Abstract
A combination of steam explosion (SE) and alkaline peroxide (AP) used to pretreat bamboo was investigated. Steam explosion at 224 °C for 4 min was applied to bamboo, and the pretreated bamboo was delignified by alkaline peroxide. Enzymatic hydrolysis was compared in the pretreated samples. Steam pretreatment led to remarkable hemicellulose solubilization (63.2%). Lignin solubilization (93.1%) was achieved by alkaline peroxide treatment of steam-pretreated bamboo at 80 °C for 1 h in 0.88% (v/v) H2O2, whereas only 33.4% of lignin was solubilized when using raw bamboo. Pretreatment methods resulted in a low degree of polymerization and increased hydrolysis of cellulose. A maximum glucose yield of 90.5% was achieved with a combined steam explosion and alkaline peroxide pretreatment. The surface structure of treated bamboo and the adsorption of enzyme on the substrate were characterized by X-ray photoelectron spectroscopy. Delignification decreased enzyme adsorption and increased enzymatic conversion. SEM analyses indicated that SE-AP pretreatment disrupted lignin networks and exposed crystalline cellulose in bamboo more effectively than SE or AP pretreatment alone.
Download PDF
Full Article
Efficient Enzymatic Hydrolysis of Bamboo by Pretreatment with Steam Explosion and Alkaline Peroxide
Yang Xing, Hailong Yu, Liwei Zhu, and Jianxin Jiang *
A combination of steam explosion (SE) and alkaline peroxide (AP) used to pretreat bamboo was investigated. Steam explosion at 224 °C for 4 min was applied to bamboo, and the pretreated bamboo was delignified by alkaline peroxide. Enzymatic hydrolysis was compared in the pretreated samples. Steam pretreatment led to remarkable hemicellulose solubilization (63.2%). Lignin solubilization (93.1%) was achieved by alkaline peroxide treatment of steam-pretreated bamboo at 80 °C for 1 h in 0.88% (v/v) H2O2, whereas only 33.4% of lignin was solubilized when using raw bamboo. Pretreatment methods resulted in a low degree of polymerization and increased hydrolysis of cellulose. A maximum glucose yield of 90.5% was achieved with a combined steam explosion and alkaline peroxide pretreatment. The surface structure of treated bamboo and the adsorption of enzyme on the substrate were characterized by X-ray photoelectron spectroscopy. Delignification decreased enzyme adsorption and increased enzymatic conversion. SEM analyses indicated that SE-AP pretreatment disrupted lignin networks and exposed crystalline cellulose in bamboo more effectively than SE or AP pretreatment alone.
Keywords: Bamboo; Combined pretreatment; Steam explosion; Alkaline peroxide; Enzymatic hydrolysis
Contact information: Department of Chemistry and Chemical Engineering, Beijing Forestry University, Beijing 100083, China; *Corresponding author: jiangjx2004@hotmail.com
INTRODUCTION
The limitations of using food crops for producing ethanol have led to the need to develop a second generation of biofuels, an issue of increasing concern. Non-food waste crops and lignocellulosic biomass have been considered as alternative feedstocks (Himmel et al. 2007). The bioconversion process for bioethanol production typically involves the following three steps: pretreatment, hydrolysis, and fermentation (Ewanick et al. 2007). Several technologies for pretreatment have been developed, and the main objective now is to overcome biomass recalcitrance and make this conversion process cost-competitive.
Steam explosion (SE) pretreatment can provide advantages with regard to energy consumption and chemical usage (Excoffier et al. 1991). Applying this pretreatment could preserve valuable components used in various other applications. Previous work has shown that SE pretreatment can result in substantially higher recovery of sugars and can minimize the inhibition of the growth of microorganisms used for fermentation. SE pretreatment is considered to have industrial prospects for the production of second-generation ethanol and other value-added products (Oliveira et al. 2012). In recent decades, considerable research has sought to combine SE with other pretreatments for efficient enzymatic hydrolysis. Pang et al. (2013) have shown that a combination of steam explosion and microwave irradiation pretreatment could inhibit the increase in biomass crystallinity.
Alkaline peroxide (AP) pretreatment is one of the most effective methods for the removal of lignin present in materials such as furfural residues, yielding a cellulose-rich insoluble residue that can be converted to glucose for the production of chemicals and ethanol. AP pretreatment can be potentially used for a wide range of Gramineae samples, such as rapeseed straw (Karagöz et al. 2012), sugarcane bagasse (Rabelo et al. 2011), and wheat straw (Chen et al. 2008). Overall, lower biomass loading was found to give higher glucose yields, and very little furfural and hydroxymethyl furfural were produced in the liquid fraction during AP pretreatment (Costa et al. 2013). Those are formed during SE pretreatment and are degraded during AP pretreatment.
Bamboo is a key biomass material that maintains the balance of oxygen and carbon dioxide in the atmosphere. Unlike other grasses, bamboo is resistant to pretreat-ment because of its high density and lignin content. The rate and extent of enzymatic hydrolysis is inversely related to the lignin and hemicellulose content (Limayem and Ricke 2012). Lignin is thought to prevent the degradation of cellulose mainly by acting as a physical barrier between the cellulase and its substrate. Substantial research related to oxygen-alkali delignification of bamboo has been carried out, indicating its competitive advantage in bamboo applications (Salmela et al. 2008). The removal of hemicellulose appears to be critical to the enzymatic digestibility of a substrate when delignification is not extensive (Li et al. 2012). Therefore, an effective pretreatment is essential to lower the cost of downstream operations. Both SE and AP pretreatments can partly remove hemicelluloses and lignin from the biomass and enhance the accessibility of cellulose. A novel pretreatment process that combines steam explosion and an alkaline peroxide pretreatment (named SE-AP pretreatment) has been proposed to separate lignocellulosic components and to improve the sugar yield.
The objective of this study was to evaluate the effectiveness and feasibility of the combination of steam explosion and alkaline peroxide (SE-AP) pretreatments as a new pretreatment method for lignocellulosic biomass to enhance its enzymatic hydrolysis yield. Bamboo was pretreated by SE, AP, and SE-AP methods, and the three pretreatment methods were evaluated and compared on the basis of the sugar yields from subsequent enzymatic hydrolysis using cellulase and β-glucosidase. The structures of raw bamboo, pretreated bamboo, and samples after enzymatic hydrolysis were characterized by X-ray photoelectron spectroscopy (XPS), and morphological studies were performed using scanning electron microscopy (SEM).
EXPERIMENTAL
Materials
The bamboo sample (Phyllostachys edulis cv. Pachyloe) was obtained from Jiangxi Province, China. Air-dried stems of bamboo were cut into small pieces with a relatively homogenous size of 50 mm × 5 mm for SE pretreatment and ground to nominal sizes of 40- to 60-mesh for determination of composition and for the controlled AP pretreatment. All chemicals were of analytical grade and were purchased from Beijing Chemical Reagent Company, China, unless otherwise noted.
Steam Explosion-Alkaline Peroxide (SE-AP) Pretreatment
Substantial research related to the influence of SE pretreatment time and pressure on the enzymatic hydrolysis and fermentation for ethanol has been carried out (Jiang et al. 2011; Wang et al. 2009). Previous work using SE pretreatment showed that the highest enzymatic conversion rate was obtained at 224 °C, and the highest yields of SSF ethanol from pretreated Lespedezaand Salix were 77.53% and 62.79% of the theoretical values when the residence time of the SE pretreatment was 4 and 5 min, respectively. The residence time of 4 min for bamboo was chosen for further study of enzymatic hydrolysis. SE pretreatment was carried out in a batch pilot unit equipped with a 7.5-L reaction vessel and exposed to saturated steam at 224 °C for 4 min. After exposure to the saturated steam, a ball valve at the bottom of the reactor was suddenly opened to bring the reactor rapidly to atmospheric pressure. The AP pretreatment was carried out by mixing dry SE-pretreated bamboo, hydrogen peroxide (30% w/w), and water at a ratio of 1:0.38:30, using 4 M sodium hydroxide to adjust to pH 11.5, and heating the mixture to 80 °C in a thermal shaker at 150 rpm for 1 h. After pretreatment, the biomass was carefully collected and washed with distilled water until the pH was neutral. Then, pretreated materials were stored at 4 °C before enzymatic hydrolysis.
Enzymatic Hydrolysis
Enzymatic hydrolysis was performed in a 100-mL flask using 50 mL of 0.05 M sodium acetate buffer (pH 4.8) at 47 °C. The flasks were agitated at 150 rpm in a rotary shaker (Certomat-R, B-Braun, Germany) for 120 h. Never-dried materials were added at a concentration of 2.5% (w/v) on a dry basis. Celluclast 1.5L (Sigma Co., St. Louis) was supplemented with Novozym 188 (Sigma Co., St. Louis) in an activity ratio of 1 FPU to 1.5 IU. For each sample, enzymatic hydrolysis was stopped by plunging the tube into boiling water for 5 min and then cooling it to room temperature in water. The mixture was then centrifuged at 5000 rpm for 5 min at 25 °C, and the supernatant was collected for glucose analysis, which was performed by a high-performance liquid chromatography system (Waters 2695e, USA) using an Aminex HPX-87P (Bio-Rad) at 85 °C. The injection volume of the sample was 10 μL, and water was used as the eluent, at a flow rate of 0.6 mL min-1. The enzymatic hydrolysis experiments were duplicated (standard deviations were in all cases < 3%), and average results are given. The glucose yield was calculated using Eq. (1):
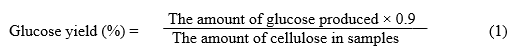
Bamboo Composition and DP of the Cellulose
The composition of the bamboo was analyzed using a two-step acid hydrolysis according to the procedure published by the National Renewable Energy Laboratory (NREL) (Sluiter et al.2004). The neutral sugars in the bamboo samples were obtained by hydrolysis with 87 mL of 4% H2SO4 at 121 °C for 1 h. After hydrolysis, the samples were filtered and analyzed by a high-performance liquid chromatography system (Waters 2695e, USA) using an Aminex HPX-87P (Bio-Rad) at 85 °C. The injection volume of the sample was 10 μL, and water was used as the eluent. The total analysis time was 50 min, and the flow rate was 0.6 mL min-1. Calibration was performed with standard solutions of glucose, xylose, arabinose, mannose, and galactose. The lignin in the raw and various pretreated samples was analysed according to the NREL lignin analysis method for biomass (Sluiter et al. 2004).
The cellulose preparations were obtained by the HNO3-ethanol method. The viscosity of the cellulose preparations was determined by British Standard Methods for determination of the limiting viscosity number of cellulose in dilute solutions (Cupriethylene-diamine method). The viscosity average DP (degree of polymerization) of the cellulose samples was estimated from their intrinsic viscosity [η] in cupriethylene-diamine hydroxide (cuene) solution,
P0.90=1.65[η]/mL•g-1 (2)
where P is an indeterminate average DP (Evans and Wallis 2003). The molecular weight of the cellulosic preparations was then calculated from the P, multiplying by 162, which is the molecular weight of an anhydroglucose. At least three parallel samples were used in all analytical procedures, and data are presented as the means of the triplicates.
Surface Analysis by X-ray Photoelectron Spectroscopy (XPS)
Four kinds of samples (raw bamboo (RM) and solid residues following SE-AP pretreatment (SE-AP) and enzymatic hydrolysis (RM-Enzyme and SE-AP-Enzyme)) were prepared. The selected samples were washed 3 times with 50 mL of deionized water in a G3 glass filter to avoid the influence of free components and then dried in a vacuum drying oven at 60 °C for 24 h. The surface analysis studies were performed by XPS employing a scanning X-ray microprobe (Quantera SXM, ULVAC-PHI INC) equipped with a monochromatic Al Kα X-ray source (hv =1486.6 eV, energy width 280 eV) operated at 23.7 W with a background pressure of 5×10-8 Torr. The low-resolution survey scans were taken with a 1-eV step and 160-eV analyzer pass energy, and high-resolution spectra were taken with a 0.1-eV step and 40-eV analyzer pass energy. The deconvolution of the high-resolution spectra was performed by means of least-squares peak analysis software, XPSPEAK version 4.1, using the Gaussian/Lorenzian sum function. The analyzed sample area was approximately 9 μm × 1.5 mm. The calibration of the binding energy scale was made by setting the C1s of the neutral carbon (C-C and C-H bonds) peak at 284.8 eV. Element atomic percentages were calculated from the integrated intensities of the XPS peaks, taking into account the atomic sensitivity factors of the instrument’s data system.
Morphological Study (SEM)
Prior to image acquisition, treated bamboo samples were prepared by lyophilization to eliminate potential structural distortion caused by the surface tension of liquid water during evaporation at atmospheric conditions. Lyophilized samples were sputtered with a thin layer of gold. SEM images were acquired of untreated and pretreated bamboo at different magnifications, such as 500, 1000, and 2000 times, using a S-3400N (HITACHI, Japan) SEM at 5 kV.
Protein Analysis of the Hydrolyzate
The protein content of the hydrolyzate was determined with the Bradford method. After 0.5 mL of hydrolyzate was combined with 5 mL of Bradford reagent in a test tube, the sample was mixed well and incubated at room temperature for 5 min. Then, the absorbance of each sample was measured at 595 nm using a UV-visible spectrophoto-meter (Techcomp, UV2300). The Bradford reagent was made by dissolving 100 mg of Coomassie Blue G-250 in 50 mL of 95% ethanol, adding 100 mL of 85% (w/v) phosphoric acid to this solution, and diluting the mixture to 1 L with water. The mixture of 0.5 mL of deionized water and 5 mL of Bradford reagent was prepared as the blank, and bovine serum albumin (BSA) was selected as the standard protein.
RESULTS AND DISCUSSION
Bamboo Composition and DP of Cellulose
The solid yield after pretreatment and the content of the polysaccharides (xylan and glucan), total lignin, and ash (based on the weight of original materials) of bamboo pretreated with SE, AP, and SE-AP are illustrated in Fig. 1. The chemical composition of raw bamboo (45% glucan, 17% xylan, and 26% lignin) was found to be within the range of previously published data (Scurlock et al. 2000; Sun et al. 2011).
Fig. 1. Retention of the main components of bamboo with different pretreatment procedures. RM represents raw bamboo, while SE, AP, and SE-AP represent bamboo that has been pretreated with steam explosion, alkaline peroxide, and combined steam explosion and alkaline peroxide methods, respectively. Solid yields after SE, AP, and SE-AP pretreatment were 75.01%, 75.53%, and 54.98%, respectively.
However, the content of polysaccharides and lignin after pretreatment varied to different degrees. It was apparent that the “blocking effect” of xylan was one of the major mechanisms that limited the accessibility of cellulase to cellulose (Hu et al. 2011). Therefore, xylan solubilization is vital in bamboo at the pretreatment step. After SE pretreatment, the xylan content of bamboo was reduced by a significant 63.2%. This suggests that SE pretreatment could effectively remove hemicelluloses in bamboo (Liu et al. 2013). The SE pretreatment process also removed 33.4% of lignin. This could be due to autohydrolysis with saturated steam, which is catalyzed by organic acids, mainly acetic acid derived from the acetylated polysaccharides present. Substantial research has been done on AP pretreatment of biomass (Gould 1984; Saha and Cotta 2006; Yamashita et al. 2010). It has been reported that AP pretreatment is an optimized delignification method for lignocelluloses to improve enzymatic hydrolysis (Xing et al. 2012). In alkaline environments, lignin can be soluble. In this study, AP pretreatment led to the reduction of lignin from 26.0% to 12.7%. Peroxide is not a strong oxidant unless H2O2 decomposes to form [OH·]. This is why Fig. 1 showed that AP pretreatment led to delignification, but did not lead glucan and xylan significant reduction. Yang et al. (2002) found that alkaline peroxide enhanced the enzymatic digestibility of SO2-catalyzed, steam-exploded softwood substrates. After SE-AP pretreatment, the bamboo samples contained 78.33% cellulose and about 6.9% lignin. This can be attributed to the substantial degradation of lignin and hemicellulosic polymers in steam explosion-treated bamboo and is undoubtedly due to a significant removal of low-molecular-weight lignin and hemicellulosic polymers by degradation of cell walls. It is reported that the rate-limiting step during hydrolysis is not the catalytic cleavage of cellulose chains, but rather the limited accessibility of the enzymes to the cellulose chains due to the physical structure of the cellulosic substrate (Arantes and Saddler 2011). Cellulose-rich samples obtained by SE-AP pretreatment might be better suited for robust enzymatic hydrolysis. As compared to the research of Yang et al. (2002), similar lignin removal was also obtained in SE-AP pretreatment without SO2. The solid yield of treated bamboo ranged from 54.9% to 75.5%, depending on the weight of the original untreated bamboo, and the same solid yield (75%) was obtained in both SE and AP pretreatments.
One of the major barriers faced by cellulase is its limited access to much of the cellulose, which is buried within the highly ordered and tightly packed fibrillar architecture of cellulose microfibrils (Arantes and Saddler 2010). It is important to increase the cellulose surface area, making it more accessible to the cellulase enzyme complex. The influence of different pretreatment methods on the degree of polymerization of cellulosic molecules needs to be investigated.
Fig. 2. DP and Mw values of cellulose-rich fractions
As can be seen from Fig. 2, SE pretreatment led to a decrease in the average degree of polymerization (DP), from 304 to 118, and a decrease in molecular weight (MW), from 49,390 to 19,166 g/mol, in the cellulose-rich fractions. SE can be considered an acid-catalyzed hydrolysis process. SE treatment resulted in the degradation of cellulose macromolecules, revealing that autohydrolysis reactions are dominant during SE pretreatment. In particular, little glucose loss took place during pretreatment (Fig. 1). In contrast to SE treatment, AP treatment had a lesser effect on the degradation of cellulose. Cellulose had a high DP in the AP-treated bamboo, showing that peroxide in the alkaline condition did not seriously damage the fiber. The cellulose-rich fraction from SE-AP-treated bamboo had the lowest DP.
Enzymatic Hydrolysis of Bamboo Samples
Irreversible adsorption of enzymes to lignin restrains their movement and inhibits the efficient hydrolysis of cellulose. This inhibitory effect can be overcome using high amounts of enzymes, but the high cost of enzymes restricts this strategy. In other words, efficient enzymatic saccharification of cellulose at low cellulase loadings continues to be a challenge for the commercialization of the bioconversion of lignocellulose to ethanol. The effect of enzyme loading on the hydrolysis of bamboo and treated bamboo is presented in Fig. 3.
Fig. 3. Glucose yield for raw and treated bamboo at enzyme loadings of 5, 18, and 30 FPU/g-cellulose after 120 h of enzymatic hydrolysis. RM, SE, AP, and SE-AP represent raw bamboo and bamboo pretreated by steam explosion, alkaline peroxide, and combined steam explosion-alkaline peroxide, respectively.
As expected, the glucose yield increased with an increase in enzyme dose for treated bamboo. However, little change can be seen in the glucose yields of untreated bamboo with an increase in enzyme loading. Moreover, untreated bamboo had a lower glucose yield of 5%, compared to other lignocellulosic materials such as Lespedeza stalks (Su et al. 2012) and furfural residues (Bu et al. 2012) under the same conditions. It appears that the complex and rigid structure of bamboo makes it difficult for biological degradation to occur. Strangely enough, the enzymatic hydrolysis of SE-treated bamboo no longer improved with an increase in enzyme loading from 18 FPU/g-cellulose to 30 FPU/g-cellulose. This finding indicates that the hydrolysis of SE bamboo reached a saturation level at 18 FPU/g-cellulose, and the efficiency of hydrolysis could not be increased with more enzyme. The enzymatic hydrolysis yield of AP bamboo improved with an increase in enzyme loading. In particular, the hydrolysis yield of AP bamboo with 30 FPU/g-cellulose enzyme loading was lower than that of SE bamboo with an enzyme loading of 18 FPU/g-cellulose. This strongly suggests that the biodegradation ability of SE bamboo under this condition is higher than that of AP bamboo. Generally, a sample with a lower DP can lead to a higher enzymatic hydrolysis yield. Thus, bamboo was pretreated with alkaline peroxide after SE treatment to improve enzymatic hydrolysis. The hydrolysis yield of SE-AP bamboo with 5 FPU/g-cellulose was significantly higher than the yields obtained from AP and SE bamboo. The maximum glucose yield of 90.5% was achieved when SE-AP-treated bamboo was hydrolyzed for 120 h with an enzyme loading of 30 FPU/g-cellulose. SE-AP pretreatment of bamboo increased the hydrolysis yield by a factor of 13.0 compared to native bamboo. This strongly indicated that the SE-AP delignification treatment significantly enhanced the enzymatic hydrolysis efficiency of bamboo. This observation can be attributed to a combination of hemicellulose reduction and delignification, which results in a dramatic increase in fiber swelling and the amount of cellulose surface area available to the enzyme.
The time course of enzymatic hydrolysis (18 FPU/g-cellulose) of bamboo with SE, AP, and SE-AP treatment is presented in Fig. 4. In the early phase of enzymatic hydrolysis, the glucose yield of SE-AP bamboo increased sharply. The yield reached 55% at 12 h, which was higher than the highest yield obtained from AP- or SE-treated bamboo after 120 h of enzymatic hydrolysis. SE- and AP-treated bamboo showed the same trends in the first 24 h of enzymatic hydrolysis. After that, the glucose yield of SE bamboo increased remarkably. However, the glucose yield of raw bamboo did not increase, even after 12 h of hydrolysis.
Fig. 4. Glucose yield variation of raw (RM) and treated bamboo (SE, AP, and SE-AP) during enzymatic hydrolysis
X-ray Photoelectron Spectroscopy Analysis of Bamboo and SE-AP Bamboo
X-ray photoelectron spectroscopy (XPS) has been used to analyze the chemical composition of lignocellulosic surfaces. Ju et al. (2013) established an XPS method to quantify surface lignin on biomass substrates. In this study, XPS was performed to characterize the effect of the pretreatment method and subsequent enzymatic hydrolysis on chemical bonds.
Fig. 5. X-ray photoelectron spectra of raw bamboo (RM) and solid residues from SE-AP pretreatment (SE-AP) and enzymatic hydrolysis (RM-Enzyme and SE-AP-Enzyme)
As shown in Fig. 5, the three zones corresponding to the carbon, oxygen, and nitrogen atoms were compared. There are small N signals in the XPS spectra from RM-enzyme and SE-AP-enzyme, which can be attributed to cellulase adsorption. Table 1 summarizes the XPS results. The contents of carbon and oxygen on the bamboo surface are 63.92% and 36.08%, respectively. After SE-AP pretreatment, the O content on the bamboo surface increased, while the C content decreased, i.e., there was an increase in the O/C ratio. Generally, the O/C ratios in cellulose, lignin, and extractives are 0.83, 0.33, and 0.10, respectively (Zhong et al. 2010). A higher O/C ratio indicates a higher cellulose concentration. These results show that cellulose is the major component remaining on the treated bamboo surface after a reduction in the amount of lignin after pretreatment. However, the ratio of O/C of bamboo after enzymatic hydrolysis decreased markedly due to cellulose degradation. It is well known that enzymes are proteins made up of amino acids. When an enzyme is formed, it is made by stringing together between 100 and 1,000 amino acids in a very specific and unique order. The XPS spectra showed an N signal on the surface of bamboo after enzymatic hydrolysis. This could be a consequence of enzyme adsorption on the bamboo surface. During the course of hydrolysis, enzyme adsorption and movement on solid cellulose is a prerequisite for effective degradation of the substrate. On the contrary, irreversible adsorption on lignin restrains the movement of enzymes and inhibits an efficient hydrolysis of cellulose. A reasonable estimate for a “typical” protein composition is approximately 65% C, 20% O, and 15% N, with an O/C ratio of 0.308. XPS analysis indicates an expected decrease in nitrogen content after SE-AP pretreatment. In this study, the ratio of N/C is probably an effective measure of the amount of enzyme adsorbed. The decrease in the N/C ratio can be attributed to a reduction in the amount of enzymes adsorbed on SE-AP-treated bamboo.
Table 1. XPS Elemental Composition of Bamboo Before and After Enzymatic Hydrolysis
On deconvolution, the carbon emission XPS spectra show the contributions of the differently bonded carbons in the samples. The C1s’ spectra in Fig. 6 show four distinct environments for C atoms. It is reported that the types of chemical bonds of carbon in cellulose, hemicelluloses, lignin, and extractives can be categorized into four groups: C1 (C-C, C-H), C2 (-C-O-), C3 (C=O or O-C-O), and C4 (O-C=O) (Zhong et al. 2010). C1 exists in lignin and extractives. C2 refers to the hydroxyl groups in the cellulose and hemicelluloses. C3 is from the carbonyl groups in hemicelluloses or lignin, and C4 exists in carboxylic acids from hemicelluloses or extractives.
Fig. 6. Deconvolution of high-resolution C1s’ spectra of raw bamboo (RM) and solid residues from SE-AP pretreatment (SE-AP) and enzymatic hydrolysis (RM-Enzyme and SE-AP-Enzyme)
The percentages of typical chemical bonds in RM, SE-AP, RM-Enzyme, and SE-AP-Enzyme are presented in Table 2. The C1 content increased in bamboo residue after enzymatic hydrolysis. This increase could be attributed to the degradation of cellulose, which leaves behind residual lignin that shows up as increased C1 content. The contents of C=O and O-C=O in raw bamboo were found to be higher than in bamboo after enzymatic hydrolysis, but the opposite was observed in SE-AP-treated bamboo. This may result from the SE-AP pretreatment method for modifying the amount and structure of lignin in the substrate, which produces more C=O and O-C=O. Consequently, the modified lignin structure decreases cellulase adsorption and facilitates the movement of the enzyme on solid samples for effective degradation of the substrate during the course of hydrolysis.
Table 2. Surface Functional Components Obtained from the Deconvolution of C1s’ Peaks of Raw Bamboo (RM) and Solid Residues from Pretreatment (SE-AP) and Enzymatic Hydrolysis (RM-Enzyme and SE-AP-Enzyme)
Because of certain features and analytical capabilities (high surface sensitivity, range of elements analyzed, angle-dependent XPS, freeze hydration XPS), XPS is particularly well suited for characterizing the adsorption of proteins to biomaterials (Griesser et al. 2003). The N1s’ XPS signal can also be used to obtain quantitative information on the adsorbed protein molecules. After enzymatic hydrolysis, it was found that the N content of the SE-AP residue was lower than that of untreated bamboo residue. The deconvolution of N1s’ core level spectra (Fig. 7) reveals two components centered at 399 and 400 eV, characteristic of H-C-N=O and C-NH2 groups, respectively (Advincula et al. 2005). The N1s’ photoelectron peaks recorded close to 400 eV are typical for nitrogen in an organic matrix (Serro et al. 2006). The cellulose content of SE-AP bamboo was found to be higher than that of raw bamboo; consequently, the enzyme loading for hydrolysis was also higher in SE-AP than it is in raw bamboo. However, the N content of SE-AP residue was lower than that of raw bamboo residue after enzymatic hydrolysis. The adsorbed protein was associated with either cellulose or lignin. Most of the retained protein was in the hydrolysate, as seen in Fig. 9. An explanation for cellulase adsorption is that the amino groups in the enzyme (NH2) can form new hydrogen bonds (C-OH…NH) with the hydroxyl groups of cellulose (Zhang et al. 2010). Cellulose had no effect on the peptide bond during enzymatic hydrolysis. These results imply that the structure and composition of cellulase adsorbed on the residue surface of treated and untreated bamboo are different. Most enzymatic hydrolysis processes follow one of two possible general mechanisms. These are called the retaining and inverting mechanisms (Petersen 2010). Both catalytic mechanisms employ two carboxylate groups, provided by either a glutamate or an aspartate amino acid residue. The irreversible enzyme adsorption on lignin has been shown to be a major contributor to its inhibition. Therefore, the SE-AP pretreatment could decrease the amount of enzyme adsorption.
Fig. 7. Deconvolution of high-resolution N1s’ spectrum from the enzymatic hydrolysis residues of raw bamboo (RM-Enzyme) and SE-AP bamboo (SE-AP-Enzyme)
Morphological Changes in Bamboo and SE-AP Bamboo
SEM images of raw bamboo and SE-, AP-, and SE-AP-pretreated bamboo (before and after enzymatic hydrolysis) are shown in Fig. 8. Raw bamboo showed compact and rigid fibril structures (Fig. 8 A-1). Cellulose microfibrils were observed between the disordered lignin structures. The treated bamboo powder had a smooth surface, although scaling was observed due to breakdown of ester bonds in the lignin-carbohydrate complex. The removal of lignin from the cell wall surface results in the exposure of carbohydrates. The SE-AP-treated bamboo sample was much smoother than SE or AP bamboo. These SEM analyses indicated that SE-AP pretreatment could disrupt the lignin networks, exposing crystalline cellulose more effectively than SE or AP pretreatment. The most exposed cell wall structure allows for a greater accessibility to hydrolytic enzymes, which facilitates the hydrolysis of lignocellulosic biomass (Cao and Aita 2013). This is observed in SE-AP-pretreated samples, which had the maximum lignin loss and the highest cellulose digestibility. After enzymatic hydrolysis (18 FPU/g-cellulose for 120 h), the glucose yield of raw bamboo was less than 5%. From the SEM image (Fig. 8 A-2), it is seen that only a small amount of cellulose on the face of the residues is degraded. However, scattered fibers were observed (Fig. 8 B-2, C-2, and D-2), and the SE-AP-treated bamboo showed severe breakage, causing a more effective enzymatic hydrolysis.
Protein Content of Hydrolyzate
A significant proportion of hydrolytic enzymes remain tightly bound to lignin-rich hydrolysis residues. Figure 9 presents the proportion of protein in the hydrolyzate as a function of time on raw and SE-AP-treated bamboo. About 78% of cellulolytic enzymes remained bound to raw bamboo residue after 120 h of hydrolysis, and glucose yield remained low (Fig. 4). On the contrary, 25% of enzymes remained bound to the cellulose-rich hydrolysis residue (SE-AP-treated bamboo) after 120 h. Interestingly, enzymes were able to desorb from treated bamboo, whereas visible desorption did not occur in raw bamboo. Lignin has an inhibitory effect during enzymatic hydrolysis of cellulose and part of this effect is caused by irreversible cellulose adsorption on lignin. Moreover, alteration of the chemical structure of residual lignin in the SE-AP-treated bamboo probably contributes to its weakened affinity for hydrolytic enzymes.
Fig. 8. SEM images of bamboo samples: (1) before enzymatic hydrolysis, (2) after enzymatic hydrolysis, A: untreated, B: SE-pretreated, C: AP-pretreated, and D: SE-AP-pretreated. All images were taken at 1000× magnification.
The extent of inhibition could be dependent on several factors, such as the origin of the lignocellulosic substrate (Berlin et al. 2005) and the pretreatment method, which affects the amount and structure of lignin in the substrate (Tu et al. 2007). In this study, the SE-AP pretreatment method was developed to efficiently remove lignin prior to enzymatic hydrolysis.
Fig. 9. Protein proportion in hydrolyzate during hydrolysis on raw bamboo (RM) and SE-AP-treated bamboo (SE-AP) (enzyme dosage 18 FPU/g-cellulose with 2.5% (w/v) substrate concentration)
CONCLUSIONS
- There is 93.1% lignin removal and 77.06% hemicellulose solubilization with the combined steam explosion and alkaline peroxide (SE-AP) pretreatment. The SE-AP pretreatment process has the attractive advantages of removing lignin and improving glucose yields compared with SE or AP pretreatments each by itself.
- The samples obtained by combined SE-AP pretreatment had a smoother surface and a looser structure, as observed by SEM, resulting in the most exposed cell wall structure.
- The maximum glucose yield of SE-AP pretreated bamboo was 90.5% when SE-AP-treated bamboo was hydrolyzed for 120 h with an enzyme loading of 30 FPU/g-cellulose and pH 4.8 at 47 °C. SE-AP pretreatment also showed attractive advantages in decreasing the cellulase adsorption on samples, as measured by X-ray photoelectric spectroscopy (XPS).
- Further research could focus on evaluating the effectiveness and feasibility of SE-AP pretreatment on the lignin structure for lignin-derived degradation products and exploring enzyme-lignin interactions.
ACKNOWLEDGMENTS
The authors are grateful for the financial support of this research from the National Science Foundation of China (31070510) and China’s Ministry of Education (NCET-07-0082).
REFERENCES CITED
Advincula, M., Fan, X., Lemons, J., and Advincula, R. (2005). “Surface modification of surface sol-gel derived titanium oxide films by self-assembled monolayers (SAMs) and non-specific protein adsorption studies,” Colloid Surface. B. 42(1), 29-43.
Arantes, V., and Saddler, J. N. (2010). “Access to cellulose limits the efficiency of enzymatic hydrolysis: The role of amorphogenesis,” Biotechnol. Biofuels. 3, 1-11.
Arantes, V., and Saddler, J. N. (2011). “Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates,” Biotechnol. Biofuels. 4(1), 1-17.
Berlin, A., Gilkes, N., Kurabi, A., Bura, R., Tu, M., Kilburn, D., and Saddler, J. (2005). “Weak lignin-binding enzymes,” Appl. Biochem. Biotechnol. 121(1), 163-170.
Bu, L. X., Xing, Y., Yu, H. L., Gao, Y. X., and Jiang, J. X. (2012). “Comparative study of sulfite pretreatments for robust enzymatic saccharification of corn cob residue,” Biotechnol. Biofuels. 5(1), 1-8.
Cao, S., and Aita, G.M. (2013). “Enzymatic hydrolysis and ethanol yields of combined surfactant and dilute ammonia treated sugarcane bagasse,” Bioresour. Technol. 131, 357-364.
Chen, H. Z., Han, Y. J., and Xu, J. (2008). “Simultaneous saccharification and fermentation of steam exploded wheat straw pretreated with alkaline peroxide,” Process Biochem. 43(12), 1462-1466.
Costa, J. A. d., Júnior, J. E. M., Gonçalves, L. R. B., and Rocha, M. V. P. (2013). “Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: Study of parameters,” Bioresour. Technol. 139, 249-256.
Evans, R., and Wallis, A. F. (2003). “Cellulose molecular weights determined by viscometry,” J. Appl. Polym. Sci. 37(8), 2331-2340.
Ewanick, S. M., Bura, R., and Saddler, J. N. (2007). “Acid-catalyzed steam pretreatment of lodgepole pine and subsequent enzymatic hydrolysis and fermentation to ethanol,” Biotechnol. Bioeng. 98(4), 737-746.
Excoffier, G., Toussaint, B., and Vignon, M.R. (1991). “Saccharification of steam-exploded poplar wood,” Biotechnol. Bioeng. 38(11), 1308-1317.
Gould, J. M. (1984). “Alkaline peroxide delignification of agricultural residues to enhance enzymatic saccharification,” Biotechnol. Bioeng. 26(1), 46-52.
Griesser, H. J., Mcarthur, S. L., Wagner, M. S., Castner, D. G., Kingshott, P., and Mclean, K. M. (2003). “XPS, ToF-SIMS, and MALDI-MS for characterizing adsorbed protein films,” in: Malmsten M. (ed.), Surfactant Science Series: Biopolymers at Interfaces, Marcel Dekker Inc., New York, pp. 641-669.
Himmel, M. E., Ding, S. Y., Johnson, D. K., Adney, W. S., Nimlos, M. R., Brady, J. W. and Foust, T. D. (2007). “Biomass recalcitrance: Engineering plants and enzymes for biofuels production,” Science. 315(5813), 804-807.
Hu, J. G., Arantes, V., and Saddler, J. N. (2011). “The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: Is it an additive or synergistic effect?,” Biotechnol. Biofuels. 4(1), 1-14.
Jiang, J. X., Tang, Y., Wang, K., and Bu, L. X. (2011). “Influence of steam pretreatment time on chemical composition and simultaneous saccharification and fermentation for ethanol from pruning shrub stalks,” J. Biobased Mater. Bio. 5(2), 258-264.
Karagöz, P., Rocha, I. V., Özkan, M., and Angelidaki, I. (2012). “Alkaline peroxide pretreatment of rapeseed straw for enhancing bioethanol production by same vessel saccharification and co-fermentation,” Bioresour. Technol. 104, 349-357.
Li, Z. Q., Yang, Q., Jiang, Z. H., Fei, B. H., Cai, Z. Y., and Pan, X. J. (2012). “Comparative study of sulfite (SPORL), dilute acid and NaOH pretreatments of bamboo for enzymatic saccharification,” J. Biobased Mater. Bio. 6(5), 544-551.
Limayem, A., and Ricke, S.C. (2012). “Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects,” Prog. Energ. Combust. 38(4), 449-467.
Liu, Z. H., Qin, L., Jin, M. J., Pang, F., Li, B. Z., Kang, Y., Dale, B. E., and Yuan, Y. J. (2013). “Evaluation of storage methods for the conversion of corn stover biomass to sugars based on steam explosion pretreatment,” Bioresour. Technol. 132, 5-15.
Oliveira, F., Pinheiro, I. O., Souto-Maior, A. M., Martin, C., Gonçalves, A. R., and Rocha, G. J. (2012). “Industrial-scale steam explosion pretreatment of sugarcane straw for enzymatic hydrolysis of cellulose for production of second generation ethanol and value-added products,” Bioresour. Technol. 130, 168-173.
Petersen, L. (2010). Catalytic Strategies of Glycoside Hydrolases, Iowa State University, pp. 155.
Rabelo, S. C., Amezquita Fonseca, N. A., Andrade, R. R., Maciel Filho, R., and Costa, A. C. (2011). “Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide,” Biomass Bioenergy 35(7), 2600-2607.
Saha, B. C., and Cotta, M. A. (2006). “Ethanol production from alkaline peroxide pretreated enzymatically saccharified wheat straw,” Biotechnol. Prog. 22(2), 449-453.
Salmela, M., Alén, R., and Vu, M. T. H. (2008). “Description of kraft cooking and oxygen-alkali delignification of bamboo by pulp and dissolving material analysis,” Ind. Crops Prod.28(1), 47-55.
Scurlock, J. M. O., Dayton, D. C., and Hames, B. (2000). “Bamboo: An overlooked biomass resource?,” Biomass Bioenergy 19(4), 229-244.
Serro, A. P., Gispert, M. P., Martins, M. C. L., Brogueira, P., Colaco, R., and Saramago, B. (2006). “Adsorption of albumin on prosthetic materials: Implication for tribological behavior,” J. Biomed. Mater. Res. A. 78(3), 581-589.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2004). “Determination of structural carbohydrates and lignin in biomass,” LAP-002 NREL Analytical Procedure, National Renewable Energy Laboratory.
Su, Z. Q., Bu, L. X., Zhao, D. Q., Sun, R. C., and Jiang, J. X. (2012). “Processing of Lespedeza stalks by pretreatment with low severity steam and post-treatment with alkaline peroxide,” Ind. Crops Prod. 36(1), 1-8.
Sun, Z. Y., Tang, Y. Q., Iwanaga, T., Sho, T., and Kida, K. (2011). “Production of fuel ethanol from bamboo by concentrated sulfuric acid hydrolysis followed by continuous ethanol fermentation,” Bioresour. Technol. 102(23), 10929-10935.
Tu, M., Chandra, R. P., and Saddler, J. N. (2007). “Recycling cellulases during the hydrolysis of steam exploded and ethanol pretreated lodgepole pine,” Biotechnol. Prog. 23(5), 1130-1137.
Wang, K., Jiang, J. X., Xu, F., and Sun, R. C. (2009). “Influence of steaming pressure on steam explosion pretreatment of Lespedeza stalks (Lespedeza crytobotrya): Part 1. Characteristics of degraded cellulose,” Polym. Degrad. Stabil. 94(9), 1379-1388.
Xing, Y., Bu, L. X., Wang, K., and Jiang, J. X. (2012). “Pretreatment of furfural residues with alkaline peroxide to improve cellulose hydrolysis. Characterization of isolated lignin,” Cell. Chem. Technol. 46(3-4), 249-260.
Yamashita, Y., Shono, M., Sasaki, C., and Nakamura, Y. (2010). “Alkaline peroxide pretreatment for efficient enzymatic saccharification of bamboo,” Carbohydr. Polym. 79(4), 914-920.
Zhang, J. H., Zhang, B. X., Zhang, J. Q., Lin, L., Liu, S. J., and Ouyang, P. K. (2010). “Effect of phosphoric acid pretreatment on enzymatic hydrolysis of microcrystalline cellulose,” Biotechnol. Adv. 28(5), 613-619.
Zhong, L. X., Fu, S. Y., Li, F., and Zhan, H. Y. (2010). “Chlorine dioxide treatment of sisal fibre: surface lignin and its influences on fibre surface characteristics and interfacial behaviour of sisal fibre/phenolic resin composites,” BioResources. 5(4), 2431-2446.
Article submitted: July 8, 2013; Peer review completed: August 9, 2013; Revised version received and accepted: August 20, 2013; Published: September 10, 2013.
