Abstract
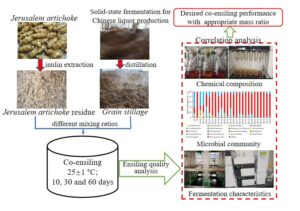
Jerusalem artichoke residue (JR) was co-ensiled with grain stillage (GS) at various weight mixing ratios (JR only, 4 to 1, 2 to 1, 1.2 to 1, 1 to 1.5, 1 to 2.7, 1 to 7, and GS only) for 10, 30, and 60 d for agricultural biomass storage. Results showed that the middle level of GS to JR ratios, e.g., 1.2 to 1 and 1 to 1.5, achieved the best co-ensiling performance among all studied ratios. The water-soluble carbohydrate (WSC) contents were significantly higher than those of the other treatments (p < 0.05), and the lignocellulose contents were significantly lower than those of other treatments (p < 0.05). The silages ensiled at the above-mentioned ratios had a higher feed value and biodegradation potential than other ratios. Lactobacillus was the dominant bacterial species during the ensiling process, and its relative abundance was significantly correlated with the content of different components, e.g., WSC, crude protein, and starch, as well as fermentation characteristics. Fungal species, e.g., Kluyveromyces and Monascus were also observed, and the relative abundance of which was positively correlated with different nutritional components. In conclusion, GS and JR can be successfully stored via co-ensiling.
Download PDF
Full Article
Investigation of Co-ensiling for the Storage of Grain Stillage and Jerusalem Artichoke Residue from Inulin Extraction
Haiwei Ren,a Li Wang,a Mei Li,a Yuchun Zhang,a Zhiye Wang,b Xiang Li,c Yi Zheng,c,* and Jinping Li a,*
Jerusalem artichoke residue (JR) was co-ensiled with grain stillage (GS) at various weight mixing ratios (JR only, 4 to 1, 2 to 1, 1.2 to 1, 1 to 1.5, 1 to 2.7, 1 to 7, and GS only) for 10, 30, and 60 d for agricultural biomass storage. Results showed that the middle level of GS to JR ratios, e.g., 1.2 to 1 and 1 to 1.5, achieved the best co-ensiling performance among all studied ratios. The water-soluble carbohydrate (WSC) contents were significantly higher than those of the other treatments (p < 0.05), and the lignocellulose contents were significantly lower than those of other treatments (p < 0.05). The silages ensiled at the above-mentioned ratios had a higher feed value and biodegradation potential than other ratios. Lactobacillus was the dominant bacterial species during the ensiling process, and its relative abundance was significantly correlated with the content of different components, e.g., WSC, crude protein, and starch, as well as fermentation characteristics. Fungal species, e.g., Kluyveromyces and Monascus were also observed, and the relative abundance of which was positively correlated with different nutritional components. In conclusion, GS and JR can be successfully stored via co-ensiling.
Keywords: Co-ensiling; Biomass storage; High-throughput sequencing; Fermentation; Microbial community structure
Contact information: a: School of Life Science and Engineering, Lanzhou University of Technology, Gansu Province Key Laboratory of Complementary Energy System of Biomass and Solar Energy, 287 Langongping Road, Lanzhou, Gansu Province 730050 P. R. China; b: Institute of Biology, Gansu Academy of Sciences, 229 South DingXi Road, Lanzhou, Gansu Province 73000 P. R. China; c: Department of Grain Science and Industry, Kansas State University, 101C BIVAP, 1980 Kimball Avenue, Manhattan, KS 66506 USA;
* Corresponding authors: yzheng@ksu.edu;lijinping77@163.com
GRAPHICAL ABSTRACT

INTRODUCTION
The food supply chain of the food industry generates a large amount of food processing waste each year (Teigiserova et al. 2019). Landfilling and incineration are the most common food waste treatment approaches, and they have caused serious environmental pollution while wasting a considerable quantity of resources. Given the rich energy content, carbon source, minerals, and nutrients of food processing waste, the valorization of such waste has attracted increasing research interest in terms of both resource recovery and utilization as well as its environmental benefits (Burgos et al. 2016). Conversion of such waste into bioenergy, animal feed, and other value-added bioproducts may offer potential technical routes for mitigating environmental pollution and creating extra profit for the food industry. Since food processing waste is characterized by broad diversity, seasonal variability, high biodegradability, and low stability, many studies have been conducted to transform food processing waste into silage for animal feed (Calabrò and Panzera 2018; Hooker et al. 2019). For example, soybean-supplemented apple pomace was used as a substitute for regular commercial feed (e.g., corn and wheat bran), which achieved good fermentation quality (Fang et al. 2020). Likewise, the co-ensiling of soybean meal, corn grain, and olive bagasse improved the fermentation characteristics compared to the silage with olive bagasse only (Kasper et al. 2019).
As a type of food and energy crop, Jerusalem artichoke has a wide number of applications for food (Díaz et al. 2019; Ko et al. 2019) and bioenergy production (Lv et al. 2019) due to its high yield, high nutritional value, minimal need of fertilizer, and resistance to adverse growth conditions, e.g., plant diseases, low temperature, drought, and saline soil (Long et al. 2016; Lv et al. 2019). During the processing of Jerusalem artichoke for inulin extraction (Díaz et al. 2019) and bioethanol production (Song et al. 2017), a large quantity of residues are generated. They quickly putrefy as their high moisture content (MC) and water-soluble carbohydrate (WSC) contents accelerate the spoilage and microbial proliferation, especially in the summer. This necessitates the immediate effective preservation of Jerusalem artichoke residue (JR) to prevent microbial decay. Ensiling may be an economical way to preserve JR for long-term storage and applications while avoiding the potential pollution issues caused by improper disposal of JR.
During the ensiling process, lactic acid bacteria (LAB) convert WSCs into organic acids, which results in a decrease in pH and the inhibition of undesirable microorganisms, e.g., Clostridium, Listeria, and Escherichia coli, thus stabilizing the organic matter for long-term preservation with minimum nutrient loss (McDonald et al. 1991). The microbial ecosystem and low pH of the ensiling system favor the long-term storage of food processing byproducts while preserving the nutrients of the silages (Gallagher et al. 2018; Rodríguez-Blanco et al. 2021). However, the ensiling process can be affected by various factors, e.g., substrate properties, storage conditions, and microbial diversity (Li et al. 2019; He et al. 2020; Ren et al. 2020). Of these factors, the substrate properties are critical in determining the success of ensiling, e.g., inappropriate feedstock compositions, such as insufficient carbohydrate contents and excessive MC, which could cause the ensiling process failure. For instance, high MC of > 70% could promote the activity of spoilage bacteria (e.g., Clostridium) which convert lactic acid (LA) to butyric acid and convert amino acids to ammonia, thus impairing the silage quality (He et al. 2020). Low MC of < 45% could suppress the overall fermentation process (Li et al. 2020). Similar to other highly perishable waste products, (e.g., sugarcane bagasse and sugar beet pulp) improper storage of JR may result in the decomposition of organic matter and energy loss (Ziemiński and Kowalska-Wentel 2015). To prepare feedstocks suitable for ensiling, co-ensiling has been developed by mixing different types of feedstocks at certain ratios to achieve favorable balances in the bacterial community, MC, and nutrient content for the ensiling process, thus producing well-preserved silages (Li et al. 2018b; Wang et al. 2019a). For example, the co-ensiling of maize straw with vegetable waste achieved desirable silage qualities (Ren et al. 2020); citrus pulp that contained highly biodegradable substrates was added to garlic stalk to facilitate the successful ensiling of garlic stalk because it was difficult to ensile garlic stalk alone (Lee et al. 2019). The MC of fresh JR is usually too high (greater than 80%) to produce quality silages when it is ensiled alone. Therefore, the co-ensiling of JR with low-MC materials could help achieve proper MC for the production of good-quality silages. Grain stillage (GS) is one of major byproducts of the wine industry, and it contains various nutrients, such as amino acids, protein, starch, fat, vitamins, and minerals (e.g., calcium, phosphorus, potassium, and others) such that GS has high potentials for value-added applications (Olstorpe et al. 2010). However, the GS is perishable and needs to be properly treated and/or utilized in time for resource recovery, while preventing the potential environmental pollution resulted from the misdisposition of the GS. In previous studies, the tubers and tops of Jerusalem artichoke have already been studied for ensiling (Kaya and Caliskan 2010; Koczoń et al. 2019). However, little information is available about the treatment of JR from the industrial processing standpoint.
In this study, the fresh JR was co-ensiled with the air-dried GS at different mass ratios, aiming to improving its long-term storage stability as well as preserving organic substances for downstream bioenergy production. The effects of the mass ratio on the silage quality at different ensiling times were evaluated in terms of nutrient components, lignocellulosic compositions, fermentation characteristics, and microbial community structure. This study will provide instructive information for further studies on the storage, conversion, and utilization of JR and other similar food processing byproducts.
EXPERIMENTAL
Materials
Air-dried GS with a MC of 15.8% (w/w), a solid waste from the production of China liquor, was collected from Jinhui Liquor Co., Ltd. (Longnan City, Gansu, China). Fresh, wet JR with a MC of 89.3% (w/w), generated during the inulin extraction process, was provided by Xirui Bioengineering Co., Ltd. (Baiyin City, Gansu, China). The chemical compositions of the GS and JR are presented in Table 1.
Table 1. Chemical Compositions of Raw Jerusalem Artichoke Residue and Grain Stillage (wt%, dry weight basis)
Co-ensiling of Jerusalem Artichoke Residue and Grain Stillage
To perform co-ensiling, the air-dried GS and wet JR were thoroughly mixed at six different mass ratios (GS to JR), including 4 to 1, 2 to 1, 1.2 to 1, 1 to 1.5, 1 to 2.7, and 1 to 7 to achieve different levels of MC, which were denoted as M3, M4, M5, M6, M7, and M8, respectively (as shown in Table 2). In addition, two single-substrate ensiling controls, i.e., GS or JR alone, were also performed together with the other co-ensiling ratios. Thus, there were 8 different substrates with different GS to JR mass ratios for the ensiling experiments. Approximately 1000 g of substrate was directly charged into plastic cylinder buckets (φ 1800 mm × 2600 mm) with a packing density of 376 kg·m-3, followed by the removal of air using a vacuum. A total of 72 plastic buckets (8 substrates × 3 storage times × 3 replicates) were tightly sealed and stored in a dark room at ambient temperature (25 °C ± 1 °C) for 10, 30, and 60 days, i.e., there were total 24 treatments [8 substrates (different GS to JR mass ratios) ×3 storage times]. At each sampling time point, the three silage buckets for each treatment were opened and sampled for subsequent analysis.
Table 2. Co-ensiling of the As-received Grain Stillage and Jerusalem Artichoke Residue
Analytical Methods
The dry matter (DM) percentage was measured by drying the samples at a temperature of 105 °C for 48 h in a forced-air oven (Hengke Instrument Co. Ltd, Beijing, China). For further analysis, the dried samples were ground to a particle size of 1 mm with a cutting mill (Jinding Machinery Manufacturing Co. Ltd, Jiangyin, China). The WSC content was determined using the anthrone-sulfuric acid method as previously described by Leyva et al. (2008). The neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) contents were determined according to the modified Van Soest method (Motte et al. 2014). The hemicellulose (HL) content was calculated as the difference between the NDF and ADF contents, while the cellulose (CL) content was determined as the difference between the ADF and ADL contents. The biodegradation potential (BDP) of the silage was estimated as the ratio of holocellulose (HoC) (HoC = HL + CL) to lignin content (Ren et al. 2020). The total nitrogen (TN) content was determined using a Kjeldahl nitrogen analyzer (K9840, Hanon instruments Co., Ltd, Jinan, China), and the crude protein (CP) content was calculated as the nitrogen content multiplied by 6.25. Starch (ST) analysis was performed by following the method of Hall and Mertens (2008). The relative feed value (RFV) was calculated according to Eq. 1,
(1)
as described by Filik (2020).
To evaluate the fermentation characteristics, the wet silage sample (30 g) was mixed with 270 mL of sterilized distilled water and was homogenized (JJ-2; Guohua Instruments Co. Ltd, Suzhou, China) at 200 rpm for 1 min. The slurry mixture was then filtered through four layers of cheesecloth and qualitative filter paper for the determination of the pH, the organic acid contents, i.e., lactic, formic, acetic, propionic, butyric, and valeric acids, and the ammonia nitrogen (AN) content. The pH was measured with a glass electrode pH meter (UB-10; Denver Instruments Co. Ltd, Beijing, China). The fermentation metabolites, i.e., ethanol and organic acids, were analyzed with a high performance liquid chromatography (HPLC) (Agilent 1200, Agilent Technology, Waldbronn, Germany) equipped with a refractive index detector, according to the method described by Li et al. (2020). Prior to the HPLC analysis, the samples were acidified with H2SO4 (50 wt.%) to decrease the pH to approximately 2 and filtered through 0.22 μm microporous membranes into HPLC vials. The analytical column was a Shodex RSpak KC-811 (300 mm × 8 mm, Showa Denko k.k., Tokyo, Japan). The mobile phase was 3 mM HClO4 at a flow rate of 1 mL·min-1. The separation temperature was 50 °C, and the sample injection volume was 5 μL. The AN content was determined using the phenol-hypochlorite colorimetric method, as described by Broderick and Kang (1980), and expressed as g/kg TN. All of the analyses were conducted in triplicate, and the results were presented on the DM basis unless otherwise specified.
Microbial Community Analysis
Metagenomic DNA extraction
High-throughput sequencing technology was adopted to monitor the dynamic changes in the microbial community during the ensiling process. For each treatment, representative samples were randomly taken from different layers of the silages and thoroughly mixed. Then, 20 g of these samples were mixed with 180 mL of a sterilized NaCl solution (0.85 wt%) and then centrifuged at 120 rpm for 2 h. The suspension was then filtered through four layers of cheesecloth to obtain the microbial cells used for DNA extraction. Total DNA was extracted using a Water DNA extraction kit (Omega Biotek, Norcross, GA) by following the instructions supplied by the manufacturer, and the DNA quantity and quality were assessed via agarose gel electrophoresis. High-molecular-weight DNA samples (a minimal concentration of 20 ng/μL) were used for sequencing.
PCR amplification
Amplification of the V3-V4 hypervariable region of the 16S rRNA was achieved using the following primer pairs: 515F (5′-ACTCCTACGGGAGGCAGCA-3′) and 907R (5′- GGACTACH VGG GTWTCTAAT-3′). The fungal ITS1 region amplicon sequencing used the BITS (5-NNNNNNNNCTACCT GCGGARGGATCA-3) and B58S3 (5-GAGATCCRTTGYTRAAAGTT-3) universal primers. The amplification of the targeted regions was achieved using the following reagents: 5 μL of Gotaq Green master mix (Promega, Madison, WI), 11.9 μL of DNase-free water, 0.5 μL of MgCl2 (50 mM), 0.5 μL of deoxynucleotide triphosphates (10 mM), 1 μL of DNA forward and reverse primers (10 μM), and 5 μL of DNA template adjusted for all samples to an average final concentration of 1 ng/μL of total reaction volume. The reaction conditions for the bacterial 16S amplification were as follows: an initial temperature of 95 °C for 3 min, followed by 35 cycles at a temperature of 95 °C for 45 s, a temperature of 50 °C for 60 s, and a temperature of 72 °C for 90 s, and a final extension at a temperature of 72 °C for 10 min. The reaction conditions for the fungal ITS-1 amplification were as follows: an initial temperature of 95 °C for 3 min, followed by 35 cycles at a temperature of 95 °C for 30 s, a temperature of 55 °C for 45 s, and a temperature of 72°C for 60 s, and a final extension at a temperature of 72 °C for 10 min. After examination via gel electrophoresis, the PCR products were denatured with NaOH to generate single-strand DNA fragments and sequenced using an Illumina HiSeq 2500 via the paired-end method.
High-throughput sequencing of the metagenomic DNA
To obtain high-quality clean reads, quality filtering on the raw reads was performed under specific filtering conditions. The chimera sequences of the bacteria were removed using the UCHIME algorithm by comparing the sequences with the Silva database, while the chimera sequences of the fungus were removed using the UCHIME algorithm by comparing the sequences with the Unite database. The effective sequences (reads) were counted, and the operational taxonomic units (OTUs) were clustered at a 97% similarity level to perform taxonomic analysis of different samples and obtain the microbial community composition at different levels. Alpha diversity analysis was conducted with QIIME to analyze the species richness (Chao1 and ACE index) and diversity (Shannon and Simpson index). Beta diversity on both the weighted and unweighted UniFrac were calculated using the QIIME software (version 1.7.0). Species with a relative abundance greater than 0.1% were selected for the analysis of the microbial community composition. The correlation thermograms between the fermentation quality and the microbial flora were obtained and analyzed.
Statistical Analysis
The ensiling experiment used a 3 × 8 full factorial design with 3 ensiling times (factor 1) and 8 substrates (with different GS to JR mass ratios) (factor 2). There were 24 treatments with three replicates for each treatment. The statistical model is shown in Eq. 2,
(2)
where Y represents the response variable, μ is the overall mean, αi is the effect of the ensiling time, βj is the effect of the substrate, (α × β)ij is the effect of the interaction between the ensiling time and the substrate, and εij is the residual error (Li et al. 2020). The chemical compositions (CL, HL, ST, and WSC), fermentation characteristics (pH, organic acids, and AN), and microbial community diversity after 10 d, 30 d, and 60 d were correlated with the ensiling time and substrate type using the general linear model (GLM) procedure of SPSS (V18.0, SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test at a significant level of α = 0.05 was used.
RESULTS AND DISCUSSION
Characterization of the Raw Grain Stillage and Jerusalem Artichoke Residue
The raw JR and GS were characterized according to chemical composition. As shown in Table 1, JR had a low DM content (10.7%), which was much lower than the desirable DM (30% to 35%) for silage (He et al. 2020). Such a high MC can cause the raw JR to rapidly deteriorate upon exposure to air and could also fail the ensiling process, especially at the high ambient temperatures during summer (He et al. 2020). The CP and ST contents of the JR reached 17.7% and 21.1%, respectively, while the JR contained abundant WSCs (13.3%, approximately twice as high as the WSC content in GS), which met the recommended WSC level (greater than 6%) for ensiling processes (Ali et al. 2020). In contrast, the raw GS had a much higher DM (84.2%) than JR, but its WSC content (7.75%) was less than the WSC content of the raw JR. The CP (16.2%) and ST (26.1%) contents of raw the GS were similar to those of raw JR. In addition, the raw JR had a slightly higher structural carbohydrate content (HoC) than the raw GS (45% versus 39%, respectively). The lignification index (expressed as the ratio of ADL to NDF or ADL to ADF) of GS was higher than the lignification index of the JR, which indicated the lower degradability of GS. Based on the chemical compositions of the JR and GS, co-ensiling of these two materials could be a suitable strategy to achieve good-quality silages due to the complementary benefits in the MC and WSC balance: i) the addition of GS reduces the MC of the JR to achieve an appropriate MC for ensiling and avoid the risk of effluent run-off; ii) mixing JR with GS would increase the MC and WSC of GS, thus stimulating the fermentation and hydrolysis of GS during ensiling; and iii) a balanced ratio of MC and WSC for co-ensiling would promote favorable fermentation and produce organic acids which would lower the pH to less than 4.5, resulting in well-preserved silages.
Dynamic Changes in the Nutrient Components During Ensiling
As important nutrient components, the DM, WSC, CP, and ST are important parameters for silage quality evaluation. Since the raw JR had a lower DM and ST and a higher WSC and CP than the raw GS, the initial DM and ST and WSC and CP contents of the substrates for co-ensiling were reduced and elevated, respectively, with an increasing proportion of JR in the ensiling substrates.
As shown in Table S1, during the ensiling process, the ensiling time, substrate, and the interaction between these two factors exhibited significant effects on the contents of the nutrient components, i.e., the DM, CP, WSC, and ST. The DM content is particularly important for silage fermentation, for LABs need water to grow and reproduce. Due to the relatively high DM content (84.17%) in GS (Table 1), the DM content decreased as the proportion of JR increased (p-value less than 0.05). After 60 d of silage, the DM of the M3, M4, and M7 groups increased significantly, while the DM of the M6 and M8 groups decreased significantly. The DM changes of the other groups were not significant. Water-soluble carbohydrates are a vital substrate for microbial metabolism during ensiling, with 60 to 80 g/kg DM being the recommended range to ensure good preservation of silages (Wang et al. 2019b; He et al. 2020). The WSC content for all substrates decreased considerably in the first 10 d of ensiling due to microbial degradation. Afterwards, it fluctuated slightly until the end of the ensiling process, which could be due to the decomposition of starch and/or structural carbohydrates, i.e., hemicellulose and cellulose, by microbes and/or acids produced additional soluble carbohydrates to compensate for the consumed WSC (Köhler et al. 2019).
The overall CP content of most substrates (except for JR alone) decreased significantly, especially during the first 10 d of ensiling. At different time points, the substrates with a higher proportion of JR primarily showed higher CP contents, suggesting that the incorporation of JR was beneficial to the preservation of proteins (Sikora et al. 2019).
Starch can hardly be utilized by LAB (except for amylolytic LAB), which primarily functions on monosaccharides, e.g., glucose and fructose (Sikora et al. 2019). The ST content variation in the final silage product was mostly related to the initial ST content of the feedstock, and the ST degrading microbes grown during the ensiling process. In the ensiling of GS or JR alone, no obvious pattern in the change in ST content was observed during ensiling. For co-ensiling, higher ST contents were obtained after storage with lower JR proportions, i.e., M3 and M4, during the first 10 d, while the ST content increased during the first 30 d, and then decreased after 60 d for the middle level of JR proportions, i.e., M5, M6, and M7). In addition, only a slight variation in ST was observed in the M8 ratio, which had the highest JR proportion. Within the same ensiling time, a greater reduction in the ST content was generally observed in the silages with a higher JR proportion.
Dynamic Changes in the Lignocellulosic Components during Ensiling
The lignocellulosic components considered in this study included ADF, NDF, ADL, CL, and HC. These components have important influences on the silage quality, especially its digestibility. The variation in the structural carbohydrates during storage are shown in Table S2. There was a slight increase in the overall compositions of NDF, ADF, ADL, CL, and HC. Stable ADL and decreased fiber compositions, i.e., NDF, ADF, CL, and HC, were reported by Köhler et al. (2019) and Dong et al. (2020), who co-ensiled grass with maize and Broussonetia papyrifera with perennial ryegrass, respectively. The ADF content in this study was found to gradually decrease in the M5, M6, and M7 samples. However, an increase in the amount of substrates was found in the high and low JR ratio samples, i.e., GS, M3, M4, M8, and JR, which indicated that adjusting the JR to GS ratio was helpful in improving the degradability of silage. The final NDF contents after 60 d of ensiling were greater than the corresponding initial values for most substrates except for M5. For the CL and HC contents, the CL content of the M6 samples was reduced significantly, and the HC content of the M5 group decreased significantly, while the CL and HC contents of the other groups increased by varying degrees after 60 d. These results indicated that the JR to GS ratios of the M5 and M6 samples were more suitable for the degradation of lignocellulosic components during the co-ensiling of JR and GS. Overall, after 30 and 60 d of ensiling, the ADF and NDF contents of all the groups increased to different degrees compared to the raw GS and JR, except for the M5 and M6 samples. With an increase in JR proportion and an elongation of ensiling time, the ADF and NDF contents of the M5 and M6 samples decreased and reached the lowest levels among all ensiling groups, which can be attributed the acid hydrolysis produced by microorganisms during the fermentation process of the ensilage (Chen et al. 2020).
As a primary barrier for the degradation of structural carbohydrates, i.e., CL and HC, a high ADL content can prevent microbials from attacking biodegradable fibers and reducing the digestibility of biomass (Li and Zheng 2018). Overall, the ADL content first decreased and then increased during the 60 d ensiling process. The co-ensiling (M4 through M8 samples) of GS and JR was beneficial to ADL decomposition and efficiently decreased the ADL content compared to its initial value. The RFV represents the digestibility (from a percentage of ADF) and intake potential (from a percentage of NDF) of a feed stuff. As shown in Table S2, the RFV scores of most treatments were assessed as fair (87 to 102) according to the quality grading standard outlined in Firsoni et al. (2019). In addition, the RFVs of all the tested groups were higher than the RFVs of quinoa stalks (Filik 2020) and rice straw (Firsoni et al. 2019). Additionally, when the mixing ratio was 1.2 to 1 (M5) and 1 to 1.5 (M6), the RFV of the silage was significantly greater than the RFVs of the other treatment groups, which may be due to the appropriate proportion of silage fermentation helping to reduce the content of the various lignocellulose components, thus increasing the RFV of the silage. The biodegradation potential is also an important indicator of biomass degradability before and after the silage process. In this study, the BDP of all treatment groups (except only JR) showed an increasing trend as the ensiling time increased, indicating that undergoing silage for a longer period of time was conducive to the promotion of the degradation performance of silages.
Dynamic Changes in the Fermentation Characteristics during Ensiling
The pH value is an important parameter for indicating the quality of a silage. A high-quality silage usually has a pH of 3.8 to 4.2, which can effectively inhibit the growth of undesired microorganisms, e.g., Clostridium butyricum, Listeria, and E. coli (McDonald et al. 1991). Silages with a pH greater than 4.7 easily decompose and decay. In this study, the pH of the silages of all eight groups of silages rapidly decreased to lower than 3.8 in the first 10 d due to the generation of organic acids, and then the pH continuously increased during the remaining time in storage (Table S3), which could be due to yeasts, molds, and/or other aerobic microorganisms consuming LA and other nutrients for their metabolism (Zeng et al. 2020). The pHs of the GS and co-silages of M3 through M7 after 60 d of ensiling were all less than 4.0, which indicated well-preserved silages, The result was similar to a previous report (Mu et al. 2020) in which rice straw was co-ensiled with high-moisture amaranth. However, the pH of the M8 and JR silages on day 60 rose to 4.32 and 4.55, respectively (approaching the threshold of putrefaction), which could be ascribed to the high MC derived from the high JR proportion. The high pH was consistent with the low LA contents in the M8 and JR silages, since LA is one of the major organic acids that can efficiently induce a pH drop. Meanwhile, the pH of the silages showed an upwards trend as the proportion of JR in the silages increased over the course of the ensiling period, which could be due to the fact that a high MC can lead to clostridial fermentation and dilute the LAB concentration, thus counteracting the decrease in pH (He et al. 2020).
The AN content reflects the degradation of protein and amino acids, i.e., a higher AN content usually suggests higher proteolytic activity and worse hygienic quality of a silage (He et al. 2020). In the current study, the AN content in all the silage groups throughout 60 d were lower than the threshold standard (7 g/100 g TN), which was an indicator of successful ensiling (as shown in Table S3). Such low AN contents were possibly related to the rapid reduction in pH to less than 4.0, which is known to greatly inhibit proteolysis. In most cases, the AN content showed an upward trend as the JR proportion increased, which was consistent with the pH variations. This result could be due to the fact that AN has a pH buffering capacity, thus mitigating the pH reduction (Desta et al. 2016). As such, the increased AN content of the silages in this study alleviated the pH reduction. These findings indicated that a high proportion of JR in the silages resulted in effective acidification via LAB, as well as other beneficial bacteria, which could metabolize and utilize carbohydrates to produce organic acids, e.g., LA and AA. This effect resulted in a reduction in the pH value and as such inhibited the decomposition and utilization of proteins via spoilage microorganisms. In addition, the relatively lower AN content in silages with a low proportion of JR suggested that the lower MC of silages could possibly suppress the protein degrading microorganisms by elevating the osmotic pressure.
Lactic acid bacteria are known to be a primary consumer of WSC during the ensiling process by converting WSC to LA, which as a result decreases the silage pH. Therefore, the rapid establishment of LA fermentation is critical to efficient ensiling. In addition, other small-molecule organic compounds can be produced from microbial metabolism during ensiling, e.g., acetic acid (AA), butyric acid (BA), and propionic acid (PA), and their contents can also affect the fermentative characteristics and quality of silages. A high LA content (greater than 3% DM) and a low BA content (less than 2% DM) are usually desired for high-quality silage (Hillion et al. 2018). In the present study, the LA content fell to the desired ranges and dominated the organic acids followed by AA. The AA content was found to increase as the proportion of the JR in the silages increased. Specifically, the LA contents of the M6 and M7 silages were greater than the LA contents of the other silages (Table S3). The LA to AA ratio decreased when the proportion of JR was increased in the silages; however, this ratio was still higher than 3 in most treatments (Table S3). This result indicated that LA fermentation was the dominant microbial metabolism during the ensiling process, which was beneficial in producing good-quality silage (Keshri et al. 2018; Xu et al. 2018). In addition, a small amount of formic acid (FA) was observed on day 10 and day 30 but disappeared by day 60, except in the silages with JR alone due to its unsuitable silage fermentation environment with a high MC. Due to its high MC and fermentability, agro-industrial organic waste is usually unsuitable for ensiling alone. No PA or BA was found in any silages in this study. This is consistent with the result reported by Mu et al. (2020), who revealed that propionic bacteria do not tolerate low pH. According to Hillion et al. (2018), good-quality silage should possess a pH less than 4.5, a LA content greater than 3% DM, a BA content greater than 2% of the total volatile fatty acids, and an AN content less than 10% of the TN. Against these criteria, all silages with different substrates in this study were considered well-preserved given their high LA content (3.6% to 9.8% DM), low AN content (less than 5.5% of the TN), and no detectable BA content. Therefore, GS can be a good organic material to be used in co-ensiling with JR to produce quality silages.
Dynamic Changes in the Bacterial Diversity during Ensiling
During ensiling, both the microbial community composition and diversity can have considerable influence on the silage quality, which was analyzed via high-throughput sequencing. For bacteria, both the Chao1 and ACE index, which reflect species richness, decreased in the first 30 d for most silages (except M5) and increased afterwards, except for GS only, in which the Chao1 and ACE index kept decreasing (as shown in Table S4). According to the microbial diversity indicated by the Shannon and Simpson index, the bacterial diversity of the silages with a relatively high proportion of JR, i.e., M5 through M8 and JR only, kept increasing during the entire ensiling process, while the bacterial diversity of the silages with a low JR content, i.e., M3 and M4, decreased in the first 30 d followed by an increase until the end of the ensiling process on day 60 (Table S4). For GS alone, the bacterial diversity decreased throughout the entire ensiling process. These results may suggest that the addition of JR provided more water and carbohydrates to the ensiling system, which benefited bacterial growth and fermentation during the ensiling process.
Figures 1a and 1b show the composition and relative abundance of the bacterial community at phylum and genus levels, respectively. At the phylum level (as shown in Fig. 1a), raw GS primarily contained Proteobacteria (62.41%), Firmicutes (19.24%), Bacteroidetes (9.30%), Cyanobacteria (3.86%), and Actinobacteria (3.07%). During the ensiling process, the relative abundance of Proteobacteria gradually decreased from 53.0% (on day 10) to 50.8% (on day 30) and to 9.4% (on day 60), while Firmicutes kept growing. The relative abundance of Bacteroidetes first increased to 11.7% on day 10 and to 18.6% on day 30, and then dramatically decreased to 1.36% on day 60. At the end of the ensiling process, Firmicutes and Cyanobacteria became the dominant species. A similar bacterial community and a shift of dominant species from Proteobacteria to Firmicutes were also observed in alfalfa ensiling processes (McGarvey et al. 2013; Yang et al. 2019), due to the proliferation of LAB, which belong to the Firmicutes phylum. Raw JR primarily contained Firmicutes (97.8%) with a small amount of Cyanobacteria (1.15%) (Fig. 1a). During the ensiling process, the relative abundance of Firmicutes gradually decreased to 89.4% on day 60, while the relative abundance of Proteobacteria and Cyanobacteria were elevated to 6.83% and 1.98%, respectively.
For the co-ensiling of GS and JR, the dominant species was observed to be Firmicutes, while its relative abundance in different silages varied in different trends (Fig. 1a). With the extension of the ensiling time, the relative abundance of Firmicutes further increased in the M3 and M8 samples, while it decreased in the M6 and M7 samples. In the M4 and M5 samples, the relative abundance of Firmicutes experienced a decrease during the early phase but increased during the later stage. Overall, a higher relative abundance of Firmicutes was observed in response to a higher proportion of JR. Proteobacteria, Cyanobacteria, and Bacteroidetes were also detected in co-ensiled silages with different relative abundances. These bacteria, which primarily originated from GS, initially had a higher relative abundance in the M3 and M4 samples compared to the other groups. After 30 d, the relative abundance of these bacteria in the M3 samples decreased along with the increased abundance of Firmicutes. The relative abundance of Cyanobacteria showed a significant increase in the M4 through M7 samples, which was followed by a decrease to lower than 10% on day 60. The relative abundance of Proteobacteria in the M5 through M7 samples and the relative abundance of Bacteroidetes in the M4 and M6 samples also increased in the later stage of the ensiling process. In the M8 samples, with the highest proportion of JR for co-ensiling, the relative abundance of these bacteria was always below 1%. Under anaerobic conditions, Firmicutes and Proteobacteria can effectively degrade fibrous materials in an ensiling system and provide substrates to support microbial growth, thus changing the lignocellulosic components during ensiling.
At the genus level (Fig. 1b), raw GS primarily contained LAB [Lactobacillus (11.3%), Enterococcus, Staphylococcus, and Streptococcus] and some non-LAB bacteria, e.g., Burkholderia (25.0%), Acinetobacter (15.4%), and Bacteroidales (4.1%). Raw JR contained LAB [Lactobacillus (94.5%)] and a small amount of non-LAB bacteria, e.g., Bacillus (3.2%). In the ensiling with GS only, Lactobacillus had a relatively low abundance at the beginning (5.5% on day 10 and 0.27% on day 30) but turned into the dominant species by day 60 (87.5%). In addition, other genera were also detected in the GS silages, e.g., Sphingomonas (8.1%) and Bacteroidales (4.9%) on day 10, and Bacteroides (15.7%), Faecalibacterium (8.0%), Citrobacter (6.0%), and Lactococcus (2.44%) on day 30. During the ensiling with JR only, Lactobacillus was the dominant bacteria at the genus level throughout all 60 d, with a relative abundance of 98.2%, 89.4%, and 87.5% on day 10, 30, and 60, respectively. In addition, more LABs were found after 60 d, including Bifidobacterium, Enterococcus, and Pediococcus. For non-LAB bacteria, the relative abundance of Acetobacter slightly increased from 0.18% on day 10 to 5.1% on day 30 and to 4.3% on day 60.
For co-ensiling, Lactobacillus was also the dominant species in all silages (Fig.1b), which was similar to the result reported by Ren et al. (2020). After 10 d of ensiling, the relative abundance of Lactobacillus increased gradually as the JR content increased in the silages (from M3 to M8). The relative abundance of Lactobacillus in the M3 and M4 samples was 46.8% and 56.4%, respectively, which was significantly lower than the relative abundance of Lactobacillus in the M5 through M8 samples (88.7% to 95.2%). From day 10 to 30, the relative abundance of Lactobacillus in the M3 samples increased to 86.9%, while it decreased in different extents in the M4 to M7 samples. On day 60, the relative abundance of Lactobacillus in the M3 and M8 samples still maintained a high level (greater than 90%), while it fell to 70% to 80% in the other silage groups. Similarly, Lactobacillus was also the dominant genera in all silages when amaranth was co-ensiled with rice straw (Mu et al. 2020). More diverse LABs were found in the M3 through M7 samples, including Lactobacillus, Pediococcus, Bifidobacterium, Enterococcus, Lactococcus, Staphylococcus, and Streptococcus, while only Lactobacillus was found as a LAB in the M8 samples. In addition, Bacillus and Acinetobacter were also found in the M3 through M8 samples. They can consume nutritional components, e.g., protein, and cause deterioration in the silages through oxidation-reduction reactions. However, the relative abundance of these undesired species were all less than 10%, which indicated that these undesirable microbes were suppressed by the LAB, leading to good conditions for long-term preservation (Luo et al. 2021).
Dynamic Changes in the Fungal Diversity during Ensiling
Controlling the MC is critical to the ensiling process. A low MC, e.g., 25% to 35%, can limit the release of leachate and inhibit undesirable microbial activities, e.g., Clostridia fermentation. However, fungi can still flourish under low MC conditions (Cheli et al. 2013). As a result, the dynamic changes in the fungal diversity were also analyzed during the ensiling process. After ensiling, both the fungal species richness and diversity became higher in most silages with different treatments, which was consistent with the results of the reads and OTUs. It is noteworthy that the M5 samples showed the most dramatic increase in the Chao1 and Shannon index, possibly because the GS to JR mass ratio was appropriate for facilitating fungal growth (Table S5).
At the phylum level (Fig. 1c), both the raw GS and raw JR primarily contained Ascomycota, with a relative abundance of 76.1% and 98.7%, respectively. A small amount of Basidiomycota (3.54%) and some other unclassified fungi were also found in the GS. The fungal community did not significantly change after the ensiling of GR and JR alone, with 72.2% Ascomycota and 6.2% Basidiomycota in GS and 96.3% Ascomycota in JR. During the co-ensiling process, Ascomycota was still the dominant species with a relative abundance greater than 90% for most silages. However, its relative abundance in the M5 samples particularly decreased to 68.9% on day 30 due to the presence of Basidiomycota and Aphelidiomycota, while the relative abundance of Ascomycota inversely increased to 89.4% on day 60.
At the genus level (as shown in Fig. 1d), raw GS primarily contained unclassified organism (74.9%), Kluyveromyces (8.2%), and Verticillium (2.9%), while JR primarily contained Kluyveromyces (96.7%) and a small number of unclassified organisms (1.8%). Yeast has been reported to have better survivability in organic acid-rich environments compared to other fungal species, as they are more resistant to a low pH and able to metabolize a wide range of substrates (McDonald et al. 1991). During the ensiling process, the yeast-dominated fungal community became more diverse as the pH increased through LA assimilation (Ávila et al. 2010). The dominant species in the GS silages changed from unclassified (81.1%) on day 10 to Lasiodiplodia (57.90%), and then back to unclassified (30.2%) on day 30. Afterwards, the relative abundance of the unclassified organism increased to 40.7% after 60 d of ensiling, while more Alternaria (8.8%), Verticillium (6.7%), and Kluyveromyces (6.1%) appeared. For the ensiling process with JR only, the dominant species was always Kluyveromyces with a relative abundance of 99.3%, 90.2%, and 85.5% on day 10, 30, and 60, respectively. The relative abundance of the unclassified organism increased with time, from 0.12% on day 10 to 3.96% on day 30, and to 6.89% on day 60.
During the co-ensiling process, the dominant fungal genus was also unclassified in the M3 and M4 samples on day 10 with a relative abundance of 93.0% and 99.5%, respectively. An increased proportion of JR turned the dominant species to Kluyveromyces in the M5 through M8 samples. From day 10 to 30, the relative abundance of the dominant fungi all decreased by various extents and even resulted in a change in dominant species. In the M3 samples, the relative abundance of the unclassified organism decreased to 3.2%, and Penicillium became the new dominant species, with a relative abundance of 87.5%, while Monascus was found for the first time, with a relative abundance of 7.8%. In the M5 and M6 samples, the relative abundance of Kluyveromyces decreased to 9.0% and 9.1%, respectively, while the unclassified organism became the new dominant species with a relative abundance of 72.3% and 90.6% on day 30 and 60, respectively. On day 60, the unclassified organism became the dominant fungi in all silages except for the M8 samples, which was consistent with findings from recent studies (Zhou et al. 2019). A relatively higher proportion of Monascus was also detected in the M3 through M5 samples, and some Kluyveromyces was detected in the M3 through M7 sample. The relative abundance of Kluyveromyces in the M8 samples further decreased to 57.5%, while the relative abundance of the unclassified organism increased to 23.9%. In addition to those dominant species, Penicillium (9.0%) and Aspergillus (4.8%) were also found in the M3 samples and Scedosporium (9.5%) was found in the M7 samples. In conclusion, the unclassified organism became the dominant fungi in the low JR silages, i.e., GS, M3, M4, M5, and M6, while the dominant fungi was Kluyveromyces in the high JR silages, i.e., M7, M8, and JR.
Fig. 1. Relative abundance of the microbial community: (a) bacteria at the phylum level; (b) bacteria at the genus level; (c) fungi at the phylum level; and (d) fungi at the genus level (the label, e.g., GS-10, of the x-axis is the substrate name, e.g., GS, followed by the ensiling time in days, e.g., -10)
Correlations between the Fermentation Quality and the Microbial Flora of the Silages
Correlations between the fermentation quality and the bacterial community
The correlations between the fermentation quality and the microbial flora of the silages are shown in Fig. 2. The color gradient bars on the right side of each figure show the correlation coefficients. Bacillus, Acetobacter, and Lactobacillus showed significant positive correlations with the contents of multiple nutritional components, e.g., DM, ADF, NDF, WSC, ST, CP, and ADL on day 10, while only Lactobacillus continued to have a significant positive correlation with the content of these nutritional components on day 30 and 60. Lactobacillus, Acetobacter, and Bacillus were responsible for the production of organic acids during the ensiling process (Fig. 2b) on day 10, which reduced the silage pH through the fermentation of WSC. As a result, the activities of undesired microorganisms can be inhibited, e.g., Enterobacteria and Clostridia, which can degrade proteins. Thus, the organic matter of the feedstock can be preserved (Sikora et al. 2019). With the extension of the ensiling time, Lactobacillus evolved into the dominant bacteria in the silages at later ensiling stages and produced a large amount of LA to reduce the pH of the silages and preserved nutritional components while inhibiting the growth and reproduction of other bacteria (Ali et al. 2020). On the contrary, the relative abundance of Streptococcus, Clostridium, and Pediococcus was negatively correlated with the DM content of the silages, as they consumed the organic matter of the silages during the ensiling process, which led to a reduction in the DM. In addition, negative correlations (p-value less than 0.05) were found between the WSC content and the relative abundance of the genus Bacteroides (r = -0.657), Alistipes (r = -0.730), and Lachnospiraceae (r = -0.748). This result indicated that these bacteria might be responsible for the depletion of the WSC.
For the fermentation characteristics, Lactobacillus and Acetobacter exhibited strong positive correlations with the LA and AA contents on day 10 (as shown in Fig. 2b). The LA and AA contents were positively correlated with Lactobacillus and bacillus on day 30 (as shown in Fig. 2d), while only Lactobacillus had a significant positive correlation with LA and AA on day 60 (as shown in Fig. 2f). These findings suggested that Lactobacillus was the major contributor for producing LA and AA during the late stage of the ensiling process. The FA content showed a high positive correlation with Lactobacillus during the entire silage process. On day 10 of the ensiling process, FA was detected in most silages and several bacterial species were found to negatively correlate with the FA content, e.g., Bacteroidales (r = -0.760), Acinetobacter (r = -0.751), Rheinheimera (r = -0.751), and Enterobacter (r = -0.633). The AN content and pH were positively correlated with Acetobacter (r = 0.884 and r = 0.891, respectively) and Lactobacillus (r = 0.862 and r = 0.894, respectively) on day 10 and were negatively correlated with Escherichia (r = -0.870 and r = -0.810, respectively). However, the AN content and pH were only positively correlated with Lactobacillus on day 30 and 60, which could be due to the fact that the low pH suppressed the growth of Lactobacillus, which started to proliferate again when the pH increased (Zeng et al. 2020).
Fig. 2. Correlations between the bacteria diversity and the fermentation quality: (a) the chemical composition at day 10; (b) the fermentation characteristics at day 10; (c) the chemical composition at day 30; (d) the fermentation characteristics at day 30; (e) the chemical composition at day 60; and (f) the fermentation characteristics at day 60 (*** denotes a p-value less than 0.001; ** denotes a p-value less than0.01; and *:denotes a p-value less than 0.05; The color gradient bars on the right side of each figure show the correlation coefficients, e.g., top red means highly positive correlation and bottom blue means highly negative correlation. ). Note: DM – dry matter; ADF – acid detergent fiber; NDF – neutral detergent fiber; WSC – water-soluble carbohydrates; ST – starch; CP – crude protein; ADL – Acid detergent lignin; FA – formic acid; AN – ammonia nitrogen; LA – lactic acid; AA – acetic acid
The homo-fermentation of LA was the absolutely dominant process, while some heterofermentative LAB were able to convert LA (pKa = 3.86) into AA (pKa = 4.76), which resulted in a slight increase in pH (Ávila et al. 2010). Therefore, the pH of the silages were primarily affected by the type of LAB fermentation. In addition, Alistipes was found to negatively correlate with all analyzed fermentation characteristics on day 60, although its relative abundance was low. The final pH of the silages could be affected by multiple species, including Alistipes, Bacteroides, Escherichia-Shigella, Pediococcus, etc. An increase in the relative abundance of these species can increase the pH of the silage.
Correlations between the fermentation quality and the fungal community
Strong correlations between the fungal species and fibrous components were found in the ensiling process (as shown in Fig. 3a). The color gradient bars on the right side of each figure show the correlation coefficients. On day 10, the contents of multiple components, i.e., ADF, ST, NDF, CP, ADL, and WSC, positively correlated with the relative abundance of Aspergillus and Kluyveromyces. As the ensiling process continued, Monascus, whose relative abundance increased from 0% to 32.66%, became the most important species in terms of affecting the DM, ADL, WSC, and ST contents. Negative correlations were found between the DM content and the relative abundance of Mortierella (r = -0.723), Sarocladium (r = -0.764), Phialosimplex (r = -0.629), Alternaria (r = -0.646), and Russula (r = -0.773), which indicated that these fungi were the major contributors of DM loss. Moreover, the WSC content was negatively correlated with Mortierella (r = −0.653) and Xerochrysum (r = −0.743). Alternaria was observed to negatively correlate with all analyzed components except for the DM content on day 60, although its relative abundance was low (less than 8.84%).
Highly positive correlations were found between the FA and the relative abundance of the genus Kluyveromyces (r = 0.824) on day 10, but the relative abundance of Monascus positively correlated with the organic acid contents, including FA, AA, and LA on day 30. In addition, on day 60, the FA content was negatively correlated with the relative abundance of Malassezia (r = -0.799), Aspergillus (r = -0.722), and Penicillium (r = -0.763) which were considered the most important colonizing fungi and producers of mycotoxins in silages (Cheli et al. 2013). Kluyveromyces was observed to positively correlate with the pH and AN content on day 10; in addition, the pH was positively correlated with the relative abundance of Monascus on day 30 and 60. In addition, Malassezia (r = -1.000), Chaetomium (r = -0.938), Aspergillus (r = -0.817), Penicillium (r = -0.830), and Alternaria (r = -1.000) were found to be negatively correlated with the pH because these fungi could degrade and consume fibrous materials and compete with LAB, and therefore impair the quality of the silages.
Fig. 3. Correlations between the fungi diversity and the fermentation quality. (a) the chemical composition at day 10; (b) the fermentation characteristics at day 10; (c) the chemical composition at day 30; (d) the fermentation characteristics at day 30; (e) the chemical composition at day 60; and (f) the fermentation characteristics at day 60 (*** denotes a p-value less than 0.001; ** denotes a p-value less than0.01; and *:denotes a p-value less than 0.05; The color gradient bars on the right side of each figure show the correlation coefficients, e.g., top red means highly positive correlation and bottom blue means highly negative correlation. ). Note: DM – dry matter; ADF – acid detergent fiber; NDF – neutral detergent fiber; WSC – water-soluble carbohydrates; ST – starch; CP – crude protein; ADL – Acid detergent lignin; FA – formic acid; AN – ammonia nitrogen; LA – lactic acid; AA – acetic acid
CONCLUSIONS
- The present study illustrated that the co-ensiling of grain stillage (GS) with Jerusalem artichoke residue (JR) could effectively preserve the water-soluble carbohydrates (WSC), crude protein (CP), and starch (ST), as well as other nutrients. The GS to JR mass ratios of 1.2 to 1 and 1 to 1.5 were the best among the studied mixing ratios in terms of the silage quality. Both mass ratios effectively preserved important nutrients, e.g., WSC, CP, and ST, and had a higher relative feed value (RFV) and biodegradation potential (BDP). According to the microbial community analysis, Lactobacillus dominated throughout the entire ensilage process and its relative abundance positively correlated with the content of multiple nutritional components, e.g., ADF, WSC, CP, and ST. The relative abundance of multiple fungal species, e.g., Kluyveromyces and Monascus, was also positively correlated with multiple nutritional components, e.g., DM, ST, and WSC.
- These results suggested that the co-ensiling of GS with JR could be a successful approach to preserve nutrients and optimize the fermentation qualities of silages. Co-ensiling provides a promising solution for the long-term storage of perishable organic waste in response to the need of timely treatment and sustainable utilization of such wastes. The authors will focus on the internal mechanism of the effects of moisture on the components and microbial changes, and adjust other components (e.g., WSC) to make co-ensiling feasible in future work.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 51666010 and 51366009), the China Postdoctoral Science Foundation Funded Project (Grant No. 2018M631217 and 2019T120961), the West Light Foundation of Chinese Academy of Sciences (Grant No. 2018XBZG XBQNXZ A), and the Red Willow First-class Discipline Project of Lanzhou University of Technology (Grant No. 0807J1).
REFERENCES CITED
Ali, N., Wang, S., Zhao, J., Dong, Z., Li, J., Nazar, M., and Shao, T. (2020). “Microbial diversity and fermentation profile of red clover silage inoculated with reconstituted indigenous and exogenous epiphytic microbiota,” Bioresource Technology 314, 1-10. DOI: 10.1016/j.biortech.2020.123606
Ávila, C. L. S., Martins, C. E. C. B., and Schwan, R. F. (2010). “Identification and characterization of yeasts in sugarcane silages,” Journal of Applied Microbiology 109(5), 1677-1686. DOI: 10.1111/j.1365-2672.2010.04796.x
Broderick, G. A., and Kang, J. H. (1980). “Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media,” Journal of Dairy Science 63(1), 64-75. DOI: 10.3168/jds.S0022-0302(80)82888-8
Burgos, N., Valdés, A., and Jiménez, A. (2016). “Valorization of agricultural wastes for the production of protein-based biopolymers,” Journal of Renewable Materials 4(3), 165-177. DOI: 10.7569/JRM.2016.634108
Calabrò, P. S., and Panzera, M. F. (2018). “Anaerobic digestion of ensiled orange peel waste: Preliminary batch results,” Thermal Science and Engineering Progress 6, 355-360. DOI: 10.1016/j.tsep.2017.12.011
Cheli, F., Campagnoli, A., and Dell’Orto, V. (2013). “Fungal populations and mycotoxins in silages: From occurrence to analysis,” Animal Feed Science and Technology 183(1-2), 1-16. DOI: 10.1016/j.anifeedsci.2013.01.013
Chen, L., Qu, H., Bai, S., Yan, L., You, M., Gou, W., Li, P., and Gao, F. (2020). “Effect of wet sea buckthorn pomace utilized as an additive on silage fermentation profile and bacterial community composition of alfalfa,” Bioresource Technology 314, 123773. DOI: 10.1016/j.biortech.2020.123773
Desta, S. T., Yuan, X., Li, J., and Shao, T. (2016). “Ensiling characteristics, structural and nonstructural carbohydrate composition and enzymatic digestibility of Napier grass ensiled with additives,” Bioresource Technology 221, 447-454. DOI: 10.1016/j.biortech.2016.09.068
Díaz, A., Bomben, R., Dini, C., Viña, S. Z., García, M. A., Ponzi, M., and Comelli, N. (2019). “Jerusalem artichoke tuber flour as a wheat flour substitute for biscuit elaboration,” Food Science and Technology 108, 361-369. DOI: 10.1016/j.lwt.2019.03.082
Dong, L., Zhang, H., Gao, Y., and Diao, Q. (2020). “Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass,” Bioresource Technology 310, 1-9. DOI: 10.1016/j.biortech.2020.123396
Fang, J., Xia, G., and Cao, Y. (2020). “Effects of replacing commercial material with apple pomace on the fermentation quality of total mixed ration silage and its digestibility, nitrogen balance and rumen fermentation in wethers,” Grassland Science 66(2), 124-131. DOI: 10.1111/grs.12258
Filik, G. (2020). “Biodegradability of quinoa stalks: The potential of quinoa stalks as a forage source or as biomass for energy production,” Fuel 266, 1-8. DOI: 10.1016/j.fuel.2020.117064
Firsoni, Hardani, S. N. W., and Wahyono, T. (2019). “Fiber content and relative feed value estimation of gamma irradiated rice straw,” IOP Conference Series: Materials Science and Engineering 546(4), 1-7. DOI: 10.1088/1757-899X/546/4/042008
Gallagher, D., Parker, D., Allen, D. J., and Tsesmetzis, N. (2018). “Dynamic bacterial and fungal microbiomes during sweet sorghum ensiling impact bioethanol production,” Bioresource Technology 264, 163-173. DOI: 10.1016/j.biortech.2018.05.053
Hall, M. B., and Mertens, D. R. (2008). “Technical note: Effect of sample processing procedures on measurement of starch in corn silage and corn grain,” Journal of Dairy Science 91(12), 4830-4833. DOI: 10.3168/jds.2008-1183
He, L., Wang, C., Xing, Y., Zhou, W., Pian, R., Chen, X., and Zhang, Q. (2020). “Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower,” Bioresource Technology 296, 1-8. DOI: 10.1016/j.biortech.2019.122336
Hillion, M.-L., Moscoviz, R., Trably, E., Leblanc, Y., Bernet, N., Torrijos, M., and Escudié, R. (2018). “Co-ensiling as a new technique for long-term storage of agro-industrial waste with low sugar content prior to anaerobic digestion,” Waste Management 71, 147-155. DOI: 10.1016/j.wasman.2017.10.024
Hooker, K., Forwood, D. L., Caro, E., Huo, Y., Holman, D. B., Chaves, A. V., and Meale, S. J. (2019). “Microbial characterization and fermentative characteristics of crop maize ensiled with unsalable vegetables,” Scientific Reports 9(1), 1-12. DOI: 10.1038/s41598-019-49608-w
Kasper, N. F., Tadielo, L. E., Altermann, O. D. C., Rosa, F. Q. d., Echeverria, A. D., Azevedo, E. B. d., Segabinazzi, L. R., and Castagnara, D. D. (2019). “Fermentation times and feed additives improve the quality of olive bagasse silage,” Semina: Ciências Agrárias 40(3), 1263-1274. DOI: 10.5433/1679-0359.2019v40n3p1263
Kaya, S., and Caliskan, M. E. (2010). “Effects of molasses and ground wheat additions on the quality of groundnut, sweet potato, and Jerusalem artichoke tops silages,” African Journal of Agricultural Research 5(9), 829-833. DOI: 10.5897/AJAR.9000262
Keshri, J., Chen, Y., Pinto, R., Kroupitski, Y., Weinberg, Z. G., and Sela, S. (2018). “Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant,” Applied Microbiology and Biotechnology 102(9), 4025-4037. DOI: 10.1007/s00253-018-8903-y
Ko, H., Bae, J.-H., Kim, M.-J., Sung, B. H., and Sohn, J.-H. (2019). “Microbial production of difructose anhydride III from Jerusalem artichoke tuber powder by recombinant yeast Saccharomyces cerevisiae and Kluyveromyces marxianus,” Industrial Crops and Products 135, 99-106. DOI: 10.1016/j.indcrop.2019.04.026
Koczoń, P., Niemiec, T., Bartyzel, B. J., Gruczyńska, E., Bzducha-Wróbel, A., and Koczoń, P. (2019). “Chemical changes that occur in Jerusalem artichoke silage,” Food Chemistry 295, 172-179. DOI: 10.1016/j.foodchem.2019.05.121
Köhler, B., Taube, F., Ostertag, J., Thurner, S., Kluß, C., and Spiekers, H. (2019). “Dry-matter losses and changes in nutrient concentrations in grass and maize silages stored in bunker silos,” Grass and Forage Science 74(2), 274-283. DOI: 10.1111/gfs.12430
Lee, Y. H., Ahmadi, F., Kim, Y. I., Oh, Y.-K., and Kwak, W. S. (2019). “Co-ensiling of garlic stalk with citrus pulp improves the fermentation quality and feed-nutritional value,” Asian-Australasian Journal of Animal Sciences 33(3), 436-445. DOI: 10.5713/ajas.19.0464
Leyva, A., Quintana, A., Sánchez, M., Rodríguez, E. N., Cremata, J., and Sánchez, J. C. (2008). “Rapid and sensitive anthrone–sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation,” Biologicals 36(2), 134-141. DOI: 10.1016/j.biologicals.2007.09.001
Li, D., Wang, Y., Zhang, Y., Lin, Y., and Yang, F. (2018a). “Evaluation of lactic acid bacteria isolated from alfalfa for silage fermentation,” Grassland Science 64(3), 190-198. DOI: 10.1111/grs.12198
Li, F., Ding, Z., Ke, W., Xu, D., Zhang, P., Bai, J., Mudassar, S., Muhammad, I., and Guo, X. (2019). “Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification,” Bioresource Technology 282, 211-221. DOI: 10.1016/j.biortech.2019.03.022
Li, F., Ke, W., Ding, Z., Bai, J., Zhang, Y., Xu, D., Li, Z., and Guo, X. (2020). “Pretreatment of Pennisetum sinese silages with ferulic acid esterase-producing lactic acid bacteria and cellulase at two dry matter contents: Fermentation characteristics, carbohydrates composition and enzymatic saccharification,” Bioresource Technology 295, 1-10. DOI: 10.1016/j.biortech.2019.122261
Li, X., and Zheng, Y. (2018). “Investigation of dynamic changes of substrate features on enzymatic hydrolysis of lignocellulosic biomass,” Industrial Crops and Products 111, 414-421. DOI: 10.1016/j.indcrop.2017.10.063
Li, X., Tian, J., Zhang, Q., Jiang, Y., Wu, Z., and Yu, Z. (2018b). “Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling,” Journal of Dairy Science 101(10), 8954-8964. DOI: 10.3168/jds.2018-14763
Long, X.-H., Shao, H.-B., Liu, L., Liu, L.-P., and Liu, Z.-P. (2016). “Jerusalem artichoke: A sustainable biomass feedstock for biorefinery,” Renewable and Sustainable Energy Reviews 54, 1382-1388. DOI: 10.1016/j.rser.2015.10.063
Luo, R., Zhang, Y., Wang, F., Liu, K., Huang, G., Zheng, N., and Wang, J. (2021). “Effects of sugar cane molasses addition on the fermentation quality, microbial community, and tastes of alfalfa silage,” Animals 11(2), 355. DOI: 10.3390/ani11020355
Lv, S., Wang, R., Xiao, Y., Li, F., Mu, Y., Lu, Y., Gao, W., Yang, B., Kou, Y., Zeng, J., et al. (2019). “Growth, yield formation, and inulin performance of a non-food energy crop, Jerusalem artichoke (Helianthus tuberosus L.), in a semi-arid area of China,” Industrial Crops and Products 134, 71-79. DOI: 10.1016/j.indcrop.2019.03.064
McDonald, P., Henderson, A. R., and Heron, S. J. E. (1991). The Biochemistry of Silage (Second Edition), Chalcombe Publications, Buckinghamshire, United Kingdom.
McGarvey, J. A., Franco, R. B., Palumbo, J. D., Hnasko, R., Stanker, L., and Mitloehner, F. M. (2013). “Bacterial population dynamics during the ensiling of Medicago sativa (alfalfa) and subsequent exposure to air,” Journal of Applied Microbiology 114(6), 1661-1670. DOI: 10.1111/jam.12179
Motte, J.-C., Escudié, R., Beaufils, N., Steyer, J.-P., Bernet, N., Delgenès, J.-P., and Dumas, C. (2014). “Morphological structures of wheat straw strongly impacts its anaerobic digestion,” Industrial Crops and Products 52, 695-701. DOI: 10.1016/j.indcrop.2013.11.038
Mu, L., Xie, Z., Hu, L., Chen, G., and Zhang, Z.(2020). “Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw,” Bioresource Technology 315, 1-9. DOI: 10.1016/j.biortech.2020.123772
Olstorpe, M., Axelsson, L., Schnurer, J., and Passoth, V. (2010). “Effect of starter culture inoculation on feed hygiene and microbial population development in fermented pig feed composed of a cereal grain mix with wet wheat distillers’ grain,” Journal of Applied Microbiology 108(1), 129-138. DOI:10.1111/j.1365-2672.2009.04399.x
Ren, H., Feng, Y., Liu, T., Li, J., Wang, Z., Fu, S., Zheng, Y., and Peng, Z. (2020). “Effects of different simulated seasonal temperatures on the fermentation characteristics and microbial community diversities of the maize straw and cabbage waste co-ensiling system,” Science of Total Environment 708, 1-12. DOI: 10.1016/j.scitotenv.2019.135113
Rodríguez-Blanco, M., Ramos, A. J., Sanchis, V., and Marín, S. (2021). “Mycotoxins occurrence and fungal populations in different types of silages for dairy cows in Spain,” Fungal Biology 125(2), 103-114. DOI: 10.1016/j.funbio.2019.08.006
Sikora, M. C., Hatfield, R. D., and Kalscheur, K. F. (2019). “Fermentation and chemical composition of high-moisture lucerne leaf and stem silages harvested at different stages of development using a leaf stripper,” Grass and Forage Science 74(2), 254-263. DOI: 10.1111/gfs.12423
Song, Y., Oh, C., and Bae, H. J. (2017). “Simultaneous production of bioethanol and value-added d-psicose from Jerusalem artichoke (Helianthus tuberosus L.) tubers,” Bioresource Technology 244, 1068-1072. DOI: 10.1016/j.biortech.2017.08.079
Teigiserova, D. A., Hamelin, L., and Thomsen, M. (2019). “Review of high-value food waste and food residues biorefineries with focus on unavoidable wastes from processing,” Resources, Conservation and Recycling 149, 413-426. DOI: 10.1016/j.resconrec.2019.05.003
Wang, C., He, L., Xing, Y., Zhou, W., Yang, F., Chen, X., and Zhang, Q. (2019a). “Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves,” Bioresource Technology 284, 240-247. DOI: 10.1016/j.biortech.2019.03.129
Wang, M., Wang, L., and Yu, Z.(2019b). “Fermentation dynamics and bacterial diversity of mixed lucerne and sweet corn stalk silage ensiled at six ratios,” Grass and Forage Science 74(2), 264-273. DOI: 10.1111/gfs.12431
Xu, Z., Zhang, S., Zhang, R., Li, S., and Kong, J. (2018). “The changes in dominant lactic acid bacteria and their metabolites during corn stover ensiling,” Journal of Applied Microbiology 125(3), 675-685. DOI: 10.1111/jam.13914
Yang, L., Yuan, X., Li, J., Dong, Z., and Shao, T. (2019). “Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant,” Bioresource Technology 275, 280-287. DOI: 10.1016/j.biortech.2018.12.067
Zeng, T., Li, X., Guan, H., Yang, W., Liu, W., Liu, J., Du, Z., Li, X., Xiao, Q., Wang, X., et al. (2020). “Dynamic microbial diversity and fermentation quality of the mixed silage of corn and soybean grown in strip intercropping system,” Bioresource Technology 313, 1-8. DOI: 10.1016/j.biortech.2020.123655
Zhou, Y.-M., Chen, Y.-P., Guo, J.-S., Shen, Y., and Yang, J.-X. (2019). “The correlations and spatial characteristics of microbiome and silage quality by reusing of citrus waste in a family-scale bunker silo,” Journal of Cleaner Production 226, 407-418. DOI: 10.1016/j.jclepro.2019.04.075
Ziemiński, K., and Kowalska-Wentel, M. (2015). “Effect of enzymatic pretreatment on anaerobic co-digestion of sugar beet pulp silage and vinasse,” Bioresource Technology 180, 274-280. DOI: 10.1016/j.biortech.2014.12.035
Article submitted: June 19, 2021; Peer review completed: August 28, 2021; Revised version received and accepted: September 12, 2021; Published: September 14, 2021.
DOI: 10.15376/biores.16.4.7300-7336
SUPPLEMENTAL
Table S1. Dynamic Changes of Nutrient Components during Ensiling Fermentation (% DM1)
Table S2. Dynamic Changes of Lignocellulosic Components during Ensiling (% DM1)
Table S3. Dynamic Changes of pH, Organic Acids (% DM1), and Ammonia-N Contents during Ensiling
Table S4. Alpha Diversity of Bacteria during Ensiling
Table S5. Alpha Diversity of Fungi during Ensiling
