Abstract
Facile green synthesis of gold nanoparticles (AuNPs) on cellulose fiber was successfully achieved by reducing chloroauric acid (HAuCl4·3H2O) by means of unbleached kraft pulp. A significant color change in pulp fiber indicating the in-situ formation of gold was observed with one-step synthesis in an autoclave. As-prepared AuNP-cellulose fiber nanocomposites were thoroughly characterized by UV–Vis diffuse reflectance spectroscopy (DRS), X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS). Gold nanoparticles were uniformly dispersed on the surface of the fiber by the bio-reduction of Au3+ from metal salt to Au0 with the α-carbonyl group and conjugated carbonyl of phenolic groups of lignin. The AuNPs formed on cellulose fibers were estimated to have average sizes of approximately 12.5, 12.4, 16.4, and 21.0 nm, depending on the concentration of Au3+ involved in the synthesis.
Download PDF
Full Article
In-situ Green Synthesis of Gold Nanoparticles using Unbleached Kraft Pulp
Nattinee Bumbudsanpharoke and Seonghyuk Ko*
Facile green synthesis of gold nanoparticles (AuNPs) on cellulose fiber was successfully achieved by reducing chloroauric acid (HAuCl4·3H2O) by means of unbleached kraft pulp. A significant color change in pulp fiber indicating the in-situ formation of gold was observed with one-step synthesis in an autoclave. As-prepared AuNP-cellulose fiber nanocomposites were thoroughly characterized by UV–Vis diffuse reflectance spectroscopy (DRS), X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS). Gold nanoparticles were uniformly dispersed on the surface of the fiber by the bio-reduction of Au3+ from metal salt to Au0 with the α-carbonyl group and conjugated carbonyl of phenolic groups of lignin. The AuNPs formed on cellulose fibers were estimated to have average sizes of approximately 12.5, 12.4, 16.4, and 21.0 nm, depending on the concentration of Au3+ involved in the synthesis.
Keywords: Green synthesis; Gold nanoparticles; Unbleached kraft pulp
Contact information: Department of Packaging, Yonsei University, 1 Yonseidaegil, Wonju-si, Gangwon-do, 220-710 Korea; *Corresponding author: s.ko@yonsei.ac.kr
INTRODUCTION
Metal nanoparticles have garnered enormous attention in multidisciplinary science and engineering because of their distinctive optical, electronic, chemical, magnetic, and catalytic properties (Eustis and El-Sayed 2006; Hutchings and Edwards 2012). Gold nanoparticles (AuNPs) offer dramatically different physicochemical and catalytic properties compared to bulk gold (Kolska et al. 2010) and have been extensively used in various applications, including environmental catalysis (Carabineiro et al. 2011), energy processing (Oelhafen and Schuler 2005), chemical synthesis (Zhang et al. 2012), chemical and biological sensors (Saha et al. 2012), surface-enhanced Raman spectroscopy (SERS) (Lee et al. 2010), and optoelectronic applications (Mukherjee et al. 2012). A wide range of methods have been used to produce AuNPs with a multitude of chemical and physical processes (Haas 2013). The traditional and most widely used method for the synthesis of AuNP commonly involves the reduction of a soluble metal salt by a reducing agent or high-temperature gaseous conditions (Raveendran et al. 2003). Hydrazine, tri-sodium citrate, sodium borohydride, and dimethylformamide are examples of reported reducing and stabilizing agents (Pinto et al. 2012; Raveendran et al. 2003). However, exposure to these highly reactive chemicals poses potential risks to human health and the environment (He et al. 2009).
Thus, enormous efforts have been made to integrate green chemistry principles into metal nanoparticle synthesis to avoid accumulating an enormous quantity of toxic chemicals in the environment. Biological resources such as plant extracts, microorganisms, biopolymers, and polysaccharides are known to be effective natural materials for alternative green synthesis of metallic nanoparticles (Iravani 2011; Hutchings and Edwards 2012). Among these ecological materials, polysaccharides are quite advantageous when utilized in green synthesis because they have an abundance of hydroxyl groups and hemiacetal reducing ends; thus, they can be used as both reducing and stabilizing agents for metal nanoparticles (Johnston and Nilsson 2012). Various polysaccharides such as buffered glucose incorporated with starch (Engelbrekt et al. 2009), chitosan associated with organic acid (Di Carlo et al.2012), cellulose in ionic liquid (Li et al. 2008), guar gum (Pandey et al. 2013), dextran (Wang et al.2010), pullulan (Choudhury et al. 2014), and arabinoxylan (Amin et al. 2013) have been employed for AuNP synthesis.
However, because gold colloids easily aggregate to yield agglomerated materials, the resulting AuNP clusters primarily serve to reduce the specific surface area, consequently reducing the sensitivity and selectivity (Azetsu et al. 2011; Li et al. 2015b). Therefore, there is intense scientific interest in the single-step integration of synthesis and immobilization of AuNPs. Azetsu et al. (2013) demonstrated the direct synthesis of AuNPs by TEMPO-oxidized softwood kraft paper. The aldehydic moieties in carbohydrates were reported to act as a reducing agent, while the obtained AuNPs were deposited on cellulose fibers. Johnston and Nilsson (2012) reported a mechanism of green reduction and immobilization of nanogold using lignin-containing wood fiber. They used unbleached kraft fibers and proposed that the aromatic methoxy and phenol groups of lignin play a significant role not only in reduction of gold ion (Au3+) to Au0, but also in binding gold nanoparticles directly to the fiber surface without an external linker.
Despite the many pathways for AuNP green synthesis, there is still great interest in developing a simple and sustainable method to prepare well-defined gold nanoparticles on renewable cellulose-based fibers. To date, few studies have focused on such techniques. The present study investigated a novel, simple, one-step, “green” approach to synthesize gold nanoparticles on unbleached kraft pulp that simultaneously acts as a reducing-stabilizing agent and a substrate for AuNP immobilization. Optical, morphological, and chemical properties of the as-prepared AuNPs composited fibers were characterized using UV–Vis diffuse reflectance spectroscopy (DRS), X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS). Notably, the results presented in this study offer unique insights into a facile preparative method to develop novel natural fiber composites, which can possibly be functionalized with nanomaterials to impart particular optical, catalytic, and antimicrobial properties.
EXPERIMENTAL
Materials
American Chemical Society (ACS) reagent-grade hydrogen tetrachloroaurate (III) trihydrate (HAuCl4.3H2O; Mw. 393.83 g/mol, ACS, 99.99%) was used as received from Alfa Aesar (Ward Hill, IL, USA) without any further purification. Unbleached softwood kraft pulp was obtained in dry form from Dongil Paper (Ansan, Korea). Ultra-pure water with a specific resistivity of 18 MΩ·cm was used in this study.
Synthesis of AuNP-Cellulose Fiber Nanocomposite
An aqueous solution (50.0 mM) of chloroauric acid was prepared as the nanogold precursor at room temperature. Unbleached softwood kraft pulp (UBK) was first disintegrated with a valley beater, followed by beating to an approximately 450 mL Canadian standard freeness (CSF) according to the Technical Association of the Pulp and Paper Industry (TAPPI) test methods TAPPI T200 sp-01 (2007) and TAPPI T227 om-04 (2007). After water drainage, the moisture content of the obtained pulp was approximately 83%, as determined by TAPPI T412 om-11 (2011).
Various concentrations of nanogold on UBK were prepared by adding different amounts of 50 mM chloroauric acid, as illustrated in Fig. 1. In a typical synthesis, 0.5 g of oven-dried (O.D.) pulp was suspended in 35 mL of ultra-pure water with vigorous shaking until the fibers were completely dispersed. Following preparation of the pulp suspension, a set amount of chloroauric acid solution (50 mM) was added to the pulp slurry with constant stirring and subsequently placed in an autoclave at 101.35 kPa and 121 °C for 30 min to facilitate AuNP synthesis.
Fig. 1. Preparation of the AuNP-cellulose fiber nanocomposite
AuNP-cellulose fibers were then centrifuged and rinsed three times with ultra-pure water to remove the residual ions. Finally, diverse and colored AuNP-fiber composites were obtained and retrieved as wet pulp for further preparation of composite sheets.
The nominal Au content in the composite fibers was calculated using the weight ratio of Au3+ and O.D. pulp. For example, in the case of 1.0 mL of 50 mM HAuCl4.3H2O(aq) dropped onto 0.5 g of O.D. pulp, HAuCl4.3H2O had a molarity of 5.0×10-5 mol, which amounts to 0.0197 g. Because the atomic weight of Au is 196.93 g and the molar mass of HAuCl4.3H2O is 393.83 g, the net amount of Au3+ in this slurry is calculated to be 9.85 x 10-3 g. Therefore, the Au3+ concentration of the prepared AuNP-cellulose fiber nanocomposite is 1.97 wt%. Table 1 shows the weight ratio percentage of Au3+ on cellulose fibers with various amounts of chloroauric acid in 0.5 g of O.D. pulp slurry.
Table 1. Nominal Ratio of Au (wt%) in Composite Fibers
Preparation of AuNP-Cellulose Fiber Nanocomposite Sheets
To prepare AuNP-fiber composite sheets, 2.0 g of wet AuNP-UBK pulp with approximately 90% moisture content was mixed with 300 mL of deionized (DI) water (about 0.05% consistency). The mixture was then vacuum-suctioned on a 200-mesh wire to form a wet sheet and dried in a vacuum oven at 60 °C for 8 h. Paper made of only unbleached kraft pulp, denoted blank, was also prepared for use as a reference sample. As-prepared AuNP-cellulose fiber nanocomposite sheets were named as listed in Table 1, and the calculated value of Au wt% on the basis of the relative percentage of weight ratio of chloroaurate ion (Au3+) against O.D. pulp weight was used in the preparation of AuNP-fibers. In addition, for the comparison of bleached fibers, 1.97 wt% of AuNP-bleached pulp composite sheet was simply prepared.
Analytical Characterization
UV−Vis diffuse reflectance spectra of as-prepared AuNP-cellulose fiber sheets were acquired using a double-beam V-600 spectrophotometer (Jasco, Japan) with an attached integrating sphere in the wavelength range of 200 to 800 nm. X-ray diffraction patterns were obtained on an X-ray diffractometer (D2 Phaser, Bruker, Germany) with Ni-filtered CuKa radiation (λ=1.5406 Å). The surface morphology of the composite sheet was observed with a field-emission scanning electron microscope (FE-SEM Quanta FEG 250, FEI, USA) in backscattered electron (BE) mode after sputtering platinum/palladium onto the specimen using a Cressington Sputter Coater 108 auto (Cressington Scientific Instruments, Watford, UK). Further elemental analysis was carried out with an energy-dispersive X-ray spectrometer (EDS, Bruker AXS, Japan) attached to the FESEM. FTIR analyses were performed using a Spectrum 65 FTIR (Perkin Elmer, USA). Each spectrum was collected in attenuated total reflectance (ATR) mode with 16 scans and 2 cm-1 resolution in the range of 4000 to 400 cm-1. The surface chemical compositions of both blank and AuNP-cellulose fiber nanocomposites were analyzed with X-ray photoelectron spectroscopy (XPS K-alpha, Thermo VG, UK) with a monochromated Al X-ray source (hν = 1486.6 eV).
RESULTS AND DISCUSSION
Characterization of AuNP-Cellulose Fiber Nanocomposites
This study established a simple green reduction method to prepare AuNP-cellulose fiber nanocomposites using unbleached kraft pulp. As described in detail in the experimental section, upon the addition of 50 mM chloroauric acid to a pulp slurry, the color of the pulp gradually changed to a darker shade after a 30-min incubation in an autoclave. Figure 2 represents the apparent color change of AuNP-cellulose fiber nanocomposites as a function of the weight ratio of Au3+. The color went through a series of changes from pale yellow (blank), gray (0.20 wt%), purple (0.98 wt%), and wine-red (2.36 wt%) to dark wine-red brown (5.91 wt%). This diverse color spectrum is attributed to the surface plasmon resonance (SPR) of AuNPs (Kumari and Philip 2013), implying the direct synthesis of nanogold on the fiber surface. It is generally known that the SPR effect is a result of the resonance interaction of incoming visible electromagnetic radiation with collective plasmon oscillations on the metal surface (Johnston and Lucas 2011).
Fig. 2. AuNP-cellulose fiber nanocomposite sheets prepared with various concentrations of Au(III)
To verify the formation of AuNPs on cellulose fiber, the blank sample (pulp fibers only), and AuNP-cellulose fiber nanocomposites were characterized using UV-Vis spectroscopy because AuNPs are known to show a plasmon resonance band in the region of 530 to 550 nm (Agnihotri et al. 2009; Kumari and Philip 2013). Figure 3 presents the UV-Vis diffuse reflectance spectra of composite samples. The blank sample presents only the multiple peaks in the region of 200 to 300 nm, which can be assigned to hexenuronic acid and aromatic lignin (Boonstra and Tjeerdsma 2006; Lähdetie et al.2009). In contrast, AuNP-cellulose fiber composite samples exhibit an additional absorption peak in the region of 530 to 540 nm that can be attributed to the SPR band of AuNPs. The intensity of the absorption band gradually increased with increasing concentration of Au3+, and the peak broadened at a relatively higher concentration of Au3+. These results suggest that the AuNPs are highly dispersed on the cellulose fiber, and this increase in metal nanoparticle dispersion results in increased interaction among AuNPs, leading to broadening of the SPR. A similar result was observed by Pulit and Banach (2014) in the preparation of AuNPs using Dog Rose aqueous extract as a reducing agent. They found that the higher concentrations of gold ions involved in the synthesis resulted in lower and broader UV-Vis absorption spectra of AuNPs because of the formation of larger particles as well as a polymodal size distribution. In addition, as can be seen in Fig. 3, the absorption peak of AuNPs in bleached kraft pulp composite is not discernable compared to that of unbleached kraft pulp nanocomposite. This indicates the Au nanoparticles were not effectively produced, due to the lack of a chemical group that was susceptible to being oxidized. Johnston and Nilsson (2012) have reported that cellulose shows lower effectiveness in forming and bonding AuNPs than UBK, which has abundant reducing ends from phenolic and methoxy groups attached to the electron-rich aromatic rings from lignin.
Fig. 3. UV/Vis diffuse reflectance spectra of AuNP-cellulose fiber nanocomposite sheets
XRD was used for further characterization of the crystallographic structure and AuNP grain size. The XRD patterns of blank and AuNP-cellulose fiber composites are shown in Fig. 4. The dominant peak at a 2-theta near 23.1° was observed for all samples; this originates from the parallel chain structure of cellulose crystalline (002) (Dinand et al. 2002; Zhao et al. 2007). In addition, AuNP-fiber composite sheets showed four Bragg reflection peaks at 2-theta values of nearly 38.6, 44.9, 65.0, and 78.0, which are indexed to the (111), (200), (220), and (311) planes of the face-centered cubic (fcc) lattice of gold
(JCPDS no. 04-0784), respectively (Sen et al. 2013). The XRD results indicate that the pure metallic gold crystals were effectively formed on cellulose fiber.
Average gold particle sizes in AuNP-cellulose fiber nanocomposites were estimated based on the Scherrer equation from the full width at half maximum (FWHM) intensity as,
![]()
where D is the grain size, K is the Scherrer constant value (0.9 to 1), is the wavelength of the X-ray radiation, b is the FWHM, and is the Bragg angle (Aromal and Philip 2012). Using this formula, the average size of AuNPs was calculated to be 12.5, 12.4, 16.4, and 21.0 nm in order of increasing concentration of chloroauric acid, i.e., 0.59, 0.98, 1.97, and 5.90 wt%, respectively. This difference in grain size influences the color of the AuNP-cellulose fiber nanocomposites, as shown in Fig. 2. A similar result was reported by Johnston and Lucas (2011), wherein the differences in particle size of the AuNP imparted various colors to AuNP-wool fiber.
Fig. 4. XRD patterns of blank and AuNP-cellulose fiber nanocomposite sheets
The formation and deposition of gold particles on the surface of cellulose fiber were clearly revealed by FESEM in BE mode. Images are shown in Fig. 5, along with representative EDS elemental spectra. In Figs. 5(b-d), AuNPs can be readily identified as white dots dispersed over the surface compared to the smooth and clean surface of cellulose fiber (Fig. 5(a)). These images confirm that AuNPs were uniformly formed on the surface of fibers and distinctly indicate that more chloroaurate ions (Au3+) involved in the preparation led to increases in the number and coverage of AuNPs on cellulose fibers. This result is consistent with the gradual broadening of SPR spectra and sharpness in diffraction peaks, as shown in Figs. 3 and 4. Similar results were observed by Ngo et al. (2012): the population of AuNPs on cellulose fiber increased linearly with the concentration of AuNP precursor solution.
(a)
(b)
(c)
(d)
(e)
Fig. 5. Backscattered electron FESEM images at 3,000x magnification and corresponding EDS spectra of (a) blank and AuNP-fiber composites of (b) 0.98 wt%, (c) 1.97 wt%, and (d) 5.90 wt%; (e) EDS analysis of the Au component
Figure 5(e) presents the average content of Au obtained by EDS microanalysis. The average was determined from five different areas on the blank and AuNP-cellulose fiber nanocomposites. The results are consistent with the proposed weight percent of Au3+ precursor and tended to increase in proportion to the concentration of Au3+, implying that the gold ions were effectively reduced to AuNPs.
Figure 6 shows the FTIR spectra of the blank and AuNP-cellulose fiber nanocomposites. No distinguishing peaks were observed when comparing the blank and AuNP composite sheets, with the exception of one visible peak integrated over the range 1550 to 1650 cm-1, which is assigned to the aromatic vibration of lignin, conjugated C=O stretching of the phenolic group, and C-O stretching from lignin (Popescu et al. 2010; Wang et al. 2009). In particular, as shown in Fig. 6(b), the intensity of the peak at 1580 to 1585 cm-1, which is associated with the -carbonyl group on lignin (Bykov 2008), was reduced with higher Au3+ concentration. This is compatible with the clustering and movement of Au0 species resulting from Au3+ bio-reduction occurring in the electron-rich lignin component and is indicative of participation of the lignin component in AuNP synthesis.
Fig. 6. (a) FTIR spectra of blank and AuNP–cellulose fiber nanocomposites; (b) the spectra at wavenumbers 1500 to 1800 cm-1
Previous studies have reported similar mechanisms of nanometal synthesis. Tagad et al. (2014) demonstrated that the aldehyde groups and hemiacetal reducing ends of polysaccharides facilitate the reduction of Au3+ to Au0. Li et al. (2015a) observed that the vibration peak of C=O from cellulose fibers slightly decreased with higher concentration of silver ions. Kan et al. (2005) proposed that metal ions attract electrons from carbonyl to form metal crystal nuclei and subsequently grow into metal particles. Thus, it is believed that the conjugated carbonyl groups in lignin play a significant role in the reduction of gold ions into metallic gold, as depicted in Fig. 7.
Figure 8 displays the XPS survey scan spectra of blank and AuNP-fiber composite sheets. As seen in Fig. 8(a), both samples exhibited C1s and O1s core level spectra that can be attributed to the carbon and oxygen present in the cellulose fiber (Johansson 2002). An additional peak corresponding to gold appeared at a binding energy of 83 to 88 eV in the gold composite sheet. Figure 8(b) shows the XPS spectrum of the Au4f region of the AuNP-fiber composite. Doublet peaks were observed at BE positions of 84.08 and 87.78 eV, assigned to the Au4f7/2 and Au4f5/2 core levels of metallic gold (Au0), respectively (Vitale et al. 2011; Zhao et al. 2006). This result is in accordance with the XRD characterization and provides further evidence that gold ions (Au3+) were successfully reduced to metallic pure Au bound to the cellulose fibers.
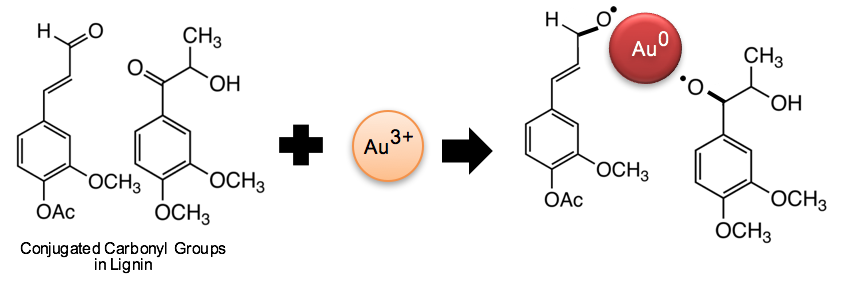
Fig. 7. Schematic of a reaction mechanism for AuNP synthesis on the conjugated carbonyl groups of lignin
Fig. 8. (a) XPS survey scan spectra and (b) the Au4f region spectra of blank and AuNP-cellulose fiber nanocomposite sheets
CONCLUSIONS
- AuNP-cellulose fiber nanocomposites were successfully prepared through a facile green method. The resulting purple-colored fiber composite showed a typical UV-Vis absorbance spectra at approximately 530 nm, which is attributed to SPR of AuNPs.
- FESEM images clearly revealed that AuNPs were formed and distributed over the surface of the fiber and were identified as metallic gold (Au0) by an XPS narrow scan of the Au4f region.
- It was also found that the -carbonyl group on lignin decreased with AuNP synthesis, suggesting that lignin plays an important role in efficient bio-reduction of gold ions into metallic gold and binding to the fiber surface.
- The preparation procedure presented here provides a simple and sustainable route to synthesize and immobilize AuNPs in an environmentally friendly manner. The comparative study of AuNP synthesis on cellulose fiber with a different synthetic condition such as reflux system under normal pressure is a topic for future work.
REFERENCES CITED
Agnihotri, M., Joshi, S., Kumar, A. R., Zinjarde, S., and Kulkarni, S. (2009). “Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589,” Mater. Lett. 63(15), 1231-1234. DOI: 10.1016/j.matlet.2009.02.042
Amin, M., Iram, F., Iqbal, M. S., Saeed, M. Z., Raza, M., and Alam, S. (2013). “Arabinoxylan-mediated synthesis of gold and silver nanoparticles having exceptional high stability,” Carbohydr. Polym. 92(2), 1896-900. DOI: 10.1016/j.carbpol.2012.11.056
Aromal, S. A., and Philip, D. (2012). “Benincasa hispida seed mediated green synthesis of gold nanoparticles and its optical nonlinearity,” Physica E 44(7), 1329-1334. DOI: 10.1016/j.physe.2012.02.013
Azetsu, A., Koga, H., Isogai, A., and Kitaoka, T. (2011). “Synthesis and catalytic features of hybrid metal nanoparticles supported on cellulose nanofibers,” Catalysts 1(1), 83-96. DOI: 10.3390/Catal1010083
Azetsu, A., Koga, H., Yuan, L. Y., and Kitaoka, T. (2013). “Direct synthesis of gold nanocatalysts on tempo-oxidized pulp paper containing aldehyde groups,” BioResources 8(3), 3706-3717. DOI: 10.15376/biores.8.3.3706-3717
Boonstra, M. J., and Tjeerdsma, B. (2006). “Chemical analysis of heat treated softwoods,” Holz Roh Werkst. 64(3), 204-211. DOI: 10.1007/s00107-005-0078-4
Bykov, I. (2008). “Characterization of natural and technical lignins using FTIR spectroscopy,” Luleå University of Technology, Luleå, Sweden
Carabineiro, S. A. C., Bogdanchikova, N., Avalos-Borja, M., Pestryakov, A., Tavares, P. B., and Figueiredo, J. L. (2011). “Gold supported on metal oxides for carbon monoxide oxidation,” NanoRes.4(2), 180-193. DOI: 10.1007/s12274-010-0068-7
Choudhury, A. R., Malhotra, A., Bhattacharjee, P., and Prasad, G. S. (2014). “Facile and rapid thermo-regulated biomineralization of gold by pullulan and study of its thermodynamic parameters,” Carbohydr. Polym. 106(1), 154-159. DOI: 10.1016/j.carbpol.2014.01.072
Di Carlo, G., Curulli, A., Toro, R. G., Bianchini, C., De Caro, T., Padeletti, G., Zane, D., and Ingo, G. M. (2012). “Green synthesis of gold-chitosan nanocomposites for caffeic acid sensing,” Langmuir28(12), 5471-5479. DOI: 10.1021/la204924d
Dinand, E., Vignon, M., Chanzy, H., and Heux, L. (2002). “Mercerization of primary wall cellulose and its implication for the conversion of cellulose I→ cellulose II,” Cellulose 9(1), 7-18. DOI: 10.1023/A:1015877021688
Engelbrekt, C., Sørensen, K. H., Zhang, J., Welinder, A. C., Jensen, P. S., and Ulstrup, J. (2009). “Green synthesis of gold nanoparticles with starch–glucose and application in bioelectrochemistry,” J. Mater. Chem. 19(42), 7839-7847. DOI: 10.1039/b911111e
Eustis, S., and El-Sayed, M. A. (2006). “Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes,” Chem. Soc. Rev. 35(3), 209-217. DOI: 10.1039/B514191e
Haas, K. H. (2013). “Industrial relevant production processes for nanomaterials and nanostructures,” in: Safety aspects of engineered nanomaterials, W. Luther and A. Zweck (eds). CRC Press, Boca Raton, FL, 29-62. DOI: 10.1201/b15261-3
He, F., Liu, J., Roberts, C. B., and Zhao, D. (2009). “One-step “green” synthesis of Pd nanoparticles of controlled size and their catalytic activity for trichloroethene hydrodechlorination,” Ind. Eng. Chem. Res. 48(14), 6550-6557. DOI: 10.1021/ie801962f
Hutchings, G. J., and Edwards, J. K. (2012). “Application of gold nanoparticles in catalysis,” in: Metal Nanoparticles and Nanoalloys, R. L. Johnston and J. P. Wilcoxon (eds). Elsevier Ltd, Oxford, UK, 249-293. DOI: 10.1016/B978-0-08-096357-0.00006-6
Iravani, S. (2011). “Green synthesis of metal nanoparticles using plants,” Green Chem. 13(10), 2638-2650. DOI: 10.1039/C1gc15386b
Johansson, L. S. (2002). “Monitoring fibre surfaces with XPS in papermaking processes,” Mikrochim. Acta 138(3-4), 217-223. DOI: 10.1007/s006040200025
Johnston, J. H., and Lucas, K. A. (2011). “Nanogold synthesis in wool fibres: novel colourants,” Gold Bull. 44(2), 85-89. DOI: 10.1007/s13404-011-0012-y
Johnston, J. H., and Nilsson, T. (2012). “Nanogold and nanosilver composites with lignin-containing cellulose fibres,” J. Mater. Sci. 47(3), 1103-1112. DOI: 10.1007/s10853-011-5882-0
Kan, C. X., Cai, W. P., Li, C. C., and Zhang, L. D. (2005). “Optical studies of polyvinylpyrrolidone reduction effect on free and complex metal ions,” J. Mater. Res. 20(2), 320-324. DOI: 10.1557/Jmr.2005.0039
Kolska, Z., Riha, J., Hnatowicz, V., and Svorcik, V. (2010). “Lattice parameter and expected density of Au nano-structures sputtered on glass,” Mater. Lett. 64(10), 1160-1162. DOI: 10.1016/j.matlet.2010.02.038
Kumari, M. M., and Philip, D. (2013). “Facile one-pot synthesis of gold and silver nanocatalysts using edible coconut oil,” Spectrochim. Acta A 111, 154-160. DOI: 10.1016/j.saa.2013.03.076
Lähdetie, A., Liitiä, T., Tamminen, T., and Jääskeläinen, A.-S. (2009). “Reflectance UV-Vis and UV resonance Raman spectroscopy in characterization of kraft pulps,” BioRes. 4(4), 1600-1619.
Lee, C. H., Tian, L., and Singamaneni, S. (2010). “Paper-based SERS swab for rapid trace detection on real-world surfaces,” ACS Appl. Mater. Inter. 2(12), 3429-3435. DOI: 10.1021/am1009875
Li, R., He, M., Li, T., and Zhang, L. (2015a). “Preparation and properties of cellulose/silver nanocomposite fibers,” Carbohydr. Polym. 115, 269-275. DOI: 10.1016/j.carbpol.2014.08.046
Li, Y., Liu, Y., Liu, J., Liu, J., Tang, H., Cao, C., Zhao, D., and Ding, Y. (2015b). “Molecularly imprinted polymer decorated nanoporous gold for highly selective and sensitive electrochemical sensors,” Sci. Rep. 5, 7699(1-8). DOI: 10.1038/srep07699
Li, Z., Friedrich, A., and Taubert, A. (2008). “Gold microcrystal synthesis via reduction of HAuCl4 by cellulose in the ionic liquid 1-butyl-3-methyl imidazolium chloride,” J. Mater. Chem. 18(9), 1008-1014. DOI: 10.1039/B716135M
Mukherjee, P., Nandi, S., and Nandi, A. K. (2012). “Optoelectronic properties of RNA–polyaniline–dendritic gold nanobiocomposite,” Synth. Met. 162(11), 904-911. DOI: 10.1016/j.synthmet.2012.04.004
Ngo, Y. H., Li, D., Simon, G. P., and Garnier, G. (2012). “Gold nanoparticle–paper as a three-dimensional surface enhanced raman scattering substrate,” Langmuir 28(23), 8782-8790. DOI: 10.1021/la3012734 |
Oelhafen, P., and Schuler, A. (2005). “Nanostructured materials for solar energy conversion,” Solar Energy 79(2), 110-121. DOI: 10.1016/j.solener.2004.11.004
Pandey, S., Goswami, G. K., and Nanda, K. K. (2013). “Green synthesis of polysaccharide/gold nanoparticle nanocomposite: An efficient ammonia sensor,” Carbohydr. Polym. 94(1), 229-234. DOI: 10.1016/j.carbpol.2013.01.009
Pinto, R. J., Neves, M. C., Neto, C. P., and Trindade, T. (2012). “Composites of cellulose and metal nanoparticles,” in: Nanocomposites–New Trends and Developments, F. Ebrahimi (eds). InTech, Croatia, 73-96. DOI: 10.5772/3389
Popescu, C. M., Popescu, M. C., and Vasile, C. (2010). “Characterization of fungal degraded lime wood by FT-IR and 2D IR correlation spectroscopy,.” Microchem. J. 95(2), 377-387. DOI: 10.1016/j.microc.2010.02.021
Pulit, J., and Banach, M. (2014). “Preparation of nanosilver and nanogold based on Dog Rose aqueous extract,” Bioinorg. Chem. Appl. 2014(1), 1-14 (Article ID 658935). DOI: 10.1155/2014/658935
Raveendran, P., Fu, J., and Wallen, S. L. (2003). “Completely “green” synthesis and stabilization of metal nanoparticles,” J. Am. Chem. Soc. 125(46), 13940-13941. DOI: 10.1021/ja029267j
Saha, K., Agasti, S. S., Kim, C., Li, X., and Rotello, V. M. (2012). “Gold nanoparticles in chemical and biological sensing,” Chem. Rev. 112(5), 2739-2779. DOI: 10.1021/cr2001178
Sen, I. K., Maity, K., and Islam, S. S. (2013). “Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity,” Carbohydr. Polym. 91(2), 518-528. DOI: 10.1016/j.carbpol.2012.08.058
Tagad, C. K., Rajdeo, K. S., Kulkarni, A., More, P., Aiyer, R. C., and Sabharwal, S. (2014). “Green synthesis of polysaccharide stabilized gold nanoparticles: chemo catalytic and room temperature operable vapor sensing application,” RSC Adv. 4(46), 24014-24019. DOI: 10.1039/C4ra02972k
TAPPI T200 sp-01. (2007). “Laboratory beating of pulp (Valley beater method),” TAPPI Press, Atlanta, GA.
TAPPI T227 om-04. (2007). “Freeness of Pulp (Canadian Standard Method),” TAPPI Press, Atlanta, GA.
TAPPI T412 om-11. (2011). “Moisture in pulp, paper and paperboard,” TAPPI Press, Atlanta, GA.
Vitale, F., Fratoddi, I., Battocchio, C., Piscopiello, E., Tapfer, L., Russo, M. V., Polzonetti, G., and Giannini, C. (2011). “Mono- and bi-functional arenethiols as surfactants for gold nanoparticles: synthesis and characterization,” Nanoscale Res. Lett. 6, 103. DOI: 10.1186/1556-276x-6-103
Wang, H., Huang, L., and Lu, Y. (2009). “Preparation and characterization of micro- and nano-fibrils from jute,” Fiber. Polym. 10(4), 442-445. DOI: 10.1007/s12221-009-0442-9
Wang, Y., Zhan, L., and Huang, C. Z. (2010). “One-pot preparation of dextran-capped gold nanoparticles at room temperature and colorimetric detection of dihydralazine sulfate in uric samples,” Analytical Methods 2(12), 1982-1988. DOI: 10.1039/C0ay00470g
Zhang, Y., Cui, X., Shi, F., and Deng, Y. (2012). “Nano-gold catalysis in fine chemical synthesis,” Chem. Rev. 112(4), 2467-505. DOI: 10.1021/cr200260m
Zhao, W., Xu, J. J., Shi, C. G., and Chen, H. Y. (2006). “Fabrication, characterization and application of gold nano-structured film,” Electrochem. Commun. 8(5), 773-778. DOI: 10.1016/j.elecom.2006.03.009
Zhao, H. B., Kwak, J. H., Zhang, Z. C., Brown, H. M., Arey, B. W., and Holladay, J. E. (2007). “Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis,” Carbohydr. Polym.68(2), 235-241. DOI: 10.1016/j.carbpol.2006.12.013
Article submitted: March 24, 2015; Peer review completed: July 20, 2015; Revised version received: July 29, 2015; Accepted: July 30, 2015; Published: August 10, 2015.
DOI: 10.15376/biores.10.4.6428-6441
