Abstract
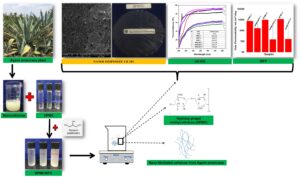
Hydroxypropylmethylcellulose (HPMC) is popularly known as a hydrocolloid for potential use as a biopolymer film. The films of HPMC exhibit brittleness, lacking flexibility, but they can provide a gas barrier. With the aim of improving the HPMC film properties, nanofibrillated cellulose (NFC) from the succulent plant Agave americana L was incorporated as reinforcement material using the solution casting method. The films were prepared with three different amounts of NFC with glycerol as a plasticizer. The incorporation of the NFC into the nanocomposite films showed a 1,000-fold reduction in the gas permeability. However, significant improvements in the tensile strength (TS), the elongation at break (EAB), and Young’s modulus (YM) were only observed with 1% NFC. A higher moisture content (24.5%) and a higher solubility (59.5%) were observed in the HPMC/NFC-1 film, which also exhibited the best biodegradability loss of the films that were observed with a 92.8% degradation rate in 15 d of soil burial studies. Therefore, the results evidence that the HPMC/NFC films might be potentially suitable as food wrap packaging on perishable produce of fruits and vegetables to maintain their quality attributes and prolong the storage life.
Download PDF
Full Article
Hydroxypropyl Methylcellulose Nanocomposites Containing Nano Fibrillated Cellulose (NFC) from Agave americana L. for Food Packaging Applications
Paladugu Krishnadev,a Kizhaeral S. Subramanian,b,* Arunachalam Lakshmanan,a Shunmugam Ganapathy,c Kalimuthu Raja,a and Subramania Krishnaraj Rajkishore d
Hydroxypropylmethylcellulose (HPMC) is popularly known as a hydrocolloid for potential use as a biopolymer film. The films of HPMC exhibit brittleness, lacking flexibility, but they can provide a gas barrier. With the aim of improving the HPMC film properties, nanofibrillated cellulose (NFC) from the succulent plant Agave americana L was incorporated as reinforcement material using the solution casting method. The films were prepared with three different amounts of NFC with glycerol as a plasticizer. The incorporation of the NFC into the nanocomposite films showed a 1,000-fold reduction in the gas permeability. However, significant improvements in the tensile strength (TS), the elongation at break (EAB), and Young’s modulus (YM) were only observed with 1% NFC. A higher moisture content (24.5%) and a higher solubility (59.5%) were observed in the HPMC/NFC-1 film, which also exhibited the best biodegradability loss of the films that were observed with a 92.8% degradation rate in 15 d of soil burial studies. Therefore, the results evidence that the HPMC/NFC films might be potentially suitable as food wrap packaging on perishable produce of fruits and vegetables to maintain their quality attributes and prolong the storage life.
Keywords: Hydroxypropyl methylcellulose; Nanofibrillated cellulose; Nanocomposite films; Mechanical properties; Barrier properties
Contact information: a: Department of Nano Science & Technology, Tamil Nadu Agricultural University, Coimbatore-641003, Tamil Nadu, India; b: Director of Research, Tamil Nadu Agricultural University, Coimbatore-641003, Tamil Nadu, India; c: Department of Food & Agricultural Process Engineering, Tamil Nadu Agricultural University, Coimbatore-641003, Tamil Nadu, India; d: Department of Environmental Sciences, Tamil Nadu Agricultural University, Coimbatore-641003;
* Corresponding author: kss@tnau.ac.in
GRAPHICAL ABSTRACT

INTRODUCTION
Presently, there is great attention from people throughout the world on various issues associated with environmental conservation. This has spurred a push to utilize eco-friendly agricultural waste. Such biomass is underutilized, particularly in India, which is the second-largest agricultural nation after China (Chandra et al. 2012). The term “packaging” is commonly used to define a material that can protect various food products from physical, chemical, and biological damage. Recently, the use of petroleum-based plastic materials is widely under attack due to improper recycling facilities or lack of infrastructure, non-biodegradability, non- renewability, non-recyclability, or addition of toxic additives.
Currently, the total share of plastics in the food packaging sector accounts (85%). The global perspective in the packaging market increased the revenues from $42.5 billion in 2014 to nearly $48.3 billion by 2020 (Huang et al. 2020). Plastics are the most widely preferred packaging materials, due to their light weight, good processability, good mechanical and barrier properties, and low-cost production (Sangroniz et al. 2019). The market growth rate of plastic packaging has been expanding by 20 to 25% per year (Huang et al. 2020). However, there is an environmental concern against single-use plastics (SUP), which is harmful to human health and aquatic life (Halimatul et al. 2019). In general, though many plastics that are made of petroleum-based packaging are technically recyclable, they instead are cast out as litter. They are mostly used by consumers for a short period, but then take centuries to degrade in natural ecosystems (The European Parliament and the Council of the European Union 2019). Additionally, the recycling of packaging waste has a target of a minimum of 70% by weight and about 55% for plastic, by the end of 2030 (The European Parliament and the Council of the European Union, 2018). However, recycling rates still were low in 2017 in some of the countries. The recycling percentage of plastics packaging according to the European Union is 41.7% (Eurostat, 2018). Furthermore, the most commonly followed process for impacting the final properties of the plastics is mechanical recycling (Geyer et al. 2017). Since 1950, the amount of plastic waste generated is about 6300 million tons, of which 4977 million tons are mostly accumulated in waterways and landfills (De Souza Machado et al. 2018). Microplastics (< 5.0 mm) is another major and emerging problem that is present in the water, air, and soil, which gives harmful effects on both marine and terrestrial ecosystems (Geyer et al. 2017). In response, the global biodegradable polymer market is expected to grow in revenues from $3.1 billion in 2016 to $7.1 billion by 2021, an annual growth rate of 18% (Huang et al. 2020).
Scientists across the various disciplines are working to find an alternative way to produce polymers from green sources to resolve the negative effects of petroleum-based polymers. Thermoplastic starch-based films are among some of the environmentally friendly alternatives to petroleum-based plastics. Research has shown that thermoplastic starch films can possess incredible reinforcing properties with the capability to modify or blend with other suitable polymers. This can facilitate the mass production of economical and biodegradable packaging materials (Sanyang et al. 2017; Ilyas et al. 2018; Atikah et al. 2019). Hydroxypropyl methylcellulose (HPMC) is one of the cellulose derivatives next to cellulose, cellulose diacetate, and cellulose triacetate. Hydroxypropyl methylcellulose is composed of units of β (1-4)-D-glucose linked by glycosidic bonds, and it is extensively used to produce edible films and coatings for packaging purposes. In addition, HPMC is used as an emulsifier, stabilizing, suspending, and gelling agent in the food industry (Burdock 2007). Hydroxypropyl methylcellulose is soluble in cold water and forms transparent, tasteless, odorless, flexible, and tough films from the film solutions (McGinity and Felton 2008). It is widely employed because of its abundance, good water solubility, non-toxic nature, and rapid processability (Ford 1999). Additives such as glycerol are incorporated into films with HPMC to improve the overall performance and properties (Navarro-Tarazaga et al. 2008).
Generally, glassy HPMC film surfaces are highly hydrophilic, which causes water molecules from the surroundings to induce both swelling and reduction in glass transition temperature, and this happens when temperature and humidity are combined (Laksmana et al. 2009). Moreover, the films tend to dissolve and flow. Another valid explanation is that when the films are exposed to high humidity, there is a reduction in breakdown strength and increased losses (Hui et al. 2013).
Interest in polymer nanocomposites has been recently ignited because of their use in the food packaging sector. Nano-biopolymers such as nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) are derived from various plant sources. Such nanocellulose (NC) materials can be reinforced into a polymer matrix that exhibits good physicochemical properties and does not negatively affect the environment (Iyer et al. 2015b; Iyer and Torkelson 2015; Yang et al. 2015; Dimic-Misic et al. 2017). Nanocomposites derived from plant materials can provide outstanding and unique properties that are not found in conventional composites. Nanofibrillated cellulose is derived naturally from various plant fibers, such as agave, sisal, banana pseudostem, pineapple, jute, hemp, and cotton (Ramesh et al. 2017). These cellulose biopolymers are transformed from micro to nanoscale fibrils with a long, flexible, entangled, and web-like network with a mean diameter of 1 nm to 100 nm (Krishnadev et al. 2020). Nano biopolymers are great candidates for food packaging applications due to their unique (Du et al. 2016; Li et al. 2016), biological (Wang et al. 2015), and physical properties (Yang et al. 2015; Cui et al. 2016). In particular, numerous studies on NFC as a reinforcing agent in the polymer matrix, which has been widely researched worldwide in past decades, have shown potential for food packaging applications. The prepared bio-films showed good mechanical robustness and improvement of cohesion and the homogenous surface of the films. Hay et al. (2018) successfully formulated HPMC by incorporating amylose-sodium palmitate inclusion complexes (Na-Palm) using the conventional solution casting method. The films had improved physical barrier and gas properties compared to the HPMC films. In addition to reducing the water vapor and oxygen permeability traits, the treated films improved the elongation at break (EAB), Young’s modulus (YM), and tensile strength (TS) values. Ilyas et al. (2019) prepared and studied the effect of NFC from the mixture leaves of sugar palm and sugar palm starch (SPS) by the casting method. The films with smaller diameters demonstrated strong miscibility, compatibility, and water barrier properties. Cheng et al. (2018) studied the preparation and extraction of nanofibrillated acetylated cellulose from corn stalk by chemical-mechanical processes and studied the reinforcement effect on starch films. The uniform dispersal of nano-fillers in the starch composite films showed greater tensile strength performances. Associated works on NFC-reinforced biopolymer nanocomposites will help promote the development of environmentally friendly and biodegradable packing films with good physical and mechanical properties.
Agave americana L. is known for its strong and rigid natural succulent plant fibers which are found in dry arid areas in India; it belongs to the Asparagaceae family (Krishnadev et al. 2020). Agave fibers have some unique characteristics such as high moisture, low density, and high tensile strength. Fresh leaves of A. americana contain approximately 60% to 80% cellulose. Cellulose has a micro-fiber structure with different types of chemical bonds such as covalent, hydrogen, or van der Waals bonds (Chaabouni et al. 2006). Therefore, cellulose has a strong potential for reinforcement in polymer composite materials.
In this study, NFC was used as a reinforcing filler for making biodegradable, flexible, and transparent HPMC/NFC composite films. The role of NFC as a reinforcement of the structural, thermal, barrier, and mechanical properties of the HPMC/NFC films was performed systematically. The nanocomposite films were assessed for their tensile strength (TS), EAB, and YM values. A gas permeability test (GPT) was also used to measure the mechanical and barrier properties in the biodegradable films. The functional and structural interaction of the HPMC/NFC films was performed by using Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD). The surface morphology was studied via scanning electron microscopy (SEM) to determine the HPMC/NFC composite films. The rheological properties of the HPMC/NFC composite films were extensively studied to understand the role of NFC in the formulation of the prepared films. The moisture content, water solubility, and biodegradability test of commercial polyvinyl chloride (PVC) and nanocomposite films were also studied. This demonstration was done in consideration of using NFC in polysaccharide films to address the demands of food packaging applications.
EXPERIMENTAL
Materials
The HPMC (Mw of 1261.45) was sourced from HiMedia Laboratories (Mumbai, India), while the glycerol (Mw of 92.09) was sourced from S.D. Fine-Chem Limited (Mumbai, India). Distilled and Millipore water was sourced from the Department of Nano Science and Technology (Tamil Nadu Agricultural University, Coimbatore, India). The commercial PVC (Oxywrap Cling Film; Paradise Consumer Products Ltd., Jalgaon, India) was purchased from the local market for comparative study along with the nanocomposite films.
Methods
Source of cellulose
The nanocellulose was prepared from Agave americana. L plant fiber, which was collected from the Thondamuthur village of Coimbatore city in Tamil Nadu, India. The fibers are successfully treated subsequently for alkali, bleaching, and acid hydrolysis to obtain nanocellulose. The complete methodology for cellulosic fibers extraction and transformation of microfibrils to nanofibrils was successfully carried out and reported in the authors’ previous work (Krishnadev et al. 2020). The obtained nanocellulose is used for the processing of nanocomposite films as a natural reinforcing filler material.
Preparation of the NFC from the Agave americana L.
The cellulosic fibers suspension was transferred to a petri dish and allowed to dry under a hot air oven at 60 °C for 3 h to evaporate the moisture, as shown in Fig. 1. Concurrently, 1 g (w/w) of dry cellulosic fibers mass was redistributed in 100 mL of distilled water under steady vigorous magnetic agitation for 3 h, followed by an ultra-sonication bath using a CD 4820 2.5 L digital ultrasonic cleaner (Citizen Scales, Mumbai, India) for 30 min to obtain nanofibrillated cellulose. The solids content of the NFC was estimated in a set of three by collecting 10 mL, 15 mL, and 20 mL samples from 100 mL of the liquid NFC suspension, which was allowed to dry under a hot air oven at 60° C for 5 h. The NFC suspension was hermetically sealed and stored in a refrigerator at 5 °C for subsequent use.
Preparation of the HPMC/NFC nanocomposite films
The HPMC/NFC composite film preparation procedure is shown in Fig. 1. Initially, HPMC powder was dissolved in 100 mL distilled water and stored in a refrigerator for 14 h at 5° C. Furthermore, constant stirring under a magnetic stirrer was allowed to produce a homogeneous film formulation at addition levels of 1%, 2%, and 3% (w/v) (Fig. 2b). The NFC liquid suspension prepared after homogenization followed by an ultrasonic bath is shown in Fig. 2a.
Fig. 1. Preparation steps for the HPMC/NFC nanocomposite films
Fig. 2. Digital images for the a) NFC formulation, the b) Different amounts of the HPMC formulation, and the c) HPMC/NFC formulation with different amounts of NFC
The NFC solids content for final optimization was successful in mixing 10 mL (0.053%), 15 mL (0.043%), and 20 mL (0.091%) of the NFC liquid suspension in 89 mL, 84 mL, and 79 mL of the HPMC formulation, respectively. One mL of glycerol was incorporated as a plasticizer and blended completely at 600 rpm for 1 h to obtain the final HPMC/NFC mixture at concentrations of 1%, 2%, and 3% (Fig. 2c). Subsequently, 100 mL of the final film composite of pure HPMC and HPMC/NFC final composite film was poured onto food-grade polypropylene (PP) round plastic plates with dimensions of 27.3 cm × 27.3 cm × 2.2 cm for forming nanocomposite films. The films were peeled carefully after 48 to 72 h of solvent casting at room temperature 35° C ± 72%. Moreover, all the peeled films had a uniform thickness and were stored in a plastic file for the morphological, structural, functional, barrier, mechanical, thermal degradation, moisture content, water solubility, and soil burial-based biodegradability tests.
Measurement of the moisture content
To determine the weight loss of the films, the moisture content was estimated. The HPMC and HPMC/NFC films were cut into square 2.0 cm2 × 2.0 cm2 pieces. The initial weight of all the films was weighed accurately. The final dry mass was recorded upon drying in an oven at 100 °C for 120 min to acquire the final dry weight. Each film treatment was replicated five times, and the moisture content was measured according to Eq. 1 (Kim et al. 2017),
(1)
where Wi is the initial weight taken at the beginning and Wf is the final weight after the film was dried in the oven.
Measurement of solubility in water
All the film samples were cut into square 2.0 cm2 × 2.0 cm2 pieces. The samples were weighed before they were immersed in 100 mL of distilled water for 24 h at ambient room conditions with minimal modifications (Ghasemlou et al. 2013). After they were removed from the water, the samples were dried in a hot air oven at 100 °C until the final weight did not decrease. The final dry weight of all the samples was accurately weighed and recorded. Glycerol incorporated as a plasticizer into the film has a good water solubility range from 18% to 25% (Gáspár et al. 2005). The percentage of water solubility was calculated according to Eq. 2,
(2)
where Mo is the initial dry weight taken at room temperature and Mi is the final dry weight of the films at 100 °C in the oven.
Biodegradability test
The biodegradation process of the commercial PVC (Cling film), and the prepared films was determined by the frequently used soil burial method with minimal modifications (Marichelvam et al. 2019). The soil was procured from the experimental field at Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India. Approximately 200 g of soil was placed in series of disposable paper cups with dimensions of 9 cm × 7.5 cm × 9 cm. The 2 cm × 2 cm film samples were buried at depth of 2 cm for 15 d. All the cups were incubated at ambient room conditions (25 °C to 35 °C), and the moisture content of the soil was maintained at 35% to 40% by sprinkling water twice a day. The degradation of the samples was successfully determined at 7 d intervals by carefully taking samples from the soil. The degradation measurements were assisted by polypropylene wipes moistened with distilled water to remove the soil. The sample was dried in the oven until a constant weight was obtained. The final biodegradability weight loss was calculated according to Eq. 3,
(3)
where Wo is the weight of the samples before the test and W is the weight of samples after the test.
Characterization and Analysis
Rheological behavior measurement of the nanocomposite formulations
The rheological behavior of the composite HPMC/NFC films was measured with an advanced cylinder rotating rheometer device (MCR52; Anton Paar, Graz, Austria). Approximately 2 mL of film solution was poured over the sample load and the test was measured. The shear rate varied from 1 s-1 to 100 s-1. All the film formulations were carried out using the computer-based master tool software RheoPlus (Anton Paar, Graz, Austria), which was the default automatic measuring and detection system connected to the rheometer.
Ultraviolet-visible spectroscopy (UV-Vis) transmittance studies
Ultraviolet-visible spectroscopy studies were carried out to better understand the UV transmitting properties of the commercial PVC (Cling Film) and the HPMC/NFC nanocomposite films. A double beam UV spectrophotometer (Genesys 180; Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the UV transmitting properties. The film samples were cut into 40 mm × 10 mm pieces, and the samples were placed in the cuvette for UV irradiation that was emitted via xenon flash lamp. Millipore water (Department of Nano Science and Technology, Tamil Nadu Agricultural University, Coimbatore, India) was used as a blank. The spectra were acquired from 200 to 800 nm.
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectrums of the nanocomposite film samples were recorded using a Jasco FT/IR-6800 spectrometer (Tokyo, Japan). The uniform circular size of all the film samples was punched using a stationary paper punching machine. The samples were placed on attenuated total reflection (ATR) and the scanning accumulation was performed with 64 scans and a spectral range of 4000 cm-1 to 400 cm-1 for each film sample.
X-ray diffraction (XRD)
The XRD patterns of the HPMC/NFC composite films were recorded using a Rigaku Ultima IV diffractometer (Tokyo, Japan) in a scattering range of 2θ (10° to 90°) at a scanning speed of 10 °/min. All the samples were cut to 2 cm × 2 cm, placed on the XRD glass quartz sample holder, and measured at 40 kV and 30 mA at room temperature and humidity.
Thermogravimetric analysis (TGA)
The thermogravimetric analysis (TGA) of the HPMC and HPMC/NFC composite films at different concentrations of HPMC was conducted using an EXSTAR TG-DTA SII 6300 instrument (Tokyo, Japan). All the film samples were studied under a nitrogen gas atmosphere to prevent thermo-oxidative degradation. Approximately 10 mg of the composite films were scanned from 23 °C to 430 °C at a heating rate of 10 °C/min.
Surface morphology observation of the nanocomposite films
The different concentrations of the pure HPMC and HPMC/NFC composite films were observed using a Quanta 250 SEM (FEI, Hillsboro, OR, USA) at an accelerating voltage of 10.00 kV at ambient temperature. All the film samples were cut at a uniform size and placed on a conductive tape sample stub and sputter-coated (Emitech SC7620; Quorum Technologies, East Sussex, UK) with gold and palladium directly to avoid charging.
Determination of the mechanical properties of the nanocomposite films
The HPMC and HPMC/NFC composite film samples were cut into rectangular pieces (10 mm wide × 100 mm long) before the TS, EAB, and YM values of the nanocomposite films were determined using a universal testing machine (Model No. 9052; Dak System Inc., Mumbai, India), according to the ASTM standard D882-18 (2018). The flaring area on both ends was adjusted to a 25 mm × 50 mm grip. The film samples were successfully mounted to the testing machine extension grips and the crosshead speed was set to 100 mm and the stretching rate was 50 mm/min.
Gas permeability measurement of the nanocomposite films
For the gas permeability testing (GPT) of the HPMC and HPMC/NFC films, the samples were cut to 100 mm × 100 mm pieces according to the ISO standard 15105-1 (2007). The gas permeation was performed using a manometric gas permeability tester (Lyssy L100-500; Systech Illinois, Johnsburg, IL, USA). The samples were placed carefully on the gas chamber column, and the upper and lower pressure limits were adjusted at 700 and 500 psi at a temperature of 25 °C. The results were expressed in mL/m2 per day.
RESULTS AND DISCUSSION
Moisture Content Measurement of the HPMC/NFC Nanocomposite Films
The moisture content for the commercial PVC (Cling film), the HPMC, and the HPMC/NFC films were calculated, as shown in Table 1. The results showed that there was no water adsorption in the commercial PVC film. However, the HPMC-1, HPMC-2, and HPMC-3 films showed lower moisture contents of 4%, 13%, and 9%, compared to the HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 films, which had moisture contents of 25%, 19%, and 22%, respectively. It is known that NFC derived from Agave americana fiber has a higher moisture content (Msahli et al. 2015). The increase in the film solubility is because of the strongly hydrophilic nature of the NFC. However, a proportional decrease would be due to the hydrophobic compounds (Kavoosi et al. 2013). From this, it can be concluded that the moisture content from the samples had the lowest value trend. Finally, the HPMC/NFC-1 sample possessed the ability to enhance the shelf life of the film for further applications.
Water Solubility Measurement of the HPMC/NFC Nanocomposite Films
The water solubility measurement of film gives important characteristics and quality of food products in packaging materials. It is closely related to the biodegradation properties of the films. From the results shown in Table 1, the lowest water solubility (6.7%) was observed in the commercial PVC sample. The HPMC samples had higher water solubility for the HPMC-1, HPMC-2, and HPMC-3 films at 63%, 46%, and 56%, respectively. The HPMC/NFC nanocomposite samples had slightly lower water solubility in the HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 films at 59%, 40%, and 37%, respectively. The water solubility values in the HPMC/NFC films may have been attributed to the strong water holding capacity in the NFC. Furthermore, various hydrocolloid-based biopolymers, which are largely employed as food packaging materials, should be very sensitive to moisture. Otherwise, there is a chance of losing the physical efficiency of the film material, as well as the inability to prevent microbial attack and chemical spoilage inside the food products (Hosseini et al. 2021). Another valid explanation is that the addition of the plasticizers may have reduced the polymer molecule interaction by providing a larger space in which the water molecules could be penetrated, thus maximizing the solubility of the nanocomposite films (Ibrahim et al. 2019). Finally, film materials with high water solubility can be used in single-use biodegradable packaging applications (Preechawong et al. 2005; Cai et al. 2011; Lv et al. 2018).
Biodegradability Properties of the HPMC/NFC Nanocomposite Films
Biodegradation of the polymer material occurs when the material is degraded or broken down into constituent molecules by natural processes. Among biodegradation methods, soil burial is a very frequently followed method to determine the total biodegradability loss of polymer films over a 15-d period, over which the samples are placed at a depth of 2 cm in the soil. The film’s weight loss was determined based on the total amount of degradation that could be taken as an indicator in natural soil. The results from the soil degradation test are shown in Table 1. A biodegradation weight loss of 14% was observed in the commercial PVC (Cling film), followed by 9%, 25%, and 25% in the HPMC-1, HPMC-2, and HPMC-3 samples, respectively. The surface of the film was visualized and found that there is a loss of surface uniformity, smoothness, and even a few samples are more brittle which are broken into pieces (Marichelvam et al. 2019). The biodegradation losses increased at the initial stages and then decreased in the HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 films with values of 93%, 32%, and 24%, respectively compared to the commercial PVC and HPMC films. It is strongly understood that the film materials are highly hydrophilic, which favors more water absorption in the water activity of the nanocomposite films. Glycerol, which was used as a plasticizer, could have caused the adsorption by soil, water penetration through the cell membrane, and weight loss of the films, among other things. Another valid explanation is that the rates of degradation in films can be attributed to environmental factors, such as temperature, moisture, and biological activity (Maran et al. 2014). From this research, it was concluded that the soil burial depth showed a gradual weight loss during biodegradation of the films.
Table 1. Mean Values of the Moisture Content, Water Solubility, and Biodegradability Test of the PVC, HPMC, and HPMC/NFC films
UV-Vis Transmittance Studies of the HPMC/NFC Nanocomposite Films
The transmittance values for the commercial PVC (Cling film), the HPMC, and the HPMC/NFC films were measured using a UV-Vis spectrophotometer (Table 2). The transmittance value of the PVC was observed at 278 nm with a transmittance percent of 74%. The HPMC-1, HPMC-2, and HPMC-3 films had transmittance values of 275 nm, 275 nm, and 258 nm with transmission percentages of 72%, 64%, and 36%, respectively. Moreover, the transmittance values for the HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 films were observed at 278, 274, and 274 nm with transmittance percentages of 32%, 29%, and 27%, respectively. The comparative transmittance values are shown in Fig. 3.
Fig. 3. UV-VIS transmittance studies of the commercial PVC, HPMC, and HPMC/NFC films
These results are in agreement with the work done by Lu et al. (2018) who studied transmittance spectra of BOPP/LDPE films. The untreated films were found with 86.3% and whereas NFC coated plasma-treated on BOPP/LDPE films with the value of 85.2%. Moreover, the films showed less transparency after the addition of NFC, and this may be explained because of nanofibrils and size effect (Fukuzumi et al. 2009; Aulin et al. 2010) The varying transmittance values may be attributed to the different concentrations of polymer and NFC in the films, which is supported by the SEM images. However, previous research has reported optical transparency values of approximately 90% in TEMPO-oxidized NFC cellulose films (Klemm et al. 2005). The films prepared from nanocellulose have high transparency values if the fibers are small enough between interstices to avoid light scattering (Fortunati et al. 2012). The UV-VIS transmittance properties may be affected due to the entanglement of fibers. This lower level of transmission upon the addition of NFC to the HPMC matrix is more suitable for designing biodegradable food packaging material, where NFC in the film surface will cover the pores to better serve as a gas barrier. The NFC can also minimize the UV light-induced lipid oxidation on the skins of perishable fruits and vegetables while prolonging the storage life by a few days (Fortunati et al. 2012; Gopinathan et al. 2017). The nanocomposite film, which was successfully prepared by a combination of HPMC/NFC, had a uniform distribution of NFC with a size range between 216 nm to 551 nm. This may have been due to good processing, a smaller nano range size, and uniform distribution of the NFC into the polymer matrix compared to the HPMC films (2.6 µm to 14 µm), which was further evident compared to the PVC cling film (Ponni et al. 2020).
Table 2. UV-Vis Transmittance Values and Transmittance Percentages of the Commercial PVC, HPMC, and HPMC/NFC films
Rheological Behavior of the HPMC/NFC Nanocomposite Films
The rheological measurement of behavior has been the most common and efficient characterization method to study the polymer-based internal structure as well as the processing, evaluation, and feasibility of the nanocomposite film formulation. Meanwhile, data from the rheology study focused on the interactions between the polymers and the other appropriate ingredients. The rheological behavior of the composite HPMC and HPMC/NFC film formulations was studied for different concentrations of HPMC (1%, 2%, and 3%) at 30 °C, as seen in Fig. 4. All formulations of the composite HPMC and HPMC/NFC films showed a distinct shear-thinning behavior within the shear rate range of 1 s-1 to 100 s-1. Similar results have been investigated and reported by Tang et al. (2014, 2018). Moreover, there was no major difference between the samples. The HPMC/NFC-2 film had the highest shear viscosity at 33.55 Pa-s, followed by the HPMC-3, HPMC/NFC-1, HPMC/NFC-3, HPMC-1, and HPMC-2 films, respectively. However, there was a slight decrease in the viscosity for HPMC/NFC-3 film in comparison with the HPMC-3 film. This may have been due to a strong dependence on a higher HPMC concentration and the incorporation of the NFC. Specifically, there is an increase in shear rate to 10s-1 for composite formulations from 2.65 Pa-s in HPMC-1 to 3.58 Pa-s in HPMC-3, whereas it showed a slight increase of 3.09 Pa-s in HPMC/NFC-3 to 3.27 Pa-s in HPMC/NFC-1 respectively. These results were consistent with previous research by Zhang et al. (2015), where the incorporation of NCC in polyvinylidene fluoride (PVDF) increased the shear viscosity of the sample. The findings from this study indicate that NFC acted as an effective thickening agent in the HPMC film formulation. The increased viscosity was attributed to strong interactions between the NFC and water, which is due to high polarity and an intrinsically large surface area. Thus, the NFC from Agave americana formed a network-like structure and exhibited a gel-forming behavior with high resistance to flow (Dimic-Misic et al. 2013; Mohtaschemi et al. 2014). In general, a higher viscosity is caused by the interaction of van der Waals forces. Although common with NFC and NCC, van der Waals forces contribute to a higher viscosity in other common filler systems (Iyer et al. 2015a). In particular, the improvement of interfacial adhesion provides opportunities to form hydrogen bonds due to the high presence of hydroxyl groups in HPMC biopolymer and NFC. Finally, all the nanocomposite film formulations showed no major differences, but adding NFC did increase the viscosity in the shear rate range.
Fig. 4. The rheological behavior of the HPMC and HPMC/NFC formulations
Surface Morphology Studies of the HPMC/NFC Nanocomposite Films
Digital photographs of the HPMC and HPMC/NFC nanocomposites films are shown in Fig. 5 (a, b, c, d, e, f). The three nanocomposites prepared with the HPMC polymer were well blended with NFC and their surface morphologies were analyzed via SEM. All the nanocomposite films showed a uniform distribution with sizes ranging from 247 nm to 306 nm, 175 nm to 262 nm, and 292 nm to 845 nm for the HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 films, respectively. The HPMC films without NFC showed some scattered aggregates with sizes of 4 µm to 11 µm, 1 µm to 5 µm, and 6 µm to 21 µm for the HPMC-1, HPMC-2, and HPMC-3 films, respectively (Fig. 6). The results were in agreement with those from the NFC/poly (vinyl alcohol) (PVA)/poly (acrylic acid)(PAA) nanofilms prepared from banana pseudostem (Ponni et al. 2020). Moreover, the average film thickness for HPMC-1, HPMC-2, HPMC-3, HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 were 44, 79, 34, 77, 76, and 75 µm respectively. However, the addition of NFC to the polymer matrix showed great transparency, as well as significant changes with a uniform surface morphology of the films. The surface of the films without the NFC incorporation showed a smooth, intended polymer dispersion in the water (Dehnad et al. 2014). The light bumps on the surface of the films increased progressively due to the function of the NFC in the nanocomposite films, as can be seen in Fig. 5. Similar light bumps with NCC were observed in a study by Samir et al. (2005).
Fig. 5. Digital photos of the a) HPMC-1, b) HPMC-2, c) HPMC-3, d) HPMC/NFC-1, e) HPMC/NFC-2, and HPMC/NFC-3 nanocomposite films
Fig. 6. SEM micrographs for the a) HPMC-1, b) HPMC-2, c) HPMC-3, d) HPMC/NFC-1, e) HPMC/NFC-2, and HPMC/NFC-3 nanocomposite films
FTIR Analysis of the HPMC/NFC Nanocomposite Films
Fourier transform infrared spectroscopy is a useful analysis for investigating and measuring the functional properties of materials. In this study, the films with or without the incorporation of the Agave americana NFC were recorded, and the results are shown in Fig. 7. For the HPMC films, absorbance maxima appeared in the range of 3407 to 3441 cm-1 with a maximum peak at 3441 cm-1 among the HPMC films. Similar results were outlined by Celebi and Kurt (2015) and Tang et al. (2018). These features correspond to OH vibration and NH stretching. Moreover, the symmetrical and asymmetrical peak shifts at 2922 cm-1 were attributed to CH stretching. The transmittance bands are recorded at the 1645 to 1652 cm-1 peak corresponding to the C=O stretching of the respective amide groups I and II. The C−O stretching vibration gives rise to a peak at 1044 cm-1. The small peaks at 655 cm-1 are assigned to C−OH out of plane bending and similar results were reported by Krishnadev et al. (2020). The FTIR spectrum of the HPMC/NFC films exhibited sharp peaks at a range of 3345 to 3373 cm-1, with the highest peak recorded at 3373 cm-1. The peak at 3373 cm-1 was attributed to OH vibrations due to a strong intra-molecular bond of hydrogen. The strong peaks at 1037, 1037, and 1044 cm-1 are related to the C−O stretching at the C-3 position (Khan et al. 2012; Krishnadev et al. 2020). However, there was an increase in the HPMC/NFC FTIR spectra in comparison with the HPMC composite film samples. The peak value at 3373 cm-1 of the HPMC/NFC-3 composite film had a higher transmittance intensity than the HPMC films, which corresponded to hydrogen bonding between the HPMC and NFC (Khan et al. 2013). Meanwhile, there was a slight change to the peaks of the film samples without incorporating NFC. From these observations, the addition of NFC in the polymer matrix showed good interaction between the NFC and the polymer formulation, which can improve the overall mechanical strength of HPMC/NFC films plasticized with glycerol.
Fig. 7. FTIR spectra of the HPMC and HPMC/NFC nanocomposite films
XRD analysis of the HPMC/NFC Nanocomposite Films
The XRD profiles on the HPMC and HPMC/NFC composite films are shown in Fig. 8. The HPMC-1, HPMC-2, and HPMC-3 films had diffraction peaks of 2θ = 20.74°, 19.96°, and 20.35°, respectively. It was understood that the peak values are associated with the distinctive I structure of cellulose. Among all the peaks, there was an increase in the peak intensity at the beginning, followed by a decrease and then an increase in the intensity.
The changes in the intensity are ascribed to the transformation of the structure from amorphous to crystalline. Another factor to consider was the HPMC/NFC nanocomposite films. The HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 films had peaks at 2θ = 20.88°, 21.27°, and 21.27°, respectively. These peaks can be strongly related to the characteristic band of cellulose (Aulin et al. 2013) and it satisfactorily showed that there was a strong interaction between the two blended components which was confirmed through rheological studies. Similar findings for polylactic acid (PLA) and NFC in the PLA/starch/NFC biocomposites were observed by Mao et al. (2019). More specifically, the XRD patterns showed a peak increase from 273 with the HPMC film to 338, 348, and 402 with the HPMC/NFC at 1%, 2%, and 3% NFC, respectively. This increase may be due to the higher NFC content in the polymer matrix and the fact that all the diffraction patterns showed an increase in the peak intensity compared to the pure HPMC films. However, as more amorphous HPMC is introduced, the peak intensity of the HPMC/NFC films may show an overall decrease in the peak intensity (Luo et al. 2019).
Fig. 8. XRD patterns of the HPMC and HPMC/NFC nanocomposite films
Thermal Analysis of the HPMC/NFC Nanocomposite Films
Derivative thermogravimetry (DTG) is a common thermal analysis technique common to TGA, which measures the weight loss at a weight increase with heating, cooling, or stable temperature. In general, DTG shows the process of decay or degradation at various stages. The DTG curves for the HPMC films with and without the addition of NFC are shown in Fig. 9. The results showed that all the nanocomposite films were usually divided into three-stage degradation curves. The first part of the weight loss was due to the removal and evaporation of the solvent (water) at a temperature of 26 °C to 180 °C in the samples without incorporating NFC. These findings support similar research reported by Yang et al. (2014) and Tang et al. (2018). Moreover, water desorption and glycerol decomposition in the NFC-loaded polymer matrix were recorded at temperatures between 74 and 182 °C (Lassoued et al. 2021). In addition, the minor peaks in the curve were consistent with the degradation of the cellulose surface and internal structure (Roman and Winter 2004). The second mass loss in the film samples was found from 214 to 220 °C, which is attributed to the dehydration, deamination, depolymerization, and breakdown of the cellulose glycosidic linkages (Cao et al. 2009; Karakoti et al. 2020). Rapid weight loss above 370 °C is associated with oxidation and breakdown of char (Gopinathan et al. 2017; Tang et al. 2018; Krishnadev et al. 2020). Moreover, the HPMC/NFC films showed a decrease in thermal stability compared to the neat HPMC films. Consequently, the HPMC/NFC nanocomposite films showed a gradual and inevitable decrease of weight loss for an increase in NFC liquid suspension into the polymer matrix. However, there was a gradual change in weight loss percentage, because it showed a slight increase and then a complete decline in weight loss, which indicates a complete breakdown of NFC and other cellulose substitutes present in the films. Similar types of reinforced nanocomposites with NCC and NFC are reported in the literature (Celebi and Kurt 2015; Tang et al. 2018; Lassoued et al. 2021). Overall, NFC decreased the thermal degradation due to the resistance of the films to the higher temperature.
Fig. 9. The DTG curves of the HPMC and HPMC/NFC nanocomposite films
Mechanical Properties of the HPMC/NFC Nanocomposite Films.
The packaging films need adequate mechanical strength with good performance. Tensile strength is well connected to the mechanical strength of films, whereas the elongation at break and young’s modulus is related to the flexibility and rigidity of the films, respectively (Khan et al. 2014). Table 3 and Fig. 10 compare the mechanical properties of HPMC and HPMC/NFC nanocomposite films. In general, it was found that the HPMC films treated with 1% NFC had better TS properties compared to the HPMC/NFC-2 and HPMC/NFC-3 films (Fig. 10a).
Fig. 10. The (a) TS, (b) EAB, and (c) YM values of the HPMC and HPMC/NFC nanocomposite films
The HPMC/NFC 1%, 2%, and 3% films had TS values of 13.68 MPa, 2.36 MPa, and 2.77 MPa, respectively. In addition, the EAB values increased moderately in all the films (Fig. 10b). However, the TS of the HPMC films decreased as the HPMC content increased. The HPMC 1%, 2%, and 3% films had TS values of 10.97 MPa, 4.96 MPa, and 1.54 MPa, respectively. It is well understood that the mechanical properties of the pure HPMC films exhibit brittleness and poor flexibility of the films. The use of a lower concentration (10 mL) of NFC with the HPMC imparted good TS and film flexibility properties. There was a gradual decrease in tensile strength, elongation at break (%), and Young’s modulus by increasing the concentration of NFC in the HPMC nanocomposite films. The gain in the TS for the HPMC/NFC-1 sample represented a 24.6% increase compared to the other films. Lu et al. (2019) studied the addition of NFC on BOPP/LDPE on mechanical properties by assisting plasma treatment. The results revealed that plasma-treated BOPP/LDPE with and without NFC found no major mechanical difference as compared to untreated films. Aulin et al. (2013) reported that the addition of the NFC as a reinforcement material in the PLA matrix significantly increased the mechanical properties of the films. However, the TS values decreased after the nano fibrillated cellulose addition rate increased to 15 mL and 20 mL. This may have been due to the weakening of the nano fibrillated cellulose as reinforcement in the polymer matrix. Moreover, it was found that the YM value was 10.98 MPa in HPMC-1 film at 1%, which was then drastically reduced to 3.41 MPa, and 1.22 MPa for the HPMC-2 and HPMC-3 samples, respectively. The HPMC/NFC nanocomposite films had YM values of 13.16, 3.66, and 2.73 MPa with respective NFC addition rates of 1%, 2%, and 3% (Fig. 9c). Therefore, the YM value of the HPMC/NFC-1 film was 16.56% higher than that of the HPMC-1 film. Similar results have been reported from related literature (Klangmuang and Sothornvit 2016; Tang et al. 2018). In addition, there was a negative effect on the EAB values with the addition of NFC to the composite samples, which was supported by findings in reported literature (Samir et al. 2004; Fortunati et al. 2015; Tang et al. 2018). The EAB values for the HPMC 1%, 2%, and 3% films were recorded with values of 7.87%, 8.60%, and 10.82%, respectively, while the EAB values for the HPMC/NFC films were recorded at 8.75%, 9.35%, and 10.40%, respectively. A change in the EAB values implies that adding NFC to the HPMC film showed good interaction between the filler and the matrix with moderate restriction of the matrix motion (Samir et al. 2004).
Table 3. Average TS, EAB, and YM Values of the HPMC, and HPMC/NFC films
Gas Permeability of the HPMC/NFC Nanocomposite Films
The most fundamental requirement for any packaging material is “oxygen resistivity” which may help to trigger several changes in food such as deterioration of nutrients and prevent microbial growth, which overall influences the shelf life of packaged food products (Soni et al. 2016). One of the essential properties of food packaging is the need for a high-level oxygen barrier. For comparative OTR with the existing commercial packaging material is 1 to 10 cc/m2·day for obtaining a good oxygen barrier (Abdellatief and Welt 2013). Other commercial packaging films prepared from polyethylene terephthalate/ polyethylene and (PET/PE) polyethylene terephthalate (PET) have been reported to be around 1.24 cc/m2·day and 110 cc/m2·day, respectively (Brody et al. 2008). In this study, the addition of NFC as reinforcement in three different concentrations of the HPMC polymer matrix was investigated. The results for gas permeability of O2 through the films are shown in Fig. 11. The oxygen barrier in any composite film can improve the quality and help to stretch the shelf life of food (Sothornvit and Pitak 2007). Based on the findings, it is inferred that the amount of NFC in the HPMC polymer matrix showed a clear beneficial effect in decreasing the gas permeability of the film samples. The gas permeability of the HPMC-1 composite film was 67,840 mL/m2·day, followed by HPMC-2 (101,620 mL/m2·day) and HPMC-3 (122,049 mL/m2·day). However, HPMC is very susceptible to oxygen permeability due to the presence of hydroxypropyl side groups, greater free volume, greater pores, lower crystallinity, and a lower cohesive energy density compared to similar cellulose-based materials like methylcellulose and ether (Miller and Krochta 1997). In addition, the 1%, 2%, and 3% reinforced HPMC/NFC matrix had gas permeability values of 17,415.5 (mL/m2·day), 392.62 (mL/m2·day), and 395.46 (mL/m2·day), respectively. Compared to the pure HPMC films, the HPMC/NFC-1 nanocomposite films showed a 74.1% decrease in the O2 permeability, followed by a 1,000-fold decrease in the HPMC/NFC 2% and 3% films. This remarkable decrease in the gas permeability could be attributed to the imposition of the NFC and a reduction in the diffusion coefficient (Tang et al. 2018). These results are with the agreement of BOPP/LDPE/NFC films which displayed the lowest OTR (24.02 cc/m2·day) (Lu et al. 2018). The main mechanism behind the gas permeability of the HPMC/NFC nanocomposite films is due to the inclusion of up to 3% of highly fibrillar material is likely to contribute only a few percentages of increase in the diffusion path of an oxygen molecule, assuming that is the only effect of the nanocellulose. It follows that the nanocellulose must be affecting the film structure. For instance, it may be preventing the formation of cracks in the film after it has been formed. The high entanglements are expected when stirred and tend to agglomerates depending upon their structural details. The NFC was also shown to close the pores in the film, which was revealed under the SEM with the dimensions from 247 to 306 nm, 175 to 262 nm, and 292 to 845 nm for the HPMC/NFC-1, HPMC/NFC-2, and HPMC/NFC-3 samples, respectively. According to chemistry, all nano-sized particles will display a higher surface-to-volume ratio, leading to high efficiency. The better interaction, crosslinking, and uniform distribution of NFC can reduce the gas barrier against air, O2, and CO2 through film sheets (Siró and Plackett 2010; Laxmeshwar et al. 2012; Ponni et al. 2020).
Fig. 11. The GPT results of the pure HPMC and HPMC/NFC samples
CONCLUSIONS
- Nanofibrillated cellulose derived from succulent plant fiber Agave americana L was successfully used as a reinforcing composite material to develop highly transparent nanocomposite films.
- The hydroxypropylmethylcellulose/ nanofibrillar cellulose (HPMC/NFC-1) film sample with 1% NFC addition had the best mechanical properties, the highest moisture content (25.4%), and the highest water solubility rate (59.5%).
- The uniform dispersion, nano-sized dimensions, and good compatibility between the filler and matrix were confirmed by SEM.
- The UV-Vis transmittance analysis showed a lower optical transmittance for all the HPMC/NFC films.
- The gas transmission rates of the HPMC/NFC 2% and 3% samples showed a 1,000-fold reduction compared to their HPMC counterpart films.
- The best biodegradability weight loss of 92.8% was observed in the HPMC/NFC-1 film sample buried in the soil for 15 d.
- The prepared nanocomposite films are suitable for packaging wrapping material for preserving perishable fruits and vegetables as a protection from UV radiation.
ACKNOWLEDGMENTS
The authors are grateful to all for their technical assistance in carrying out this research work. The authors wish to thank K. S. Subramanian and A. Lakshmanan for their financial assistance in conducting experimental work from the DST-Nanomission pulses and EID-Parry schemes.
REFERENCES CITED)
“The European Parliament and the Council of the European Union. Directive (EU) 2019/904 of the European parliament and of council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment”, Off. J. Eur. Union 2019, L155, 19
“The European Parliament and the Council of the European Union. Directive (EU) 2018/852 of the European parliament and of council of 30 May 2018 amending Directive 94/62/EC on packaging and packaging waste”, Off. J. Eur. Union 2018, L150, 14.
Abdellatief, A., and Welt, B. A. (2013). “Comparison of new dynamic accumulation method for measuring oxygen transmission rate of packaging against the steady‐state method described by ASTM D3985,” Packaging Technology and Science 26(5), 281-288. DOI:10.1002/PTS.1974
ASTM D882-18 (2018). “Standard test method for tensile properties of thin plastic sheeting,” ASTM International, West Conshohocken, PA.
Atikah, M. S. N., Ilyas, R. A., Sapuan, S. M., Ishak, M. R., Zainudin, E. S., Ibrahim, R., Atiqah, A., Ansari, M. N. M., and Jumaidin, R. (2019). “Degradation and physical properties of sugar palm starch/sugar palm nanofibrillated cellulose bionanocomposite,” Polimery 64(10), 27-36. DOI: 10.14314/polimery.2019.10.5
Aulin, C., Gällstedt, M., and Lindström, T. (2010). “Oxygen and oil barrier properties of microfibrillated cellulose films and coatings,” Cellulose 17(3), 559-574. DOI:10.1007/s10570-009-9393-y
Aulin, C., Karabulut, E., Tran, A., Wågberg, L., and Lindström, T. (2013). “Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties,” ACS Applied Materials and Interfaces 5(15), 7352-7359. DOI: 10.1021/am401700n
Brody, A. L., Bugusu, B., Han, J. H., Sand, C. K., and McHugh, T. H. (2008). “Innovative food packaging solutions,” Journal of Food Science 73(8), 107-116. DOI:10.1111/J.1750-3841.2008.00933.X
Burdock, G. A. (2007). “Safety assessment of hydroxypropyl methylcellulose as a food ingredient,” Food and Chemical Toxicology 45(12), 2341-2351. DOI: 10.1016/j.fct.2007.07.011
Cai, J., Liu, M., Wang, L., Yao, K., Li, S., and Xiong, H. (2011). “Isothermal crystallization kinetics of thermoplastic starch/poly(lactic acid) composites,” Carbohydrate Polymers 86(2), 941-947. DOI: 10.1016/ j.carbpol.2011.05.044
Cao, X., Habibi, Y., and Lucia, L. A. (2009). “One-pot polymerization, surface grafting, and processing of waterborne polyurethane-cellulose nanocrystal nanocomposites,” Journal of Materials Chemistry 19(38), 7137-7145. DOI: 10.1039/b910517d
Celebi, H., and Kurt, A. (2015). “Effects of processing on the properties of chitosan/cellulose nanocrystal films,” Carbohydrate Polymers 133, 284-293. DOI: 10.1016/j.carbpol.2015.07.007
Chaabouni, Y., Drean, J.-Y., Msahli, S., and Sakli, F. (2006). “Morphological characterization of individual fiber of Agave americana L.,” Textile Research Journal 76(5), 367-374. DOI: 10.1177/0040517506061965
Chandra, R., Takeuchi, H., and Hasegawa, T. (2012). “Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production,” Renewable and Sustainable Energy Reviews 16(3), 1462-1476. DOI: 10.1016/j.rser.2011.11.035
Cheng, L., Zhang, D., Gu, Z., Li, Z., Hong, Y., and Li, C. (2018). “Preparation of acetylated nanofibrillated cellulose from corn stalk microcrystalline cellulose and its reinforcing effect on starch films,” International Journal of Biological Macromolecules 111, 959-966. DOI: 10.1016/j.ijbiomac.2018.01.056
Cui, S., Zhang, S., Ge, S., Xiong, L., and Sun, Q. (2016). “Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis,” Industrial Crops and Products 83, 346-352. DOI: 10.1016/j.indcrop.2016.01.019
De Souza Machado, A. A., Kloas, W., Zarfl, C., Hempel, S., and Rillig, M. C. (2018). “Microplastics as an emerging threat to terrestrial ecosystems,” Global Change Biology 24(4), 1405-1416. DOI: 10.1111/gcb.14020
Dehnad, D., Mirzaei, H., Emam-Djomeh, Z., Jafari, S.-M., and Dadashi, S. (2014). “Thermal and antimicrobial properties of chitosan-nanocellulose films for extending shelf life of ground meat,” Carbohydrate Polymers 109, 148-154. DOI: 10.1016/j.carbpol.2014.03.063
Dimic-Misic, K., Maloney, T., Liu, G., and Gane, P. (2017). “Micro nanofibrillated cellulose (MNFC) gel dewatering induced at ultralow-shear in presence of added colloidally-unstable particles,” Cellulose 24(3), 1463-1481. DOI: 10.1007/s10570-016-1181-x
Dimic-Misic, K., Puisto, A., Gane, P., Nieminen, K., Alava, M. J., Paltakari, J., and Maloney, T. (2013). “The role of MFC/NFC swelling in the rheological behavior and dewatering of high consistency furnishes,” Cellulose 20(6), 2847-2861. DOI: 10.1007/s10570-013-0076-3
Du, H., Liu, C., Mu, X., Gong, W., Lv, D., Hong, Y., Si, C., and Li, B. (2016). “Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis,” Cellulose 23(4), 2389-2407. DOI: 10.1007/s10570-016-0963-5
Eurostat (2018). Available online: https://ec.europa.eu/eurostat/statistics explained/index.php/Packaging_waste_ statistics#Recycling_and_recovery_rates (accessed on 14 October 2021).
Ford, J. L. (1999). “Thermal analysis of hydroxypropylmethylcellulose and methylcellulose: Powders, gels and matrix tablets,” International Journal of Pharmaceutics 179(2), 209-228. DOI: 10.1016/s0378-5173(98)00339-1
Fortunati, E., Luzi, F., Puglia, D., Petrucci, R., Kenny, J. M., and Torre, L. (2015). “Processing of PLA nanocomposites with cellulose nanocrystals extracted from Posidonia oceanica waste: Innovative reuse of coastal plant,” Industrial Crops and Products 67, 439-447. DOI: 10.1016/j.indcrop.2015.01.075
Fortunati, E., Puglia, D., Monti, M., Peponi, L., Santulli, C., Kenny, J. M., and Torre, L (2012). “Extraction of cellulose nanocrystals from Phormium tenax fibres,” Journal of Polymers and the Environment 21(2), 319-328. DOI: 10.1007/s10924-012-0543-1
Fukuzumi, H., Saito, T., Iwata, T., Kumamoto, Y., and Isogai, A. (2009). “Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation,” Biomacromolecules 10(1), 162-165. DOI:10.1021/bm801065u
Gáspár, M., Benkő, Z., Dogossy, G., Réczey, K., and Czigány, T. (2005). “Reducing water absorption in compostable starch-based plastics,” Polymer Degradation and Stability 90(3), 563-569. DOI: 10.1016/j.polymdegradstab.2005.03.012
Geyer, R., Jambeck, J. R., and Law, K. L. (2017). “Production, use, and fate of all plastics ever made,” Science Advances 3(7), article no. e1700782. DOI: 10.1126/sciadv.1700782
Ghasemlou, M., Aliheidari, N., Fahmi, R., Shojaee-Aliabadi, S., Keshavarz, B., Cran, M. J., and Khaksar, R. (2013). “Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils,” Carbohydrate Polymers 98(1), 1117-1126. DOI: 10.1016/j.carbpol.2013.07.026
Gopinathan, P., Subramanian, K. S., Paliyath, G., and Subramanian, J. (2017). “Genotypic variations in characteristics of nano-fibrillated cellulose derived from banana pseudostem,” BioResources 12(4), 6984-7001, DOI: 10.15376/biores.12.4.6984-7001
Halimatul, M. J., Sapuan, S. M., Jawaid, M., Ishak, M. R., and Ilyas, R. A. (2019). “Effect of sago starch and plasticizer content on the properties of thermoplastic films: Mechanical testing and cyclic soaking-drying,” Polimery 64(6), 422-431. DOI: 10.14314/polimery.2019.6.5
Hay, W. T., Fanta, G. F., Peterson, S. C., Thomas, A. J., Utt, K. D., Walsh, K. A., Boddu, V. M., and Selling, G. W. (2018). “Improved hydroxypropyl methylcellulose (HPMC) films through incorporation of amylose-sodium palmitate inclusion complexes,” Carbohydrate Polymers 188, 76-84. DOI: 10.1016/j.carbpol.2018.01.088
Hosseini, S. N., Pirsa, S., and Farzi, J. (2021). “Biodegradable nano composite film based on modified starch-albumin/MgO; antibacterial, antioxidant and structural properties,” Polymer Testing 97, 107182. DOI: 10.1016/j.polymertesting.2021.107182
Huang, J., Ma, X., Yang, G., and Alain, D. (2020). “Introduction to nanocellulose,” in: Nanocellulose: From Fundamentals to Advanced Materials, J. Huang, D. Alain, and L. Ning (eds.), Wiley, pp. 1-20. DOI: 10.1002/9783527807437.ch1
Hui, L., Schadler, L. S., and Nelson, J. K. (2013). “The influence of moisture on the electrical properties of crosslinked polyethylene/silica nanocomposites,” IEEE Transactions on Dielectrics and Electrical Insulation 20(2), 641-653. DOI: 10.1109/TDEI.2013.6508768.
Ibrahim, M. I. J., Sapuan, S. M., Zainudin, E. S., and Zuhri, M. Y. M. (2019). “Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers,” International Journal of Food Properties 22(1), 925-941. DOI: 10.1080/10942912.2019.1618324
Ilyas, R. A., Sapuan, S. M., Ibrahim, R., Abral, H., Ishak, M. R., Zainudin, E. S., Atikah, M. S. N., Mohd Nurazzi, N., Atiqah, A., Ansari, M. N. M., et al. (2019). “Effect of sugar palm nanofibrillated cellulose concentrations on morphological, mechanical and physical properties of biodegradable films based on agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch,” Journal of Materials Research and Technology DOI: 10.1016/j.jmrt.2019.08.028
Ilyas, R. A., Sapuan, S. M., Ishak, M. R., and Zainudin, E. S. (2018). “Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites,” Carbohydrate Polymers 202, 186-202. DOI: 10.1016/j.carbpol.2018.09.002
ISO 15105-1 (2007). “Plastics – Film and sheeting – Determination of gas transmission rate – Part 1: Differential-pressure methods,” International Organization for Standardization, Geneva, Switzerland.
Iyer, K. A., and Torkelson, J. M. (2015). “Importance of superior dispersion versus filler surface modification in producing robust polymer nanocomposites: The example of polypropylene/nanosilica hybrids,” Polymer 68, 147-157. DOI: 10.1016/j.polymer.2015.05.015
Iyer, K. A., Flores, A. M., and Torkelson, J. M. (2015a). “Comparison of polyolefin biocomposites prepared with waste cardboard, microcrystalline cellulose, and cellulose nanocrystals via solid-state shear pulverization,” Polymer 75, 78-87. DOI: 10.1016/j.polymer.2015.08.029
Iyer, K. A., Schueneman, G. T., and Torkelson, J. M. (2015b). “Cellulose nanocrystal/ polyolefin biocomposites prepared by solid-state shear pulverization: Superior dispersion leading to synergistic property enhancements,” Polymer 56, 464-475. DOI: 10.1016/j.polymer.2014.11.017
Karakoti, A., Biswas, S., Aseer, J. R., Sindhu, N., and Sanjay, M. R. (2020). “Characterization of microfiber isolated from Hibiscus sabdariffa var. altissima fiber by steam explosion,” Journal of Natural Fibers 17(2), 189-198. DOI: 10.1080/15440478.2018.1477085
Kavoosi, G., Dadfar, S. M. M., and Purfard, A. M. (2013). “Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing,” Journal of Food Science 78(2), E244-E250. DOI: 10.1111/1750-3841.12015
Khan, A., Khan, R. A., Salmieri, S., Tien, C. L., Riedl, B., Bouchard, J., Chauve, G., Tan, V., Kamal, M. R., and Lacroix, M. (2012). “Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films,” Carbohydrate Polymers 90(4), 1601-1608. DOI: 10.1016/j.carbpol.2012.07.037
Khan, R. A., Beck, S., Dussault, D., Salmieri, S., Bouchard, J., and Lacroix, M. (2013). “Mechanical and barrier properties of nanocrystalline cellulose reinforced poly(caprolactone) composites: Effect of gamma radiation,” Journal of Applied Polymer Science 129(5), 3038-3046. DOI: 10.1002/app.38896
Khan, A., Salmieri, S., Fraschini, C., Bouchard, J., Riedl, B., and Lacroix, M. (2014). “Genipin cross-linked nanocomposite films for the immobilization of antimicrobial agent,” ACS Applied Materials & Interfaces 6(17), 15232-15242. DOI: 10.1021/am503564m
Kim, H.-Y., Jane, J.-L., and Lamsal, B. (2017). “Hydroxypropylation improves film properties of high amylose corn starch,” Industrial Crops and Products 95(4) 175-183. DOI: 10.1016/j.indcrop.2016.10.025
Klangmuang, P., and Sothornvit, R. (2016). “Combination of beeswax and nanoclay on barriers, sorption isotherm and mechanical properties of hydroxypropyl methylcellulose-based composite films,” LWT – Food Science and Technology 65, 222-227. DOI: 10.1016/j.lwt.2015.08.003
Klemm, D., Heublein, B., Fink, H.-P., and Bohn, A. (2005). “Cellulose: Fascinating biopolymer and sustainable raw material,” Angewandte Chemie (International Edition) 44(22), 3358-3393. DOI: 10.1002/anie.200460587
Krishnadev, P., Subramanian, K. S., Janavi, G. J., Ganapathy, S., and Lakshmanan, A. (2020). “Synthesis and characterization of nano-fibrillated cellulose derived from green Agave americana L. fiber,” BioResources 15(2), 2442-2458. DOI: 10.15376/biores.15.2.2442-2458
Laksmana, F. L., Kok, P. J. A. H., Vromans, H., and Van der Voort Maarschalk, K. (2009). “Predicting the diffusion coefficient of water vapor through glassy HPMC films at different environmental conditions using the free volume additivity approach,” European Journal of Pharmaceutical Sciences 37(5), 545-554. DOI:10.1016/j.ejps.2009.04.011
Lassoued, M., Crispino, F., and Loranger, E. (2021). “Design and synthesis of transparent and flexible nanofibrillated cellulose films to replace petroleum-based polymers,” Carbohydrate Polymers 254, article no. 117411. DOI: 10.1016/j.carbpol.2020.117411
Laxmeshwar, S. S., Kumar, D. J. M., Viveka, S., and Nagaraja, G. K. (2012). “Preparation and properties of biodegradable film composites using modified cellulose fibre-reinforced with PVA,” ISRN Polymer Science article no. 154314, 1-8. DOI: 10.5402/2012/154314
Li, Y., Liu, Y., Chen, W., Wang, Q., Liu, Y., Li, J., and Yu, H. (2016). “Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation,” Green Chemistry 18(4), 1010-1018. DOI: 10.1039/c5gc02576a
Lu, P., Guo, M., Xu, Z., and Wu, M. (2018). “Application of nanofibrillated cellulose on BOPP/LDPE film as oxygen barrier and antimicrobial coating based on cold plasma treatment,” Coatings, 8(6), 207. DOI:10.3390/coatings8060207
Luo, K., Wang, Y., Xiao, H., Song, G., Cheng, Q., and Fan, G. (2019). “Preparation of convertible cellulose from rice straw using combined organosolv fractionation and alkaline bleaching,” IOP Conference Series: Earth and Environmental Science 237(5), 1-6. DOI: 10.1088/1755-1315/237/5/052053
Lv, S., Zhang, Y., Gu, J., and Tan, H. (2018). “Physicochemical evolutions of starch/poly (lactic acid) composite biodegraded in real soil,” Journal of Environmental Management 228, 223-231. DOI: 10.1016/ j.jenvman.2018.09.033
Mao, J., Tang, Y., Zhao, R., Zhou, Y., and Wang, Z. (2019). “Preparation of nanofibrillated cellulose and application in reinforced PLA/starch nanocomposite film,” Journal of Polymers and the Environment 27(4), 728-738. DOI: 10.1007/s10924-019-01382-6
Maran, J. P., Sivakumar, V., Thirugnanasambandham, K., and Sridhar, R. (2014). “Degradation behavior of biocomposites based on cassava starch buried under indoor soil conditions,” Carbohydrate Polymers 101, 20-28. DOI: 10.1016/j.carbpol.2013.08.080
Marichelvam, M. K., Jawaid, M., and Asim, M. (2019). “Corn and rice starch-based bio-plastics as alternative packaging materials,” Fibers 7(4), 32. DOI: 10.3390/fib7040032
McGinity, J. W., and Felton, L. A. (2008). Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms, CRC Press, Boca Raton, FL.
Miller, K. S., and Krochta, J. M. (1997). “Oxygen and aroma barrier properties of edible films: A review,” Trends in Food Science & Technology 8(7), 228-237. DOI: 10.1016/S0924-2244(97)01051-0
Mohtaschemi, M., Dimic-Misic, K., Puisto, A., Korhonen, M., Maloney, T., Paltakari, J., and Alava, M. J. (2014). “Rheological characterization of fibrillated cellulose suspensions via bucket vane viscometer,” Cellulose 21(3), 1305-1312. DOI: 10.1007/s10570-014-0235-1
Mondal, S. (2018). “Review on nanocellulose polymer nanocomposites,” Polymer-Plastics Technology and Engineering 57(13), 1377-1391. DOI:10.1080/03602559.2017.1381253
Msahli, S., Jaouadi, M., Sakli, F., and Drean, J.-Y. (2015). “Study of the textile potential of fibres extracted from Tunisian Agave americana L.,” Journal of Natural Fibers 12(6), 552-560. DOI: 10.1080/15440478.2014.984046
Navarro-Tarazaga, M. L., del Río, M. A., Krochta, J. M., and Pérez-Gago, M. B. (2008). “Fatty acid effect on hydroxypropyl methylcellulose-beeswax edible film properties and postharvest quality of coated ‘ortanique’ mandarins,” Journal of Agricultural and Food Chemistry 56(22), 10689-10696. DOI: 10.1021/jf801967q
Ponni, P., Subramanian, K. S., Janavi, G. J., and Subramanian, J. (2020). “Synthesis of nano-film from nanofibrillated cellulose of banana pseudostem (Musa spp.) to extend the shelf life of tomatoes,” BioResources 15(2), 2882-2905. DOI: 10.15376/biores.15.2.2882-2905
Preechawong, D., Peesan, M., Supaphol, P., and Rujiravanit, R. (2005). “Preparation and characterization of starch/poly(L-lactic acid) hybrid foams,” Carbohydrate Polymers 59(3), 329-337. DOI: 10.1016/j.carbpol.2004.10.003
Ramesh, M., Palanikumar, K., and Reddy, K. H. (2017). “Plant fibre based bio-composites: Sustainable and renewable green materials,” Renewable and Sustainable Energy Reviews 79, 558-584. DOI: 10.1016/j.rser.2017.05.094
Roman, M., and Winter, W. T. (2004). “Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose,” Biomacromolecules 5, 1671-1677. DOI: 10.1021/bm034519
Samir, M. A. S. A., Alloin, F., and Dufresne, A. (2005). “Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field,” Biomacromolecules 6(2), 612-626. DOI: 10.1021/bm0493685
Samir, M. A. S. A., Alloin, F., Sanchez, J.-Y., and Dufresne, A. (2004). “Cellulose nanocrystals reinforced poly(oxyethylene),” Polymer 45(12), 4149-4157. DOI: 10.1016/j.polymer.2004.03.094
Sangroniz, A., Zhu, J. B., Tang, X., Etxeberria, A., Chen, E. Y. X., and Sardon, H. (2019). “Packaging materials with desired mechanical and barrier properties and full chemical recyclability,” Nature Communications 10(1), 1-7. DOI:10.1038/s41467-019-11525-x
Sanyang, M. L., Ilyas, R. A., Sapuan, S. M., and Jumaidin, R. (2017). “Sugar palm starch-based composites for packaging applications,” in: Bionanocomposites for Packaging Applications, M. Jawaid, and S. K. Swain (eds.), Springer International Publishing, Basel, Switzerland, pp. 125-147.
Siró, I., and Plackett, D. (2010). “Microfibrillated cellulose and new nanocomposite materials: A review,” Cellulose 17(3), 459-494. DOI: 10.1007/s10570-010-9405-y
Soni, B., Schilling, M. W., and Mahmoud, B. (2016). “Transparent bionanocomposite films based on chitosan and TEMPO-oxidized cellulose nanofibers with enhanced mechanical and barrier properties,” Carbohydrate Polymers 151, 779-789. DOI:10.1016/j.carbpol.2016.06.022
Sothornvit, R., and Pitak, N. (2007). “Oxygen permeability and mechanical properties of banana films,” Food Research International 40(3), 365-370. DOI: 10.1016/j.foodres.2006.10.010
Tang, Y., Yang, S., Zhang, N., and Zhang, J. (2014). “Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis,” Cellulose 21(1), 335-346. DOI: 10.1007/s10570-013-0158-2
Tang, Y., Zhang, X., Zhao, R., Guo, D., and Zhang, J. (2018). “Preparation and properties of chitosan/guar gum/nanocrystalline cellulose nanocomposite films,” Carbohydrate Polymers 197, 128-136. DOI: 10.1016/j.carbpol.2018.05.073
Wang, C., Huang, H., Jia, M., Jin, S., Zhao, W., and Cha, R. (2015). “Formulation and evaluation of nanocrystalline cellulose as a potential disintegrant,” Carbohydrate Polymers 130, 275-279. DOI: 10.1016/j.carbpol.2015.05.007
Yang, L., Lu, S., Li, J., Zhang, F., and Cha, R. (2015). “Nanocrystalline cellulose-dispersed AKD emulsion for enhancing the mechanical and multiple barrier properties of surface-sized paper,” Carbohydrate Polymers 136, 1035-1040. DOI: 10.1016/j.carbpol.2015.10.011
Yang, S., Tang, Y., Wang, J., Kong, F., and Zhang, J. (2014). “Surface treatment of cellulosic paper with starch-based composites reinforced with nanocrystalline cellulose,” Industrial & Engineering Chemistry Research 53(36), 13980-13988. DOI: 10.1021/ie502125s
Zhang, Z., Wu, Q., Song, K., Lei, T., and Wu, Y. (2015). “Poly(vinylidene fluoride)/ cellulose nanocrystals composites: Rheological, hydrophilicity, thermal and mechan-ical properties,” Cellulose 22(4), 2431-2441. DOI: 10.1007/s10570-015-0634-y
Article submitted: September 1, 2021; Peer review completed: October 4, 2021; Revised version received and accepted: October 16, 2021; Published: October 20, 2021.
DOI: 10.15376/biores.16.4.8125-8151
