Abstract
Fast pyrolysis is an important technology in biological waste disposal. It can produce fuels (bio-gas and bio-oil) and high-value chemicals. In this paper, camphor wood powder was subjected to fast pyrolysis, and the influence of the pyrolysis and torrefaction pretreatment temperature on the pyrolysis product distribution was investigated. The results showed that camphor wood powder produced higher phenol, 2-methoxy, 2-methoxy-4-vinyl-phenol, and 1, 2, 4-trimethoxybenzene compared with corn straw, rice husk, and wheat straw. The temperature of torrefaction pretreatment and fast pyrolysis simultaneously affected the yield of bio-oil and the composition of the pyrolysis product. The yield of bio-oil was the highest at 500 °C. Additionally, the yield of different high-value chemicals was the highest at 500 °C. When the camphor wood powder was pretreated under conditions of torrefaction, the yield of 3-furfural decreased and that of phenol, 2-methoxy, 2-methoxy-4-vinyl-phenol, 2-furancarboxaldehyde, 5-hydroxymethylfurfural, and 1, 2, 4-trimethoxybenzene increased, especially phenol, 2-methoxy, and 1, 2, 4-trimethoxybenzene. The yields of bio-oil and high-value chemicals were the highest at 500 °C of catalytic pyrolysis and at 300 °C of torrefaction treatment during the fast pyrolysis of camphor wood powder.
Download PDF
Full Article
Influence of Pyrolysis and Torrefaction Pretreatment Temperature on the Pyrolysis Product Distribution
Dongmei Bi,* Bozheng Li, Shanjian Liu, Weiming Yi, Mei Jiang, and Zhidong Lin
Fast pyrolysis is an important technology in biological waste disposal. It can produce fuels (bio-gas and bio-oil) and high-value chemicals. In this paper, camphor wood powder was subjected to fast pyrolysis, and the influence of the pyrolysis and torrefaction pretreatment temperature on the pyrolysis product distribution was investigated. The results showed that camphor wood powder produced higher phenol, 2-methoxy, 2-methoxy-4-vinyl-phenol, and 1, 2, 4-trimethoxybenzene compared with corn straw, rice husk, and wheat straw. The temperature of torrefaction pretreatment and fast pyrolysis simultaneously affected the yield of bio-oil and the composition of the pyrolysis product. The yield of bio-oil was the highest at 500 °C. Additionally, the yield of different high-value chemicals was the highest at 500 °C. When the camphor wood powder was pretreated under conditions of torrefaction, the yield of 3-furfural decreased and that of phenol, 2-methoxy, 2-methoxy-4-vinyl-phenol, 2-furancarboxaldehyde, 5-hydroxymethylfurfural, and 1, 2, 4-trimethoxybenzene increased, especially phenol, 2-methoxy, and 1, 2, 4-trimethoxybenzene. The yields of bio-oil and high-value chemicals were the highest at 500 °C of catalytic pyrolysis and at 300 °C of torrefaction treatment during the fast pyrolysis of camphor wood powder.
Keywords: Fast pyrolysis; Torrefaction pretreatment; Bio-oil; High-value chemicals; Pyrolysis product distribution
Contact information: School of Agricultural Engineering and Food Science, Shandong University of Technology, Zibo, Box 255000, China; *Corresponding author: dongmei070719@163.com
INTRODUCTION
Fossil fuels, such as oil and coal, are non-renewable resources consumed at a high rate, causing huge CO2 emissions and environment damage (Chen et al. 2016). Biomass can be used for clean and renewable energy that replaces fossil fuels (Chen et al. 2017). Fast pyrolysis is an important technology to convert biomass into fuels or chemicals. Camphor wood is used in some furniture and in mothballs. During the processing of camphor wood, a large amount of wood powder is wasted. Recycling the camphor wood powder waste is important for environmental protection and waste utilization. Fast pyrolysis technology can convert the camphor wood into high-value chemicals.
The components of biomass are complex. The pyrolysis of cellulose, hemicellulose, and lignin produces many high-value chemicals that are used in medical care, cosmetics, spices, and other industries. Levoglucosterone (LGO) is one of the main substances in the high-value chemicals produced from the pyrolysis of cellulose. LGO is widely used as an intermediate of antibiotics, immunosuppressants, natural organics, chemical spice, and drugs, and can composite carbohydrate derivatives (Cai et al. 2016; Cui et al. 2017). Xia et al. (2013) used FePO4 as a catalyst to investigate the effect of FePO4 on the pyrolysis of LGO and found that the LGO is produced during pyrolysis of microcrystalline cellulose (Hu et al. 2018). Gas chromatography-mass spectrometry (GC-MS) analysis showed that the addition of FePO4 catalyst promoted the production of bio-oil and had high selectivity for LGO. When the temperature of pyrolysis is 350 °C, the proportion of the FePO4 catalyst and cellulose is 1:1 and the output of LGO had the highest production rate (32.7%) of bio-oil.
Furfural (FF) is a furan compound pyrolyzed from hemicellulose. It is used in the production of drugs, resins, food additives, and fuel additives (Lu et al. 2018). Zhang et al. (2016) established the degradation kinetics model under the condition of dilute acid catalysis. They studied the extraction of hemicellulose from rapidly growing poplar and their conversion into furfural by acid degradation. There are many factors affecting the yield of furfural, such as temperature, acidity, and reaction time. At the situation of 181 °C for 15 min of reaction time with 1.0 wt% sulfuric acid and 17.9 g/L pentose, the maximum conversion rate of furfural is 52.0 mol%. Thus, the pretreatment of hemicellulose improves the yield and selectivity of furfural and reduces the occurrence of side reactions. Wang et al. (2016) used the mesoporous ZSM-5 catalyst to catalyze the pyrolysis of furan materials. When no catalyst is added, FF is the main bio-oil, accounting for 67.8% of bio-oil, and approximately 14.2% phenolic substances accompany the FF. After adding the ZSM-5 catalyst, the content of aldehyde, acid, alcohol, ester, ketone, ether, and furan in the bio-oil decreases significantly, and the phenolic content increases from 14.2% to 54.1%.
Many researchers had done studies on extraction of the target product (high-value chemicals) from biomass. Most wood, however, is used as cheap fuel or as indiscriminate storage. Only a small amount of lignin is used in the production of antirust agent, dye dispersant, and adhesive, resulting in a large amount of resource waste (Wang et al. 2016, 2017). Currently, the target products of lignin are aromatic compounds including phenolic compounds and aromatic hydrocarbons. There have been recent studies on lignin pyrolysis for aromatic hydrocarbons. The extraction of aromatic hydrocarbons using the lignin from pulping black liquor by fast pyrolysis was studied (Shen et al. 2013). The products of lignin pyrolysis in black liquor can be divided into five types: aromatic compounds, guaiacol compounds, lilac phenols, phenol compounds, and catechol compounds. The amount of the five compounds reached maximum at 700 °C. After adding four catalysts, 1% HZSM-5(25), 3% Co/HZSM-5(25), 3% Fe/HZSM-5(25), and 5% Pd/HZSM-5(25), the maximum amount of aromatic compounds is produced at 900 °C after using the catalyst 1% HZSM-5(25). The other three catalysts increase first and then decrease when the temperature increases, peaking at approximately 700 °C.
The rapid development of torrefaction technology has led to its application in biomass pyrolysis. Yu et al. (2017) conducted torrefaction pyrolysis experiments at different temperatures, using microcrystalline cellulose as raw material (Yu et al. 2017). The torrefaction deoxygenation pretreatment can reduce the volatile and oxygen content of cellulose and increase the content of fixed carbon and the calorific value of cellulose. In the pyrolytic oil of cellulose, the content of l-dextran and furfural increases, and the quality of cellulose products improves. The combined torrefaction and organic-acid leaching process could enrich the phenols and sugars and concentrate the chemical compounds of C6 and C9 using the rice husk (Zhang et al. 2018a). When the Fe-modified ZSM-5 zeolite was the catalyst, the washing- torrefaction pretreatment could enrich the hydrocarbon and BTX (Benzene, Toluene, and Xylenes) in the bio-oil. The mild torrefaction temperature was 210 °C and 240 °C (Zhang et al. 2018b).
In this study, torrefaction technology was used to conduct a pre-experimental treatment of camphor wood powder. The effects of the fast pyrolysis temperature and the torrefaction pretreatment temperature on the distribution of the pyrolysis products of camphor wood powder were examined.
EXPERIMENTAL
The experiments were grouped into two categories: the raw material was or was not torrefied before the fast pyrolysis.
Raw Material
Camphor wood powder samples were screened before fast pyrolysis. The particle size selected for fast pyrolysis ranged from 0.250 mm (60-mesh diameter) to 0.425 mm (40-mesh diameter). The proximate and elemental analysis results of camphor wood powder (Zhang et al. 2014) are shown in Table 1.
Table 1. Proximate and Elemental Analysis of Camphor Wood Powder
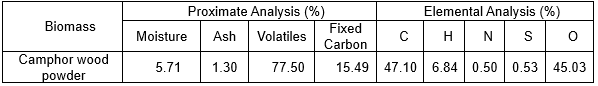
Experimental Apparatus
First, the camphor wood powder was dried in a thermostatic drying box for more than 24 h at 105 °C. The dried camphor wood powder was put in hermetic bags to wait for pyrolysis. The fast pyrolysis equipment included a horizontal fixed-bed reactor (Yilong, YL201; Suzhou Yilong Oven Equipment Manufacturing Co., Ltd., Suzhou, China.
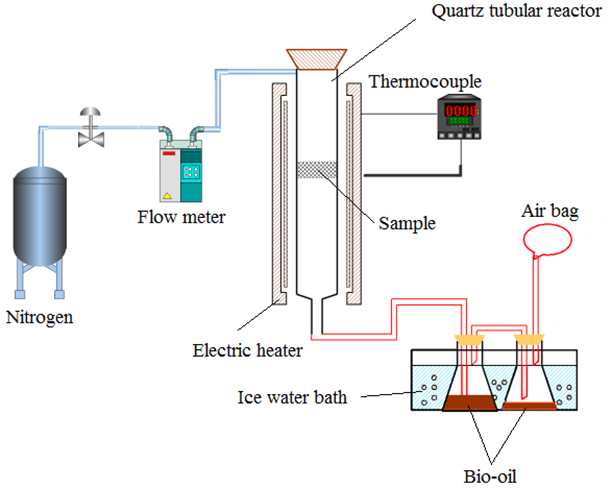
Fig. 1. Experimental setup
The heating rate of the horizontal fixed-bed reactor was 50 °C/s. The residence time of the fast pyrolysis was 2 s. The temperature of the horizontal fixed-bed reactor was set at 400 °C, 450 °C, 500 °C, 550 °C, and 600 °C and was filled by nitrogen with a velocity of 40 L/h. The dried camphor wood powder was placed into the reactor to pyrolyze; the fast pyrolysis lasted 5 min. The bio-oil was collected by a condensing pipe. The environment temperature of the condensing pipe was controlled at -10 °C in an ice-water bath. The experimental setup is shown in Fig. 1.
The camphor wood powder was torrefied in the horizontal fixed tubular furnace (Kejing, GSL-1100X-XX-S; Hefei Ke Jing Materials Technology Co., Ltd, Hefei, China), as shown in Fig. 2. The nitrogen was charged for the entire torrefaction experiment, and the interior of the horizontal fixed tubular furnace had atmospheric pressure. The heating rate of the horizontal fixed tubular furnace was 10 °C/min, and the furnace temperature was set at 200 °C, 250 °C, and 300 °C. After the camphor wood powder was torrefied, it was placed in a drying box to pyrolyze.

Fig. 2. Horizontal fixed tubular furnace
The distribution of the bio-oil was analyzed by GC-MS on Agilent model 5972N equipment (Santa Clara, CA, USA), as shown in Fig. 3.

Fig. 3. GC-MS system
RESULTS AND DISCUSSION
Thermogravimetric Analysis of Camphor Wood Powder
The thermogravimetry of camphor wood powder was analyzed. The heating rate of the camphor wood powder was 10 °C/min. The nitrogen was charged during the experiment.
The thermogravimetry (TG) and derivative thermogravimetry (DTG) are shown in Fig. 4. The best pyrolysis temperature for camphor wood powder was in the range of 350 °C to 550 °C.
The pyrolysis temperatures for cellulose, hemicellulose, and lignin were in the ranges of 320 °C to 360 °C, 220 °C to 320 °C, and 240 °C to 600 °C, respectively. The pyrolysis temperature was set above 400 °C to ensure the adequacy of the pyrolysis.
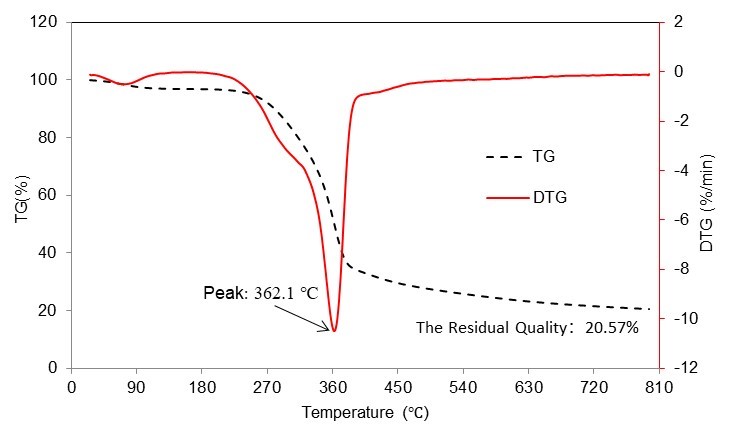
Fig. 4. Thermogravimetric analysis of camphor
Analysis of Pyrolysis Products of Camphor Wood Powder
The camphor wood powder bio-oil was analyzed after pyrolysis, and the composition of the products at 500 ℃ is shown in Table 2. The components of the pyrolysis products of camphor wood powder were mainly aromatics, alcohols, acids, aldehydes, ketones, lipids, phenols, and other inorganic substances. These components increased the range and difficulty when selecting target products. However, the pyrolysis produced a variety of high-value chemicals.
The area described in Table 2 is the relative peak area; this relative peak area can indirectly reflect the size of yield. A variety of biomass base platform compounds (Zhang et al. 2013) such as 2, 6-dimethoxyphenol, 1, 2, 4-trimethoxybenzene, 2-methoxyphenol, 3-furfural, ethyl-guaifenol, and 5-hydroxymethylfurfural were selected as the target products, considering the high yield and high added value of the chemicals.
Table 2. Main Products from Camphor Pyrolysis
Effect of Temperature on Pyrolysis of Camphor Wood Powder
Pyrolysis temperature influences the yield and composition of camphor wood powder bio-oil, as shown in Fig. 5. This experiment investigated five pyrolysis temperatures of 400 °C, 450 °C, 500 °C, 550 °C, and 600 °C. The reaction time of pyrolysis was 5 min. The yield of bio-oil collected by a condensing pipe was compared between experiments. The calculated quantity of the achieved bio-oil was the total quantity of the condensing pipe and bio-oil minus the quantity of the condensing pipe after pyrolysis.
Figure 5 shows the yield of bio-oil at different temperatures. When the pyrolysis temperature was 400 °C, which is relatively low, the yield of bio-oil was low. The bio-oil yield first increased and then decreased with increasing pyrolysis temperature, with a maximum yield of 45.4% at 500 °C. The change in the production rates was not obvious when the pyrolysis temperature was 450 °C and 500 °C. Above 500 °C, the yield of bio-oil decreased remarkably. High temperatures caused some of the macromolecular polymers in the bio-oil to split into small molecules of gas and evaporate, increasing the gas production rate and reducing the bio-oil yield; this explains the rise in the production of gas at 550 °C.
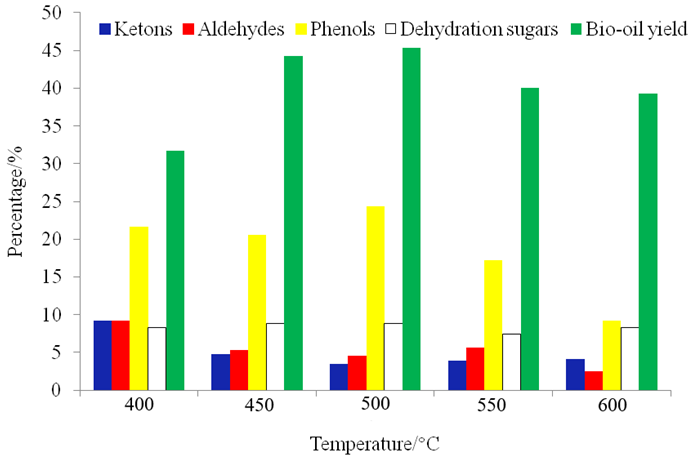
Fig. 5. Pyrolysis product distribution and yield of bio-oil at different temperatures
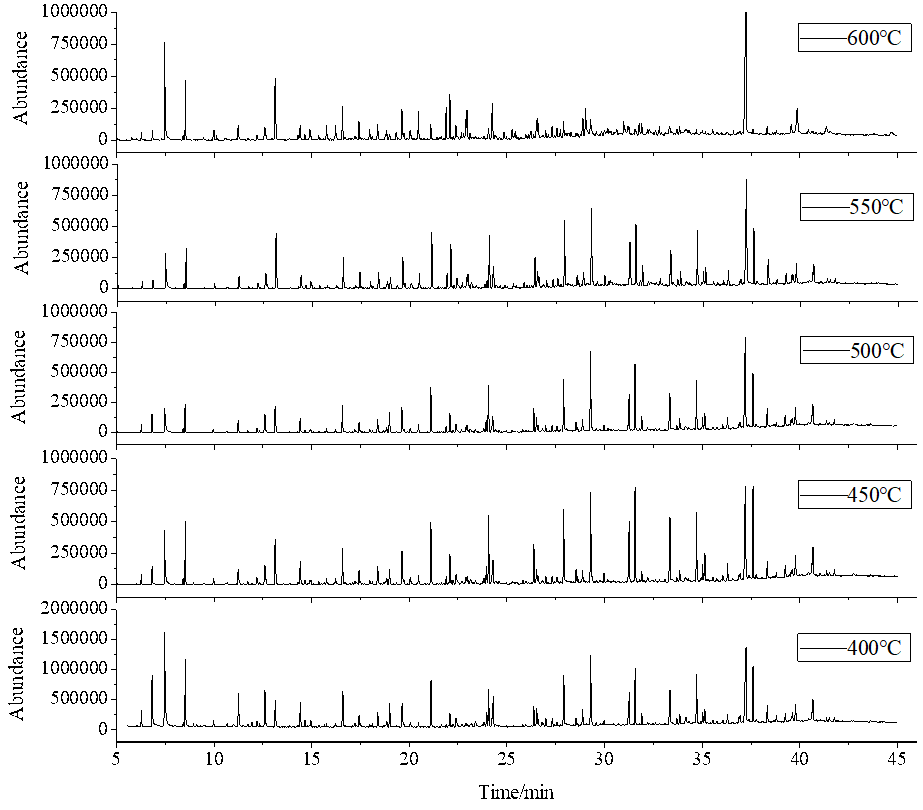
Fig. 6. Total ion chromatograms from fast pyrolysis of camphor wood powder vs. temperature
With increased pyrolysis temperature, the chemical composition of the bio-oil changed, as shown in Fig. 6. When the temperature was 400 °C, the most abundant chemicals in the bio-oil were acetic acid and levoglucosan at the peak times of 7.47 min and 37.24 min, respectively. There were many macromolecular polymers and phenols above the C8 structure because there was a high content of lignin in the camphor wood powder, and the main products of lignin pyrolysis are phenolic compounds. However, the types of phenols obtained by pyrolysis were varied. Many high value phenolic compounds were obtained (Sui et al. 2010), and lignin pyrolysis was accompanied by a small number of aldehydes and acids. The furan content was low during the low temperature pyrolysis. The maximum content of phenolic derivatives was 26.9% at 450 °C. The content of phenolics fell sharply with increasing temperature and was reduced to 15.9% at 600 °C. Many phenolic substances produced by lignin might be intermediates at a lower pyrolysis temperature. With an increase in the pyrolysis temperature, phenolic compounds such as guaiacol and 2,3-dimethoxyphenol derivatives underwent a secondary cracking, which reduced the yield of phenol derivatives. The content of levoglucosan increased with the increase of temperature, and the yield of aromatic hydrocarbons reached its maximum value at 450 °C.
This study compared the effects of different pyrolysis temperatures on the yield of target products, as shown in Fig. 7. The composition of bio-oil changed with the varying pyrolysis temperatures, and some of the value chemicals changed inversely when the pyrolysis temperature rose.
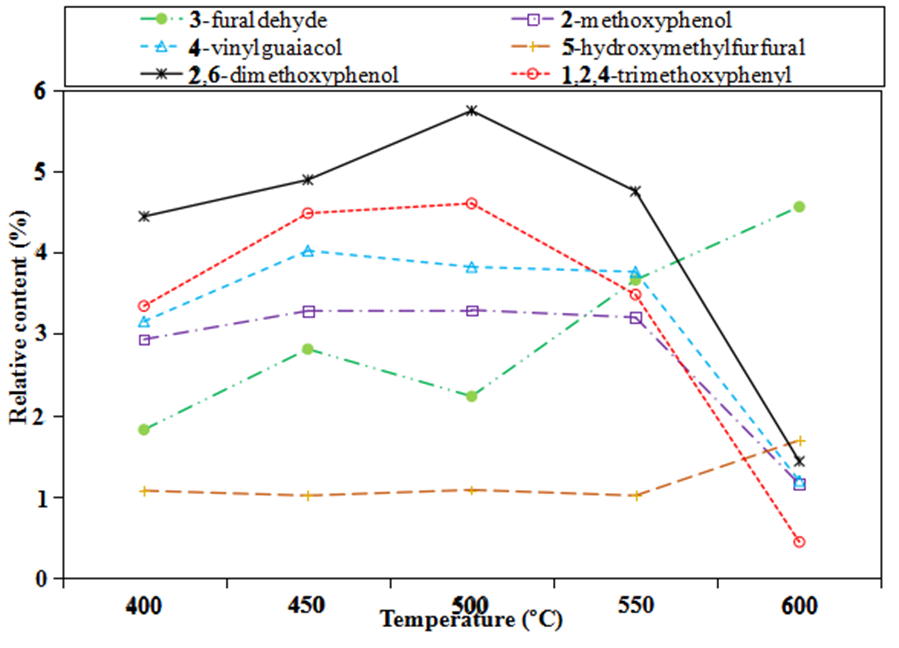
Fig. 7. Effects of temperature on high-value chemicals
The yield of 3-furfural was between 2% and 3% before 500 °C. The yield of 3-furfal rose when the temperature was more than 500 °C, and the yield of 3-furfural was 4.56% at 600 °C. The production rate of the four types of chemicals declined at more than 500 °C because at that point the high-value chemicals are cracking or gasifying, reducing the bio-oil yield. At 500 °C, a variety of the high-value chemicals reached their maximum production. The yield of phenol, 2-methoxy, furfural, 2, 6-peroxide, and phenol, 1,2,4-trimethoxy reached 3.29%, 5.74% and 4.6%, respectively, and the maximum production rate of 2-methoxy-4-vinylphenol was 4.02% at 450 °C. The yield of 2-methoxy-4-vinylphenol and phenol, 2-methoxy changed little from 450 °C to 550 °C.
Coupling Effect of Pyrolysis and Torrefaction Pretreatment Temperature on the Pyrolysis of Camphor Wood Powder
The application of torrefaction in biomass pyrolysis is new. This experiment subjected camphor wood powder to biomass torrefaction at 200 °C, 250 °C, and 300 °C in an anaerobic environment. The torrefaction product was pyrolyzed at 400 °C, 450 °C, and 500 °C. Figure 8 shows the yield of the bio-oil at different temperatures. The highest production rate of bio-oil (45.4%) occurred with 200 °C torrefaction pretreatment and 450 °C pyrolysis. At the same torrefaction pretreatment temperature (200 °C), the yield of pyrolysis oil increased first and then decreased. However, at the same pyrolysis temperature (450 °C), the yield of pyrolysis oil decreased with the increasing torrefaction pretreatment temperature. Compared with the pyrolytic oil yield without torrefaction pretreatment, the bio-oil production rate of camphor wood powder remained unchanged at the 200 °C torrefaction pretreatment temperature. However, after increasing the torrefaction pretreatment temperature, the yield of bio-oil decreased. When the torrefaction pretreatment temperature and the pyrolysis temperature were 300 °C, the yield of bio-oil was the lowest, at 13.74%. Thus, the torrefaction pretreatment temperature had an impact on the yield of the bio-oil.
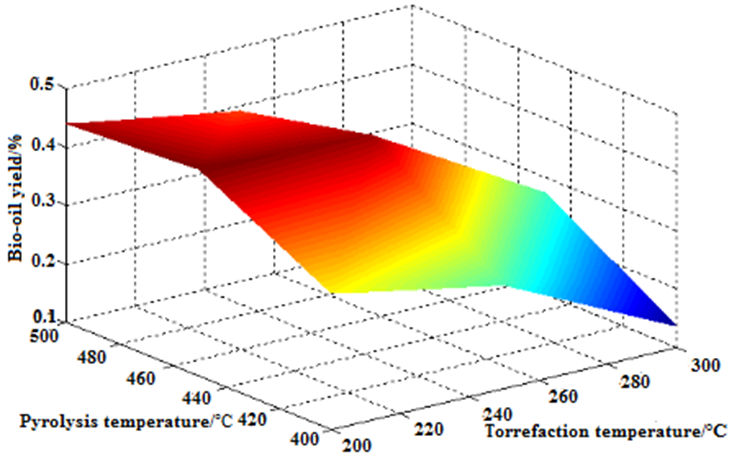
Fig. 8. Effect of torrefaction pretreatment temperature and pyrolysis temperature on pyrolysis of camphor
Figure 9 shows the yields of various high-value chemicals at different torrefaction and pyrolysis temperatures. The highest yield of 4.01% for 3-furfural appeared with in a torrefaction pretreatment temperature of 250 °C and pyrolysis temperature of 400 °C. The yield decreased without torrefaction treatment; the GC-MS could not detect the content of 3-furfural at a 300 °C torrefaction pretreatment temperature and at a 400 °C pyrolysis temperature. Torrefaction pretreatment had a remarkable inhibitory effect on the production of 3-furfural. Additionally, the torrefaction pretreatment temperature influenced the yield of phenol, phenol, 2-methoxy.
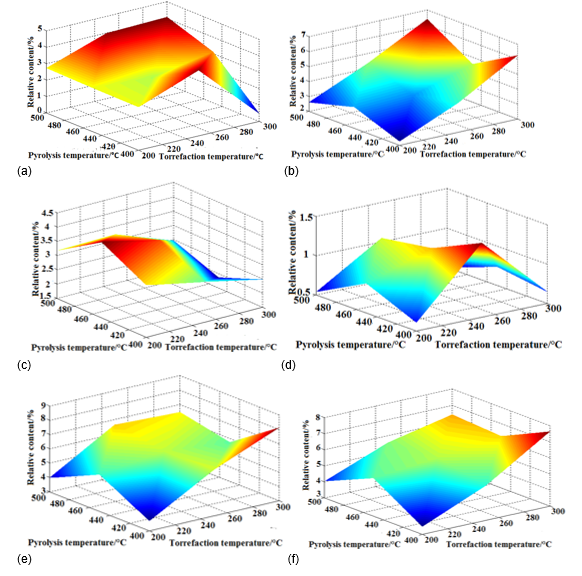
Fig. 9. Effect of torrefaction pretreatment temperature and pyrolysis temperature on high-value chemicals
When the torrefaction pretreatment temperature increased, the yield of phenol, 2-methoxy increased, reaching 6.3%, and was higher than the result without torrefaction pretreatment. Therefore, torrefaction pretreatment promotes the pyrolysis of phenol, 2-methoxy. The highest yield of 2-methoxy-4-vinyl-phenol was at a 200 °C torrefaction pretreatment temperature and at a 450 °C pyrolysis temperature. However, the yield of 2-methoxy-4-vinyl-phenol did not vary with the change in the pyrolysis temperature remarkably at a 200 °C torrefaction pretreatment temperature, which showed that the torrefaction pretreatment had little effect on the yield of 2-methoxy-4-vinyl-phenol. The yield of 5-hydroxymethylfurfural was affected by both the torrefaction pretreatment temperature and the pyrolysis temperature. When the torrefaction pretreatment temperature was at 200 °C or 300 °C, the yield of 5-hydroxymethylfurfural declined, but when dealing with camphor wood powder at the 250 °C torrefaction pretreatment temperature, the content of 5-hydroxymethylfurfural in pyrolysis oil is higher than that without the torrefaction pretreatment. The yield of 1,2,4-trimethoxybenzene increased remarkably after torrefaction pretreatment and reached 7.67%, and the yield of 1,2,4-trimethoxy-benzene increased with the increase of the torrefaction pretreatment temperature. It is apparent that the torrefaction pretreatment before the pyrolysis of camphor wood powder had a directional, catalytic effect on the bio-oil composition of the pyrolysis of camphor wood powder.
CONCLUSIONS
Camphor wood powder was analyzed by GC-MS, and the high-value characteristics of the chemicals were considered. The chemicals 2,6-dimethoxyphenol, 1,2,4-trimethoxybenzene, 2-methoxyphenol, 3-furfural, 2-methoxy-4-vinyl-phenol, and 5-hydroxymethylfurfural were selected as the target products of higher added value.
The temperature had an influence on the pyrolysis products of camphor wood powder. The yield of 3-furfural improved when the pyrolysis temperature exceeded 550 °C, but the most other high-value chemicals declined at this temperature. To obtain more high-value chemicals (2,6-dimethoxyphenol, 1,2,4-trimethoxybenzene, 2-methoxyphenol, 3-furfural, 2-methoxy-4-vinyl-phenol, and 5-hydroxymethylfurfural), the pyrolysis temperature range of 450 °C to 500 °C is practical. The yield of target products was high, although there are many types of high-value chemicals.
The oil yield of camphor wood powder after the torrefaction pretreatment temperature did not improve and had almost no change. However, the high-value chemicals in the bio-oil increased, therefore improving the quality of the bio-oil.
Torrefaction pretreatment and pyrolysis temperature can affect the yield and composition of the pyrolysis oil of camphor wood powder at the same time. The yield of 3-furfural was inhibited after torrefaction treatment of camphor powder, the yield of phenol was increased. The chemicals 2-methoxy, 2-methoxy-4-vinyl-phenol, 5-hydroxymethylfurfural, and 1,2,4-trimethoxybenzene were in different degrees of temperature, and the increase in 2-methoxyphenol and 1, 2, 4-trimethoxybenzene was the most obvious. In conclusion, to obtain a high yield rate of high-value chemicals, choosing 300 °C for the torrefaction pretreatment temperature and 500 °C for the pyrolysis temperature increases the content of high-value chemicals.
ACKNOWLEDGEMENTS
The research was sponsored by China National Natural Science Fund (No. 51606113, 51406108, and 51536009), SDUT and Zibo City Integration Development Project (No. 2016ZBXC104), Key Research and Development Program of Shandong Province (No. 2017GGX40108), and Distinguished Expert of the Taishan Scholars of the Shandong Province.
REFERENCES CITED
Cai, Y., Fan, Y., Li, X., Yu, N., Chen, L., and Wang, J. (2016). “Preparation of refined bio-oil by catalytic transformation of vapors derived from vacuum pyrolysis of rape straw over modified HZSM-5,” Energy 1, 70-76. DOI: 10.1016/j.energy.2016.02.051
Chen, Y., Wang, X., Han, H., An, H., Wang, H., and Zhang, L. (2016). “Preparation of aromatic compounds from catalytic pyrolysis of lignin by complex metal oxide,” Industrial Catalysis 24(9), 69-74. DOI: 10.3969/j.issn.1008-1143.2016.09.015
Chen, Y., Wang, X., Han, H., Wang, H., An, H., Song, H., Gong, X., and Zhang, J. (2017). “Production of phenolic compounds from bagasse lignin via catalytic pyrolysis of CaZr1-xFexO3,” Chemical Journal of Chinese Universities 38(2), 252-260. DOI: 10.7503/cjcu20160515
Cui, X., Zhao, X., and Liu, D. (2017). “Pyrolysis characteristics and kinetics of five isolated lignin from sugarcane bagasse,” Chemical Industry and Engineering Progress 36(8), 2910-2915. DOI: 10.16085/j.issn.1000-6613.2017-0002
Hu, E., Zhao, L., Wu, J., Meng, H., Zhao, Z., Cong, Z., and Wu, Y. (2018). “Research advance on influence factors and technologies of biomass pyrolysis,” Transactions of the Chinese Society of Agricultural Engineering 34(14), 212-220. DOI: 10.11975/j.issn.1002-6819.2018.14.027
Lu, Y., Zheng, Z., Huang Y., and Li, W. (2018). “Research advances in preparation of furfural and its derivatives by selective catalytic conversion of hemicellulose,” Chemistry and Industry of Forest Products 38(3), 1-16. DOI: 10.3969/j.issn.0253-2417.2018.03.001
Shen, D., Hu, J., Xiao, R., and Zhang, H. (2013). “Isolation and characterization of lignin in black liquor,” Journal of Southeast University (Natural Science Edition) 43(1), 120-124. DOI: 10.3969/j.issn.1001-0505.2013.01.023
Sui, X., Wang, Z., Ruan R., and Zhang, Y. (2010). “Progress in research of levoglucosenone,” Modern Chemical Industry 30(11), 29-36. DOI: 10.16606/j.cnki.issn0253-4320.2010.11.009
Wang, F., Zheng, Y., Huang, Y., Yang, X., Liu, C., Xu, G., and Zheng, Z. (2016). “Component analysis of pyrolysis bio-oil from three major components of biomass and Pinus yunnanensis by ZSM-5 catalytic,” Transactions of the Chinese Society of Agricultural Engineering 32(2) 331-337. DOI: 10.11975/j.issn.1002-6819.2016.z2.047
Wang, J., Liu, X., and Zhu, J. (2017). “Research progress on the synthesis of bio-based aromatic platform chemical 2,5-furandicarboxylic acid,” Chemical Industry and Engineering Progress 36(2), 672-682. DOI: 10.16085/j.issn.1000-6613.2017.02.038
Xia, H., Huang, C., Xiao, Y., and Yang, L. (2013). “Catalytic pyrolysis of cellulose into levoglucosenone using FePO4 as catalyst,” Guangdong Chemical Industry 18(40), 15-16. DOI: 10.3969/j.issn.1007-1865.2013.18.007
Yu, X., Cen, K., Mei, J., Li, H., and Chen, D. (2017). “Effect of torrefaction pretreatment on the pyrolysis characteristics of cellulose,” Science Technology and Engineering 17(35), 240-244. DOI: 10.3969/j.issn.1671-1815.2017.35.039
Zhang, L., Liu, R., Yin, R., and Mei, Y. (2013). “Upgrading of bio-oil from biomass fast pyrolysis in China: A review,” Renew. Sust. Energ. Rev. 24(10), 66-72. DOI: 10.1016/j.rser.2013.03.027
Zhang, Z., Dong, C., Ye, X., Lu, Q., and Liu, Y. (2014). “Preparation of levoglucosenone by catalytic pyrolysis of cellulose over solid phosphoric acid,” CIESC Journal 65(3), 913-920. DOI: 10.3969/j.issn.0438-1157.2014.03.021
Zhang, C., Xu, T., Li, H., Fu, S., Li, B., Zhan, H., Zhang, F., and Li, C. (2016). “Hemicelluloses extraction from fast-growing poplar and their conversion into furfural,” Paper Science and Technology 35(6), 12-17. DOI: 10.19696/j.issn1671-4571.2016.06.003
Zhang, S., Su, Y., Xu, D., Zhu, S., Zhang, H., and Liu, X. (2018a). “Effects of torrefaction and organic-acid leaching pretreatment on the pyrolysis behavior of rice husk,” Energy 149, 804-813. DOI: 10.1016/j.energy.2018.02.110
Zhang, S., Zhang, H., Liu, X., Zhu, S., Hu, L., and Zhang, Q. (2018b). “Upgrading of bio-oil from catalytic pyrolysis of pretreated rice husk over Fe-modified ZSM-5 zeolite catalyst,” Fuel Processing Technology 175, 17-25. DOI: 10.1016/j.fuproc.2018.03.002
Article submitted: October 15, 2018; Peer review completed: December 1, 2018; Revised version received: December 7, 2018; Accepted: December 15, 2018; Published: December 19, 2018.
DOI: 10.15376/biores.14.1.1185-1197
