 24 h soaking in distilled water.
Table 6. Mean Values and Standard Deviation of SV (%) of Nano-Ag-Treated and Untreated Samples after 24 h Soaking in Distilled Water
The lowest SV was in the N-Ag-treated-RSPK samples by spraying treatment (11.98%) after 24 h. The highest SV with respect to the control after 24 h soaking in distilled water was in the HSAZK-treated samples by spraying, vacuum desiccator, immersion 2 h, and immersion 24 h, with values of 64.14%, 45.19%, 39.61%, and 38.99%, respectively. The highest and lowest SV in the untreated samples was for UT-HSAZK and UT-RSPK with values of 56.06% and 21.07% after 24 h soaking in distilled water, respectively. In both treated inlay samples with different methods and untreated inlay samples, the lowest SV was in the RSPK samples and the highest was in the HSAZK samples.
The best treatment method in the treated samples with the least volumetric swelling after 24 h soaking in distilled water was VD for SPK, I/D (24 h) for OK and HSAZK, and spraying for RSPK.
Anti-shrinkage efficiency of treated and untreated-inlay samples after 24 h soaking in distilled water
Table 7 presents the anti-shrinkage efficiency (AShE, %) of the treated inlay samples after 24 h.
Table 7. Change in AShE (%) of Inlay Samples as Affected by Nano-Ag Treatment after 24 h Soaking in Distilled Water
The highest AShE was found in the Nano-Ag-treated-OK samples, with values of 111.03% after 24 h. The lowest AShE with respect to the control after 24 h was found in the Nano-Ag-treated-HSAZK (-7.56%).
Anti-swelling efficiency of treated and untreated-inlay samples after 24 h soaking in distilled water
Table 8 presents the anti-swelling efficiency (ASwE, %) of the treated inlay samples after 24 h soaking in water.
Table 8. Change in ASwE (%) of Inlay Samples as Affected by Nano-Ag Treatment after 24 h Soaking in Distilled Water
The highest ASwE was in the Nano-Ag-treated-SPK samples, with values of 145.39% after 24 h. The lowest ASwE with respect to the control after 24 h was in the Nano-Ag-treated-HSAZK (-11.60%).
Totally, the best treatment method in the treated samples with the highest anti-swelling and anti-shrinkage efficiencies, respectively, after 24 h soaking in distilled water was VD for SPK (145% and 17%), I/D (24 h) for OK (142% and 91%) and HSAZK (54% and 34%), and spraying for RSPK (86% and 71%).
Cellulose and hemicelluloses are the two main components of wood cell wall structure that are responsible for absorbing moisture in wood. However, with increasing contents of these two components the water sorption of wood will be increased. Consequently, the values of radial, tangential, and volumetric swelling of wood is increased. According to Fengel and Wegener (2003), the content of lignin in maple wood of different species is in the range of 20.7% to 25.3%; and according to Pettersen (1984), the cellulose content in different species of maple wood varies from 34% to 49%. The hemicellulose content of maple (Acer campestre L.) wood is in the range of 13.3% to 15.7% (Antczak et al. 2013). Jovicic et al. (2022) stated that the biggest difference between walnut shell biomass and wood is in the contents of cellulose and lignin (cellulose content from 39.18% to 48.38%, polyoses content from 22.69% to 32.41%, and lignin content from 21.82% to 27.96%).
The moisture content of heartwood of sugar maple, American sycamore, and black walnut is approximately 65%, 114%, and 90%, respectively, and the moisture content of sapwood of sugar maple, American sycamore, and black walnut is approximately 72%, 130%, and 73%, respectively (Simpson and TenWolde 1999). The highest wettability for sycamore may be attributed to a capillary effect related to its large fibers or a high amount of amorphous cellulose components on its thick cell wall (De Peres et al. 2020).
The radial shrinkage of sugar maple, American sycamore, and black walnut is approximately 4.9%, 5.1%, and 5.5%, respectively; the tangential shrinkage of sugar maple, American sycamore, and black walnut is approximately 9.5%, 7.6%, and 7.8%, respectively, and the volumetric shrinkage of sugar maple, American sycamore, and black walnut is approximately 14.7%, 14.1%, and 12.8%, respectively (Eckelman 1998; Glass and Zelinka 2010).
The immersion/dipping (24 h) method was one of the most successful methods for treating inlay samples with nanosilver preservative and increasing anti-swelling and anti-shrinkage efficiencies because immersion and impregnation are the most adequate processes to improve wood properties (Bak and Németh 2018).The spraying method was one of the most successful methods for treating inlay samples and increasing decay resistance because nanosilver-based disinfectant solutions are useful for disinfecting surfaces, learning tools in kindergartens, schools, workplaces, various types of equipment and computers, toys, all kinds of furniture and the like, in industrial and domestic, and public utilities (Tran et al. 2013).
CONCLUSIONS
- According to the obtained results, among the untreated inlay samples, the lowest weight loss was observed in OK and the lowest volumetric shrinkage and volumetric swelling was in the RSPK, but among the treated samples, the lowest weight loss, volumetric shrinkage, and volumetric swelling was observed in the OK and this issue is even important for some buyers of inlay items to know which of them are more durable and stable, but due to the use of high percentage of brass wire and variety of materials used in the HSAZK, it has a colorful and beautiful appearance and attract more buyers.
- The best treatment method in the treated samples with the highest anti-swelling and anti-shrinkage efficiencies after 24 h soaking in distilled water was VD for SPK, I/D (24 h) for OK and HSAZK, and spraying for RSPK.
- The immersion/dipping (24 h) was one of the most successful methods for treating and improving of anti-swelling and anti-shrinkage efficiencies of all types of inlay samples with nanosilver preservative.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Department of Wood Science and Paper Technology, Karaj Branch, Islamic Azad University.
REFERENCES CITED
Antczak, A., Michaluszko, A., Klosinska, T., and Drozdzek, M. (2013). “Determination of the structural substances content in the field maple wood (Acer campestre L.) –comparison of the classical methods with instrumental,”Ann. Warsaw Univ. Life Sci.-SGGW for Wood Technol. 82, 11-17.
ASTM D143 (2007). “Standard test methods for small clear specimens of timber,” ASTM International, West Conshohocken, PA, USA.
Bak, M., and Németh, R. (2018). “Effect of different nanoparticle treatments on the decay resistance of wood,” BioResources 13(4), 7886-7899. DOI: 10.15376/biores.13.4.7886-7899
BS 838 (1961). “Methods of test for toxicity of wood preservatives to fungi,” British Standards Institution, London, UK.
BS EN 350-2 (1994). “Durability of wood and wood-based products. Natural durability of solid wood. Guide to natural durability and treatability of selected wood species of importance in Europe,” British Standards Institution, London, UK.
Crossman, M., and Simm, J. D. (2004). Manual on the Use of Timber in Coastal and River Engineering,Thomas Telford Ltd., London, UK.
Damann, F. E., and Jans, M. M. E. (2017). “Microbes, anthropology and bones,” in: Forensic Microbiology, D. O. Carter, J. K. Tomberlin, M. E. Benbow, and J. L. Metcalf (eds.), John Wiley & Sons Ltd., Hoboken, NJ, USA.
De Peres, M. L., de Avila Delucis, R., Beltrame, R., and Gatto, D. A. (2020). “Hydrothermal treatments to promote surface inactivation and increased flexibility in three hardwoods,”Maderas. Cienc. Tecnol. 22(4), 439-446. DOI: 10.4067/S0718-221X2020005000402439
Devi, R. R., Ali, I., and Maji, T. K. (2003). “Chemical modification of rubber wood with styrene in combination with a crosslinker: Effect on dimensional stability and strength property,” Bioresource Technol. 88(3), 185-188. DOI: 10.1016/S0960-8524(03)00003-8
Dorau, B., Arango, R., and Green, III, F. (2004). “An investigation into the potential of ionic silver as a wood preservative,” in: Proceeding of the Woodframe Housing Durability and Disaster Issues,2004 Conference, Las Vegas, NV, USA, pp. 133-145.
Eckelman, C. A. (1998). The Shrinking and Swelling of Wood and its Effect on Furniture, Report FNR 163, Department of Forestry & Natural Resources, Purdue University, West Lafayette, IN, USA.
Fengel, D., and Wegener, G. (2003). Wood: Chemistry, Ultrastructure, Reactions, Springer-Verlag, Kessel, Germany.
Fierascu, R. C., Fierascu, I., Baroi, A. M., Brazdis, R. I., Fistos, T., Nicolae, C. A., Raditoiu, V., Inel, I. C., and Sava, V. (2020). “Characterization of historical ceramics: Acase study,”Rom. Rep. Phys. 72(801), Article Number 801.
Galpperin, A. S., Kuleshov, G. G., Tarashkevich, V. I., and Smtov, G. M. (1995). “Manufacturing and properties of modified wood: A review of 25 years work,” Holzforschung 49(1), 45-50. DOI: 10.1515/hfsg.1995.49.1.45
Giraldo, A., Gené, J., Sutton, A., Madrid, H., de Hoog, G. S., Cano, J., Decock, C., Corous, P. W., and Guarro, J. (2015). “Phylogeny of Sarocladium (Hypocreales),” Persoonia. 34, 10-24. DOI: 10.3767/003158515X685364
Glass, S. V., and Zelinka, S. L. (2010). “Moisture relations and physical properties of wood,” in: Wood Handbook: Wood as an Engineering Material, Forest Products Laboratory, United States Department of Agriculture Forest Service, Madison, WI, USA, pp. 1-19.
Harte, A. M. (2009). “Introduction to timber as an engineering material,” in: ICE Manual of Construction Materials, Institution of Civil Engineers, London, UK, pp. 1-9. DOI: 10.1680/mocm.00000.0001
Highley, T. L. (1975). “Inhibition of celluloses of wood decay fungi,” in: US Forest Service Research Paper FPL 247, Madison, WI, USA, pp. 1-8.
Hosseini Hashemi, S. K., Jahan Latibari, A., Khademi-Eslam, H., and Faraji Alamuti, R. (2010). “Effect of boric acid treatment on decay resistance and mechanical properties of poplar wood,” BioResources 5(2), 690-698. DOI: 10.15376/biores.5.2.690-698
Hosseini Hashemi, S. K., and Jahan Latibari, A. (2011). “Evaluation and identification of walnut heartwood extractives for protection of poplar wood,” BioResources 6(1), 59-69. DOI: 10.15376/biores.6.1.59-69
Islam, M. S., Hamdan, S., Rusop, M., Rahman, M. R., Ahmed, A. S., and Mohd Idrus, M. A. M. (2012). “Dimensional stability and water repellent efficiency measurement of chemically modified tropical light hardwood,” BioResources 7(1), 1221-1231. DOI: 10.15376/biores.7.1.1221-1231
Jovicic, N., Antonovic, A., Matin, A., Antolovíc, S., Kalambura, S., and Kricka, T. (2022). “Biomass valorization of walnut shell for liquefaction efficiency,”Energies 15(2), Article ID 495. DOI: 10.3390/en15020495
Kumar, S. (1994). “Chemical modification of wood,” Wood Fiber Sci. 2, 270-280.
Kurtzman, C. P., and Robnett, C. J. (2013). “Relationships among genera of Saccharomycotina (Ascomycota) from multigene phylogenetic analysis of type species,” FEMS Yeast Res. 13(1), 23-33. DOI: 10.1111/1567-1364.12006
Liu, Y., Laks, P., and Heiden, P. (2001). “Use of nanoparticles for controlled release of biocides in solid wood,” J. Appl. Polym. Sci. 79(3), 458-465. DOI: 10.1002/1097-4628(20010118)79:3%3C458::AID-APP80%3E3.0.CO;2-H
Liu, Y., Laks, P., and Heiden, P. (2002a). “Controlled release of biocides in solid wood: I. Efficacy against brown rot wood decay fungus (Gloeophyllum trabeum),” J. Appl. Polym. Sci. 86(3), 596-607. DOI: 10.1002/app.10896
Liu, Y., Laks, P., and Heiden, P. (2002b). “Controlled release of biocides in solid wood: II. Efficacy against Trametes versicolor and Gloeophyllum trabeum wood decay fungi,” J. Appl. Polym. Sci. 86(3), 608-614. DOI: 10.1002/app.10897
Liu, Y., Laks, P., and Heiden, P. (2002c). “Controlled release of biocides in solid wood: III. Preparation and characterization of surfactant-free nanoparticles,” J. Appl. Polym. Sci. 86(3), 615-621. DOI: 10.1002/app.10898
Mahapatra, I., Clark, J., Dobson, P. J., Owen, R., and Lead, J. R. (2013). “Potential environmental implications of nano-enabled medical applications: Critical review,” Environ. Sci. Process. Impacts 15(1), 123-144. DOI: 10.1039/C2EM30640A
Mahdavi, A. (2022). “Shiraz Khatam or Marquetry,” (https://www.irangazette.com/en/12/331-shiraz-khatam-or-marquetry.html), Accessed 02 June 2022.
Moya, R., Berrocal, A., Rodriguez-Zuñiga, A., Vega-Baudrit, J., and Chaves-Noguera, S. (2014). “Effect of silver nanoparticles on white-rot wood decay and some physical properties of three tropical wood species,” Wood Fiber Sci. 46(4), 527-538.
Pettersen, R. (1984). “Chemical composition of wood,” in: The Chemistry of Solid Woods, R. M. Rowell (ed.), Advances in Chemistry Series 207, American Chemical Society, Washington D.C., USA, pp. 57-126.
Piepenbrink, H. (1986). “Two examples of biogenous dead bone decomposition and their consequences for taphonomic interpretation,”J. Archaeol. Sci. 13(5), 417-430.DOI: 10.1016/0305-4403(86)90012-9
Rozi Talab, G. R., and Jalali, N. (2003). Khatam Art, SAMT, Tehran, Iran.
Safra, J. (2010). The New Encyclopedia Britannica, 15th ed., Britannica Inc., Chicago, IL, USA.
Shojanoori, N. (2014). “A background of Khatam art,” Eur. Online J. Nat. Soc. Sci. 3(4), 330-344.
Siavoshan, S. (2019). “Khatamkari,” (https://en.shivar.org/khatamkari/), Accessed 05 Febr 2019.
Simpson, W., and TenWolde, A. (1999). “Physical properties and moisture relations of wood,” in: Wood Handbook, Wood as Engineering Material (FPL-GTR-113), USDA Forest Service, Forest Products Laboratory, Madison, WI, USA, pp. 3-17.
Taghiyari, H. R. (2012). “Fire-retarding properties of nanosilver in solid woods,” Wood Sci. Technol. 46(5), 939-952. DOI: 10.1007/s00226-011-0455-6
Taghiyari, H. R., and Bibalan, O. F. (2013). “Effect of copper nanoparticles on permeability, physical, and mechanical properties of particleboard,” Eur. J. Wood Wood Prod. 71(1), 69-77. DOI: 10.1007/s00107-012-0644-5
Tran, Q. H., Nguyen, V. Q., and Le, A. T. (2013). “Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives,” Advances in Natural Sciences: Nanoscience and Nanotechnology 4(3), Article ID: 033001, DOI: 10.1088/2043-6262/4/3/033001
United Nations Educational, Scientific, and Cultural Organization (UNESCO) (2012). (http://www.taghribnews.com/en/news/115861/unesco-award-of-excellence-granted-to-iranian-handicrafts), Accessed 19 Nov 2012.
Velmurugan, N., Kumar, G. G., Han, S. S., Nahm, K. S., and Lee, Y. S. (2009). “Synthesis and characterization of potential fungicidal silver nano-sized particles and chitosan membrane containing silver particles,” Iran Polym. J. 18(5), 383-392.
Wanderly, M. C. D. A., Neto, J. M. W. D., Filho, J. L. D. L., Lima, C. D. A., Teixeira, J. A. C., and Porto, A. L. F. (2017). “Collagenolytic enzymes produced by fungi: A systematic review,” Braz. J. Microbiol. 48(1), 13-24. DOI: 10.1016/j.bjm.2016.08.001
Yakovleva, M. B., Khoang, T. L., and Nikitina, Z. K. (2006). “Collagenoytic activity in several species of Deuteromycetes under various storage conditions,” Appl. Biochem. Microbiol. 42(4), 431-434. DOI: 10.1134/S000368380604017X
Article submitted: May 17, 2022; Peer review completed: July 23, 2022; Revised version received and accepted: July 24, 2022; Published: July 26, 2022.
DOI: 10.15376/biores.17.3.5268-5284
