Abstract
The production of cashew nuts has been increasing globally, leading to a greater volume of waste materials that require proper management. Nevertheless, cashew nutshells (CNS), currently considered waste by most processors, offer a noteworthy opportunity for alternative applications owing to their distinct physical, chemical, and thermal properties. This article reviews alternative applications for CNS that can leverage these properties, while evaluating research gaps. The potential uses are classified into three categories: material development, energy production, and substance absorption. In the materials segment, various examples are discussed where CNS serves as raw material to synthesize biopolymers, cementitious materials, and a broad range of composites. The energy production section discusses various processes that utilize CNS, including pyrolysis, gasification, and briquette production. The absorption section presents CNS and activated carbon derived from CNS as effective absorbents for liquid-phase and gas-phase applications. While this review highlights numerous research-level possibilities for CNS utilization, only a few of these options have been implemented within the industry. Consequently, further research is essential, particularly in CNS characterization, economic and environmental assessment, and real-life implementation, to broaden and enhance the integration of this biomass into applications that can contribute to the value of both its production and processing chain.
Download PDF
Full Article
Exploring the Potential of Cashew Nutshells: A Critical Review of Alternative Applications
Tatiana Cruz,a Alejandro Maranon,b Camilo Hernandez,c Oscar Alvarez,a Camilo Ayala-García,d and Alicia Porras a,*
The production of cashew nuts has been increasing globally, leading to a greater volume of waste materials that require proper management. Nevertheless, cashew nutshells (CNS), currently considered waste by most processors, offer a noteworthy opportunity for alternative applications owing to their distinct physical, chemical, and thermal properties. This article reviews alternative applications for CNS that can leverage these properties, while evaluating research gaps. The potential uses are classified into three categories: material development, energy production, and substance absorption. In the materials segment, various examples are discussed where CNS serves as raw material to synthesize biopolymers, cementitious materials, and a broad range of composites. The energy production section discusses various processes that utilize CNS, including pyrolysis, gasification, and briquette production. The absorption section presents CNS and activated carbon derived from CNS as effective absorbents for liquid-phase and gas-phase applications. While this review highlights numerous research-level possibilities for CNS utilization, only a few of these options have been implemented within the industry. Consequently, further research is essential, particularly in CNS characterization, economic and environmental assessment, and real-life implementation, to broaden and enhance the integration of this biomass into applications that can contribute to the value of both its production and processing chain.
DOI: 10.15376/biores.19.3.Cruz
Keywords: Cashew nutshells (CNS); Energy production; Substance adsorption; Materials development
Contact information: a: Grupo de Diseño de Productos y Procesos (GDPP), Department of Chemical and Food Engineering, Universidad de los Andes, CR 1 ESTE 19A-40, Bogotá, 111711, Colombia; b: Structural Integrity Research Group (GIE), Department of Mechanical Engineering, Universidad de los Andes, CR 1 ESTE 19A-40, Bogotá, 111711, Colombia; c: Sustainable Design in Mechanical Engineering Research Group (DSIM), Mechanical Engineering, Escuela Colombiana de Ingeniería Julio Garavito, AK 45 205-59, Bogotá, 111166, Colombia; d: Department of Design, Universidad de los Andes, CR 1 ESTE 19A 40, Bogotá, 111711, Colombia; *Corresponding author: n-porras@uniandes.edu.co
GRAPHICAL ABSTRACT
INTRODUCTION
The utilization of agro-industrial waste has recently emerged as an essential focus within diverse sectors concerned with sustainability. As global food demand, production, and consumption steadily increase, the amount of waste generated by the agro-industrial sector in the form of husks, peels, shells, and seeds inevitably accumulates in landfill sites (Kumar et al. 2022). This situation has exacerbated various environmental problems, including the increase in methane and carbon dioxide emissions, water pollution, and soil degradation (Mafakher et al. 2010; Shin et al. 2016).
Nevertheless, agro-industrial residues also offer promising opportunities for valorization through diverse applications. These applications vary depending on the residue type and can include energy production, biochemical and pharmaceutical applications, the creation of water absorbents, and the development of biopolymers and materials (Yaashikaa et al. 2022). A prime example of such residues is cashew nutshells (CNS), a lignocellulosic fiber derived from the cashew tree (Anacardium occidentale L.) that remains as waste after cashew kernel harvesting.
The cashew tree (Anacardium occidentale L.) is native to South America and was introduced to India and Mozambique in the 16th century (Tola and Mazengia 2019; Malik and Bhadauria 2020; Orduz-Rodríguez and Rodríguez-Polanco 2022). Since then, cashew
farming has spread to several parts of Asia and Africa. Although the cashew fruit consist of a visually striking yellow or red peduncle, the actual fruit is the cashew nut (Oliveira et al. 2020), which contains an edible kernel (Malik and Bhadauria 2020) within a distinctive kidney-shaped grayish shell, or pericarp (Orduz-Rodríguez and Rodríguez-Polanco 2022). The cashew nutshell (CNS) comprises the following layers: the epicarp, an external leathery layer; the mesocarp, a spongy layer that has a honeycomb structure containing a caustic and flammable liquid called cashew nutshell liquid (CNSL) (Oliveira et al. 2020); the endocarp, the innermost and hardest layer; and the testa, a thin and papery coat adhered to the kernel. Both the endocarp and the testa protect the kernel from contact with the CNSL (Orduz-Rodríguez and Rodríguez-Polanco 2022) (Fig. 1).
The main traded products are raw cashew nuts, cashew kernels, and CNSL (Malik and Bhadauria 2020), while by-products including CNS, CNS cake (CNS after CNSL extraction), testa, and the pseudo-fruit are often wasted (Sawadogo et al. 2018). Ivory Coast, India, Cambodia, Vietnam, and Tanzania were the top cashew producers in the 2022/23 period, during which global production of raw nuts reached 5 million tons (International Nut and Dried Fruit Council 2023). Other countries in the Americas, Asia, and Africa also contribute to cashew production to a lesser extent (Nair 2021). The demand for cashews is rising due to their nutritional properties, flavor, and versatility (Dendena and Corsi 2014). Global raw cashew nut production is expected to increase as cultivated hectares expand and crop management improves, leading to higher yields (Oliveira et al. 2020; Nair 2021; Orduz-Rodríguez and Rodríguez-Polanco 2022).
However, cashew production generates significant waste and by-products, with less than 30% of the nut being edible (Van Hoof et al. 2020). Around 70% of the nut is discarded, resulting in approximately 3.5 million tons of CNS waste globally in 2022/23 (International Nut and Dried Fruit Council 2023). The current management of these residues presents environmental and economic challenges. Some plants incinerate CNS residues for energy generation, emitting harmful vapors (Oliveira Galvão et al. 2014). In other cases, this waste is left on the ground due to the high transportation cost for disposal, resulting in soil acidification and fire hazards (Dendena and Corsi 2014; Sawadogo et al. 2018; Nair 2021). Therefore, it becomes crucial to find effective and profitable solutions for CNS waste management in the context of the growth of cashew production, global concerns regarding sustainability, circular economy, and the Sustainable Development Goals.
Table 1. Some Properties of CNS
Current research on cashew nut processing mainly concentrates on kernel and pseudo-fruit production and applications (Oliveira et al. 2020; Chen et al. 2023), CNSL extraction and valorization (Kyei et al. 2023), as well as testa utilization (Sruthi and Naidu 2023). However, CNS management receives less attention despite its promising physical, chemical, and thermal properties (Table 1). Further investigation into these properties is warranted. While Table 1 showcases extensive studies on proximate and ultimate analysis, and thermal behaviors, only three studies have explored CNS chemical composition while writing this article. The results from these studies also exhibit significant disparity (Ocheja et al. 2015; Paternina Reyes et al. 2023; de Paiva et al. 2024).
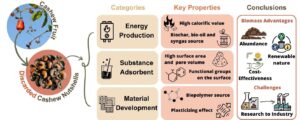
To the authors’ knowledge, no comprehensive review article has been dedicated solely to CNS. Therefore, this article aims to address this gap by reviewing alternative applications of cashew nutshells (CNS) waste that could enhance the value chain of cashew nut production and mitigate its environmental impact. This review categorizes CNS applications into three groups: material development, energy production, and substance adsorption. This review explores the properties of CNS relevant to each application, compares findings from various studies, discusses potential opportunities and challenges, and proposes future research directions.
EXPERIMENTAL
Bibliometric Analysis
A comprehensive literature research was conducted using three online databases: Scopus, Web of Science, and Google Scholar. The search terms employed were: (cashew) AND (nutshell OR nut-shell OR “nut shell”) AND (character* OR property*) in the title, abstract, or keywords of the articles. The search included articles published up to December 2023 with no restrictions on country, journal ranking, or publication type to ensure a thorough review of all relevant sources. This resulted in the identification of 1091 articles.
Studies focusing on CNS as a raw material for various applications were included during the screening process. Articles solely discussing CNSL or written in languages other than English, Spanish, or Portuguese were excluded. Finally, only studies with a well-described methodology for CNS utilization were considered after a full-text review. Additionally, the reference lists of the selected articles were examined to identify further relevant sources. In the end, 108 publications were included in this review article.
The compiled data were categorized based on application area, processing methods, CNS states (ground, ash, etc.), and investigated properties. This categorization ensured a complete analysis of the available literature and facilitated including high-quality and relevant studies in the review.
Based on the gathered information and the most frequently co-occurring topics (refer to Fig. 2a), this revision proposes three main categories for the alternative uses of CNS (refer to Fig. 2b), corresponding to the clusters depicted on the map. The green cluster, centered on the term “Cashew nutshells”, regroups terms related to activated carbon applications, which also connect to the three proposed categories. The red cluster associates the keywords related to energy production, while the yellow cluster presents terms linked to material development, such as starch, cellulose, and mechanical properties. Finally, the blue cluster presents terms related to substance adsorption. An additional trend observed among all the depicted keywords is a focus on agro-industrial waste, waste management, and sustainability.
The bibliometric analysis reveals a rising trend in research on this topic, with the highest number of publications occurring in 2018. Furthermore, among the reviewed articles, India had the highest number of publications, followed by Brazil and Nigeria. Interestingly, while the most cited articles focused on substance adsorption, the most published papers address energy production; this bibliometric analysis strengthens the authors’ claim of novelty, as no prior comprehensive study of this scope has been documented.
a)
b)
Fig. 2. a) Co-occurrence map of cashew nutshells related topics. Made with VOSViewer. b) Main cashew nutshell alternative applications
MATERIALS DERIVED FROM CASHEW NUTSHELLS
Construction Industry
The construction industry relies heavily on materials, particularly cement composites (Tantri et al. 2021). Unfortunately, cement production contributes significantly to CO2 emissions and incurs high energy costs (Thirumurugan et al. 2018; Oyebisi et al. 2019, 2021; Mendu and Pannem 2021). To address this problem, researchers have explored CNS-derived supplementary cementitious materials (SCMs) pretreated at different temperatures such as CNS ash (CNSA) (Lima and Rossignolo 2010; Pandi and Ganesan 2015; Thirumurugan et al. 2018; Oyebisi et al. 2019, 2021; Tantri et al. 2021, 2022), uncalcined CNS (burned below 400 °C), and CNS powder (CNSP) (Pavithra et al. 2020).
Among the studies, examination of CNSA particles below 10 µm using SEM revealed a variety of sizes ranging from 2 to 10 µm. These particles exhibited lamellar features with overlapping layers. Concrete samples containing 15% CNSA also showed similar characteristics (Mendu and Pannem 2021). Field emission scanning electron microscopy (FE-SEM) analysis of CNSA revealed a dense structure with flat medium to small-sized particles. Some voids were observed, likely due to burned-out and unburnt carbon (Tantri et al. 2022).
CNSA exhibits promise as a pozzolanic material and a potential SCM due to its oxide composition, allowing partial cement replacement in mortar and concrete applications. Studies indicated that a 15% replacement with CNSA is ideal for structural purposes, while a 20% substitution is suitable for non-load-bearing applications, resulting in denser and stronger concrete (Thirumurugan et al. 2018; Oyebisi et al. 2019; Mendu and Pannem 2021; Tantri et al. 2021, 2022). Additional research suggests that up to 25% CNSA substitution reduces water absorption, improves curing, minimizes pores, and enhances strength (Pandi and Ganesan 2015; Oyebisi et al. 2019). In contrast, uncalcined CNS is inadequate as a cement substitute due to its weak mechanical properties. Calcining CNS biomass increases its effectiveness as a substitute, allowing for a higher replacement ratio than uncalcined CNS. Specifically, studies suggest that 15 to 20% substitution with CNSA is possible, compared to only 8 to 10% with raw or uncalcined CNS (Tantri et al. 2021, 2022). Additionally, research on CNSP suggests that a maximum substitution percentage of 8% in cement is possible without compromising properties when combined with chicken feathers (Pavithra et al. 2020).
Similarly, in the construction industry, achieving soils with high bearing capacity is crucial, often requiring soil stabilization. In this context, James et al. (2022) utilized CNSA combined with lime to enhance the strength of a highly expansive soil, replacing 0.5% of CNSA with 5.5% of lime, which led to a significant increase in the compressive strength of the soil. Likewise, Bhat et al. (2019) incorporated CNSA and glass industrial waste into a lateritic soil, but only marginal improvements in soil properties were observed. Although the CNSA enhanced soil cohesion and reduced the friction angle, decreasing the soil’s maximum dry density. Further research is needed to determine the optimal percentage of CNSA for soil stabilization, as well as to evaluate its long-term effects and cost-effectiveness in comparison to other stabilizers.
In conclusion, CNSA is presented as a promising partial replacement for cement in construction applications and lime in soil stabilization. However, further research is necessary to assess its viability fully. When considering CNSA as a cement substitute, it is crucial to investigate its thermal properties, long-term durability, cost-effectiveness, and CO2 emissions compared to traditional materials (Jannat et al. 2021). Additionally, exploring the impact of CNSA on other critical soil properties, such as permeability, swelling potential, or even mineral contribution to the soil matrix, would be valuable.
Biopolymers
To counter environmental challenges posed by non-biodegradable polymers from petrochemicals, researchers are developing biopolymers from plant-based materials (Yuliana et al. 2012; Harini et al. 2018; Minh et al. 2019; Bamgbola et al. 2020). Utilizing polysaccharides, proteins, lipids, and polyesters derived from renewable agricultural sources, biodegradable plastics can be produced (Harini et al. 2018). Starch and cellulose are especially crucial in this biopolymer research, with starch standing out as a renewable, biodegradable, and non-toxic alternative to fossil fuels in the polymer industry.
CNSc starch was isolated through wet milling (Yuliana et al. 2012), resulting in a starch with 85.0% starch content, along with protein, fiber, ash, and impurities in the form of polymeric resins. The CNSc starch had 75.4% amylopectin and 24.6% amylose, which influence solubility and swelling, which are crucial for industrial applications. Despite high amylopectin content, CNSc starch exhibited low swelling due to the identified polymeric resins. CNSc starch showed twice the solubility of maize starch, high crystallinity, and the presence of agglomerated resins. These characteristics make it a promising renewable material in the polymer industry. However, its thermal processing temperature should not exceed 174 °C.
Additionally, CNSc was utilized to obtain pure cellulose (Bamgbola et al. 2020) through a modified acid hydrolysis (HNO3) method, followed by alkali (NaOH) treatment and bleaching (NaOCl). SEM images revealed that the isolated cellulose presented a porous structure resulting from lignin degradation during alkali treatment. Successful removal of lignin was confirmed by micrographs from morphological characterization and FTIR analysis. TGA analysis supported these findings, revealing that obtained cellulose decomposed in a single stage between 300 and 400 °C. X-ray diffraction analysis the extracted cellulose had a crystallinity index of 77.7%, and energy-dispersive X-ray (EDX) spectra confirmed the presence of essential elements in the cellulose fibers. These cellulose fibers hold potential applications in industries such as binders, reinforcements, and packaging.
In contrast, Minh et al. (2019) developed a phenolic resin using CNSc as the main component. Liquefaction of high lignin content biomasses is a process wherein formaldehyde can be substituted with biomass, in the present of specific catalysts, to produce phenolic resins in a more environmentally friendly manner (Alma et al. 1998). CNSc’s high lignocellulosic content makes it a desirable feedstock for liquefaction and transformation into valuable chemicals and products (Minh et al. 2019). The liquefaction process involved heating grounded CNSc and phenol with sulfuric acid. The 2:1 phenol to CNSc ratio yielded the highest amount of phenolic resin. Gel permeation chromatography indicated the resin was an oligomer based on its polydispersity. Further characterization using FTIR analysis and nuclear magnetic resonance (NMR) analysis confirmed the success of liquefaction process, revealing functional groups and chemical structure typical of a phenolic compound. This innovative work paves the way for further research on synthesizing CNS-derived resins through liquefaction, while more comprehensive characterizations in future investigations are recommended.
In conclusion, CNS is a valuable source of starch, cellulose, and lignin essential components of biopolymers. Starch-based biopolymers serve as eco-friendly substitutes for petroleum-based plastics in various applications like packaging, cutlery, bags, and films, offering biodegradability and reduced environmental impact (Bertolini 2009; Yuliana et al. 2012). Plant-based biopolymers are also used in adhesives, coatings, paints, and fabric production (Bertolini 2009; Harini et al. 2018). In agriculture, biopolymers find applications in soil stabilization, seed coating, fertilizers, and biodegradable pots, providing sustainable alternatives to conventional materials (Majeed et al. 2016).
When working with CNS, it is crucial to consider the biomass composition percentage to isolate the appropriate components. The environmental impact and cost of isolation procedures should also be considered, aiming to maximize biomass utilization (Alma et al. 1998; Bertolini 2009). Utilizing CNS as a low-cost and readily available raw material allows for the mitigation of environmental and economic implications, enhancing its overall benefits (Yuliana et al. 2012).
Composites
Various forms of CNS have been used in composites as fillers, reinforcements, and matrix constituents for structural materials, coatings, and packaging. For example, Harini et al. (2018) synthesized a composite using CNSc starch to create bio-thermoplastic films. Walnut shell cellulose served as reinforcement, with pomegranate antioxidants and antimicrobial compounds added for intelligent packaging purposes. The composite showed improved oxygen transfer rates with higher CNSc starch concentration, and mechanical properties increased with CNSc starch content, which are relevant features for packaging. Moisture retention decreased with higher CNSc starch concentration, and solubility of CNS starch films ranged from 40% to 48%. The addition of pomegranate peel extract to the best-performing cellulose-reinforced film showed favorable properties for intelligent packaging.
Researchers explored the use of polymeric matrices to create composites with CNS. Gomes et al. (2018) combined CNSP with a recycled high-density polyethylene (rrHDPE) matrix to develop Wood Plastic Composites. Different CNS concentrations were investigated, and the composite properties were evaluated. TGA and DSC analyses revealed a two-stage degradation with decreasing temperature as CNSP percentage increased, hindering crystalline structure formation, and resulting in lower crystallinity. Due to residual CNSL, the fluidity of the composite increased, and FTIR analysis showed deterioration of the composite interface. SEM images exhibited voids after processing, and tensile tests indicated decreased elastic modulus and increased elongation at fracture. Potential causes for the poor performance included incomplete CNSL extraction, low resin absorption by the fiber, inadequate control of CNSP particle size, and void formation during processing. The author suggested that CNS-reinforced polymer composites could be suitable for less demanding applications, but complete CNSL extraction is essential for structural use.
Consequently, some researchers have explored the use of CNSA as an alternative to address the negative effects of residual CNSL. For instance, Saravanan et al. (2017) developed an environmentally friendly composite by combining epoxy resin, CNSA, rice husk (RH), and sawdust (SD). The composite consisted of varying proportions of ashes: CNSA (0 to 40%), RH (20 to 30%), and SD (0 to 40%), although the initial state of the CNSA was unclear. The composite was prepared by blending predetermined amounts of biomass ash with an epoxy resin and hardener using the hand-lay-up technique to form rectangular specimens and casting cylindrical samples. Mechanical properties, including tensile strength, torsion, hardness (Rockwell), and impact strength (Izod), were measured. Among all tested mechanical properties, the second-best performing sample was the one with 40% CNSA, coming after the sample that incorporated ashes from all three biomasses.
Similarly, Sundarakannan et al. (2019) developed unsaturated polyester resin composites reinforced with CNS-derived biochar after pyrolysis. The impact of pyrolysis carbonization time and the percentage of biochar in the composite was studied. The biochar treated for 3 h showed higher crystallinity (28.4%) compared to 1-h treatment (26.4%).
Mechanical tests revealed that the composite with 10% biochar treated for 3 h exhibited the highest tensile, flexural, impact, and hardness strengths, showing improvements compared to the unfilled matrix. Samples with 15% biochar showed the highest flexural strength with a 40% increase. The enhanced mechanical properties were attributed to the increased crystallinity of the 3-hour-treated biochar, facilitating better resin penetration and generating a stronger interface, crack resistance, and improved impact energy absorption. SEM images confirmed that the best-performing sample had a smooth surface; other samples showed voids and microcracks. The study further supports the benefits of using CNSA in composite materials.
Another example of CNS composites is shown in the work of Mari and Villena (2016), where ground CNSc was combined with wood residues to create particle boards. Adhesive type and CNS/wood ratio influenced strength and dimensional stability. These two properties were negatively affected by CNSc replacement due to uneven particle geometry and residual CNSL. However, boards exhibited reduced flammability compared to pure wood, taking longer to ignite and extinguishing in a shorter time, resulting in less damage to the wood board area. Based on the results, CNSc is suitable for less demanding applications with cost-saving benefits.
Finally, studies were conducted on natural rubber filled with CNSP as a renewable and cost-effective additive (Mamza et al. 2016; Okele et al. 2016, 2018). Natural rubber requires vulcanization to enhance its mechanical properties, often using fillers such as carbon black derived from depletable and costly petrochemicals. However, researchers attempted to replace it with agricultural residues including CNSP. Findings revealed that carbon black exhibited better compatibility with rubber, while CNSP contained reinforcing particles of silicon dioxide and silicon carbide (Okele et al. 2016). CNSP composites had comparable rheological properties but longer curing times due to their acidic nature, with lower viscosity and torques than carbon black composites (Mamza et al. 2016). Neutralizing the acidic nature of CNSP and enhancing particle size and surface area could improve CNSP composite performance. Thus, in the last study (Okele et al. 2018), semi-nano CNSc powder (25%) mixed with carbon black (5%) improved hardness, abrasion resistance, and tensile strength, surpassing the solo carbon black sample. Therefore, semi-nano CNSc powder combined with carbon black presents a potential low-cost and eco-friendly reinforcing filler for natural rubber.
Overall, CNS has shown promise as a renewable and cost-effective component in composite materials, offering opportunities to enhance mechanical, physical, and chemical properties while reducing costs in specific applications. Table 2 illustrates the changes in mechanical properties resulting from CNS inclusion in composites. It is important to note that the impact on properties can vary, with enhancements observed in some cases, while decreases occur in others. Therefore, a comprehensive evaluation should consider additional aspects of composite processing, such as cost and other desired properties. For example, while CNS inclusion may improve flammability resistance, there might be a trade-off with a decrease in mechanical properties (Mari and Villena 2016). Further research and optimization are necessary to fully understand the potential of CNS, particularly in addressing residual CNSL, and to facilitate its expanded utilization across diverse industries.
Table 2. Mechanical Properties Comparison between Composites Made with CNS and Other Biomasses
Supercapacitors
CNSAC has been used in electrochemical double-layer capacitors (EDLCs) due to its combination of high surface area, superior porosity, excellent chemical stability, and increased electrical conductivity (Pulikkottil et al. 2022). Merin et al. (2021) successfully produced CNSAC through chemical activation with varying KOH ratios, followed by carbonization under argon. SEM revealed an extensive, 3D honeycomb-like porous structure in the CNSAC, ideal for electrolyte storage and ion transport. This porous morphology was attributed to the intercalation and removal of potassium during KOH activation and carbonization. X-ray diffraction (XRD) and FTIR analyses confirmed the presence of a graphitic carbon structure, contributing to its electrochemical activity. The high surface area of CNSAC provides numerous interfaces for charge storage, making it a desirable electrode material for supercapacitors. Interestingly, the pore size increases with higher KOH activation ratios, although this effect becomes less predominant beyond a 1:2 activation ratio. The reported specific surface area ranged from 772 to 1150 m²/g, with pore size distribution between 0.47 and 0.56 cm³/g. CNSAC with a 2:1 activating agent ratio showed higher capacitance (214 F/g), faster charge transfer, and 98% capacitance retention after 1000 cycles, making it a promising alternative to market products for supercapacitor applications.
In general, CNSAC exhibits favorable properties for electrochemical applications, particularly due to its high porosity and surface area. This material sustained high capacitance even after 1000 cycles, suggesting its potential for long-term performance in real-world devices. However, a comprehensive evaluation of the complete device incorporating supercapacitors made of CNSAC is necessary. Future research should focus on assessing key parameters like energy density, power density, cycle efficiency, and long-term stability. This area lacks in-depth investigation, presenting a significant opportunity for further exploration of CNSAC. Additionally, exploring environmentally friendly activation methods, such as physical activation, could minimize the use of hazardous substances and may be a valuable avenue for sustainable production of CNSAC.
Other Materials Applications
More et al. (2018) studied the use of silane-treated ash from CNS, groundnut shells, tamarind shells, rice husks, and sugarcane bagasse to enhance epoxy-amine coatings on metal sheets. Ashless epoxy resin was compared with amine-modified ash resins at various percentages, and ash effects on the chemical and mechanical properties were investigated. Characterization results for CNSA showed successful introduction of amine groups on its surface. The coating exhibited good chemical resistance to acid, alkali, and solvents without wrinkling, softening, or blistering. However, scratch hardness and anticorrosive effects were enhanced with different CNSA percentages. The study concluded that the excellent interaction between the epoxy resin and the CNSA improved the coating’s properties, making it a promising additive for improving coating performance.
In another study, Rajendran et al. (2022) explored CNSP as an eco-friendly alternative in sound-emitting pyrotechnic formulations, aiming to reduce the reliance on sulfur usage in fireworks. Sound-emitting pyrotechnic formulations were developed by incorporating CNSP as a replacement for sulfur. The study compared the acoustic properties, sound intensity, thermal performance, safety (impact and friction sensitivity), and eco-friendliness of the CNS-based pyrotechnics with conventional sulfur-containing compositions. The findings indicated that 5% CNSP showed the best performance across all properties. SEM images revealed a flake-shaped structure of CNSP at micrometer scales, with measurements aligned with the average particle size data (43 µm) of CNSP. By using CNS in pyrotechnic compositions, the study suggests a potential pathway to reduce the environmental impact of fireworks, specifically lowering SO2 gas emissions and making use of CNS residues, while still maintaining their desired sound-emitting properties.
In conclusion, CNS demonstrates great potential for diverse material developments, thanks to its components including starch, cellulose, and lignin. These components make CNS a versatile and renewable resource suitable for various applications, including biopolymers, composites, cementitious materials, rubber additives, activated carbon, coatings, and adhesives. Despite its advantages, challenges exist, such as complex and energy-intensive processes to component extraction and processing, composition variability, limited advanced processing technologies, and performance limitations compared to petrochemical-derived materials. Nevertheless, CNS offers benefits such as abundance, renewability, cost-effectiveness, versatility, and eco-friendliness. These advantages lead to waste reduction, lower production costs, expanded material applications, and reduced environmental impact. To fully utilize CNS’s potential in different industries, further research, and technological advancements are necessary to overcome challenges and enhance its overall viability.
ENERGY PRODUCTION FROM CASHEW NUTSHELLS
While petroleum derivatives still dominate global energy production, exploring clean and renewable alternatives is becoming increasingly crucial. In this context, biomass derived from industrial and agricultural waste has emerged as a promising alternative in replacing fossil fuels (dos Santos et al. 2022). Agricultural crop processing generates substantial amounts of biomass waste that can serve as an energy source, addressing power supply challenges for agriculture-dependent economies (Chungcharoen and Srisang 2020). One such example is cashew nut processing waste, consisting of the CNS, CNS cake (CNSc), and CNSL, which can be valorized as fuel.
CNS by-products present an opportunity to convert process waste into energy, especially benefiting energy-intensive stages such as roasting and drying during cashew processing (Sawadogo et al. 2018). Currently, CNS are burned in boilers for energy; however, this method generates smoke and exposes individuals to anacardic acid, found in the CNSL, which is irritating and potentially carcinogenic (Sawadogo et al. 2018). Thus, it is essential to study energy production methods involving CNS that have minimal negative effects on the environment and human health. In the following sections, three applications (briquette fabrication, pyrolysis, and gasification) that utilize CNS as a sustainable energy source are reviewed.
Briquettes
Biomass exhibits promise as a sustainable energy source, yet certain forms require treatment to enhance their overall efficiency. In general, thermomechanical treatments, drying, roasting, and densification improve the suitability of biomass for power generation (dos Santos et al. 2022). Biomass briquettes, compacted cylinders made through densification, offer an effective alternative. Densification is a simple process, and the resulting briquettes can meet heating and cooking needs in rural areas (Sawadogo et al. 2018).
Briquettes have many advantages over charcoal, lignite, and firewood. Their higher energy density, calorific power, and combustion efficiency make them easier to store, transport, and incinerate (Chungcharoen and Srisang 2020; dos Santos et al. 2022). Furthermore, briquettes offer environmental benefits and superior quality compared to coal, as they are derived from renewable resources. Their use can reduce deforestation and provide a way to dispose of agro-industrial wastes (Sawadogo et al. 2018).
Before densification, biomass waste undergoes various treatments depending on the waste type and manufacturing process. These treatments typically involve cleaning, grinding, and pressing (densification). Biomass densification may also require biowaste carbonization and binder addition (Sawadogo et al. 2018). Carbonization techniques influence briquette properties, with carbonized biomass briquettes exhibiting higher thermal efficiency and lower pollutant emissions (Chungcharoen and Srisang 2020). Furthermore, biomass and binder content, particle size, and pressing technique all impact briquette quality (Sawadogo et al. 2018; Chungcharoen and Srisang 2020). While high binder percentages can improve cohesion, they can also decrease calorific value (CV), which is usually determined with use of a bomb calorimeter (Sawadogo et al. 2018).
In comparison to charcoal, lignite, and firewood, briquettes offer distinct advantages, including higher energy density, convenient storage, and cleaner incineration (Chungcharoen and Srisang 2020; dos Santos et al. 2022). Furthermore, briquettes present environmental benefits, as they are derived from renewable resources, contributing to reduced deforestation and the efficient management of agro-industrial waste (Sawadogo et al. 2018).
Several studies have explored the potential of CNS as a raw material for briquette production. For instance, cylindrical briquettes with CNS, cassava starch, as a binder, and water were developed. The aim was to identify optimal processing parameters. The analysis revealed that a starch content of 12.9%, water content of 50%, and a drying period of 7 days yielded the most favorable results. The study found a positive correlation between binder content and the compressive strength and durability of the briquettes. However, an increase in both binder and moisture content led to a decrease in the overall calorific value of the briquette. Conversely, extending the drying duration significantly reduced moisture content, leading to improved compressive strength and water resistance of the briquettes. Interestingly, the moisture content did not appear to have a substantial impact on the durability and shatter resistance of the briquettes (Ajith Kumar and Ramesh 2022). Similarly, Arulkumar et al. (2019) conducted experiments using a mix of 80% sawdust and 20% other biomasses, such as rice straw, tamarind shell, neem leaf, and CNS. Among these, 20% CNS exhibited the highest CV (4.28 MJ/kg) indicating a potential as a high-energy fuel source.
Mohod et al. (2008) prepared briquettes using CNSc, sawdust, cow dung, and waste wheat flour. The goal was to determine the optimal composition for maximizing CV and durability. The results indicated that while increasing the CNSc content resulted in a higher CV, it also negatively affected durability due to the lower compaction capacity of the biomass. The optimal composition was 55% CNSc, 25% sawdust, 10% cow dung, and 10% waste wheat flour. Briquettes produced using this formula demonstrated minimal energy consumption during production and low water absorption. Furthermore, a test measuring shatter and durability revealed good shock and impact resistance, good handling and transportation properties, a favorable energy density ratio and degree of densification, and a strong calorific value (21 MJ/kg). Additionally, a water boiling test confirmed that these briquettes burned entirely with a consistent flame, leaving minimal ash residue. In contrast, Santos et al. (2022) produced pellets without binders using densification instead of briquetting. CNS pellets were compared with coconut shell pellets. The CNS pellets exhibited a lower combustion temperature but released more combustion gases than the coconut shell pellets.
To address the emissions problems, some authors proposed to pretreat the biomass before forming the briquettes. For example, Tuates et al. (2020) produced briquettes from three forms of CNSc: untreated CNSc, CNSc treated with hexane, and carbonized CNSc. The briquettes were then assessed for their physical and mechanical properties, including density, shatter resistance and compressive strength. The hexane treated CNSc briquettes with 10% binder exhibited the best overall physical and mechanical properties. However, the carbonized CNSc briquettes produced less smoke during burning. Similarly, other studies thermally treated CNS and mixed it with cassava/tapioca starch as binder to produce briquettes. For instance, Sawadogo et al. (2018) determined the optimal percentage of components and evaluated briquettes’ mechanical, physical, and thermal properties. CV for these briquettes was 25.7 MJ/kg (higher than wood, similar to charcoal), with good mechanical properties (compressive strength index 383 kPa, impact resistance index 61.1), and thermal efficiency (33.9%). However, Ifa et al. (2020) used the remaining carbonized CNS from pyrolysis and found a CV of 29.5 MJ/kg. An economic analysis revealed a 37% cost savings in CNS briquette production compared to liquefied petroleum gas. In a study by Chungcharoen and Srisang (2020), combining 65% CNS with carbonized areca nuts resulted in briquettes that emitted lower greenhouse gases than charcoal combustion, achieving a CV of up to 21 MJ/kg, making it suitable for cooking applications. Additionally, Huko et al. (2015) utilized carbonized CNS and mango seeds, using banana peel as a binder, to produce briquettes and evaluated the impact of particle size on their properties. Density, moisture content, ash content, durability index, compressive strength, and carbon monoxide emissions decreased with larger particle size, while the CV remained constant.
Table 3. Calorific Value (CV) of Briquettes and Other Fuels
In summary, all of the cited studies confirmed that CNS briquettes could be a viable alternative energy source, comparable to other commonly used biomass briquettes. Both untreated and carbonized CNS exhibit promising combustion properties (CV). However, there is still ample room for further research on CNS briquettes, including determining the best form of CNS (cake, carbonized, etc.), identifying optimal synergies with other biomasses, selecting suitable binders, and optimizing processing parameters. Table 3 displays the CV of the mentioned briquettes, highlighting CNS’s potential and performance in this context.
Pyrolysis
Pyrolysis is a thermochemical process that converts biomass into valuable products such as biochar, bio-oil, and biogas (Moreira et al. 2017; Ifa et al. 2018). During pyrolysis, biomass is heated in a sealed reactor or chamber without air or with limited oxygen. The high temperature, typically from 400 to 900 °C, induces thermal decomposition of the biomass (Nguyen et al. 2021). As the biomass heats, it breaks down into various products: gaseous components (e.g., methane, hydrogen, carbon monoxide), liquid bio-oil, and solid char. The gases and bio-oil can be utilized as fuel sources, while the solid char can be further processed or directly employed (Moreira et al. 2017; Melzer et al. 2018).
The specific type of pyrolysis and equipment used to influence product quantities (Moreira et al. 2017). For instance, fast pyrolysis is more likely to yield more bio-oil, whereas slow pyrolysis produces more biochar (Melzer et al. 2013). CNS is considered a promising material for pyrolysis owing to its high CV, approximately 20 MJ/kg (Moreira et al. 2017; Nguyen et al. 2021; Ajith Kumar and Ramesh 2022).
Melzer et al. (2013) studied product yield of lab-scale fast pyrolysis of CNS. Four different states of CNS by-products were subjected to pyrolysis: CNSc (after mechanical extraction), de-oiled CNSc (mechanical and chemical extraction using petroleum ether), two-step extracted CNSc (mechanical and chemical extraction using water and petroleum ether), and CNSL. Fast pyrolysis was conducted under a nitrogen atmosphere at 500 °C for 12 minutes. Bio-oil yields increased with higher extractive content (CNSL), while biochar and biogas yields decreased. Pyrolysis gas composition (CO2 and CO) remained relatively unchanged, resulting in higher CV. CNS and its by-products showed promise as alternative biomass sources for bio-oil production with diesel fuel-like properties. In a related study, Ifa et al. (2018) investigated the potential of CNS as a source of liquid smoke for varnish production. This work identified 300 °C as the optimal temperature for generating liquid smoke. This finding highlights CNS as a potential valuable alternative resource.
Nguyen et al. (2021) also investigated CNS pyrolysis and the relation of process parameters (temperature, heating rate, and residence time) on char characteristics. In this study, predicting synthesis gas product properties was challenging due to varying hemicellulose and cellulose quantities. The authors found low-temperature, slow-heating, and short-duration pyrolysis optimal for high-energy coal, while high-temperature (>600 °C) and fast-heating rates (>1000 °C/min) were suitable for activated carbon generation.
Moreira et al. (2017) further explored CNS pyrolysis in nitrogen and air atmospheres. Pyrolysis under nitrogen yielded higher amounts of bio-oil, while pyrolysis under air increased biogas yield. Biochar proved suitable for energy generation due to high its CV (25 to 28 MJ/kg) and high carbon content (70 to 75%), and as fertilizer owing to the presence of essential elements. Bio-oils had a high CV (32 MJ/kg) but required mixing with diesel for effective use. Biogas composition (CO, CO2, and H2) depended on the pyrolysis temperature. Below 400 °C, CO, and CO2 were dominant, while H2 became more prevalent at higher temperatures (above 400 °C). These gas products have various applications, from producing organic chemicals to serving as an energy source for pyrolysis process itself. Amaliyah and Eka Putra (2021) also investigated CNS pyrolysis products using microwaves, achieving faster and more efficient production of biochar (35%) and bio-oil (45%) while maintaining favorable properties like high H:C content, low oxygen content, and a high CV. Both studies employed SEM analyses. Moreira et al. (2017) presented micrographs showing biochars with no defined morphology and lacking pores. On the other hand, Amaliyah and Eka Putra (2021) noted that untreated CNS displayed an irregular and porous surface, which changed to a smoother and more porous structure after pyrolysis treatment. Pyrolysis also generates volatile organic compounds that may impact the porous structure of resulting biochars. Specifically, biochar derived from microwave-assisted pyrolysis exhibited a porous structure covering the range of macropores (>50 nm).
Finally, Tsamba et al. (2007) compared pyrolysis of CNS products, wood, and Miscanthus giganteus (grass commonly used for pyrolysis) at low heating rates (10 to 100 K/min). CNS generated more biochar and fewer volatile combustibles than wood. Table 4 presents additional biomass comparisons and pyrolysis product yields.
To conclude, CNS and CNSc are viable biomass options for pyrolysis, producing biochar, bio-oil, and biogas under various process conditions. While the thermal characteristics of these by-products are competitive with other biomasses, pyrolysis is a complex process that can release toxic compounds and gases if it is not set up correctly. Process parameters need to be studied in depth, especially when investigating alternative biomasses such as CNS. Additionally, given their high energy consumption and costs of pyrolysis, it is important to use abundant biomasses with high CV, such as CNS, to mitigate these drawbacks.
Gasification
Gasification is a thermochemical process converting solid biomass into synthesis gas (syngas). The biomass is prepared (shredded, dried) and fed into a gasifier, which is a high-temperature reactor where thermochemical processes occur. Inside the gasifier, in a controlled oxygen-starved environment, the biomass undergoes pyrolysis and gasification, which can be defined as the partial oxidation of the solid biomass char and volatile compounds generated from pyrolysis, leading to the formation of syngas, primarily composed of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and traces of other gases. The syngas may contain impurities, such as tars, particulates, sulfur compounds, and trace contaminants, which must removed or reduced to avoid corrosion, fouling, and environmental emissions. Syngas can be utilized in heat exchangers or for direct combustion. Cooling and purification remove such tar and dust particles, making it suitable for engines or electricity generation. While wood is commonly used, exploring alternative biomass sources like agricultural waste, forestry residues, energy crops, and municipal solid waste is essential due to wood’s high demand in various industries (Alcócer et al. 2015; Muthu Dineshkumar et al. 2021; Nguyen et al. 2021; Singh et al. 2006; Tippayawong et al. 2011).
Various studies have explored CNS as a feedstock for gasification. Singh et al. (2006) investigated CNS’s combustible properties and its suitability for gasification in an open-core downdraft gasifier. CNS demonstrated favorable properties for gasification, comparable to biomass briquettes and wood. Gasification performance was assessed at different gas flow rates (61 to 130 m3/h), revealing key parameters such as fuel consumption rate (47 kg/h), gas CV (4.5 MJ/m3), gas composition, and maximum gasification efficiency (70% at the highest flow rate).
Table 4. Products Yields and Calorific Values (CV) of CNS and other Biomasses Pyrolysis
In a similar study, Muthu Dineshkumar et al. (2021) utilized CNS in a downdraft gasifier for power generation. Findings revealed that CNS contains catalytic components (Al2O3, CaO, MgO, SnO2) important for biomass gasification. CNS also possesses a heat capacity of 20 MJ/kg and suitable thermal properties for gasification. Additionally, Alcócer et al. (2015) optimized CNS biomass utilization for gasification. The gasifier’s operation was analyzed to find the most favorable conditions. Using thermodynamic analysis and data from a fluidized bed gasifier, the gasifier’s efficiency and potential were determined. When processing 150 kg/h of CNS, at an average temperature of 850 °C, the gasifier achieved a 50.4% syngas yield with a CV of approximately 5.5 MJ/m3. Overall, results showed the viability of utilizing the generated gas as an energy source with lower pollutant emissions than direct combustion methods.
Previous investigations (Ramanan et al. 2008) highlighted challenges with CNS gasification, such as clogging and corrosion due to residual CNSL. To overcome this, CNS were charred before gasification. A theoretical model predicting gas composition was compared to experimental data, and outcomes were similar. Overall, CNS gasification was successful with minimal oil residue issues after charring. Nguyen et al. (2021) investigated the changes in the properties of CNS biochar during gasification with CO2 and H2O. The physical properties of the resulting biochar were compared to the initial biochar. Micropores and mesopores were detected in the biochar gasified with H2O, while only micropores were found in the biochar gasified with CO2. The Brunauer–Emmett–Teller (BET) surface area increased in both cases, from 0.10 to 527 m2/g with CO2 and 721 m2/g with H2O. The total pore volume increased from 0 to 0.24 cm3/g for CO2 and 0.33 cm3/g for H2O. The study also revealed that higher temperature and gas concentration favored the conversion rate.
Additionally, SEM analysis was used to investigate the morphology of biochar particles after the gasification process. Macroscopic observations revealed particle shrinkage affecting heat transfer and gas flow. Microscopic analysis showed increased porosity with gasification progression, notably more pronounced with H2O than CO2 due to molecular size differences affecting diffusion and surface reactions. Finally, Tippayawong et al. (2011) conducted a social and economic study by employing a CNS-fed downdraft fixed-bed gasifier to heat water at a cashew nut processing facility. The positive technical results led to monthly plant savings of 150 USD.
In summary, gasification presents a compelling alternative for efficient energy generation with advantages like fuel versatility, reduced emissions, multiple syngas applications, and feedstock flexibility. CNS is considered a promising feedstock for gasification by various authors. Table 5 compares syngas composition, CV, and gasification efficiency of CNS and other biomasses. Understanding syngas composition aids process optimization and energy content determination. This information is essential for safety, environmental impact evaluation, product applications, and process monitoring in gasification systems.
While the reviewed research did not delve into managing post-gasification residues like char and ash, these elements require proper handling to prevent environmental concerns. Fortunately, CNS offers an advantage with its inherently low ash content, minimizing this potential issue.
Table 5. Syngas Composition and Calorific Values (CV), Gasification Efficiency and Temperature of CNS and other Biomasses
In summary, utilizing CNS as feedstock for briquette production, pyrolysis, or gasification processes presents significant environmental and economic advantages. CNS offers a renewable and readily available biomass resource, contributing to reduced emissions with a promising calorific value. This approach promotes the development of cleaner energy sources and fosters decentralized energy production, particularly in rural areas. Furthermore, value-added products like biochar, bio-oil, and syngas present commercial opportunities, supporting bioenergy markets and reducing reliance on imported fossil fuels.
SUBSTANCE ADSORPTION USING CASHEW NUTSHELLS
Industrial wastewater discharge containing heavy metals and organic compounds harms aquatic life, causes water eutrophication, and disrupts biological cycles (Coelho et al. 2018; Jain et al. 2022; Kalaba et al. 2022). These contaminants can enter the food chain through poorly sanitized water, posing health risks to humans (Nuithitikul et al. 2020). In developing countries, restricted access to safe drinking water due to these pollutants leads to diseases affecting multiple bodily systems, hampering community development (Kalaba et al. 2022).
To treat polluted water, several separation techniques are available, such as filtration, coagulation, and reverse osmosis. However, these methods often require expensive and energy-intensive processes. Moreover, most generated hazardous wastes, including sludge, filters can be most effective in water with high pollutant concentrations. On the other hand, adsorption has been recommended by many authors as the best decontamination option due to its efficiency, low cost, and simplicity. Adsorption can remove contaminants almost entirely, and in many cases the adsorbent can be reused, sometimes with the recovery of adsorbates, mainly metals. Additionally, adsorption processes can exhibit high removal efficiency even at low solute concentrations, below 100 mg/L for heavy metals. Since adsorption utilizes a wide range of adsorbents to address various contaminants, it is a highly flexible process. However, the use of commercial adsorbents such as polymers, minerals (e.g., zeolites), and other materials can be expensive and offer limited possibilities for regeneration and longer equilibrium times. Consequently, the research for abundant and cost-effective alternatives has gained popularity, leading to the study of biosorbents such as coconut shells, neem leaves, rice straw, corn cob, Pinus ash, chitosan, and CNS (Prabu et al. 2016; Coelho et al. 2018; Senthil Kumar et al. 2018; Nuithitikul et al. 2020; Geczo et al. 2021; Wang 2021; Jain et al. 2022; Kalaba et al. 2022).
Simultaneously, polluting compounds from combustion and anthropogenic activities pose environmental threats such as the greenhouse effect, acid rain, and ozone layer depletion. To safeguard the environment, it is crucial to remove harmful components from gases before release. Studies have investigated gas adsorption using biomasses and activated carbon, including those derived from CNS (Suresh et al. 2012; Serafin et al. 2017; Garg and Das 2020).
Adsorption involves the attachment of molecules or ions from an aqueous or gaseous solution onto the surface of a solid adsorbent (biomass, such as CNS in this case) (Nuithitikul et al. 2020). Then, the biomass is often pretreated to enhance its adsorption capacity (Coelho et al. 2018). Pretreatment processes may include drying, grinding, sieving, and activation (e.g. physical activation with steam or chemical activation with acids or bases). Next, the prepared biomass is brought into contact with the solution containing the target molecules. This can be achieved through various methods, such as batch adsorption (stirring biomass in a solution, usually with a liquid phase) (Jain et al. 2022) or column adsorption (flowing solution through a packed bed of biomass) (Suresh et al. 2012; Yahya et al. 2020). Adsorption occurs as molecules come into contact with the surface of the biomass material. The molecules adhere to the surface through various mechanisms, including physical adsorption (Van der Waals forces, electrostatic interactions) (Senthil Kumar et al. 2011c), chemical adsorption (formation of chemical bonds) (Senthil Kumar et al. 2010), and complexation (formation of complexes between target substances and functional groups on the biomass surface) (Coelho et al. 2014). Over time, a balance is reached between the rate of adsorption (molecules adhering to the biomass surface) and desorption (molecules detaching from the surface back into the solution). This equilibrium is influenced by factors such as temperature, the concentration of the target substances, and the properties of the biomass material (Jain et al. 2022). The adsorption capacity of biomass materials depends on factors such as the surface area, pore size distribution, and surface chemistry of the biomass (Garg and Das 2020). The efficiency of the adsorption process can be affected by parameters like adsorbent dosage, contact time, and the concentration of target substances in the solution (Jain et al. 2022; Senthil Kumar and Ramalingam 2013). After adsorption, the biomass may undergo desorption to recover the adsorbed substances or to regenerate the biomass for reuse. Desorption can be achieved through chemical elution, thermal treatment, or solvent extraction (Coelho et al. 2018; Nuithitikul et al. 2020; Senthil Kumar et al. 2011c).
CNS have been extensively studied as adsorbents for various substances in both liquid and gas-phase applications. Their adsorption capacity is influenced by their porous structure and the chemical reactivity of the functional groups on their surfaces (Senthil Kumar et al. 2010, 2011c, 2012b; Coelho et al. 2014; Nuithitikul et al. 2020; Jain et al. 2022).
Liquid-phase Applications
Cashew nutshells and cashew nutshell cake
Ground CNSc has demonstrated effectiveness as an adsorbent for various heavy metals. Under optimal conditions for each study, CNSc achieved a maximum monolayer adsorption capacity of 18.9 mg/g for Ni(II) (Senthil Kumar et al. 2011c), 29.0 mg/g for Zn(II) (Senthil Kumar et al. 2012a), 20.2 mg/g for Cu(II) (Senthil Kumar et al. 2011e), 22.1 mg/g for Cd(II) (Senthil Kumar et al. 2012b), and 28.6 mg/g and 8.4 mg/g for Pb(II) and Cr(III), respectively (Coelho et al. 2014). Maximum adsorption capacity is highly influenced by adsorption parameters such as initial concentration, pH, contact time, adsorbent dosage. The presence of surface functional groups on CNS and CNSc, such as hydroxyl and carboxyl, indicates the presence of lignin, which facilitate metal removal from aqueous solutions. Additionally, amine and carbonyl groups further enhance metal ions binding (Coelho et al. 2014; Senthil Kumar et al. 2011c).
In addition, several studies employed SEM analysis to examine the surface morphology and microstructure of the CNS-derived adsorbents. These analyses revealed that the CNS surface exhibits a lamellar, spongy, heterogeneous, irregular, and porous structure, making it highly suitable for the adsorption of Ni (II), Zn (II), Cd (II), Pb (II), and Cr (III) ions adsorption (Senthil Kumar et al. 2011c, 2012a,b; Coelho et al. 2014). Notably, SEM analysis of Cd (II) loading by Coelho et al. (2014) suggested that adsorption occurs within the walls of the CNS surface pores.
Moreover, treating CNSc with substances including H2SO4, H2O2, NaOH, HNO3, and nano-zero-valent iron enhanced their adsorption capacity for metal ions such as Pb (II), Cd (II), Cr (II), Mn (II), Cu (II), and Zn (II). Adsorption studies were performed by varying the solution pH, adsorbent dose, initial concentration, contact time, and temperature. The maximum improvement observed is a 14-fold increase in adsorption capacity for Pb (II) (Senthil Kumar et al. 2011b, 2018; Prabu et al. 2016, 2021; Coelho et al. 2018; Nuithitikul et al. 2020; Yahya et al. 2020). These chemical treatments modify CNSc surface functional groups and eliminate impurities, creating more active sites for adsorption and potentially enhancing surface area and pore volume (Nuithitikul et al. 2020). However, Coelho et al. (2018) stated that changes in these properties after treatment are not significantly improved, suggesting adsorption mainly occurs superficially rather than through diffusion.
Furthermore, SEM analysis revealed that sulfuric acid treatment resulted in irregular and porous surfaces on CNS, confirming their suitability for adsorption of Pb (II), Cr (II), and Mn (II) ions (Senthil Kumar et al. 2011b; Yahya et al. 2020). Moreover, Nuithitikul et al. (2020) observed a collapsed CNS structure after NaOH treatment using SEM imaging, indicating lignin dissolution. This collapsed structure presented an irregular pattern and porosity, containing evident pores suitable for adsorption. Post-adsorption images showed these pores filled with the adsorbed ions. Additionally, SEM-EDX analyses confirmed the presence of adsorbed metallic ions by demonstrating a complete filling of the pore spaces after adsorption (Nuithitikul et al. 2020).
Overall, the treatment choice depends on the type of metal ions present in the solution and their affinity for the CNS surface under specific conditions. For instance, CNS treated with H2SO4 exhibits higher efficiency in removing Pb (II), while those treated with NaOH are more effective in removing Cr (III) (Coelho et al. 2018; Nuithitikul et al. 2020).
Some studies discussed the desorption of metal ions from spent CNS-derived adsorbents, primarily focusing on heavy metals. The desorption process involved treating the spent adsorbent with an HCl solution, followed by filtration and analysis of metal concentrations. In the study by Senthil Kumar et al. (2011c), desorption of Ni (II) ions was achieved using chemical regeneration methods. The recovery rate of these ions was reported at 72.0%. Senthil Kumar et al. (2012a) demonstrated 75.1% and 67.7% desorption percentages for Zn(II) ions at concentrations of 25 and 50 mg/L, respectively. Similarly, 73.8% and 66.6% desorption percentages were achieved for Cd(II) ions at the same concentrations (Senthil Kumar et al. 2012b). Coelho (2014) found high desorption percentages for Cd(II) and Pb(II) ions but low desorption for Cr (III).
The desorption studies aimed to elucidate the nature of the adsorption and recycling of spent adsorbents, which is crucial for water treatment. Chemical regeneration methods were preferred over thermal activation due to energy considerations and adsorbent loss. The desorption process involves increasing the ion force of H+ in the solution, leading to the exchange of metal cations adsorbed on the adsorbent surface. While desorption percentages varied for different metal ions, CNS showed potential for the reutilization of adsorbates such as Cd (II) and Pb (II). However, the desorption capacity for Cr (III) was relatively low, limiting its reusability in other adsorption processes.
CNS have also shown high efficiency in removing dyes from wastewater generated by the textile industry. CNSP used as an adsorbent, achieved remarkable removal efficiencies: around 99% for Congo Red dye (Senthil Kumar et al. 2010) and 96% for Acid Green 25 dye (Jain et al. 2022). Additionally, CNSP showed adsorption capacities of 5.18 mg/g for Congo Red and 76.34 mg/g for Acid Green 25. Adsorption is favored at low pH (≤ 3) and with spontaneous reactions. Senthil Kumar et al. (2010) observed that the adsorption kinetics followed a pseudo-second-order equation, while Jain et al. (2022) suggested physical adsorption based on thermodynamic parameters. Finally, Jain et al. (2022) further investigated the CNS adsorbent by performing SEM analysis before and after dye adsorption. Pre-adsorption SEM images revealed a porous structure, suggesting favorable conditions for dye uptake. However, post-adsorption images showed noticeable alterations, indicating that pore blockage occurred due to dye adsorption. This observation suggests a significant interaction between Acid Green 25 and the CNS adsorbent.
In addition, CNS have been used to remove phenols from wastewater. Phenol, an organic compound, poses risks to humans and aquatic life. CNSc powder (Senthil Kumar et al. 2011a) and sulfuric acid treated CNSP (Kulkarni et al. 2018) were employed for phenol removal. In the CNSc powder experiment, the Langmuir isotherm provided the best correlation of phenol sorption onto CNS, indicating monolayer adsorption with a maximum adsorption capacity of 5.41 mg/g. Thermodynamic parameters for phenol adsorption on untreated CNSc powder revealed a spontaneous and exothermic reaction. Regarding adsorption kinetics, the adsorption exhibited a better fit with a pseudo-second-order model, potentially indicating chemisorption. Conversely, with treated CNSP, the adsorption equilibrium was better described by the Freundlich isotherm, which had multilayer adsorption with a maximum uptake capacity of 35.08 mg/g. Also, a pseudo-first-order model provided a better fit for sulfuric acid-treated CNSP. Both studies aimed to optimize parameters such as adsorbent dosage, contact time, pH, phenol concentration and temperature.
Cashew nutshells derived activated carbon
Activated carbon, obtained through wood or coal carbonization followed by activation, is a widely used and versatile adsorbent (Subramaniam and Senthil Kumar 2015) with high porosity and surface area. It is cost-effective, regenerable, and effective at low pollutant concentrations in both liquid and gas phases (Geczo et al. 2021; Hoc Thang et al. 2021; Smith et al. 2021; Kalaba et al. 2022). Activated carbon efficiently removes various contaminants from water, including taste, odor, color, organic pollutants, and some heavy metals (Kouassi Brou et al. 2020). However, the cost of raw materials and energy consumption during processing are potential drawbacks (Hoc Thang et al. 2021; Nuithitikul et al. 2020). To reduce costs, agricultural residues have been evaluated as alternative raw material sources (Tangjuank et al. 2009a; Nuithitikul et al. 2020).
Several authors have documented the production of CNS-based activated carbon (CNSAC) for the removal of heavy metals, dyes, and fluoride. CNSAC, similar to CNS, relies on its surface functional groups, high surface area, and porosity for effective adsorption. For instance, two related studies demonstrated the successful removal of heavy metals (Cr (II), Pb (II), and Cd (II)) from aqueous solutions using CNSAC activated with KOH (chemical activation) and CO2 (physical activation). Notably, modifying impregnation time and ratio during chemical activation enhanced the development of mesoporous structures and increased the BET surface area (up to 1120 m2/g). In both studies, the adsorption equilibrium behavior was accurately described by the Freundlich and Langmuir isotherms, and it was established that CNSAC achieved approximately 99% removal efficiency for all metal ions tested. Maximum adsorption capacity attained was 28.9 mg/g, 14.3 mg/g, and 13.9 mg/g for Pb(II), Cd(II), and Cr(III), respectively (Tangjuank et al. 2009a,b). In addition, SEM images depicted irregular surfaces with slit pores (10 to 30 µm), evolving into rough textures with shallow cavities upon activation with KOH. Increasing activation time and KOH ratio, resulted in more uniform pore structures, forming honeycomb shapes indicative of increased porosity (Tangjuank et al. 2009a, b).
Similarly, CNSAC was employed to remove Brilliant Green (BG) and Methylene Blue (MB) dyes from water. The study focused on BG removal (Samiyammal et al. 2022), used CNSAC activated with KOH, and evaluated the impact of the impregnation ratio on the adsorption capacity of the activated carbon. The results showed a maximum adsorption capacity of 244 mg/g with a 1:1 impregnation ratio, corresponding to higher surface area (408 m2/g) and pore volume (0.29 cm3/g). This experiment exhibited a better fit with the Langmuir isotherm model. SEM micrographs of CNSAC and EDS analysis show a honeycomb-like porous surface. A 1:1 ratio yields numerous smaller pores, correlating with a larger BET surface area. Despite size differences, both ratios produce nearly spherical pores, crucial for effective adsorption.
On the other hand, MB removal studies focused on chemical, physical, and mixed activation. In the chemical activation study (Spagnoli et al. 2017), CNS was treated with ZnCl2 at 400 °C for 2 hours (h). Impregnation ratios (ZnCl2/CNS) were examined, and 1,5:1 ratio resulted in the highest surface area (1100 m2/g), pore volume (0.565 cm3/g), and maximum adsorption capacity of MB (456 mg/g). This phenomenon was best described by the Langmuir equation. Likewise, CNSAC physical activation study (Hoc Thang et al. 2021) explored various temperatures and activation time ranges using steam at a constant rate. Optimal conditions were found at 850 °C for 50 minutes (min), achieving the highest pore volume (0.342 cm3/g) and surface area (679 m2/g) while preventing carbon decomposition. The maximum adsorption capacity of MB was approximately 45 mg/g.
Finally, the two related studies on mixed activation (Senthil Kumar et al. 2011d; Subramaniam and Senthil Kumar 2015) used KOH as the chemical activating agent, followed by physical activation using CO2. CNSAC characterization revealed a specific surface area of 984 m2/g, pore volume of 0.55 cm3/g, and average pore diameter of 2.52 nm, resulting in 99.97% MB efficiency and a maximum monolayer adsorption capacity of 68.7 mg/g. The adsorption of MB was adequately described by the pseudo-second-order equation (Senthil Kumar et al. 2011d).
Ragupathy et al. (2015) also studied BG and MB removal using KOH-activated CNSAC further enhanced by incorporating TiO2 particles. FTIR analysis revealed increased active sites and vibrational peaks indicating titanium incorporation. Optimal parameters such as catalyst amount (0.20 g/L), initial dye concentration (10 mg/L), pH (6.7), and contact time (120 min) achieved 96% MB and 99% BG removal efficiency. The adsorption of BG and MB on TiO2/CNSAC was better described by a pseudo-second-order kinetic model. SEM analyses of CNSAC, TiO2, and TiO2/CNSAC were performed. Pure TiO2 displayed a spherical morphology, while CNSAC exhibited a honeycomb-like structure with surface pores. TiO2/CNSAC displayed a rough, porous surface due to the growth of TiO2 nanoparticles on the CNSAC surface.
Alagumuthu and Rajan (2010) used CNSAC impregnated with zirconium to remove fluoride from water. Fluoride in water can cause fluorosis in high concentrations (del Bello 2020). The CNSAC was prepared with H2SO4 activation and zirconium impregnation. The process achieved a maximum fluoride removal percentage of 80.3%. The Langmuir isotherm provided the best description of the adsorption mechanism, and the pseudo-second-order model exhibit the best fit. The thermodynamic parameters indicated a spontaneous and endothermic reaction. Co-ions in water, like sulfate, chloride, bicarbonate, and nitrate, influenced the adsorption process, with bicarbonate decreasing fluoride adsorption. Desorption study showed 96.2% weight desorption, decreasing with subsequent cycles.
Finally, Geczo et al. (2021) studied the effectiveness of CNSAC in removing acetaminophen from water, examining the role of surface chemistry, porosity in the adsorption process, and activation temperatures (400 to 700 °C). Activation with H3PO4 at 600 °C showed the highest adsorption capacity (146 mg/g) after 4 h. The results also demonstrated that the Langmuir model provided the best fit for equilibrium adsorption data at all activation temperatures. The pseudo-second-order model was the most suitable for describing the adsorption process. Interestingly, surface functional groups played a vital role in the adsorption process through hydrogen bonding or acid hydrolysis, outweighing surface area and pore volume.
In summary, these investigations demonstrate the versatility of CNS-derived adsorbents for removing various contaminants, including heavy metals, dyes, and phenols from aqueous solutions. The influence of chemical and physical treatments on their adsorption capacity is noteworthy. Further research on the long-term stability and reusability of these adsorbents is crucial. Additionally, in-depth investigations into the adsorption mechanisms with CNS-derived adsorbents are needed to gain a deeper understanding of the removal process. For real-world applications, studies focusing on selective adsorption from binary or ternary mixtures and real samples are necessary before practical implementation. Finally, comparative studies evaluating different adsorption methods from environmental, efficiency, and economic perspectives would be valuable.
Gas-phase Applications
CNSAC activated with H3PO4 was used to remove benzene vapor and CO2. The best results achieved a surface area of 903 m2/g, pore volume of 0.492 cm3/g, and carbon content of 87.5%, which led to a maximum adsorption capacity of 1095 mg/g. The adsorption kinetics followed a second-order rate expression, and the equilibrium adsorption data fit the Langmuir isotherm. SEM images showed that a 1:1 ratio generated a few cylindrical and slit-shaped pores, while a 2:1 ratio led to larger honeycomb-like pores. After benzene adsorption, surface area reduction and partial pore blockage were observed (Suresh et al. 2012).
Furthermore, for CO2 removal, CNS biochar from pyrolysis activated by chemical and physical methods was used (Garg and Das 2020). Chemical activation with K2CO3 resulted in the highest surface area (1225 m2/g) and pore volume (0.66 cm3/g). Physical activation achieved a surface area of 287 m2/g, while CO2 activation produced a surface area of 701 m2/g. Ultra-micropores in CNSAC activated with K2CO3 provided significant advantages, reaching CO2 adsorption between 183 mg and 274 mg CO2/g of CNSAC at atmospheric pressure and 0 °C.
Overall, removing pollutants in gas-phase applications using CNS-derived adsorbents remains a relatively unexplored area. More detailed studies are needed addressing topics similar to those suggested for liquid-phase applications in the previous subsection. These topics include adsorbent reusability, adsorption mechanisms, sustainability concerns, and real-life case studies.
In summary, CNS, CNSc, and CNSAC are effective adsorbents for pollutants in liquid and gas phases. These biomasses have high porosity, surface area, available active sites, and superficial functional groups, enabling them to adsorb heavy metals, dyes, organic pollutants, gases, and other harmful substances. CNS and its by-products are abundant, sustainable, and renewable resources. They offer advantages like cost-effectiveness, high adsorption capacity, versatility in pollutant types, and regenerability, reducing the need for frequent replacement. However, the use of chemicals during carbon activation should be carefully evaluated, as there is a risk of generating other waste and increase the substance treatment problem. In addition, the impact of thermal treatments should also be considered and utilize CNSAC from other processes, such as pyrolysis, should be preferred to avoid unnecessary energy expenditure.
CONCLUSIONS
This article has provided an overview of the available literature on the potential applications of cashew nutshells (CNS) in three categories: material development, energy generation, and substance adsorption. Various alternatives utilizing CNS are presented for each field. The following conclusions have been reached:
- Several studies have partially characterized CNS for various applications. However, there is a need for a comprehensive characterization focused on understanding all CNS properties, independent of specific applications, and not as a means for other applications, which closes the gaps in the characterization results has not been carried out. As presented in Table 1, the values show much dispersion, and important information such as the type of clones, places of origin, or the shelling process is not usually detailed in the articles since all of these are factors that can alter the properties of CN, and likewise their performance in different applications.
- The potential of CNS in the materials sector is significant, as the processing of these nutshells can yield various types of materials, including starch, cellulose, phenolic resins, and fillers for ceramics. These materials can serve as feedstock for the development of composite materials. Utilizing CNS to produce valuable materials generates economic benefits and promotes sustainability by reducing the carbon footprint. In addition, it can create an alternative source of income for cashew farmers in developing countries, contributing to poverty alleviation and economic development. Therefore, further research in this area can lead to the development of sustainable solutions in diverse fields. Future research should include additives to formulation, study of additional processing methods, evaluate material prototypes for real-world application performance, cost-efficiency compared to existing materials, and life cycle assessment.
- In energy production applications, the review highlights the remarkable thermal properties of CNS, which are comparable to those of wood. The high calorific value of CNS makes it a viable option as a source of energy for the cashew production chain in developing countries, where other traditional energy sources may be scarce or expensive. However, implementing systems like gasifiers or reactors requires significant technological expertise and upfront investment, which might be challenging for cashew farmers. Therefore, further research is crucial to explore cost-efficient alternatives that avoid combustion. This is also important because using CNS for briquettes, pyrolysis, and gasification can reduce dependence on non-renewable energy sources. Promoting CNS as a bioenergy source holds potential for the cashew industry by providing a sustainable and potentially cost-effective alternative.
- The porosity and functional groups on the CNS surface, especially after its pyrolytic conversion to biochar or activated carbon, make it a promising material for an adsorbent in both aqueous and gaseous media. The porous structure of the CNS provides a large surface area for adsorption, while the various functional groups on its surface offer sites for chemical interaction with target molecules. This makes CNS an attractive alternative to traditional adsorbents, especially in water treatment and air pollution control applications. The use of CNS as an adsorbent has the potential to be an economically viable and environmentally friendly solution to various pollution problems.
- Despite numerous potential applications, large-scale industrial use of cashew nut shells (CNS) remains elusive. Effective CNS management strategies are crucial to unlocking their full potential and preventing environmental hazards. These strategies should consider the techno-economic, environmental, and social aspects of new developments.
- Minimizing waste after each application is ideal. This can be achieved by creating synergies between different uses. For instance, biochar or ash residues from energy production or adsorption processes can be repurposed in material generation.
- Finally, CNS offers a compelling combination of abundance, affordability, thermal properties, porosity, and surface area. This review highlights its potential as an economic driver in cashew-growing regions, adding another layer to its overall value proposition.”
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to Ministerio de Ciencia, Tecnología e Innovación de Colombia and OCAD de CTeI, who carried out the viability, prioritization, and approval of this research with resources from Sistema General de Regalías – SGR in the call No. 6 of the Project “Aprovechamiento de los subproductos Agroindustriales en la producción del marañón en el departamento del Vichada – BPIN 2020000100571”. Likewise, the authors thank the government and the community of the department of Vichada in general for their interest and participation.
REFERENCES CITED
Ajith Kumar, T. T., and Ramesh, S. T. (2022). “Sustainable production of cashew nutshell briquettes: Experimental assessment and optimization of factors affecting the physical and fuel characteristics,” Biomass Conversion and Biorefinery 1-22. DOI: 10.1007/S13399-021-02234-X/METRICS
Alagumuthu, G., and Rajan, M. (2010). “Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon,” Chemical Engineering Journal 158(3), 451-457. DOI: 10.1016/J.CEJ.2010.01.017
Alcócer, J. C. A., Duarte, J. B. F., Pereira, M. C., de Oliveira, M. L. M., de Lima, R. K. C., Benevides, D., and Barros, G. (2015). “Mass and energy balance of a cashew nut shell gasification pilot unit utilized in power generation,” IEEE Latin America Transactions 13(10).
Alma, M. H., Maldas, D., and Shiraishi, N. (1998). “Liquefaction of several biomass wastes into phenol in the presence of various alkalis and metallic salts as catalysts,” Journal of Polymer Engineering 18(3), 161-178. DOI: 10.1515/POLYENG.1998.18.3.161
Amaliyah, N., and Eka Putra, A. E. (2021). “Microwave-assisted pyrolysis of cashew nut shell,” International Journal of Design and Nature and Ecodynamics 16(2), 227-232. DOI: 10.18280/ijdne.160213
Antwi-Boasiako, C., and Acheampong, B. B. (2016). “Strength properties and calorific values of sawdust-briquettes as wood-residue energy generation source from tropical hardwoods of different densities,” Biomass and Bioenergy 85, 144-152. DOI: 10.1016/j.biombioe.2015.12.006
Arulkumar, R., Kanagasabapathy, H., and Manickam, I. (2019). “Combination of agricultural waste and saw dust into biomass material for briquette,” Indian Journal of Ecology.
Bamgbola, A. A., Adeyemi, O. O., Olubomehin, O. O., Akinlabi, A. K., Sojinu, O. S., and Iwuchukwu, P. O. (2020). “Isolation and characterization of cellulose from cashew (Anacardium occidentale L.) nut shells,” Current Research in Green and Sustainable Chemistry 3. DOI: 10.1016/J.CRGSC.2020.100032
del Bello, L. (2020). “Fluorosis: An ongoing challenge for India,” The Lancet Planetary Health 4(3), 94-95.
Bertolini, A. C. (2009). Starches: Characterization, Properties, and Applications, CRC Press, Boca Raton, FL, USA.
Bhat, A. K., Marathe, S., and Ashmitha, N. M. (2019). “Stabilization of locally available soil using CNSA and glass industry waste,” International Journal of Recent Technology and Engineering 8(3), 4245-4249. DOI: 10.35940/IJRTE.C5151.098319
Cao, W., Guo, L., Yan, X., Zhang, D., and Yao, X. (2018). “Assessment of sugarcane bagasse gasification in supercritical water for hydrogen production,” International Journal of Hydrogen Energy 43(30), 13711-13719. DOI: 10.1016/j.ijhydene.2017.12.013
Chen, Y., Li, N., Guo, X., Huang, H., Garcia‐Oliveira, P., Sun, J., Zhang, J., Prieto, M. A., Guo, Z., and Liu, C. (2023). “The nutritional and bio‐active constituents, functional activities, and industrial applications of cashew (Anacardium occidentale): A review,” Food Frontiers, 4(4), 1606-1621. DOI:10.1002/fft2.250
Chiang, K. Y., Lin, Y. X., Lu, C. H., Chien, K. L., Lin, M. H., Wu, C. C., Ton, S. S., and Chen, J. L. (2013). “Gasification of rice straw in an updraft gasifier using water purification sludge containing Fe/Mn as a catalyst,” International Journal of Hydrogen Energy 38(28), 12318-12324. DOI: 10.1016/j.ijhydene.2013.07.041
Chungcharoen, T., and Srisang, N. (2020). “Preparation and characterization of fuel briquettes made from dual agricultural waste: Cashew nut shells and areca nuts,” Journal of Cleaner Production 256. DOI: 10.1016/J.JCLEPRO.2020.120434
Coelho, G. F., Gonçalves, A. C., Schwantes, D., Rodríguez, E. Á., Tarley, C. R. T., Dragunski, D., and Conradi Junior, É. (2018). “Removal of Cd(II), Pb(II) and Cr(III) from water using modified residues of Anacardium occidentale L.,” Applied Water Science 8(3). DOI: 10.1007/s13201-018-0724-8
Coelho, G. F., Gonçalves, A. C., Tarley, C. R. T., Casarin, J., Nacke, H., and Francziskowski, M. A. (2014). “Removal of metal ions Cd (II), Pb (II), and Cr (III) from water by the cashew nut shell Anacardium occidentale L,” Ecological Engineering 73, 514-525. DOI: 10.1016/J.ECOLENG.2014.09.103
de Paiva, E. M., Mattos, A. L. A., da Silva, J. C. G., Mumbach, G. D., Arias, S., Pacheco, J. G. A., Di Domenico, M., Alves, J. L. F., and de Brito, E. S. (2024). “Pyrolysis of cashew nutshell residues for bioenergy and renewable chemicals: Kinetics, thermodynamics, and volatile products,” Journal of Analytical and Applied Pyrolysis 177, article 106303. DOI:10.1016/j.jaap.2023.106303
de Paula, Y. L., de Melo, R. R., Paula, E. A. de O., de Morais, E. R. C., Rodolfo Junior, F., Pimenta, A. S., de Oliveira, R. R. A., de Souza, J. A. G., Scatolino, M. V., and Pedrosa, T. D. (2023). “biodegradable tubes based on beeswax and cashew nut wastes: An eco-friendly solution for seedling production,” Waste and Biomass Valorization. DOI: 10.1007/s12649-023-02194-5
dos Santos, G. R., de Sousa, A. M., Lima, B. K. S., Moreira, F. L., Gondim, F. L., da Silva, G. M., Ratts, M. G. A. C., Serra, D. S., de Oliveira, M. L. M., and Cavalcante, F. S. Á. (2022). “Combustion of pellets produced from the powders of coconut and cashew nut shells: Chemical, thermal and emission analyses,” Waste Management and Research 40(4), 420-428. DOI:10.1177/0734242X20983417
Dendena, B., and Corsi, S. (2014). “Cashew, from seed to market: A review,” Agronomy for Sustainable Development 34(4), 753-772. DOI: 10.1007/S13593-014-0240-7
Garg, S., and Das, P. (2020). “Microporous carbon from cashew nutshell pyrolytic biochar and its potential application as CO2 adsorbent,” Biomass Conversion and Biorefinery 10(4), 1043-1061. DOI: 10.1007/S13399-019-00506-1
Geczo, A., Giannakoudakis, D. A., Triantafyllidis, K., Elshaer, M. R., Rodríguez-Aguado, E., and Bashkova, S. (2021). “Mechanistic insights into acetaminophen removal on cashew nut shell biomass-derived activated carbons,” Environmental Science and Pollution Research 28(42), 58969-58982. DOI: 10.1007/S11356-019-07562-0
Gomes, V. N. C., Carvalho, A. G., Furukava, M., Medeiros, E. S., Colombo, C. R., Melo, T. J. A., Araújo, E. M., Morais, D. D. S., Ueki, M. M., Paskocimas, C. A., and Santos, A. S. F. (2018). “Characterization of wood plastic composite based on HDPE and cashew nutshells processed in a thermokinetic mixer,” Polymer Composites 39(8), 2662-2673. DOI: 10.1002/PC.24257
Harini, K., Chandra Mohan, C., Ramya, K., Karthikeyan, S., and Sukumar, M. (2018). “Effect of Punica granatum peel extracts on antimicrobial properties in walnut shell cellulose reinforced bio-thermoplastic starch films from cashew nut shells,” Carbohydrate Polymers 184, 231-242. DOI: 10.1016/J.CARBPOL.2017.12.072
Hoc Thang, N., Sy Khang, D., Duy Hai, T., Thi Nga, D., and Dinh Tuan, P. (2021). “Methylene blue adsorption mechanism of activated carbon synthesised from cashew nut shells,” RSC Advances 11(43), 26563-26570. DOI: 10.1039/D1RA04672A
Van Hoof, B., Gómez, H., Duque, J., Méndez, C., and Orduz, J. (2020). “Transformación productiva de los agronegocios: La experiencia de MAS marañón Vichada,” _____
Huko, D., Kamau, D. N., and Ogola, W. O. (2015). “Effects of varying particle size on mechanical and combustion characteristics of mango seed shell cashew nut shell composite briquettes,” International Journal of Engineering Science Invention 48-58.
Ifa, L., Sabara, Z., Mandasini, Nurjannah, N., Anas, A., and Madilao, W. (2018). “Utilization of liquid smoke produced through the pyrolysis of cashew nut shells as raw materials for varnish manufacturing,” IOP Conference Series: Earth and Environmental Science 175(1), article 012034. DOI: 10.1088/1755-1315/175/1/012034
Ifa, L., Yani, S., Nurjannah, N., Darnengsih, D., Rusnaenah, A., Mel, M., Mahfud, M., and Kusuma, H. S. (2020). “Techno-economic analysis of bio-briquette from cashew nut shell waste,” Heliyon 6(9), article e05009. DOI: 10.1016/J.HELIYON.2020.E05009
International Nut & Dried Fruit Council. (2023). Nuts and Dried Fruits Statistical Yearbook 2022/2023, < https://inc.nutfruit.org/> (Mar.17, 2024).
Jain, S. N., Tamboli, S. R., Sutar, D. S., Jadhav, S. R., Marathe, J. V., and Mawal, V. N. (2022). “Kinetic, equilibrium, thermodynamic, and desorption studies for sequestration of acid dye using waste biomass as sustainable adsorbents,” Biomass Conversion and Biorefinery 12(7), 2597-2609. DOI: 10.1007/S13399-020-00780-4
James, J., Roshna, R., and Santhiya, S. (2022). “Cashew nut shell ash as a supplementary additive in lime stabilized expansive soil composites,” Materials Today: Proceedings 644-649. DOI: 10.1016/j.matpr.2022.03.627
Jannat, N., Latif Al-Mufti, R., Hussien, A., Abdullah, B., and Cotgrave, A. (2021). “Utilisation of nut shell wastes in brick, mortar and concrete: A review,” Construction and Building Materials 293, article 123546. DOI: 10.1016/J.CONBUILDMAT.2021.123546
Kalaba, G., Nyirenda, J., and Munyati, O. (2022). “Characterisation of activated carbons for removal of organic and heavy metal pollutants from water in resource limited countries,” Desalination and Water Treatment 261, 224-233. DOI: 10.5004/DWT.2022.28531
Kouassi Brou, G., Serpokrylov, N. S., Smolyanichenko, A. S., Cheblakova, E. G., and Gorina, V. A. (2020). “Preparation of activated carbon from cashew nut shells for water purification,” Russian Journal of Non-Ferrous Metals 61(1), 112-118. DOI: 10.3103/S1067821220010058
Kulkarni, K., Sudheer, V., and Girish, C. R. (2018). “Phenol adsorption from wastewater using cashew nut shells as adsorbent,” International Journal of Engineering and Technology(UAE) 7(3), 966-969. DOI: 10.14419/IJET.V7I3.9771
Kumar, V., Sharma, N., Umesh, M., Selvaraj, M., Al-Shehri, B. M., Chakraborty, P., Duhan, L., Sharma, S., Pasrija, R., Awasthi, M. K., Bhatnagar, A., and Maitra, S. S. (2022). “Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery,” Bioresource Technology 362, article 127790. DOI: 10.1016/j.biortech.2022.127790
Kyei, S. K., Eke, W. I., Nagre, R. D., Mensah, I., and Akaranta, O. (2023). “A comprehensive review on waste valorization of cashew nutshell liquid: Sustainable development and industrial applications,” Cleaner Waste Systems 6, article 100116. DOI:10.1016/j.clwas.2023.100116
Lima, S. A., and Rossignolo, J. A. (2010). “Estudo das características químicas e físicas da cinza da casca da castanha de caju para uso em materiais cimentícios,” Acta Scientiarum – Technology 32(4), 383-389. DOI: 10.4025/ACTASCITECHNOL.V32I4.7434
Mafakher, L., Mirbagheri, M., Darvishi, F., Nahvi, I., Zarkesh-Esfahani, H., and Emtiazi, G. (2010). “Isolation of lipase and citric acid producing yeasts from agro-industrial wastewater,” New Biotechnology 27(4), 337-340. DOI: 10.1016/j.nbt.2010.04.006
Majeed, A., Najar, R. A., Ul Rehman, W., Choudhary, S., Thakur, S., Singh, A., Sharma, G., and Bhardwaj, P. (2016). “Cellulose: A multifaceted biopolymer,” in: Natural Polymers: Derivatives, Blends and Composites, Volume I, Nova Science Publ.
Malik, J. A., and Bhadauria, M. (2020). “Cashew nut (Anacardium occidentale),” in: Antioxidants in Vegetables and Nuts – Properties and Health Benefits, Springer, Singapore, pp. 539-557. DOI: 10.1007/978-981-15-7470-2_28
Mamza, P. A. P., Okele. I. A., and Nkeonye, P. O. (2016). “Studies on the rheological properties of cashew nut shell powder (Anacardium accidentale) and carbon black on natural rubber vulcanisates,” Composite Materials 009-018.
Manzone, M. (2018). “Performance evaluation of different techniques for firewood storage in Southern Europe,” Biomass and Bioenergy 119, 22-30. DOI: 10.1016/j.biombioe.2018.09.004
Mari, E. L., and Villena, E. M. (2016). “Properties of particleboard from wood wastes and cashew nut shell residue,” Philippine Journal of Science 145(1), 1-8.
Melzer, M., Blin, J., Bensakhria, A., Valette, J., and Broust, F. (2013). “Pyrolysis of extractive rich agroindustrial residues,” Journal of Analytical and Applied Pyrolysis 104, 448-460. DOI: 10.1016/j.jaap.2013.05.027
Mendu, J. K., and Pannem, R. M. R. (2021). “Assessment of mechanical properties of cashew nut shell ash blended concrete,” Innovative Infrastructure Solutions 6(4). DOI: 10.1007/S41062-021-00586-X
Merin, P., Henock, A., Malamal Neelanchery, M., Gopalan, E. V., and Seema, A. (2022). “Cashew nut shell derived porous activated carbon electrodes for ‘water-in-salt’ electrolyte based symmetric supercapacitor,” ChemistrySelect 7(23), article e202200984. DOI: 10.1002/SLCT.202200984
Merin, P., Jimmy Joy, P., Muralidharan, M. N., Veena Gopalan, E., and Seema, A. (2021). “Biomass-derived activated carbon for high-performance supercapacitor electrode applications,” Chemical Engineering and Technology 44(5), 844-851. DOI: 10.1002/CEAT.202000450
Minh, D. Q., Huynh, N. M., Nguyen, V. U. N., Nguyen, N. T. H., Kieu, D. T. K., Pham, T. K., and Nguyen, H. T. (2019). “The influence of composition of raw materials on formation of phenolic resin from cashew nut shell waste (CNSW),” Defect and Diffusion Forum 394 DDF, 103-108. DOI: 10.4028/WWW.SCIENTIFIC.NET/DDF.394.103
Mohod, A. G., Khandetod, Y. P., and Powar, A. G. (2008). “Processed cashew shell waste as fuel supplement for heat generation,” Energy for Sustainable Development 12(4), 73-76. DOI: 10.1016/S0973-0826(09)60009-0
Moon, C., Sung, Y., Ahn, S., Kim, T., Choi, G., and Kim, D. (2013). “Effect of blending ratio on combustion performance in blends of biomass and coals of different ranks,” Experimental Thermal and Fluid Science 47, 232-240. DOI: 10.1016/j.expthermflusci.2013.01.019
More, A. P., Amrutkar, S. Y., and Mhaske, S. T. (2018). “Enrichment of epoxy coating system with modified shell ash,” International Journal of Environment and Waste Management 21(1), 58-79. DOI: 10.1504/IJEWM.2018.091310
Moreira, R., dos Reis Orsini, R., Vaz, J. M., Penteado, J. C., and Spinacé, E. V. (2017). “Production of biochar, bio-oil and synthesis gas from cashew nut shell by slow pyrolysis,” Waste and Biomass Valorization 8(1), 217-224. DOI: 10.1007/s12649-016-9569-2
Müller, C. M. O., Laurindo, J. B., and Yamashita, F. (2009). “Effect of cellulose fibers on the crystallinity and mechanical properties of starch-based films at different relative humidity values,” Carbohydrate Polymers 77(2), 293-299. DOI: 10.1016/j.carbpol.2008.12.030
Muthu Dineshkumar, R., Meera Sheriffa Begum, K. M., and Ramanathan, A. (2019). “Comprehensive characterization of cashew nutshell for biomass gasification,” in: Materials Today: Proceedings 9837-9843. DOI: 10.1016/j.matpr.2020.10.932
Nagarajan, J., and Prakash, L. (2021). “Preparation and characterization of biomass briquettes using sugarcane bagasse, corncob and rice husk,” in: Materials Today: Proceedings 4194-4198. DOI: 10.1016/j.matpr.2021.04.457
Nair, K. P. (2021). “Cashew nut (Anacardium occidentale L.),” in: Tree Crops, Springer, Cham, 27-77. DOI: 10.1007/978-3-030-62140-7_2
Nguyen, H. N., Khuong, D. A., Le Gia, T. T., Truong, A. H., and Ha, V. T. T. (2020). “Pyrolysis of cashew nut shell: A parametric study,” Vietnam Journal of Chemistry 58(4), 506-511. DOI: 10.1002/vjch.202000015
Nguyen, H. N., Khuong, D. A., Vu, T. T. H., Mai, T. N., Tsubota, T., Tran, V. B., Blin, J., and Van De Steene, L. (2021). “Kinetic and structural changes during gasification of cashew nut shell char particles,” Environmental Progress & Sustainable Energy 40(3), article e13580. DOI: 10.1002/EP.13580
Nuithitikul, K., Phromrak, R., and Saengngoen, W. (2020). “Utilization of chemically treated cashew-nut shell as potential adsorbent for removal of Pb(II) ions from aqueous solution,” Scientific Reports 10(1). DOI: 10.1038/S41598-020-60161-9
Ocheja, J. O., Lablable, B. C., Oguche, H. G. E., and Usman, G. O. (2015). “Vitamin composition and fibre fractions of cashew nut shell: implication for animal nutrition,” Pakistan Journal of Nutrition 14(5), 252-254.
Okele, A. I., Embu, Y. E., Marut, A. J., Leo, Y. Z., and Musa, E. (2016). “Studies on the morphology of natural rubber compound filled cashew nut shell powder (CNSP),” International Journal of Environmental Sciences 5(3), 149-153.
Okele, A.-W. I., Gimba, C. E., Mamza, P. A. P., and Abba, H. (2018). “Hybridisation of carbon black: Cashew nut shell powder as fillers on the mechanical properties of natural rubber composites,” Composite Materials 2(2), 49-54. DOI: 10.11648/j.cm.20180202.12
Oliveira, N. N., Mothé, C. G., Mothé, M. G., and de Oliveira, L. G. (2020). “Cashew nut and cashew apple: A scientific and technological monitoring worldwide review,” J. Food Science and Technology 57(1), 12-21. DOI: 10.1007/S13197-019-04051-7
Orduz-Rodríguez, J. O., and Rodríguez-Polanco, E. (2022). “Cashew (Anacardium occidentale L.) a crop with productive potential: Technological development and prospects in Colombia,” Agronomia Mesoamericana 33(2). DOI: 10.15517/AM.V33I2.47268
Oyebisi, S., Ede, A., Owamah, H., Igba, T., Mark, O., and Odetoyan, A. (2021). “Optimising the workability and strength of concrete modified with anacardium occidentale nutshell ash,” Fibers 9(7). DOI: 10.3390/FIB9070041
Oyebisi, S., Igba, T., and Oniyide, D. (2019). “Performance evaluation of cashew nutshell ash as a binder in concrete production,” Case Studies in Construction Materials 11. DOI: 10.1016/J.CSCM.2019.E00293
Pandi, K., and Ganesan, K. (2015). “Effect of water absorption and sorptivity on durability of cashew nut shell ash mortar,” International Journal of Applied Engineering Research 10(13), 33345-33349.
Park, Y. H., Park, H. Y., Kim, H. H., and Park, S. Bin. (2018). “Non-isothermal pyrolysis of cashew shell cake-bituminous coal blends,” Environmental Engineering Research, Korean Society of Environmental Engineers 23(2), 121-128. DOI: 10.4491/eer.2017.012
Paternina Reyes, M. J., Unfried Silgado, J., Santa Marín, J. F., Colorado Lopera, H. A., and Espitia Sanjuán, L. A. (2023). Cashew nutshells: A promising filler for 3D printing filaments. Polymers 15(22), article 4347. DOI:10.3390/polym15224347
Pavithra, C., Arokiaprakash, A., and Maheshwari, A. (2020). “Behaviour of concrete adding chicken feather as fibre with partial replacement of cement with cashew nut shell powder,” Materials Today: Proceedings 43, 1173-1178. DOI: 10.1016/J.MATPR.2020.08.731
Prabu, D., Parthiban, R., Senthil Kumar, P., Kumari, N., and Saikia, P. (2016). “Adsorption of copper ions onto nano-scale zero-valent iron impregnated cashew nut shell,” Desalination and Water Treatment 57(14), 6487-6502. DOI: 10.1080/19443994.2015.1007488
Prabu, D., Senthil Kumar, P., Sathiish, S., and Suresh, A. (2021). “Superhigh adsorption of cadmium(II) ions onto surface modified nano zerovalent iron composite (CNS-nZVI): Characterization, adsorption kinetics and isotherm studies,” Chemistry and Chemical Technology 15(4), 457-464. DOI: 10.23939/CHCHT15.04.457
Pulikkottil, M., Antony, H., Muralidharan, M. N., Gopalan, E. V., and Ansari, S. (2022). “Cashew nut shell derived porous activated carbon electrodes for ‘water-in-salt’ electrolyte based symmetric supercapacitor,” ChemistrySelect 7(23). DOI:10.1002/SLCT.202200984
Ragupathy, S., Raghu, K., and Prabu, P. (2015). “Synthesis and characterization of TiO2 loaded cashew nut shell activated carbon and photocatalytic activity on BG and MB dyes under sunlight radiation,” Spectrochimica Acta – Part A: Molecular and Biomolecular Spectroscopy 138, 314-320. DOI: 10.1016/J.SAA.2014.11.087
Rajendran, M., Ramanathan, R., Shanmugavel, R., Senthil Andavan, G. T., and Thiagamani, S. M. K. (2022). “Utilization of cashew nutshell biomass as eco-friendly sound-emitting pyrotechnic formulation to reduce sulfur usage in fireworks,” Biomass Conversion and Biorefinery. DOI: 10.1007/S13399-022-03228-Z
Ramanan, M. V., Lakshmanan, E., Sethumadhavan, R., and Renganarayanan, S. (2008). “Modeling and experimental validation of cashew nut shell char gasification adopting chemical equilibrium approach,” Energy and Fuels 22(3), 2070-2078. DOI: 10.1021/ef700467x
Samiyammal, P., Kokila, A., Pragasan, L. A., Rajagopal, R., Sathya, R., Ragupathy, S., Krishnakumar, M., and Minnam Reddy, V. R. (2022). “Adsorption of brilliant green dye onto activated carbon prepared from cashew nut shell by KOH activation: Studies on equilibrium isotherm,” Environmental Research 212, article 113497. DOI: 10.1016/J.ENVRES.2022.113497
dos Santos, G. R., de Sousa, A. M., Lima, B. K. S., Moreira, F. L., Gondim, F. L., da Silva, G. M., Ratts, M. G. A. C., Serra, D. S., de Oliveira, M. L. M., and Cavalcante, F. S. Á. (2022). “Combustion of pellets produced from the powders of coconut and cashew nut shells: Chemical, thermal and emission analyses,” Waste Management and Research 40(4), 420-428. DOI: 10.1177/0734242X20983417
Saravanan, S., and Ganesan, K. (2017). “Mechanical testing of epoxy bonded eco-friendly natural fibre composite material,” International Journal of Computer Aided Engineering and Technology 9(2), 241-250. DOI: 10.1504/IJCAET.2017.083396
Sawadogo, M., Tchini Tanoh, S., Sidibé, S., Kpai, N., and Tankoano, I. (2018). “Cleaner production in Burkina Faso: Case study of fuel briquettes made from cashew industry waste,” Journal of Cleaner Production 195, 1047-1056. DOI: 10.1016/J.JCLEPRO.2018.05.261
Senthil Kumar, P., Nair, A. S., Ramaswamy, A., and Saravanan, A. (2018). “Nano‐zero valent iron impregnated cashew nut shell: A solution to heavy metal contaminated water/wastewater,” IET Nanobiotechnology 12(5), 591-599. DOI: 10.1049/iet-nbt.2017.0264
Senthil Kumar, P., and Ramalingam, S. (2013). “Process optimization studies of Congo red dye adsorption onto cashew nut shell using response surface methodology,” Int. Journal of Industrial Chemistry 4(1), article 17. DOI:10.1186/2228-5547-4-17
Senthil Kumar, P., Ramakrishnan, K., Dinesh Kirupha, S., and Sivanesan, S. (2011a). “Thermodynamic, kinetic, and equilibrium studies on phenol removal by use of cashew nut shell,” Canadian Journal of Chemical Engineering 89(2), 284-291. DOI: 10.1002/cjce.20396
Senthil Kumar, P., Ramalingam, S., Abhinaya, R. V., Thiruvengadaravi, K. V., Baskaralingam, P., and Sivanesan, S. (2011b). “Lead(II) adsorption onto sulphuric acid treated cashew nut shell,” Separation Science and Technology 46(15), 2436-2449. DOI: 10.1080/01496395.2011.590174
Senthil Kumar, P., Ramalingam, S., Kirupha, S. D., Murugesan, A., Vidhyadevi, T., and Sivanesan, S. (2011c). “Adsorption behavior of nickel(II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design,” Chemical Engineering Journal 167(1), 122-131. DOI: 10.1016/j.cej.2010.12.010
Senthil Kumar, P., Ramalingam, S., and Sathishkumar, K. (2011d). “Removal of methylene blue dye from aqueous solution by activated carbon prepared from cashew nut shell as a new low-cost adsorbent,” Korean Journal of Chemical Engineering 28(1), 149-155. DOI: 10.1007/S11814-010-0342-0
Senthil Kumar, P., Ramalingam, S., Sathyaselvabala, V., Kirupha, S. D., and Sivanesan, S. (2011e). “Removal of copper(II) ions from aqueous solution by adsorption using cashew nut shell,” Desalination 266(1–3), 63-71. DOI: 10.1016/j.desal.2010.08.003
Senthil Kumar, P., Ramalingam, S., Abhinaya, R. V., Kirupha, S. D., Vidhyadevi, T., and Sivanesan, S. (2012a). “Adsorption equilibrium, thermodynamics, kinetics, mechanism and process design of zinc(II) ions onto cashew nut shell,” Canadian Journal of Chemical Engineering 90(4), 973-982. DOI: 10.1002/CJCE.20588
Senthil Kumar, P., Ramalingam, S., Sathyaselvabala, V., Kirupha, S. D., Murugesan, A., and Sivanesan, S. (2012b). “Removal of cadmium(II) from aqueous solution by agricultural waste cashew nut shell,” Korean Journal of Chemical Engineering 29(6), 756-768. DOI: 10.1007/S11814-011-0259-2
Senthil Kumar, P., Ramalingam, S., Senthamarai, C., Niranjanaa, M., Vijayalakshmi, P., and Sivanesan, S. (2010). “Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions,” Desalination 261(1–2), 52-60. DOI: 10.1016/J.DESAL.2010.05.032
Serafin, J., Narkiewicz, U., Morawski, A. W., Wróbel, R. J., and Michalkiewicz, B. (2017). “Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions,” Journal of CO2 Utilization 18, 73-79. DOI: 10.1016/J.JCOU.2017.01.006
Shin, J., Hong, S. G., Kim, S.-C., Yang, J. E., Lee, S. R., and Li, F.-Z. (2016). “Estimation of potential methane production through the mass balance equations from agricultural biomass in Korea,” Applied Biological Chemistry 59(5), 765-773. DOI: 10.1007/s13765-016-0224-1
Singh, R. N., Jena, U., Patel, J. B., and Sharma, A. M. (2006). “Feasibility study of cashew nut shells as an open core gasifier feedstock,” Renewable Energy 31(4), 481-487. DOI: 10.1016/J.RENENE.2005.04.010
Smith, V. A., Rivera, J. F. A., Bello, R., Rodríguez-Aguado, E., Elshaer, M. R., Wodzinski, R. L., and Bashkova, S. (2021). “The role of surface chemistry and polyethylenimine grafting in the removal of Cr(VI) by activated carbons from cashew nut shells,” Journal of Carbon Research 7(27). DOI: 10.3390/C7010027
Sotannde, O. A., Oluyege, A. O., and Abah, G. B. (2010). “Physical and combustion properties of charcoal briquettes from neem wood residues,” International Agrophysics 24, 189-194.
Spagnoli, A. A., Giannakoudakis, D. A., and Bashkova, S. (2017). “Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters,” Journal of Molecular Liquids 229, 465-471. DOI: 10.1016/J.MOLLIQ.2016.12.106
Sruthi, P., and Naidu, M. M. (2023). “Cashew nut (Anacardium occidentale L.) testa as a potential source of bioactive compounds: A review on its functional properties and valorization,” Food Chemistry Advances 3, article 100390. DOI:10.1016/j.focha.2023.100390
Subramaniam, R., and Senthil Kumar, P. (2015). “Novel adsorbent from agricultural waste (cashew nut shell) for methylene blue dye removal: Optimization by response surface methodology,” Water Resources and Industry 11, 64-70. DOI: 10.1016/J.WRI.2015.07.002
Sundarakannan, R., Arumugaprabu, V., Manikandan, V., and Vigneshwaran, S. (2019). “Mechanical property analysis of biochar derived from cashew nut shell waste reinforced polymer matrix,” Materials Research Express 6(12). DOI: 10.1088/2053-1591/AB6197
Suresh, S., Vijayalakshmi, G., Rajmohan, B., and Subbaramaiah, V. (2012). “Adsorption of benzene vapor onto activated biomass from cashew nut shell: Batch and column study,” Recent Patents on Chemical Engineering 5(2), 116-133. DOI: 10.2174/2211334711205020116
Tangjuank, S., Insuk, N., Tontrakoon, J., and Udeye, V. (2009a). “Adsorption of lead(II) and cadmium(II) ions from aqueous solutions by adsorption on activated carbon prepared from cashew nut shells,” International Journal of Chemical and Molecular Engineering 3(4).
Tangjuank, S., Insuk, N., Udeye, V., and Tontrakoon, J. (2009b). “Chromium (III) sorption from aqueous solutions using activated carbon prepared from cashew nut shells,” International Journal of Physical Sciences 4(8), 412-417.
Tantri, A., Nayak, G., Kamath, M., Shenoy, A., and Shetty, K. K. (2021). “Utilization of cashew nut-shell ash as a cementitious material for the development of reclaimed asphalt pavement incorporated self compacting concrete,” Construction and Building Materials 301, article 124197. DOI: 10.1016/J.CONBUILDMAT.2021.124197
Tantri, A., Nayak, G., Shenoy, A., Shetty, K. K., Achar, J., and Kamath, M. (2022). “Implementation assessment of calcined and uncalcined cashew nut-shell ash with total recycled concrete aggregate in self-compacting concrete employing Bailey grading technique,” Innovative Infrastructure Solutions 7(5), article 305. DOI: 10.1007/S41062-022-00907-8
Thirumurugan, V., George, S., Raj, V., and Dheenadhayalan, K. (2018). “Experimental study on strength of concrete by partial replacement of cement by cashew nut shell ash (CNSA) and chicken feather fiber (CFF) as fiber reinforcement,” International Journal of Advance Research and Development 3(3).
Tippayawong, N., Chaichana, C., Promwangkwa, A., and Rerkkriangkrai, P. (2011). “Gasification of cashew nut shells for thermal application in local food processing factory,” Energy for Sustainable Development 15(1), 69-72. DOI: 10.1016/J.ESD.2010.10.001
Tola, J., and Mazengia, Y. (2019). “Cashew production benefits and opportunities in Ethiopia: A review,” Journal of Agricultural and Crop Research 7(8), 18-25. DOI: 10.33495/jacr_v7i2.19.105
Tsai, W. T., Lee, M. K., and Chang, Y. M. (2007). “Fast pyrolysis of rice husk: Product yields and compositions,” Bioresource Technology 98(1), 22-28. DOI: 10.1016/j.biortech.2005.12.005
Tsamba, A. J., Yang, W., Blasiak, W., and Wójtowicz, M. A. (2007). “Cashew nut shells pyrolysis: Individual gas evolution rates and yields,” Energy and Fuels 21(4), 2357-2362. DOI: 10.1021/ef0604792
Tuates, A. M., Suligan, J. M., Ligisan, A. R., Capariño, O. A., and Elauria, J. C. (2020). “Development of de-oiled cashew nut shell as fuel briquettes,” Nihon Enerugi Gakkaishi/Journal of the Japan Institute of Energy 99(8), 123-128. DOI: 10.3775/JIE.99.123
Wang, J. (2021). “Study on the performance identification of OpenCV in cashew nut shell-based activated carbon,” IOP Conference Series: Earth and Environmental Science 769(3), article 032030. DOI: 10.1088/1755-1315/769/3/032030
Yaashikaa, P. R., Senthil Kumar, P., and Varjani, S. (2022). “Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: A critical review,” Bioresource Technology 343, article 126126. DOI: 10.1016/j.biortech.2021.126126
Yahya, M. D., Aliyu, A. S., Obayomi, K. S., Olugbenga, A. G., and Abdullahi, U. B. (2020). “Column adsorption study for the removal of chromium and manganese ions from electroplating wastewater using cashew nutshell adsorbent,” Cogent Engineering 7(1), article 1748470. DOI: 10.1080/23311916.2020.1748470
Yuliana, M., Huynh, L. H., Ho, Q. P., Truong, C. T., and Ju, Y. H. (2012). “Defatted cashew nut shell starch as renewable polymeric material: Isolation and characterization,” Carbohydrate Polymers 87(4), 2576-2581. DOI: 10.1016/J.CARBPOL.2011.11.044
Yuliana, M., Tran-Thi, N. Y., and Ju, Y.-H. (2012). “Effect of extraction methods on characteristic and composition of Indonesian cashew nut shell liquid.” Industrial Crops and Products 35(1), 230-236. DOI: 10.1016/j.indcrop.2011.07.007
Zachl, A., Soria-Verdugo, A., Buchmayr, M., Gruber, J., Anca-Couce, A., Scharler, R., and Hochenauer, C. (2022). “Stratified downdraft gasification of wood chips with a significant bark content,” Energy 261, article 125323. DOI: 10.1016/j.energy.2022.125323
Article submitted: February 5, 2024; Peer review completed: March 9, 2024; Revised version received and accepted: April 22, 2024; Published: May 6, 2024.
DOI: 10.15376/biores.19.3.Cruz
