Abstract
Wood is a biomass material that is easily eroded by wood-rotting fungi. Coptis chinensis is a natural green plant, which has an inhibitory effect on most microorganisms. Based on the highly toxic effects of the currently used wood chemical preservatives on humans, animals, and the environment, Coptis chinensis was selected to perform decay resistance experiments of wood in this paper. The active ingredients with bacteriostatic properties in Coptis chinensis were separated and screened via chemical treatment, and their structure was identified via nuclear magnetic resonance spectroscopy. The primary bacteriostatic components in Coptis chinensis were berberine hydrochloride, palmatine, and jatrorrhizine. The bacteriostatic zone experiment with a single component and different compounds for white-rot and brown-rot fungus were tested by the disc agar diffusion method. The bacteriostatic effect of berberine hydrochloride in a single active fraction was better. The three-fraction compound had the best bacteriostatic effect and was equivalent to alkaline copper quaternary. The natural active bacteriostatic fractions in Coptis chinensis had noticeable inhibitory effects on white-rot fungus (Trametes versicolor (L.) Lloyd) and brown-rot fungus (Gloeophyllum trabeum (Pers.) Murrill). The minimum bacteriostatic concentration was 0.01 g/mL. The results showed that Coptis extracts had potential as a wood protectant.
Download PDF
Full Article
Isolation and Identification of the Antibacterial Compounds in Coptis chinensis for the Preservation of Wood
Lei Wang, Binhui Li, Xiaoqi Zhao, Shiming Ren, and Yamei Wang *
Wood is a biomass material that is easily eroded by wood-rotting fungi. Coptis chinensis is a natural green plant, which has an inhibitory effect on most microorganisms. Based on the highly toxic effects of the currently used wood chemical preservatives on humans, animals, and the environment, Coptis chinensis was selected to perform decay resistance experiments of wood in this paper. The active ingredients with bacteriostatic properties in Coptis chinensis were separated and screened via chemical treatment, and their structure was identified via nuclear magnetic resonance spectroscopy. The primary bacteriostatic components in Coptis chinensis were berberine hydrochloride, palmatine, and jatrorrhizine. The bacteriostatic zone experiment with a single component and different compounds for white-rot and brown-rot fungus were tested by the disc agar diffusion method. The bacteriostatic effect of berberine hydrochloride in a single active fraction was better. The three-fraction compound had the best bacteriostatic effect and was equivalent to alkaline copper quaternary. The natural active bacteriostatic fractions in Coptis chinensis had noticeable inhibitory effects on white-rot fungus (Trametes versicolor (L.) Lloyd) and brown-rot fungus (Gloeophyllum trabeum (Pers.) Murrill). The minimum bacteriostatic concentration was 0.01 g/mL. The results showed that Coptis extracts had potential as a wood protectant.
Keywords: Coptis chinensis; Purification; Structural identification; Decay resistance; Nuclear magnetic resonance
Contact informational: College of Materials Science and Art Design, Inner Mongolia Key Laboratory of Sandy Shrubs Fibrosis and Energy Development and Utilization, Inner Mongolia Agricultural University, Hohhot 010018 China; *Corresponding author: wangym80@126.com
INTRODUCTION
Wood is a natural polymer material composed of cells. It is the only renewable material among the four most commonly used building materials in the world, and it has been used in construction since ancient times (Li et al. 2016). Because of its unique aesthetic and environmental characteristics, as well as its biodegradability (Brischke 2020), wood is considered an application material with broad development prospects (Song et al. 2017). However, it is easily degraded by microorganisms found in daily life, which affects its usage, value, and shortens its service life (Oberhofnerova et al. 2019; Sgarbossa et al. 2020). To reduce the waste of wood as much as possible and maximize the utilization rate of wood, research on wood preservation becomes a valuable tool (Johnston et al. 2016).
The purpose of wood preservation is to counteract the susceptibility of wood to decay and biological degradation, so as to protect the wood from wood rot fungi and prolong the service life of the wood (Guo et al. 2018). After many years of development, there are three current treatment methods for wood preservation: physical modification, chemical treatment, and microbial treatment (Zalamea et al. 2016). The physical modification method is primarily through the thermal modification of the wood, i.e., the wood is placed in a high-temperature (160 to 240 °C) environment for an extended period of time (Gaff et al. 2019), and the water and nutrients in the wood are reduced so that the fungus that cause decay cannot survive in the wood (Sikora et al. 2018). Thermal modification of wood improves dimensional stability and decay resistance (Mubarok et al. 2019), but this method reduces the strength of wood and makes it difficult to apply to industrial building materials (Wang et al. 2018; Kojima et al. 2020). Wood microbial treatment refers to the use of dynamic interactions between microbial species to protect the wood by antagonistic microorganisms (Hiscox et al. 2018; Lasota et al. 2019). These antagonistic microorganisms achieve the purpose of inhibiting the growth of wood-rot fungi by reducing the chance of harmful microorganisms inoculating wood and the ability to invade wood (Presley et al. 2020). Microbial treatment provides wood products with the ability to resist microbial damage, but research on microbial treatment is not comprehensive and cannot be applied to production (Joseph and Junier 2020). Currently, the most common wood treatment method is chemical treatment (Cai et al. 2020). Traditional wood chemical preservatives include copper chromium boron (CCB), copper chromium arsenic (CCA) (Oh and Kim 2020), ammonia soluble copper arsenate (ACA), fluoride chromium arsenic phenol (FCAP), and acid copper chromate (ACC) (Yanitch et al. 2020), etc. These preservatives are effective for the prevention of microorganism growth, but the harm to the environment is undoubted (Barbero-Lopez et al. 2021). With the change and development of the application range of wood in the world, new low-toxic and high-efficiency preservatives (Cai et al. 2019), including organic ammonium, inorganic boron, nano wood, organic fungicides, and tree extracts, are being researched (Usmani et al. 2018). Inspired by the field of wood bionics, the effective fractions (alkaloids, phenols, aldehydes, etc.) in plants that have bacteriostatic effects have been extracted and further developed into preservatives (Hassan et al. 2019).
Coptis chinensis Franch. is a member of the Ranunculaceae family. It is grown in Japan and China and has pharmacological effects on hypertension, gastric ulcer, diarrhea, influenza (Le et al. 2020), etc. After years of exploration and research, it was found that C. chinensis has noticeable bacteriostatic effects on various organisms, e.g., bacteria, fungi, molds, insects, and other microorganisms (Tseng et al. 2020). The main biological process involved in wood decay is fungal decay (Kim et al. 2016). White-rot fungus and brown-rot fungus are the most common species that cause fungal erosion (Kumar et al. 2020). The idea of using C. chinensis as a preservative was put forward to solve the environmental problems of existing wood preservatives. Compared with traditional preservatives, this type of preservative relies on natural materials (Farias et al. 2020) and has a remarkable environmental protection effect (Ahmed et al. 2020). But it also has the characteristics of easy degradation and a short effective period of the single active ingredient (Treu et al. 2020). At present, many functional studies on plant extracts have been reported, but few studies have reported the efficacy of the active ingredients and compounds related to active ingredients (Mkindi et al. 2020). Due to the complexity of plant raw materials, most researchers only have studied the crude extracts of plants (Cornara et al. 2020). Therefore, it is necessary to establish a fast and efficient method of separation, purification, screening, and identification of active substances as soon as possible to realize the full and effective use of plant extracts. At the same time, the effective components and contents obtained by different extraction methods were also different (Ciganovic et al. 2019). The isolation and identification of the antifungal active ingredients of C. chinensis can be helpful to characterize the extraction process and have a further understanding of the active ingredients.
In this paper, the extraction method with higher active ingredient content was selected by comparing different extraction methods. The alcohol extraction combined with microwave and ultrasonic extraction technology was used to extract the components of C. chinensis. These extractions were combined with an alumina column to separate and purify the primary extracts, and the natural active ingredients with a bacteriostatic effect were selected. The structure of these bacteriostatic components were identified via nuclear magnetic resonance (NMR) spectroscopy. Besides, the decay resistance of wood was tested. A single complex study on the bacteriostatic components of C. chinensis will more clearly define the bacteriostatic mechanism. As such, it was important to explore the bacteriostatic active components and structures of C. chinensis, which provided theoretical support for the development of C. chinensis wood preservatives (Yang et al. 2018).
EXPERIMENTAL
Materials
Coptis chinensis was purchased from the Northern Pharmacy (Hohhot, Inner Mongolia, China). Beijing Poplar (Populus x beijingensis W. Y. Hsu) and Pinus sylvestris var. mongolica Litv. specimens were obtained from Hohhot (Inner Mongolia, China). Samples with dimensions of 20 mm x 20 mm x 10 mm (Radial x Tangential x Longitudinal) were prepared for decay resistance tests in accordance with Chinese Forestry Standard LY/T1283-2011. The test bacteria, white-rot fungus (Trametes versicolor (L.) Lloyd) and brown-rot fungus (Gloeophyllum trabeum (Pers.) Murrill), were purchased from the Institute of Forest Ecology, Environment and Protection, Chinese Academy of Forestry (Beijing Shi, China), then activated via laboratory cultivation.
The berberine hydrochloride standard was kindly provided by the Guizhou Dida Biotechnology Co., Ltd. (Guizhou Province, China). The tetrahydrojatrorrhizine and tetrahydropalmatine standards were purchased from the Shanghai Yaji Biotechnology Co., Ltd. (Shanghai, China). Ethanol, acetonitrile, acetone, and all other chemical agents were all HPLC grade.
Culturing Medium
In the experiment, potato medium was selected in accordance to Chinese Forestry Standard LY/T1283-2011. 300 g of potatoes were washed and cut into small pieces. Then, 1500 mL of deionized water was added and boiled for 30 min. The mixed solution was poured into a bottle; then the mixture was decanted and filtered through cheesecloth in order to make the potato infusion. Deionized water was added to bring the total volume of the suspension to 1500 mL, and then 27 g of agar and 22 g of glucose were added to the solution. The potato culture medium was evaporated at 121 °C and 0.1 MPa for 30 min. It was poured into a glass petri dish in a sterile inoculation room after cooling. Inoculation needles were used to inoculate white-rot fungus and brown-rot fungus after the potato medium had solidified. The inoculated agar plates were cultured at a constant temperature (28 °C) and humidity (85% relative humidity (RH)) in an incubator for 9 d.
Analytical Methods
Instrumental analysis
The structure of the bacteriostatic components was identified via Fourier transform nuclear magnetic resonance (FT-NMR) spectrometry using an AC-80 spectrometer (Bruker, Billerica, MA). The measurement temperature was 25 °C, the ambient humidity was 35%, and the operating frequency was 500.13 MHz. The content of the active ingredients of Coptis chinensis were detected via high-performance liquid chromatography (HPLC) (LC-20AT, Shimadzu, Kyoto, Japan).
Berberine hydrochloride chromatographic conditions: the column used was C18 (150 mm × 4.6 mm and 5 μm), the mobile phase was acetonitrile (0.033 mol/L), potassium dihydrogen phosphate (pH = 3.0) (30:70), the total velocity was 0.8 mm/min, the detection wavelength was 263 nm, the column temperature was 25 °C, and the injection volume was 20.0 µL. The standard curve drawing was quantified by the external standard method. The detection limit concentration of berberine hydrochloride was 0.34 mg/mL.
Palmatine and jatrorrhizine chromatographic conditions: the total velocity was 1.2 mL/min, and the detection wavelength was 349 nm, while the other test conditions were the same as berberine hydrochloride. The detection limit concentration of jatrorrhizine and palmatine was 0.3 ug/mL (Gilani et al. 2019).
Extraction of berberine
Coptis chinensis rhizome contained a variety of isoquinoline alkaloids, e.g., berberine, methyl-berberine, palmatine, jatrorrhizine, etc., but the berberine content was highest, approximately 5% to 8% (Ren et al. 2007). Compared with other alkaloids, berberine had a better bacteriostatic effect on wood-rot fungus (Singh and Sharma 2018) and was more easily extracted from the Coptis rhizome (Liu et al. 2011). Therefore, berberine was selected as the material to determine the best alkaloid extraction method.
At present, the extraction methods of plant extracts include solvent extraction, ultrasonic extraction, and microwave extraction (Ji et al. 2020). The solvent extraction method adopts the principle of similarity and compatibility to extract the effective components. The effective components have good selectivity but low extraction efficiency (Busto and Vera 2019). The ultrasonic extraction method uses the strong vibration produced by ultrasonic waves to release the substances in plant cells. The extraction efficiency is high, but the purity of effective components is poor (Arafat et al. 2020). The microwave extraction method is to use the microwave to heat the target component for selective extraction, but the microwave parameters are not easy to control, and heating may change the properties of the extract (Yuan et al. 2020). In this study, the method with a higher extraction rate and suitable for industrial production was selected for the extraction of berberine through the comparison of different extraction methods.
The same batch of C. chinensis was crushed and dried through an 80-mesh sieve and set aside. According to the properties of berberine (Ye et al. 2009), the following six methods were used to extract berberine.
(1) Ethanol extraction method: 20.0000 g of dry Coptis powder was accurately weighed and placed in a beaker. After adding 160 mL of 95% ethanol, the solution was evenly stirred. Plastic wrap was placed on the mouth of the beaker to prevent the evaporation of ethanol. C. chinensis powder was soaked for 12 h and refluxed for 4 h. Most of the ethanol was recovered via distillation under reduced pressure to obtain a mobile extractive. Finally, it was dried to a constant weight at 70 °C in a blast drying furnace to obtain sample A.
(2) Microwave aided ethanol extraction method: 20.0000 g of dried Coptis powder was accurately weighed and added to 160 mL of 95% ethanol and evenly stirred. It was covered with plastic wrap and soaked for 12 h. The solution was intermittently treated by microwaving (at 320 w) for 5 min and refluxed for 4 h. Most of the ethanol under reduced pressure was recovered to obtain a stream extractive. Finally, it was dried to a constant weight in a 70 °C blast drying oven to obtain sample B.
(3) Ultrasonic aided ethanol extraction method: 20.0000 g of dried C. chinensis powder was weighed accurately and put into a beaker. Then, 160 mL of 95% ethanol was added and evenly stirred. C. chinensis powder was soaked for 12 h. The solution was treated with a 600 W ultrasonic wave and 20 kHz pulse for 30 min and refluxed for 4 h. Most of the ethanol was distilled under reduced pressure to obtain a stream extractive. Finally, it was dried in a blast drying oven at 70 °C to a constant weight to obtain sample C.
(4) Water extraction and decoction method: 20.0000 g of dried Coptis powder was accurately weighed and added to 160 mL of deionized water, stirred, and then soaked for 12 h. The solution was boiled for 0.5 h to concentrate into a thick paste. Finally, it was dried to a constant weight in a blast drying oven at 90 °C to obtain sample D.
(5) Microwave aided water extraction method: 20.0000 g of dried Coptis powder was accurately weighed and added to 160 mL of deionized water in a beaker and soaked for 12 h. The solution was intermittently treated by microwaving (at 320 w) for 5 min and then boiled for 0.5 h to obtain a thick paste. Finally, it was dried to a constant weight in a blast drying oven at 90 °C to obtain sample E.
(6) Ultrasonic aided water extraction method: 20.0000 g of dried Coptis powder was accurately weighed and added to 160 mL of 95% ethanol and soaked for 12 h. It was treated with a 600 W, 20 kHz pulse ultrasound for 30 min and boiled for 0.5 h to obtain a concentrated thick paste. The final solution was dried to a constant weight in a blast drying oven at 90 °C to obtain sample F.
Sample A to sample F were accurately weighed. After methanol was added to the volumetric flask up to 50.00 mL, the solution was diluted 10 times. The sample was filtered through a 0.45 μm microporous membrane, and the filtrate was used as the test product. The berberine content was calculated using Eq. 1,
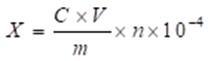
where X (g/100g) is the berberine content, C (ug/mL) is the sample concentration, V (mL) is the constant volume, m (g) is the sample weight, and n is the diluted multiples.
Screening of the bacteriostatic components
According to the properties of the components in C. chinensis and the best extraction process of berberine hydrochloride, an 8 to 1 ratio of 95% ethanol to C. chinensis powder was used as the extraction solvent in this study. The powder was sifted through a size 80 mesh screen, then soaked for 12 h. The solution was treated with an ultrasonic pulse of 600 W and 20 kHz for 30 min and refluxed for 4 h in order to separate and extract.
Rhizoma C. chinensis root powder was soaked in ethanol for 24 h and ultrasonicated for 30 min to obtain an ethanol concentrate. Then, concentrated hydrochloric acid was added for filtration and the yellow precipitate was recrystallized with water several times to obtain substance A. The filtrate was treated with NH4OH and ether, then filtered through H2SO4 to obtain a yellow precipitate, and crystalline substance B was obtained after ethanol was added. The filtrate was added to a 10% concentration of KI to obtain precipitation C. Then, the solution was stratified with the addition of a 5% concentration of KOH. The filtrate was added to acetic acid (HAc) and a 10% concentration of KI to obtain precipitation G. The precipitate was dissolved in H2SO4 and Zn to obtain a reducing solution, and then it was treated with NH4OH and chloroform, to which a 2% concentration of KOH was added. The alkaline aqueous layer was sequentially treated with HCl/NH4OH/chloroform to obtain a colorless crystalline substance D. The chloroform layer was concentrated and then treated with acetone, and the precipitate was eluted with chloroform to obtain colorless needle crystal substance E. This filtrate was eluted with acetone, and the obtained eluate was eluted with benzene to obtain the pale yellow flaky crystal substance F.
The overall flow chart for screening the bacteriostatic components of C. chinensis was visualized in Fig. 1.
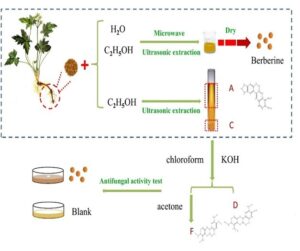
Fig. 1. The overall flow chart of screening the bacteriostatic components of Coptis chinensis via the berberine extraction method
Selection of the minimum inhibitory concentration
The minimum inhibitory concentration (MIC) is an important index for the screening of bacteriostatic components. It refers to the minimum concentration of a drug that obviously inhibits the growth of a certain microorganism in a specific environment. It is used to quantitatively determine the bacteriostatic activity of drugs (Mahmoud et al. 2019). In this study, the drug sensitivity was used to determine the MIC. Commonly used drug sensitivity test methods are the paper agar diffusion method for qualitative determination (K-B method) and the dilution method for quantitative determination. In this experiment, the paper agar diffusion method was used (Sirgel et al. 2009). A 4% concentration of a crude drug comprised of C. chinensis in a toxic medium was used as the reference for the selection of the natural active fractions. Berberine hydrochloride, palmatine, and jatrorrhizine extracted from C. chinensis were accurately weighed. They were heated and dissolved in deionized water to prepare sample solutions of 0.04, 0.02, 0.01, 0.005, and 0.001 g/mL. After mixing the three substances in a mass ratio of 1 to 1, they were heated and dissolved in deionized water to make sample solutions of 0.04, 0.02, 0.01, 0.005, and 0.001 g/mL. Three-layer filter paper with a diameter of 6 mm was put into the four sample solutions to soak and absorb the solution. White-rot fungus (Trametes versicolor (L.) Lloyd) and brown-rot fungus (Gloeophyllum trabeum (Pers.) Murrill) were used as the test bacteria to determine the minimum inhibitory concentration. At the same time every day, the bacteriostatic zone of each plate was measured via the cross method using a sterilized vernier caliper, and the data was recorded.
Decay Test
Wood decay test methods and decay resistance grades were performed according to Chinese Forestry Standard LY/T1283-2011. The samples were dried in a drying oven until the weight remained unchanged, and weight was recorded (W1). Then samples were put into extractive of Coptis coptis and immersed in a vacuum drying oven (0.01 MPa) for 30 min. The extractive of sample’s surface was absorbed with filter paper and weight (W2) was recorded. After soaking, the sample were dried in atmospheric conditions and weight (W3) was recorded. Then samples were cultured in a constant temperature and humidity chamber. Decayed sample was taken out and the mycelium of sample surface was scraped off. Then decayed samples were dried, its weight (W4) was recorded.
The extractive-loading level was calculated using Eq. 2,
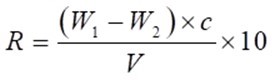
where R (kg/m3) is extractive-loading rate, C (%) is the extractive concentration, and V (cm3) is the sample volume.
The weight loss percentage was calculated using Eq. 3,
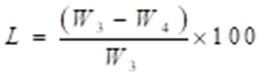
where L (%) is weight loss, W3 (g) is the constant weight of the sample before decay, W4 (g) is the constant weight of the sample after decay.
RESULTS AND DISCUSSION
Extraction of the Active Ingredient Berberine
Berberine had a high content in Coptis chinensis, and a good bacteriostatic effect on wood-rot fungus. Besides, it was the main bacteriostatic component found in C. chinensis (Yan et al. 2014). The quality and a corresponding yield of the C. chinensis extractive obtained via the above 6 extraction methods are shown in Table 1. The Coptis extractum trends and berberine content are shown in Fig. 2.
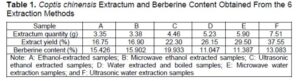
Table 1 and Fig. 2 show that under the conditions of this test, the water-soluble substances in C. chinensis was greater than 50% of the alcohol-soluble substance. Among them, the yield of the microwave water extracted samples (E) exceeded the 74.56% yield of the microwave alcohol extracted samples (B). Since the water extraction liquid had a long time to evaporate and condense during the subsequent processing, it consumed a lot of energy. Many non-alkaloids, e.g., proteins and sugars, will be dissolved in a large amount of water (Deans et al. 2018). Combining this with the fact that berberine was soluble in ethanol (Hao et al. 2020), an alcohol extraction method would be better used in industrial production. It can be seen that the berberine content obtained via alcohol extraction was noticeably greater than the berberine content obtained via water extraction, as shown in Fig. 2. The ultrasonic treatment (C) berberine content was greater than the microwave treatment (B) berberine content, which was greater than the berberine content of the untreated samples (A). The berberine content of the ethanol extracted samples via ultrasonic treatment (C) was 29.2% higher than the untreated samples (A), and the ethanol ultrasonic treatment (C) was 80.4% higher than the untreated water extraction method (D).
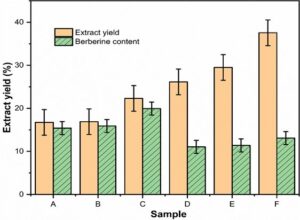
Fig. 2. Coptis chinensis extractum trend and the berberine content
To reiterate the treatment method, the C. chinensis powder was treated in an 8 to 1 ratio of 95% ethanol to powder (with ethanol as the extraction solvent), the solution was soaked for 12 h, and treated with an ultrasonic pulse at 600 W and 20 kHz for 30 min. The maximum berberine content in the extractive was 19.93% after refluxing for 4 h. Therefore, an ethanol ultrasonic treatment should be used to extract berberine from C. chinensis; the ultrasonic treatment not only has a high efficiency and simple operation, but also saves a lot of energy, so it can be widely used for the industrial extraction and production of berberine.
Bacteriostatic Effect of Coptis extractive
The recorded data of the bacteriostatic effect of Coptis extractive, prepared by six different extraction methods, on the toxic medium of white-rot fungus and brown-rot fungus are shown in Tables 2 and 3. According to the data of the bacterial circle, the growth trend of white-rot fungus and brown-rot fungus on the toxic medium are shown in Figs. 3a and 3b.
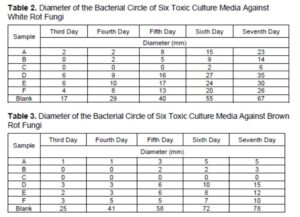
Compared to the blank control, the C. chinensis water extractive and alcohol extractive had a bacteriostatic effect on white-rot fungus and brown-rot fungus. However, they both had a more significant bacteriostatic effect on brown-rot fungus, as shown in Fig. 2.

Fig. 3. The effects of different toxin culture media on the growing tendency of white-rot fungi (a); and the effect of different toxin medium on the growth trend of brown-rot fungus (b)
The ethanol extraction had better results than the water extraction; the ultrasonic treatment (C) had a greater bacteriostatic effect than the microwave treatment (B), which had a greater bacteriostatic effect than the untreated samples (A). This may be due to the significant increase in the content of bacteriostatic active fractions after the alcohol and ultrasonic treatments, e.g., the berberine found in C. chinensis.
The next step of the experiment was to find out the specific components of C. chinensis that effected bacteriostasis, in order to make the selection of protectants found in Chinese herbal medicine more targeted. The growth of white-rot fungus and brown-rot fungus at the later stage of the bacteriostatic test was slightly improved, which could be the reason for the gradual weakening of the efficacy. In the future, more in-depth research should be performed in terms of maintaining the long-term efficacy of the pharmaceutical effect and explore non-harmful extraction methods.
Screening and Structure Determination of the Active Ingredients
Isolation of Coptis chinensis
In summary, the obtained effective components were substance A, substance D, and substance F. The calculated amount of the effective substances A, D, and F found in the root powder of C. chinensis were 5.21%, 0.91%, and 0.98%, respectively. Furthermore, its structure was identified via nuclear magnetic resonance, and its content was analyzed via high-performance liquid chromatography.
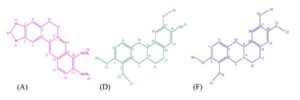
Fig. 4. The structure of the bacteriostatic components in Coptis chinensis: the structure of berberine hydrochloride (A); the structure of jatrorrhizine (D); and the structure of palmatine (F)
Structural identification and content analysis
The structure of the bacteriostatic components in Coptis chinensis are shown in Fig. 4. Substance A was described as yellow needle-like crystals, with the following results: 1H NMR (500.13 MHz, MeOD) peak was attributed to δ ppm: 9.77 (1H, s, 14-H), 8.71 (1H, s, 11-H ), 8.12 (1H, d, J = 9.5 Hz, 15-H), 8.00 (1H, d, J = 9.0 Hz, 16-H), 7.66 (1H, s, 3-H), 6.96 (1H, s, 6-H), 6.11 (2H, s, 20-OCH2O-), 4.92 (2H, t, J = 6.5Hz, 9-H), 4.20 (3H, s, 22-OCH3-), 4.11(3H, s, 23-OCH3-), 3.25 (2H, t , J = 6.0Hz, 10-H) (Bayar et al. 2019); 13C NMR (125.77 MHz, MeOD) peak was attributed to δ ppm: 152.2, 152.1, 150.0, 146.5, 145.8, 139.8, 135.2, 131.9, 128.1, 124.6, 123.4, 121.9, 121.6, 109.4, 106.6, 103.7, 62.6, 57.7, 57.2, and 28.2. The data from the 1H NMR and 13C NMR were consistent with the data reported by Sahu et al. (2016). The purity was 91%, as determined via high performance liquid chromatography. Therefore, the active ingredient was identified as berberine hydrochloride.
Substance D was described as a colorless crystal, with the following results: 1H NMR (500.13 MHz, CDCl3) peak was attributed to δ ppm: 6.89 (1H, d, J = 8.5 Hz, 15-H), 6.81 (1H, d, J = 8.0 Hz, 18-H), 6.70 (2H, d, J = 5.5 Hz, 1, 6-H), 4.26 (1H, s, 9-CH-), 3.87 (9H, m, 20, 22, 24-OCH3-), 3.56 (2H, s, 7-CH2-), 3.19 (3H, m, 10-CH2-, 12-CH2-), 2.86 (1H, s, 12-CH2-), 2.68 (1H, s, 11-CH2-); 13C NMR (125.77 MHz,CDCl3) peak was attributed to δ ppm: 150.4, 145.2, 144.1, 127.4, 123.9, 114.3, 111.2, 107.9, 60.2, 59.4, 56.1, 55.9, 54.0, 51.5, 36.2, and 28.8. The data from the 1H NMR and 13C NMR were consistent with the data reported by Liu et al. (2019). The purity was 90%, as determined via HPLC. Therefore, the active ingredient was identified as jatrorrhizine.
Substance F was described as a yellow flaky crystal, with the following results: 1H NMR (500.13 MHz, MeOD) peak was assigned to δ ppm: 6.91 (3H, m, 1, 15, 18-H), 6.70 (1H, s, 6-H), 4.20 (1H, d, J = 15.5 Hz, 9-CH-), 3.82 (12H, dd, J = 1.5 Hz , J=7.5 Hz, 20, 22, 24, 26-OCH3-), 3.55 (1H, dd, J = 3.5 Hz, J = 3.0 Hz, 7-CH2-), 3.50 (1H, d, 16 Hz, 7-CH2-) , 3.43 (1H, dd, J = 3.5 Hz, J = 4.0 Hz, 10-CH2-), 3.20 (1H, m, 10-CH2-), 3.09 (1H, m, 12-CH2-), 2.74 (2H, m, 12-CH2-, 11-CH2-), 2.63 (1H, m, 11-CH2-); 13C NMR (125.77MHz,MeOD) peak was assigned to δ ppm: 151.8, 149.2, 146.3, 130.7, 128.9, 128.7, 127.8, 125.3, 113.0, 112.7, 110.5, 60.8, 60.6, 56.7, 56.4, 54.9, 52.7, 36.6, and 29.4. The data from the 1H NMR and 13C NMR were consistent with the data reported by Fan et al. (2012). The purity was 90%, as determined via HPLC. Therefore, the active ingredient was identified as palmatine.
Bacteriostatic properties of the different active ingredients against white-rot fungus
Through the paper agar diffusion method, the different concentrations of the active ingredients and the different compound ratios of the active ingredients found in C. chinensis were used for the bacteriostatic testing of white-rot fungus. The diameter data of the bacteriostatic circle measured at different days is shown in Table 4, and the relationship between the diameter of the bacteriostatic circle on the 7th d and the concentration of the active ingredient is shown in Fig. 5a. Since the bacteriostatic effect of each active ingredient at concentrations of 0.005 g/mL and 0.001 g/mL was poor, the data were not listed in the table. The different compounds were listed as follows: Berberine hydrochloride was A, jatrorrhizine was B, palmatine was C, berberine hydrochloride and jatrorrhizine combined was D, berberine hydrochloride and palmatine combined was E, jatrorrhizine and palmatine combined was F, and a 1 to 1 to 1 ratio of the three active ingredients was G.
The bacteriostatic effect can be seen in Table 4 and Fig. 5a. Compared with the blank group, the active ingredients in C. chinensis and their various combinations had an obvious inhibitory effect on white-rot fungus at various concentrations, and the bacteriostatic effect was enhanced as the concentration of the active ingredients was increased, but the amplitude was not obvious. It was apparent that the concentration range selected in the experiment had reached the minimum inhibitory concentration of white-rot fungus. The bacteriostatic circle data of each active ingredient and its compounds were compared with alkaline copper quaternary (ACQ). The 1 to 1 to 1 mixed ratio compound (G) had the same bacteriostatic effect as ACQ, while the other groups were worse than ACQ. This may be due to the volatilization and loss of the active ingredients of traditional Chinese medicine during the process, and the efficacy was gradually weakened.
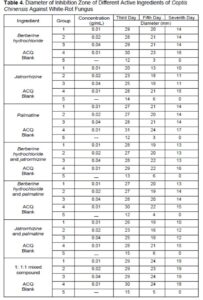

Fig. 5. The bacteriostatic properties of different active ingredients against white-rot fungus (a); and the bacteriostatic properties of different active ingredients against brown-rot fungi (b)
At the same time, it also showed that a single active ingredient of C. chinensis was inferior to a mixture of all three in terms of the bacteriostatic effect, this proved that the synergism of the active ingredients can enhance the bacteriostatic effect.
Table 5 and Fig. 5a show that in terms of a single active ingredient, berberine hydrochloride (A) had the best bacteriostatic effect, and the minimum bacteriostatic concentration was 0.01 g/mL; palmatine (C) was the second best, and the minimum bacteriostatic concentration was also 0.01 g/mL; and jatrorrhizine (B) had the worst bacteriostatic effect, and the minimum bacteriostatic concentration was 0.02 g/mL. There was no noticeable difference in the bacteriostatic effect between the two most effective components. The minimum bacteriostatic concentration of the berberine hydrochloride and jatrorrhizine compound (D) was 0.01 g/mL. The minimum bacteriostatic concentration of the berberine hydrochloride and palmatine compound (E) was 0.02 g/mL. The minimum bacteriostatic concentration of the jatrorrhizine and palmatine compound (F) was 0.02 g/mL. The minimum inhibitory concentration of the 1 to 1 to 1 mixed ratio compound (G) was 0.01 g/mL.
According to the test results, the bacteriostatic effect of the compound with a 1 to 1 to 1 ratio of the three active ingredient was the best, so it was used as the primary bacteriostatic extractive for the subsequent preparation of high-efficiency preservatives, and its performance was evaluated.
Bacteriostatic properties of different active ingredients against brown-rot fungus
The paper agar diffusion method was used to test the inhibition of brown-rot fungus (Gloeophyllum trabeum (Pers.) Murrill) with different concentrations of the active ingredients and different compound ratios of the active ingredients found in Coptis chinensis. The diameter data of the bacteriostatic circle measured on different days is shown in Table 5. The relationship between the diameter of the bacteriostatic circle and the concentration of the native active fractions on the 7th d is shown in Fig. 5b.
At the same concentration, the bacteriostatic effect of each active ingredient on brown-rot fungus was slightly worse in comparison to their effect on white-rot fungus, as shown in Fig. 5. Table 5 and Fig. 5b show that compared with the blank group, the active ingredients in C. chinensis and their various combinations had the obvious inhibitory effects on brown-rot fungus (G. trabeum) at various concentrations, but the bacteriostatic effect varied with the concentration of the active components. Among them, the berberine hydrochloride (A), palmatine (C), berberine hydrochloride and jatrorrhizine compound (D), jatrorrhizine and palmatine compound (F), and 1 to 1 to 1 mixed ratio compound (G) all had their bacteriostatic effect increased or decreased with the increase of the concentration of active ingredient. The bacteriostatic effect of jatrorrhizine (B) and berberine hydrochloride and palmatine compound (E) were increased as the concentration increased, but the changes were not noticeable. It was indicated that the concentration range in this study achieved the minimum inhibitory concentration for brown-rot fungus. It was clearly demonstrated in Fig. 5b that the 1 to 1 to 1 mixed ratio compound (G) had the best bacteriostatic effect and it was equivalent to ACQ. The bacteriostatic effect of jatrorrhizine (B) was the worst when the minimum bacteriostatic concentration was 0.01 g/mL. This was consistent with the white-rot fungus test results, and once again demonstrated that the synergistic effect of each active ingredient will enhance their efficacy.
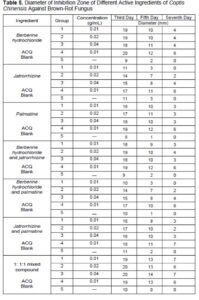
It is shown in Table 5 and Fig. 5b, that as far as a single effective ingredient was concerned, berberine hydrochloride (A) had the best bacteriostatic effect, and the minimum bacteriostatic concentration was 0.01 g/mL. The bacteriostatic effect of palmatine (C) was the second best, and the minimum inhibitory concentration was also 0.01 g/mL. The bacteriostatic effect of jatrorrhizine (B) was the worst, and the minimum inhibitory concentration was 0.04 g/mL. The bacteriostatic effect of the two active ingredient compound of jatrorrhizine and palmatine (F) was the best. The minimum inhibitory concentration was 0.01 g/mL. The bacteriostatic effect of the berberine hydrochloride and jatrorrhizine compound (D) was second best, and the minimum bacteriostatic concentration was 0.02 g/mL. The bacteriostatic effect of the berberine hydrochloride and palmatine compound (E) was the worst, and the minimum bacteriostatic concentration was 0.04 g/mL. The minimum inhibitory concentration of the 1 to 1 to 1 mixed ratio compound (G) was 0.01 g/mL.
Decay Resistance of Wood
The surface of wood not treated with active ingredients was completely covered by white-rot fungi, and the growth of white-rot fungi was vigorous in Fig. 6a. As can be seen in Fig. 6b, only small amounts of mycelia were scattered on the wood surface, and the growth of white-rot fungi was poor, indicating that the active ingredients have a good inhibitory effect on the growth of white-rot fungi. It can be found that the amount of brown rot fungi mycelium is more than that of white-rot fungi in Fig. 6d. Compared with Fig. 6c, the wood surface is not completely covered by brown-rot fungi, which indicates that the active ingredients are effective against brown-rot fungi.
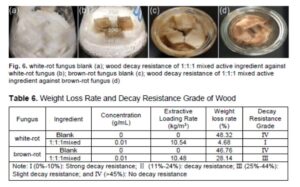
Decay resistance of wood can be measured by the weight loss of decayed samples. It can be found from Table 6 that the weight loss percentages of the white-rot blank and the brown rot blank were 48.3% and 46.8%, respectively, indicating that there was no decay resistance. The weight loss of the wood treated with 1 to 1 to 1 active ingredient against white rot fungi was 4.7%, indicating strong decay resistance. However, the weight loss against brown rot fungi was 28.1%, indicating slight decay resistance. The extract concentration is the same for white-rot fungi and brown rot fungi in Table 6, and the difference of extractive loading rate was small, indicating that the vacuum pressure leaching method has a protective effect on the extractive leaching (Clausen et al. 2010). In future studies, different factors should be changed to further consider the leaching of active ingredients in wood. The hydroxyl radical produced by brown rot fungi has a strong oxidation ability, which can oxidize the hydroxyl group on the glucose of cellulose to the carboxyl group (Faure and Nystrom 2016). It leads to the breaking of the hydrogen bonds of the cellulose molecular chain and the looseness of the cellulose structure (Lu et al. 2019). The reason for the poor decay resistance of brown rot fungus in this study may be due to the low degree of influence for the active ingredients on the formation of hydroxyl free radicals and the activity of glucosidase, which leads to the brown rot fungus still can participate in the degradation of wood cellulose. This may require further research in the field of enzymology.
CONCLUSIONS
1. The method with the highest extraction efficiency for the active components of Coptis chinensis was determined in this paper. The best extraction method of the active fractions was soaking the root powder in ethanol for 12 h, followed by an ultrasonic treatment for 30 min, and reflux for 4 h.
2. The bacteriostatic components of Coptis chinensis were berberine, jatrorrhizine, and palmatine. The bacteriostatic effect of berberine in terms of a single-component was the better of the three active components. Among the component mixtures, the bacteriostatic effect of the 1 to 1 to 1 ratio of the three active components was the best and was equivalent to ACQ. The stronger bacteriostatic effect of active component mixtures was shown by the test results.
3. The active ingredients in Coptis chinensis and their various mixtures had noticeable inhibitory effects on white-rot fungus and brown-rot fungus. The white-rot fungus was inhibited to a greater extent than the brown-rot fungus. It was shown that the synergistic effect of each active ingredient enhanced the bacteriostatic efficacy and delayed the growth of wood-rotting fungi. This article provides a reference for the extraction of Coptis chinensis active ingredients as well as the mixing of different active agents for the development of wood preservatives.
ACKNOWLEDGEMENTS
This study was financially supported by the Natural Science Foundation of Inner Mongolia (2018MS03048) and the Science and Technology Innovation Guidance Project of Inner Mongolia. The authors would also like to thank the National Key Research and Development Program of China (2016YFD0600904).
REFERENCES CITED
Ahmed, S., Fatima, R., and Hassan, B. (2020). “Evaluation of different plant derived oils as wood preservatives against subterranean termite Odontotermes obesus,” Maderas-Ciencia Y Tecnologia 22(1), 109-120. DOI: 10.4067/S0718-221X2020005000110
Arafat, Y., Altemimi, A., Ibrahim, S. A., and Badwaik, L. S. (2020). “Valorization of sweet lime peel for the extraction of essential oil by solvent free microwave extraction enhanced with ultrasound pretreatment,” Molecules 25(18), 4072. DOI: 10.3390/molecules25184072
Brischke, C. (2020). “Wood protection and preservation,” Forests 11(5), 549. DOI: 10.3390/f11050549
Barbero-Lopez, A., Akkanen, J., Lappalainen, R., Peraniemi, S., and Haapala, A. (2021). “Bio-based wood preservatives: Their efficiency, leaching and ecotoxicity compared to a commercial wood preservative,” Science of the Total Environment 753, 142013. DOI: 10.1016/j.scitotenv.2020.142013
Busto, M., and Vera, C.R. (2019). “Deacidification of vegetable oil by extraction with solvent recovery,” Adsorption 25(7), 1397-1407. DOI: 10.1007/s10450-019-00102-9
Cai, L.L., Jeremic, D., Lim, H., and Kim, Y. (2019). “Beta-cyclodextrins as sustained-release carriers for natural wood preservatives,” Industrial Crops and Products 130, 42-48. DOI: 10.1016/j.indcrop.2018.12.061
Cai, L., Lim, H., Nicholas, D. D., and Kim, Y. (2020). “Bio-based preservative using methyl-beta-cyclodextrin-essential oil complexes for wood protection,” International Journal of Biological Macromolecules 147, 420-427. DOI: 10.1016/j.ijbiomac.2020.01.056
Ciganovic, P., Jakimiuk, K., Tomczyk, M., and Koncic, M. Z. (2019). “Glycerolic licorice extracts as active cosmeceutical ingredients: Extraction optimization, chemical characterization, and biological activity,” Antioxidants 8(10), 445. DOI: 10.3390/antiox8100445
Clausen, C.A., Green, F., and Nami Kartal, S. (2010). “Weatherability and leach resistance of wood impregnated with nano-zinc oxide,” Nanoscale Research Letters 5(9), 1464-1467. DOI: 10.1007/s11671-010-9662-6
Cornara, L., Mariottini, G. L., Giordani, P., Smeriglio, A., Trombetta, D., Guida, L., Lavorano, S., and Burlando, B. (2020). “Modulatory activities of plant extracts on jellyfish cytotoxicity,” Wilderness and Environmental Medicine 31(3), 266-272. DOI: 10.1016/j.wem.2020.03.004
Deans, B. J., Olivier, W. J., Girbino, D., Bissember, A. C., and Smith, J. A. (2018). “Extraction of carboxylic acid-containing diterpenoids from Dodonaea viscosa via pressurised hot water extraction,” Fitoterapia 126, 65-68. DOI: 10.1016/j.fitote.2017.10.008
Fan, G., Zhang, M. Y., Zhou, X. D., Lai, X. R., Yue, Q. H., Tang, C., Luo, W. Z., and Zhang, Y. (2012). “Quality evaluation and species differentiation of Rhizoma coptidis by using proton nuclear magnetic resonance spectroscopy,” Analytica Chimica Acta, 747, 76-83. DOI: 10.1016/j.aca.2012.08.038
Farias, A. P. P., Monteiro, O. D., da Silva, A. A. C., Monteiro, I. N., and Maia, J. G. S. (2020). “Chemical composition and biological activities of two chemotype-oils from Cinnamomum verum J. Presl growing in North Brazil,” Journal of Food Science and Technology-Mysore 57(9), 3176-3183. DOI: 10.1007/s13197-020-04288-7
Faure, A. M., and Nystrom, L. (2016). “Effect of apotransferrin, lactoferrin and ovotransferrin on the hydroxyl radical mediated degradation of beta-glucan,” Food Chemistry 204, 1-6. DOI: 10.1016/j.foodchem.2016.02.075
Gaff, M., Kacik, F., and Gasparik, M. (2019). “Impact of thermal modification on the chemical changes and impact bending strength of European oak and Norway spruce wood,” Composite Structures 216, 80-88. DOI: 10.1016/j.compstruct.2019.02.091
Gilani, S. J., Imam, S. S., Ahmed, A., Chauhan, S., Mirza, M. A., and Taleuzzaman, M. (2019). “Formulation and evaluation of thymoquinone niosomes: Application of developed and validated RP-HPLC method in delivery system,” Drug Development and Industrial Pharmacy 45(11), 1799-1806. DOI: 10.1080/03639045.2019.1660366
Guo, H., Bachtiar, E. V., Ribera, J., Heeb, M., Schwarze, F. W. M. R., and Burgert, I. (2018). “Non-biocidal preservation of wood against brown-rot fungi with a TiO2/Ce xerogel,” Green Chemistry 20(6), 1375-1382. DOI: 10.1039/c7gc03751a
Hao, Y., Huo, J., Wang, T., Sun, G., and Wang, W. (2020). “Chemical profiling of Coptis rootlet and screening of its bioactive compounds in inhibiting Staphylococcus aureus by UPLC-Q-TOF/MS,” Journal of Pharmaceutical and Biomedical Analysis 180, 113089. DOI: 10.1016/j.jpba.2019.113089
Hassan, B., Mankowski, M. E., Kirker, G., Ahmed, S., and Bishell, A. (2019). “Ex-situ performance of extracts from naturally durable heartwood species and their potential as wood preservatives,” European Journal of Wood and Wood Products 77(5), 869-878. DOI: 10.1007/s00107-019-01443-6
Hiscox, J., O’Leary, J., and Boddy, L. (2018). “Fungus wars: Basidiomycete battles in wood decay,” Studies in Mycology 89, 117-124. DOI: 10.1016/j.simyco.2018.02.003
Ji, Y. H., Li, X. H., Wang, Z. Z., Xiao, W., He, Z. G., Xiong, Z. L., and Zhao, L. S. (2020). “Extraction optimization of accelerated solvent extraction for eight active compounds from Yaobitong capsule using response surface methodology: Comparison with ultrasonic and reflux extraction,” Journal of Chromatography A 1620, 460984. DOI: 10.1016/j.chroma.2020.460984
Johnston, S. R., Boddy, L., and Weightman, A. J. (2016). “Bacteria in decomposing wood and their interactions with wood-decay fungi,” FEMS Microbiology Ecology 92(11), 1-12. DOI: 10.1093/femsec/fiw179
Joseph, E., and Junier, P. (2020). “Metabolic processes applied to endangered metal and wood heritage objects: Call a microbial plumber!” New Biotechnology 56, 21-26. DOI: 10.1016/j.nbt.2019.11.003
Kim, N. K., Park, J. Y., Park, M. S., Lee, H., Cho, H. J., Eimes, J. A., Kim, C., and Lim, Y. W. (2016). “Five new wood decay fungi (polyporales and hymenochaetales) in Korea,” Mycobiology 44(3), 146-154. DOI: 10.5941/MYCO.2016.44.3.146
Kojima, E., Yamasaki, M., Imaeda, K., Lee, C. G., Sugimoto, T., and Sasaki, Y. (2020). “Effects of thermal modification on the mechanical properties of the wood cell wall of soft wood: Behavior of S2 cellulose microfibrils under tensile loading,” Journal of Materials Science 55(12), 5038-5047. DOI: 10.1007/s10853-020-04346-7
Kumar, A., Ryparová, P., Kasal, B., Adamopoulos, S., and Hajek, P. (2020). “Resistance of bamboo scrimber against white-rot and brown-rot fungi,” Wood Material Science & Engineering 15(1), 57-63. DOI: 10.1080/17480272.2018.1475420
Lasota, S., Stephan, I., Horn, M. A., Otto, W., and Noll, M. (2019). “Copper in wood preservatives delayed wood decomposition and shifted soil fungal but not bacterial community composition,” Applied and Environmental Microbiology 85(4), 1-13. DOI: 10.1128/aem.02391-18
Le, K., Tran, D., Nguyen, A., and Le, L. (2020). “A screening of neuraminidase inhibition activities of isoquinolone alkaloids in Coptis chinensis using molecular docking and pharmacophore analysis,” ACS Omega 5(46), 30315-30322. DOI: 10.1021/acsomega.0c04847
Li, T., Zhu, M., Yang, Z., Song, J., Dai, J., Yao, Y., Luo, W., Pastel, G., Yang, B., and Hu, L. (2016). “Wood composite as an energy efficient building material: Guided sunlight transmittance and effective thermal insulation,” Advanced Energy Materials 6(22), 1601122. DOI: 10.1002/aenm.201601122
Liu, M., Liu, Q., Chen, M., Huang, X., and Chen, X. (2019). “Large-scale separation of acetylcholinesterase inhibitors from Zanthoxylum nitidum by pH-zone-refining counter-current chromatography target-guided by ultrafiltration high-performance liquid chromatography with ultraviolet and mass spectrometry screening,” Journal of Separation Science 42(6), 1194-1201. DOI: 10.1002/jssc.201801238
Liu, Q., Qiu, S., Yu, H., Ke, Y., Jin, Y., and Liang, X. (2011). “Selective separation of structure-related alkaloids in Rhizoma coptidis with “click” binaphthyl stationary phase and their structural elucidation with liquid chromatography-mass spectrometry,” Analyst 136(20), 4357-4365. DOI: 10.1039/c1an15444c
Lu, Q. L., Lu, L. N., Li, Y. G., and Huang, B. A. (2019). “Facile manufacture of cellulose nanoparticles in high yields by efficient cleavage of hydrogen bonds via mechanochemical synergy,” Cellulose 26(13-14), 7741-7751. DOI: 10.1007/s10570-019-02647-y
LY/T 1283 (2011). “Method of laboratory test for toxicity of wood preservatives to decay fungi,” Standardization Administration of China, Beijing, China.
Mahmoud, B. S., ElMasry, S. A., Fahim, N., Abd ElSattar, M. A., and Shaker, O. A. (2019). “Detection of antibiotic susceptibility by colorimetric minimum inhibitory concentration in staphylococcal isolates,” Journal of Applied Microbiology 127(3), 693-700. DOI: 10.1111/jam.14347
Mkindi, A. G., Tembo, Y. L. B., Mbega, E. R., Smith, A. K., Farrell, I. W., Ndakidemi, P. A., Stevenson, P. C., and Belmain, S. R. (2020). “Extracts of common pesticidal plants increase plant growth and yield in common bean plants,” Plants 9(2), 149. DOI: 10.3390/plants9020149
Mubarok, M., Dumarcay, S., Militz, H., Thevenon, M. F., and Gerardin, P. (2019). “Non-biocide antifungal and anti-termite wood preservation treatments based on combinations of thermal modification with different chemical additives,” European Journal of Wood and Wood Products 77(6), 1125-1136. DOI: 10.1007/s00107-019-01468-x
Oberhofnerova, E., Panek, M., Podlena, M., Pavelek, M., and Sterbova, I. (2019). “Color stabilization of Siberian and European larch wood using UVA, HALS, and nanoparticle pretreatments,” Forests 10(1), 23. DOI: 10.3390/f10010023
Oh, J.-J., and Kim, G.-H. (2020). “The effects of pH on copper teaching from wood treated with copper amine-based preservatives,” Holzforschung 74(9), 891-897. DOI: 10.1515/hf-2019-0218
Presley, G. N., Zhang, J., Purvine, S. O., and Schilling, J. S. (2020). “Functional genomics, transcriptomics, and proteomics reveal distinct combat strategies between lineages of wood-degrading fungi with redundant wood decay mechanisms,” Frontiers in Microbiology 11, 1646. DOI: 10.3389/fmicb.2020.01646
Ren, L., Xue, X., Zhang, F., Xu, Q., and Liang, X. (2007). “High performance liquid chromatography-mass spectrometry analysis of protoberberine alkaloids in medicine herbs,” Journal of Separation Science 30(6), 833-842. DOI: 10.1002/jssc.200600246
Sahu, A., Narayanam, M., Kurmi, M., Ladumor, M.K., and Singh, S. (2016). “Quantitation of memantine hydrochloride bulk drug and its tablet formulation using proton nuclear magnetic resonance spectrometry,” Magnetic Resonance in Chemistry 54(8), 632-636. DOI: 10.1002/mrc.4421
Sgarbossa, A., Boschiero, M., Pierobon, F., Cavalli, R., and Zanetti, M. (2020). “Comparative life cycle assessment of bioenergy production from different wood pellet supply chains,” Forests 11(11), 1127. DOI: 10.3390/f11111127
Sikora, A., Kacik, F., Gaff, M., Vondrova, V., Bubenikova, T., and Kubovsky, I. (2018). “Impact of thermal modification on color and chemical changes of spruce and oak wood,” Journal of Wood Science 64(4), 406-416. DOI: 10.1007/s10086-018-1721-0
Singh, N., and Sharma, B. (2018). “Toxicological effects of berberine and sanguinarine,” Frontiers in Molecular Biosciences 5, 21. DOI: 10.3389/fmolb.2018.00021
Sirgel, F. A., Wiid, I. J. F., and van Helden, P. D. (2009). “Measuring minimum inhibitory concentrations in mycobacteria,” in: Mycobacteria Protocols T. Parish, and A. C. Brown (ed.), Springer Publishing, Berlin, Germany. DOI: 10.1007/978-1-59745-207-6_11
Song, J., Chen, C., Wang, C., Kuang, Y., Li, Y., Jiang, F., Li, Y., Hitz, E., Zhang, Y., Liu, B., Gong, A., et al. (2017). “Superflexible wood,” ACS Applied Materials & Interfaces 9(28), 23520-23527. DOI: 10.1021/acsami.7b06529
Treu, A., Nunes, L., and Larnoy, E. (2020). “Macrobiological degradation of esterified wood with sorbitol and citric acid,” Forests 11(7), 776. DOI: 10.3390/f11070776
Tseng, C. Y., Sun, M. F., Li, T. C., and Lin, C. T. (2020). “Effect of Coptis chinensis on biofilm formation and antibiotic susceptibility in Mycobacterium abscessus,” Evidence-Based Complementary and Alternative Medicine, 2020, 9754357. DOI: 10.1155/2020/9754357
Usmani, S. M., Stephan, I., Hubert, T., and Kemnitz, E. (2018). “Nano metal fluorides for wood protection against fungi,” ACS Applied Nano Materials 1(4), 1444-1449. DOI: 10.1021/acsanm.8b00144
Wang, Y., Zhang, Z., Fan, H., and Wang, J. (2018). “Wood carbonization as a protective treatment on resistance to wood destroying fungi,” International Biodeterioration & Biodegradation 129, 42-49. DOI: 10.1016/j.ibiod.2018.01.003
Yan, D., Li, J., Xiong, Y., Zhang, C., Luo, J., Han, Y., Wang, R., Jin, C., Qian, H., Li, J., et al. (2014). “Promotion of quality standard of herbal medicine by constituent removing and adding,” Scientific Reports 4(1), 3668. DOI: 10.1038/srep03668
Yang, S. B., Kim, E. H., Kim, S. H., Kim, Y. H., Oh, W., Lee, J.-T., Jang, Y.-A., Sabina, Y., Ji, B. C., and Yeum, J. H. (2018). “Electrospinning fabrication of poly (vinyl alcohol)/Coptis chinensis extract nanofibers for antimicrobial exploits,” Nanomaterials, 8(9), 734-748. DOI: 10.3390/nano8090734
Yanitch, A., Kadri, H., Joly, S., Pitre, F. E., and Labrecque, M. (2020). “A four-year phytoremediation trial to decontaminate soil polluted by wood preservatives: phytoextraction of arsenic, chromium, copper, dioxins and furans,” International Journal of Phytoremediation 22(14), 1505-1514. DOI: 10.1080/15226514.2020.1785387
Ye, M., Fu, S., Pi, R., and He, F. (2009). “Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research,” Journal of Pharmacy and Pharmacology 61(7), 831-837. DOI: 10.1211/jpp/61.07.0001
Yuan, J. F., Wang, T. T., Wang, D. H., Wang, Y., Gong, M. G., and Zhang, B. (2020). “Effect of microwave on changes of gallic acid and resveratrol in a model extraction solution,” Food and Bioprocess Technology 13(7), 1246-1254. DOI: 10.1007/s11947-020-02452-7
Zalamea, M., Gonzalez, G., and Lodge, D. J. (2016). “Physical, chemical, and biological properties of soil under decaying wood in a tropical wet forest in Puerto Rico,” Forests 7(8), 168-187. DOI: 10.3390/f7080168
Article submitted: October 21, 2020; Peer review completed: January 24, 2021; Revised version received and accepted: February 2, 2021; Published: February 5, 2021.
DOI: 10.15376/biores.16.2.2346-2368
