Abstract
The authors developed a high-throughput method for analyzing softwood lignin using tetra-n-butylphosphonium hydroxide (TBPH). Wood meal, TBPH, and hydrogen peroxide (H2O2) were introduced into a screw-capped glass test tube and allowed to react in a pressure cooker at 121 °C for 3 h to solubilize the wood meal. The solubilized polysaccharide was precipitated by the addition of a poor solvent such as methanol. After removal of the polysaccharide, the lignin concentration was measured via ultra-violet (UV) absorption spectroscopy. The series of operations performed was summarized as the TBPH method. The TBPH method was characterized as a simple and rapid procedure that used common equipment and was suitable for multiple-sample analysis. Softwood sample groups were prepared, and the lignin contents of these samples were measured by the TBPH method, the Klason method, and the acetyl bromide method to determine the accuracy of the proposed method. The TBPH method showed a high coefficient of determination (R2 = 0.94) when compared to the Klason method. By contrast, the acetyl bromide method showed a comparatively low correlation (R2 = 0.71) with the Klason method. This study revealed that the TBPH method presented high-throughput rapid analysis and good accuracy for soft wood lignin analysis.
Download PDF
Full Article
High-throughput Analysis of Softwood Lignin Using Tetra-n-butylphosphonium Hydroxide (TBPH)
Hajime Yamada,a Shiho Takahashi,a Kana Yamashita,a Hisashi Miyafuji,b Hiroyuki Ohno,c and Tatsuhiko Yamada a,*
The authors developed a high-throughput method for analyzing softwood lignin using tetra-n-butylphosphonium hydroxide (TBPH). Wood meal, TBPH, and hydrogen peroxide (H2O2) were introduced into a screw-capped glass test tube and allowed to react in a pressure cooker at 121 °C for 3 h to solubilize the wood meal. The solubilized polysaccharide was precipitated by the addition of a poor solvent such as methanol. After removal of the polysaccharide, the lignin concentration was measured via ultra-violet (UV) absorption spectroscopy. The series of operations performed was summarized as the TBPH method. The TBPH method was characterized as a simple and rapid procedure that used common equipment and was suitable for multiple-sample analysis. Softwood sample groups were prepared, and the lignin contents of these samples were measured by the TBPH method, the Klason method, and the acetyl bromide method to determine the accuracy of the proposed method. The TBPH method showed a high coefficient of determination (R2 = 0.94) when compared to the Klason method. By contrast, the acetyl bromide method showed a comparatively low correlation (R2 = 0.71) with the Klason method. This study revealed that the TBPH method presented high-throughput rapid analysis and good accuracy for soft wood lignin analysis.
Keywords: Aqueous ionic liquid; Tetra-n-butylphosphonium hydroxide; Softwood; Lignin; Cellulose; Multi-sample analysis
Contact information: a: Forestry and Forest Products Research Institute, 1 Matsunosato, Tsukuba, Ibaraki 305-8687, Japan; b: Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Hangi-cho, Shimogamo, Sakyo-ku, Kyoto 606-8522, Japan; c: President of Tokyo University of Agriculture and Technology, Naka-cho, 2-24-16, Koganei, Tokyo 184-8588, Japan;
* Corresponding author: yamadat@affrc.go.jp
INTRODUCTION
Wood is composed of three major chemical components, cellulose, hemicellulose, lignin, and a small amount of extractives. Evaluating the amount of lignin is quite important relative to optimizing the usage of wood as chemical and polymeric resources, as well as in the pulp and paper industry. The Klason method is the most popular standard method to determine the lignin content (Dence 1992; T222 om-88 standard (1992)). In this method, the solubilization of carbohydrates is performed by sulfuric acid hydrolysis, and the lignin is gravimetrically measured as the residue. However, the process is time-consuming to analyze a large number of samples, and the handling of the concentrated sulfuric acid needs extreme caution for the laboratory staff’s safety.
In contrast to the approach utilized in the direct determination of lignin, such as in the Klason method, there are indirect methods that do not isolate a lignin residue. Among the indirect analytical methods, the acetyl bromide method has received the most widespread acceptance (Dence 1992). In this method, wood is treated with acetyl bromide to solubilize lignin, and then the lignin content is calculated from the UV absorbance of the resulting solution. The acetyl bromide method is considered to be suitable for measurements of lignin contents in a small number of samples (e.g., 5 mg). Because the heating temperature of this method is as low as 75 °C and the solubilization treatment time is as short as 30 min, it is easy to process a large number of samples. However, the method uses acetyl bromide, which is hazardous, volatile, fumigating, and irritating, and requires experiments to be performed within a fume hood. Lignin quantification using nuclear magnetic resonance (Ralph et al. 1994; Capanema et al. 2004) and near infrared (Yamada et al. 2006) spectroscopies have also been proposed, but currently equipment for such analysis is expensive and cannot ordinarily be handled by inexperienced analysts.
Ionic liquids are expected to be useful as solvents and reaction media in the biorefinery of wood resources (Brandt et al. 2013). Ionic liquids are room-temperature molten salts that exhibit characteristics such as low volatility and diversity of molecular design; they are believed to have potential as a medium for green chemistry processes in the future (Rogers et al. 2012). In light of the fact that cellulose can be solubilized by ionic liquids (Swatloski et al. 2002), several attempts have been made to solubilize different combinations of cellulose, lignin, and wood using a variety of ionic liquids and their derivatives (Remsing et al. 2006; Fort et al. 2007; Kilpeläinen et al.2007). The authors searched for an ionic liquid suitable for the biorefinery of woody biomass and discovered tetra-n-butylphosphonium hydroxide (TBPH), which is the hydroxyl form of an -onium cation (Abe et al. 2012). The TBPH can solubilize wood in the presence of water viatreatments conducted at temperatures lower than those used in prior experiments.
In previous studies, the authors thoroughly investigated the solubilization of wood using TBPH (Abe et al. 2014, 2015; Yamada et al. 2016). In particular, by adding hydrogen peroxide (H2O2) to 60% TBPH and performing treatment using an autoclave at 121 °C, the authors succeeded in solubilizing all of the cellulose and lignin in woody biomass quickly (Yamada et al. 2017). This paper reports on, based on the authors’ previous results, the development of a quantitative lignin analysis method utilizing TBPH, hereinafter referred to as the TBPH method. The characteristics of the proposed method are evaluated to determine the lignin suitability as a quantitative method. The TBPH method is compared with conventional lignin analysis methods, such as the Klason and acetyl bromide methods.
EXPERIMENTAL
Materials
Six softwood trees, Japanese cedar (Cryptomeria japonica D. Don, Tsukuba, Japan), Japanese red pine (Pinus densiflora, Tsukuba, Japan), Yezo spruce (Picea jezoensis, Hokkaido-Prefecture, Japan), Hinoki cypress (Chamaecyparis obtusa, Kami, Japan), Japanese larch (Larix kaempferi, Sendai, Japan), and Sakhalin fir (Abies sachalinensis, Tama, Japan) were collected.
Seven samples of Japanese cedar wood were separately collected, which contained compression wood parts. The cedar wood discs were divided into three parts, namely the compression, opposite, and normal parts, to yield 21 total pieces of Japanese cedar wood samples. These cedar samples showed a high range of the Klason lignin contents (Maximum: 44.5%, Minimum: 33.6%, Average: 37.1%, and SD: 2.9).
To begin with, debarked wood chips were air-dried and crushed by a rotor mill (Pulverisette 14, Fritsch Japan, Yokohama, Japan). The wood meal passing through a 0.5-mm mesh sieve was collected and extracted with a mixture of ethanol-benzene solvents (v/v, 1:2) in a Soxhlet extractor (Sibata, Soka, Japan). The extractive-free wood meal thus obtained was vacuum-dried at 40 °C for 24 h.
Holocellulose and alpha-cellulose contents were obtained from the Japanese cedar samples via the Wise method (Dahlman et al. 1999). In this method, the wood meal was treated with NaClO2 and acetic acid for 4 h at 70 °C to 80 °C. The treated mixture was then filtered and washed with water and acetone, after which holocellulose was obtained as the residue. Alpha-cellulose was then prepared from the holocellulose through treatment of the residue with concentrated aqueous NaOH.
Soda lignin of Japanese cedar was prepared from its black liquor produced from the Forestry and Forest Products Research Institute bioethanol pilot plant in Akita, Japan (Ikeda et al. 2007). The black liquor was first neutralized with sulfuric acid and then filtered. The residue obtained from filtration was collected as the soda lignin.
A 40% aqueous TBPH solution was purchased from Hokko Chemical Industry (Tokyo, Japan). A 60% aqueous TBPH solution was prepared by evaporation, and its water content was checked by the Karl Fischer method. A solution of 30% aqueous H2O2 was purchased from Wako Pure Chemical Industries (Osaka, Japan). The ethanol, methanol, 1-4 dioxane, sodium hydroxide (NaOH), and acetic acid used for the experiments were purchased from Tokyo Chemical Industry (Tokyo, Japan) and Junsei Chemicals (Tokyo, Japan). All of the chemicals were used as received and without further purification.
Methods
The TBPH method
Figure 1 outlines the steps involved in the TBPH method. The TBPH method is based on the authors’ previous results (Yamada et al. 2017). Approximately 100 mg of extractive-free wood meal, 1.9 g of 60% aqueous TBPH, and 0.1 g of H2O2 were placed into glass test tubes with screwcaps. The tubes were loosely sealed, and heat-treatments were performed in an autoclave at 121 °C, 2 atm, and for 3 h. After the treatment, 4 mL of aqueous MeOH/0.2 M NaOH (1:1), was added to the glass tubes. The tubes were shaken well and centrifuged for 15 min at 3000 rpm. The total volume of the supernatant was 6.0 mL, and polysaccharide was precipitated in the bottom of the tube. The supernatant 0.2 mL, acetic acid 0.02 mL, and 1.78 mL of MeOH/water (1:1) were mixed well for 10-fold dilution. And then, the 0.2 mL of the mixed solution was diluted with 4.8 mL of MeOH/water for 25-fold dilution.
The UV absorption (UV-1800, Shimadzu, Kyoto, Japan) of the resulting solution was measured. The lignin contents were then calculated from Eq. 1,
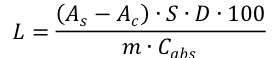
(1)
where L denotes the Lignin content (%), m represents the mass of the oven-dried wood meal (0.1 g), S denotes the total volume of supernatant (6.0・10-3 L), D represents the dilution ratio (250 times), As represents the absorbance of the sample solution (cm−1), Ac is the absorbance of the control solution (cm−1), and Cabs denotes the TBPH lignin absorbance coefficient (26.7 L·g−1·cm−1). This coefficient was calculated from the slope of the linear equation passing through the origin based on the correlation between the concentration based on Klason lignin content and the UV absorbance at 280 nm.
Fig. 1. Protocol for quantitative lignin analysis
Selection of solvents
The yields of precipitates were determined by adding six types of poor solvent. Aqueous dioxane (50%), aqueous methanol (aq. MeOH, 50%), aqueous ethanol (aq. EtOH, 50%), dioxane with 0.2 M aq. NaOH (1:1), MeOH with 0.2 M aq. NaOH (1:1), and EtOH with 0.2 M aq. NaOH (1:1) were prepared. Exactly 4 mL of these solvents were added to the treated mixture of cedar wood and TBPH. The precipitates were separated by centrifugation, and the supernatant was removed. Water was added to the precipitate, the precipitates were washed with water several times, and the supernatants were removed via centrifugation. The final residue was vacuum-dried overnight at 40 °C, and the precipitation yield was calculated from the dry weight.
To determine the best diluent and to establish whether neutralization was necessary, the UV absorbance of the three major constituents of the woody biomass was compared. The woody biomass samples were then dissolved in TBPH and subjected to treatment in the autoclave. Four process conditions were investigated: (1) mixing of the precipitate with alkaline MeOH and dilution with aq. MeOH; (2) mixing of the precipitate with alkaline EtOH and dilution with aq. EtOH; (3) mixing of the precipitate with alkaline MeOH, neutralization with acetic acid, and dilution with aq. MeOH; and (4) mixing of the precipitate with alkaline EtOH, neutralization with acetic acid, and dilution with aq. EtOH. The UV absorbances of the solutions obtained from these treatments were measured at 190 nm to 400 nm, and the absorption differences between lignin and carbohydrates were determined.
Klason method
The lignin content in wood samples was also determined by the Klason method for comparison (Dence 1992; TAPPI T222 om-88 1992). In this method, 0.3 g of the wood meal sample was treated with 5 mL of 72% sulfuric acid for 2 h with continuous stirring. Then, 190 mL of water was added to the solution, and the mixture was heated in an autoclave at 121 °C for 1 h. The residue was collected from the solution with a glass filter. After washing and oven drying at 105 °C, the weights of the residues were obtained, and the lignin contents were calculated.
Acetyl bromide method
The lignin content in the wood samples was additionally determined by the acetyl bromide method (Iiyama and Wallis 1988). In this method, 50 mg of wood meal sample was placed in glass reaction bottles, and 5 mL of 25% (w/w) acetyl bromide in acetic acid, containing 0.2 mL of perchloric acid, was added. The glass bottles were tightly sealed with a cap, and placed in a block heater at 70 °C for 30 min, with intermittent gentle shakings at 10 min intervals. Then, the solution was transferred to a 100-mL volumetric flask containing 10 mL of 2 M sodium hydroxide, and the solutions in the flask were made up to 100 mL with acetic acid. The lignin content of the samples was determined by measuring the absorbance at 280 nm, using the lignin absorbance coefficient for softwood as 20.0 L·g−1·cm−1.
RESULTS AND DISCUSSION
Process Improvement in TBPH Method
Figure 1 shows the outline of the protocol of the TBPH method for quantitative lignin analysis. In the proposed TBPH method, various steps of operations, such as polysaccharide precipitation, separation, and neutralization, were performed, and the lignin content was finally calculated from the UV absorbance of the residue.
Initially, 2.0 mL of TBPH was used per 0.1 g of sample, but the UV absorbance of the resulting solution could not be measured because of its high lignin concentration. To enable the measurement of UV absorbance, the sample solution was diluted 250 times. For dilution, the use of a general-purpose, safe, and inexpensive organic solvent was preferred to compensate for the relatively expensive TBPH used.
To determine the lignin solubilized in TBPH, the authors diluted the lysate with aqueous dioxane solution. Aqueous dioxane is a solvent frequently used for measuring lignin content (Nakano and Meshitsuka 1992). However, when TBPH was dropped into this solvent, a white layer of spheres formed around the TBPH solution. This white layer was believed to be polysaccharides (Abe et al.2014), such as cellulose, that were solubilized in TBPH and posed an obstacle for the accurate UV measurement.
To solve this problem, a diluent was added to the solution to precipitate and remove polysaccharides by centrifugation. Figure 2 illustrates the precipitation yields on the various types of diluents. The addition of an alkali to aqueous MeOH, EtOH, or dioxane increased the formation yield of the precipitate. Despite its good solubilizing ability for lignin, dioxane is toxic to the human body and has increasingly been regulated by laws on water pollution in recent years. Hence, an alkaline aqueous MeOH or EtOH was used as a precipitation solvent immediately after the TBPH treatment.
Fig. 2. The various types of solvents added and the formation yields of the precipitates; neutral- Solvent/Water:1/1 and Alkaline- Solvent/0.2M aq. NaOH:1/1
The next step involved the examination of an appropriate solvent for dilution purposes and determination of whether neutralization was required or not. Table 1 shows the UV absorbance (280 nm) values obtained after solubilizing the wood meal, soda lignin, or holocellulose in TBPH followed by precipitation and dilution steps. The sample of aq. MeOH with neutralization showed the most suitable properties for the isolated lignin. It had the highest UV absorbance at the wood and soda lignin samples, and relatively lower absorbance at holocellulose, cellulose, and TBPH control samples. The UV absorbance of lignin was highly affected by the pH of the solution, and the absorbance of the lignin fraction seemed to be stabilized by the acetic acid addition. For example, when the purity of soda lignin was determined from UV absorbance, acetic acid was added for neutralization and the pH of the solution was adjusted to approximately 5.0 (Lin 1992; Takahashi et al. 2014).
In conclusion, the effectiveness of the TBPH method was improved by the addition of an alkaline aq. MeOH solution as the poor solvent, followed by the removal of insolubilized polysaccharides via centrifugation. The neutralization of soluble fractions and dilution with an aq. MeOH solution completed the process steps.
Table 1. UV Absorbances of Wood Meal, Holocellulose, Cellulose, or Soda Lignin with Various Dilution Methods
*Note: Neutralization was performed with acetic acid
Verification of the Effectiveness of the Proposed TBPH Method
Figure 3 shows the correlations between the lignin contents measured by the TBPH method and those determined by the Klason method from the Japanese cedar samples. A total of 21 samples were used, and the lignin contents were determined as an average value from three repeated measurements. A very good coefficient of determination (R2 = 0.94) was found between the Klason and TBPH methods. Despite marked differences between the procedures involved in the TBPH and Klason methods, the measured lignin contents were similar, and the TBPH method was shown to be sufficiently useful as a substitute for the Klason method.
Fig. 3. Correlations between the lignin contents measured by the TBPH and Klason methods from Japanese cedar samples
Figure 4 shows the correlations between the lignin contents measured by the acetyl bromide method with those determined by the Klason method from the Japanese cedar samples. The correlation coefficient between the methods was R2 = 0.71. Although the Klason and acetyl bromide methods were classic methods for conventional lignin content determination, their correlation was not as high as expected.
Fig. 4. Correlations between the lignin contents measured by the acetyl bromide and Klason methods using Japanese cedar samples
Table 2 summarizes the characteristics of the TBPH, Klason, and acetyl bromide methods. Compared with the Klason method, the TBPH method required small amounts of samples. In the Klason method, lab workers need to handle sulfuric acid directly during the entire treatment time; 72% sulfuric acid soaked samples need to be stirred frequently for 2 h with a glass rod, followed by 1 h of diluted sulfuric acid treatment. In the acetyl bromide method, lab workers need to handle acetyl bromide, which is an irritating and harmful reagent. On the other hand, in the TBPH method, the samples are just soaked into a 60% TBPH solution that is not a harmful volatile organic solvent. The entire process is performed in sealed test tubes. The test tubes are placed in an autoclave for 3 h in the TBPH treatment. The correlation between the TBPH and Klason methods was R2 = 0.94, while the correlation between the acetyl bromide and Klason methods was only R2 = 0.71. Therefore, the TBPH method proved to be a better strategy.
Table 2. Comparison of the Characteristics of the Lignin Analysis Methods Applied in this Work
Figure 5 shows the linear regression plot between the lignin contents measured by the TBPH method and those determined by the Klason method using six species of softwood trees. The lignin contents were determined as an average value of three repeated measurements for each type of the softwood samples. A very good correlation was found between the Klason method and the TBPH method (R2 = 0.95). These results suggested that the proposed TBPH method can be applied not only to the measurement of lignin contents of Japanese cedar but also to any softwood trees in general. Because the softwood lignin is composed of only a guaiacyl skeleton (Marton 1971), the absorbance coefficient of lignin solubilized by this method is similar to that of Japanese cedar. However, because only six softwood species were examined in this study, further investigations with larger numbers of softwood species is recommended. Future studies are also necessary to confirm whether the TBPH method developed in this work is applicable to hardwood trees and non-woody biomass.
Fig. 5. Linear regression plot between the lignin contents measured by the TBPH and Klason methods using six species of softwood trees
CONCLUSIONS
- A novel and high-throughput quantitative lignin analysis method was developed using tetra-n-butylphosphonium hydroxide (TBPH). Wood meal was almost completely dissolved by the TBPH and H2O2 solution, followed by polysaccharide precipitation, separation, and neutralization processes that enabled the quantitative lignin content analysis from the UV absorbance of the solution.
- The lignin contents of a Japanese cedar sample group were measured, and the coefficient of determination between the TBPH and Klason methods was R2 = 0.94, while that between the acetyl bromide and Klason methods was only R2 = 0.71. Thus, the TBPH method was proven to be a better strategy than using the acetyl bromide method.
- The TBPH method can be applied to the measurement of lignin contents of six species of softwood trees, which also showed high coefficients of determination between the Klason methods (R2 = 0.95).
- The TBPH method is characterized as a simple, rapid, safe, and accurate procedure that uses common equipment and is suitable for multiple-sample analysis, compared with the Klason method or the acetyl bromide method.
ACKNOWLEDGMENTS
This study was supported by the following projects: the SIP-Lignin project, technologies for creating next-generation agriculture forestry and fisheries cross-ministerial Strategic Innovation Promotion Program (SIP), and the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (26052A).
REFERENCES CITED
Abe, M., Fukaya, Y., and Ohno, H. (2012). “Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water,” Chem. Commun. 48(12), 1808-1810. DOI: 10.1039/c2cc16203b
Abe, M., Yamada, T., and Ohno, H. (2014). “Dissolution of wet wood biomass without heating,” RSC Adv. 4(33), 17136-17140. DOI: 10.1039/C4RA01038H
Abe, M., Yamanaka, S., Yamada, H., Yamada, T., and Ohno, H. (2015). “Almost complete dissolution of woody biomass with tetra-n-butylphosphonium hydroxide aqueous solution at 60 °C,” Green Chem. 17(8), 4432-4438. DOI: 10.1039/C5GC00646E
Brandt, A., Gräsvik, J., Hallett, J. P., and Welton, T. (2013). “Deconstruction of lignocellulosic biomass with ionic liquids,” Green Chem. 15(3), 550-583. DOI: 10.1039/C2GC36364J
Capanema, E. A., Balakshin, M. Y., and Kadla, J. F. (2004). “A comprehensive approach for quantitative lignin characterization by NMR spectroscopy,” J. Agr. Food Chem. 52(7), 1850-1860. DOI: 10.1021/jf035282b
Dence, C. W. (1992). “Ultraviolet spectrophotometry,” in: Methods in Lignin Chemistry, S. Y. Lin, and C. W. Dence (eds.), Springer-Verlag, Berlin, Germany, pp. 33-61.
Iiyama, K., and Wallis, F. A. (1988). “An improved acetyl bromide procedure for determining lignin in woods and wood pulps,” Wood Sci. Technol. 22(3), 271-280. DOI: 10.1007/BF00386022
Ikeda, T., Sugimoto, T., Nojiri, M., Magara, K., Hosoya, S., and Shimada, K. (2007). “Alkali pre-treatment for the bioethanol fuel production from woody biomasses, Part1: Soda cooking conditions as an alkali pre-treatment,” TAPPI J– JPN. 61(9), 1102–1111. DOI: 10.2524/jtappij.61.1102
Kilpeläinen, I., Xie, H., King, A., Granstrom, M., Heikkinen, S., and Argyropoulos, D. S. (2007). “Dissolution of wood in ionic liquids,” J. Agr. Food Chem. 55(22), 9142-9148. DOI: 10.1021/jf071692e
Lin, S. Y. (1992). “The determination of lignin,” in: Methods in Lignin Chemistry, S. Y. Lin, and C. W. Dence (eds.), Springer-Verlag, Berlin, Germany, pp. 217-232.
Marton, J. (1971). “Reactions in alkaine pulping,” in: Lignins: Occurrence, Formation, Structure and Reactions, K. V. Sarkanen, C. H. Ludwig (eds.), John Wiley & Sons, New York, pp. 639-694.
Nakano, J., and Meshitsuka, G. (1992). “The detection of lignin,” in: Methods in Lignin Chemistry, S. Y. Lin, and C. W. Dence (eds.), Springer-Verlag, Berlin, Germany, pp. 23-32.
Ralph, J., Hatfield, R. D., Quideau, S., Helm, R. F., Grabber, J. H., and Jung, H. -J. G. (1994). “Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR,” J. Am. Chem. Soc. 116(21), 9448-9456. DOI: 10.1021/ja00100a006
Remsing, R. C., Swatloski, R. P., Rogers, R. D., and Moyna, G. (2006). “Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems,” Chem. Commun. 12, 1271-1273. DOI: 10.1039/B600586C
Rogers, R. D., Seddon, K. R., and Volkov, S. (2012). Green Industrial Applications of Ionic Liquids, Springer Science & Business Media, Berlin, Germany.
Swatloski, R. P., Spear, S. K., Holbrey, J. D., and Rogers, R. D. (2002). “Dissolution of cellulose with ionic liquids,” J. Am. Chem. Soc. 124(18), 4974-4975. DOI: 10.1021/ja025790m
Takahashi, S., Hattori, M., Morimoto, M., Uraki, Y., and Yamada, T. (2014). “Performance of softwood soda-anthraquinone lignin as water-reducing chemical admixture in concrete,” J. Wood Chem. Technol. 34(1), 31-38. DOI: 10.1080/02773813.2013.820322
TAPPI T222 om-88 (1992). “Acid-insoluble lignin in wood and pulp,” TAPPI Press, Atlanta, GA.
Yamada, H., Miyafuji, H., Ohno, H., and Yamada, T. (2016). “A simple method for separating lignin and carbohydrates from softwood biomass in a glass tube using tetra-n-butylphosphonium hydroxide,” BioResources 11(1), 839-849. DOI: 10.15376/biores.11.1.839-849
Yamada, H., Miyafuji, H., Ohno, H., and Yamada, T. (2017). “Rapid and complete dissolution of softwood biomass in tetra-n-butylphosphonium hydroxide with hydrogen peroxide,” BioResources12(3), 4515-4526. DOI: 10.15376/biores.12.3.4515-4526
Yamada, T., Yeh, T. -F., Chang, H. -M., Li, L., Kadla, J. F., and Chiang, V. L. (2006). “Rapid analysis of transgenic trees using transmittance near-infrared spectroscopy (NIR),” Holzforschung60(1), 24-28. DOI: 10.1515/HF.2006.005
Article submitted: June 23, 2017; Peer review completed: September 17, 2017; Revised version received and accepted: October 24, 2017; Published: October 30, 2017.
DOI: 10.15376/biores.12.4.9396-9406
