Abstract
This paper presents a kinetic study of fuel grade ethanol production by simultaneous saccharification and fermentation from Fe(II)-catalyzed cornstalks. The study observed the optimal conditions of ethanol production as: inoculation proportion (ratio of Pachysolen tannophilus to Saccharomyces cerevisiae) 2:1, fermentation temperature 32 °C, inoculation quantity 20%, addition amount of Fe2+ 4 mg/g (substrate), and cellulase dosage 30 U/g (substrate). An ethanol yield of 0.335 mg/g was obtained from cornstalks pretreated using liquefaction under optimum conditions. A 30.4% increase in the yield was observed when compared with the control group without the addition of Fe2+. The relationship between ethanol yield and fermentation time could be described through a Langmuir isotherm model. The findings of this study will help researchers better understand and describe the complex characteristics of ethanol production from cornstalks with Fe2+ promoter, which will be very useful in improving production yields.
Download PDF
Full Article
Kinetic Analysis of Fe(II)-promoted Ethanol Preparation from Cornstalks
Kun Chen,a,b,* Dehuan Liu,a Xu Chen,a and Zhiwen Fan a
This paper presents a kinetic study of fuel grade ethanol production by simultaneous saccharification and fermentation from Fe(II)-catalyzed cornstalks. The study observed the optimal conditions of ethanol production as: inoculation proportion (ratio of Pachysolen tannophilus to Saccharomyces cerevisiae) 2:1, fermentation temperature 32 °C, inoculation quantity 20%, addition amount of Fe2+ 4 mg/g (substrate), and cellulase dosage 30 U/g (substrate). An ethanol yield of 0.335 mg/g was obtained from cornstalks pretreated using liquefaction under optimum conditions. A 30.4% increase in the yield was observed when compared with the control group without the addition of Fe2+. The relationship between ethanol yield and fermentation time could be described through a Langmuir isotherm model. The findings of this study will help researchers better understand and describe the complex characteristics of ethanol production from cornstalks with Fe2+ promoter, which will be very useful in improving production yields.
Keywords: Cornstalks; Fe catalysis; Fractal-like; Kinetic; SSF; Fuel; Ethanol
Contact information: a: School of Safety Engineering, Chongqing University of Science and Technology, Chongqing 401331, China; b: Centre for Risk, Integrity and Safety Engineering (C-RISE), Faculty of Engineering and Applied Science, Memorial University of Newfoundland, St. John’s A1B 3X5, Canada;
* Corresponding author: ckym117@cqust.edu.cn
INTRODUCTION
Lignocellulosic ethanol is widely considered one of the most important alternatives to fossil fuel energy (Jain et al. 2016; Chen et al. 2018). In China, the annual production of cornstalks (CS) has reached 400 million tons (Mei et al. 2016). The CS are an abundant, renewable, low cost, and widely available resource that are rich in cellulose (35% to 50%) (Wong and Fikri 2014). These characteristics make CS a promising renewable feedstock for alternative fuel. For the effective conversion of lignocellulosic material into ethanol, there are three major steps involved: pretreatment, enzymatic hydrolysis, and fermentation (Zhang et al. 2014). This study focuses on parameter optimization of the fermentation process. In a previous study, the effects of various parameters on ethanol production from lignocellulosic material in the presence of diluted acid and simultaneous saccharification with fermentation (SSF) were considered (Chen et al. 2013). The study observed that the factors with the greatest influence were fermentation time, inoculation ratio, solid-liquid ratio, inoculum size, and the catalyst (Chen et al. 2013, 2014; Nguyen et al. 2015).
Iron compounds, which are influence factors for ethanol production, have been applied as promoters for the bio-processing of various biomasses. Studies have reported that a microwave-assisted Fe3+pretreatment enhanced both enzymatic saccharification and ethanol production (Lü and Zhou 2011, 2015, 2017); however, the effect of iron ions on ethanol production was not studied separately.
Several researchers reported that inorganic-salts containing Fe-ions (Fe2+ or 3+) could increase the hydrolysis efficiency of hemicellulose and cellulose in biomass during pretreatment. Yan et al. (1996), Nguyen and Tucker (2002), and Liu et al. (2009a, 2009b) have demonstrated that utilization of Fe-salts results in higher sugar yields due to the pretreatment. This was due to lower activation energy requirements (i.e., a lower temperature) in the hydrolysis of the biomass. It was observed that FeSO4 enhances the pretreatment efficiency of cellulose-rich material at lower concentrations (Monavari et al. 2011; Zhao et al. 2011), possibly by facilitating the interaction between cellulose and cellulase.
The FeSO4 pretreatment showed particularly strong effects on the hemicellulose removal and xylose release, thus enhancing the recovery of hemicellulose and the enzymatic hydrolysis of cellulose. However, the Fe2+ ion was able to promote enzyme activity in the cellulose (Lü and Zhou 2015), thus enhancing the subsequent enzymatic hydrolysis in the fermentation process. Fe ion as catalysts in pretreatment system for ethanol production was reported, but little research reported that inorganic iron salts were used in fermentation processes. Fe2+ has been used as a supplementation in fermentation (Izmirlioglu and Demirci 2016). However, the effect of Fe-salts on the activity of cellulase and the catalytic mechanism of iron salt have not been discussed in detail. Moreover, iron is an essential micronutrient, acting as a catalytic cofactor of essential enzymes, and it plays a critical structural role in enzymes and many non-catalytic proteins.
Study of the kinetic parameters involved in fermentation is important for understanding the mechanisms and the impact of process parameters on ethanol production. However, little information about kinetic models is available regarding the ethanol production from Fe (II)-catalyzed CS. Moreover, kinetic parameters coupled with mathematical models can be used to predict the dynamics of substrate utilization and ethanol production rate (Yao et al. 2011; Zhang et al.2012).
Until now, a kinetic model for the complex process of ethanol production has been far from realized. Fractal kinetic analysis provides another viewpoint regarding the heterogeneous chemical reactions used to achieve the optimal conditions of ethanol production from CS (Chen et al. 2014; Nguyen et al. 2015). In the researchers’ previous work, it was observed that the Langmuir equation can be utilized to describe the complex process of converting lignocellulosic materials to ethanol, and that the reaction process is fractal-like (Chen et al. 2013, 2014). In the preparation of fuel ethanol from CS, the liquefaction pretreatment and the addition of iron ions can theoretically improve the ethanol production efficiency of CS. Consequently, it is of great interest to explore the effect of Fe2+ on ethanol production from CS.
Therefore, the effects of FeSO47H2O (FeSO4) on ethanol production from CS with liquefaction pretreatment by SSF were investigated. The aim was to obtain the optimal conditions for ethanol production from CS by liquefaction pretreatment, mainly with fermentation temperature, fermentation time, Fe2+ addition, inoculation quantity, cellulase dosage, and inoculation proportion. In addition, the kinetic analysis of ethanol preparation from CS catalyzed by different additions of Fe2+ was considered and characterized, which will be helpful for the energy utilization of CS and the theoretical development of ethanol production.
EXPERIMENTAL
Materials
The cornstalks used in this study were collected from a household in Bishan of Chongqing (China). Cellulase (filter paper activity, 15000 U/g) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), and the yeasts (Pachysolen tannophilus 1770 (P.) and Saccharomyces cerevisiae 1001(S.)) were purchased from the China Center of Industrial Culture Collection (Beijing, China). Potassium dichromate, ethanol, sulfuric acid, glycol, FeSO47H2O (Fe2+), glucose, agar, wort, and peptone were purchased from Baimadang Chemical Storage (Chongqing, China). The oven (101-1), grinding mill (XQM-2L), portable steam sterilizer (YX280B), spectrophotometer (722), microscope (XSP-2CA), and hemocytometer (7108) were purchased from Shanghai CSOIF Company Limited (Shanghai, China).
Methods
Material pretreatment
The cornstalk material was sheared and milled until the entire sample passed through an 80-mesh sieve and then was dried to a constant weight. A total of 20 g of dried cornstalks and 120 g of glycol were placed into a three-mouth flask with a magnetic rotor. The middle mouth of the three-mouth flask was connected to a condenser tube. The flask was placed in a water bath with magnetic stirring (90 °C, 100 rpm). The side mouth of the flask had a perforated rubber stopper, through which a thermometer was inserted into the reaction mixture. The other side mouth was a feed inlet (a loaded rubber plug without a hole). After 10 min of preheating, the rubber plug of the feed inlet was removed, and 25 mmol of concentrated sulfuric acid was added to the flask. A rubber plug was inserted, and the reaction solution was stirred for 75 min at 90 °C.
After completion of the reaction, the flask and condenser pipe were separated, and the stirrer was removed. The reaction mixture in the flask was diluted with hot water. The reaction was poured into the funnel of the vacuum suction filter and filtered. The reaction mixture was filtered twice more, with 20 mL of methanol added to the filtrate each time. This process was repeated twice with 20 mL of hot water. The final product containing the fermentation substrate was obtained by drying the filter cake for 1 h at 105 °C.
Preparation of the slant, seed, fermentation medium
The media were prepared as described by Li et al. (2013). Medium I was used for slant medium, and it was composed of 10% wort with 2% agar and 2% peptone. Medium II was used for seed culture, and it was composed of 10% wort with 2% peptone. Medium III was used for fermentation and was composed of fermentation substrate (10 g), 1.0% dilute sulfuric acid, 30% NH4HCO3(NHC), and had a solid-liquid ratio of 1:15 (m:m). All of the media were adjusted to a pH of 6 and sterilized for 30 min at 121 °C. The seed culture of the strains (P. tannophilus and S. cerevisiae) was grown aerobically in a 250-mL Erlenmeyer flask containing 30 mL of the seed medium and shaken at 160 rpm for 24 h at 30 °C.
Activation and dilution of yeast strains
The P. tannophilus and S. cerevisiae were collected from the test tube slant and activated in the liquid medium at 30 °C for 24 h. Then, the mixture was stirred at 160 rpm for 2 h. The samples were then counted by a hemocytometer and diluted to 108 cell/mL with sterile water.
Design of ethanol optimization experiment and kinetic experiment
i) Orthogonal experiment design of process optimization of ethanol production
The orthogonal design L16 (45) was used to investigate the influence of factors on ethanol fermentation. Based on the researchers’ previous research results, the experimental conditions were as follows: solid-liquid ratio was 1:15, and fermentation time was 72 h. The orthogonal optimization experiment was designed with specific values of inoculation proportion (ratio of P. tannophilus to S. cerevisiae) (A), fermentation temperature (B), inoculation quantity (C), addition of Fe2+ (D), and cellulase dosage (E) as factors to optimize the process of SSF. The results of the replicated orthogonal experiments are listed in Table 1. Additionally, the control groups without the added Fe2+ were designed. Table 2 shows the results of the replicated orthogonal experiment.
ii) Kinetic experiment design based on different additions of Fe2+concentrations
Using the optimized values (inoculation quantity, inoculation proportion, cellulase dosage, and fermentation temperature) obtained from the orthogonal experiment, the kinetics and effects of different additions of Fe2+ concentrations (4 mg/g, 6 mg/g, 8 mg/g, and 10 mg/g (substrate)) on ethanol production by SSF of CS were investigated. Figure 1 shows the results of the replicated kinetic experiment.
Determination method
The collected materials were oven-dried at 105 C for 4 h before testing. The chemical composition analysis of the CS was performed according to previous literature and standards ASTM E1721-01 (2015), ASTM E1756-08 (2015), and ASTM E1755-01 (2015) (Sluiter et al. 2012). Ethanol concentrations were determined every 12 h according to literature (He et al. 2013). All experiments were performed in duplicate under the same anaerobic conditions. The function of the standard curve was,
where Y is the absorbance (g/L) and X is the ethanol concentration (g/L). The ethanol yield was calculated according to the following equation,
where C is alcohol concentration that was obtained from contrasting with a standard curve (g/L), V is the total volume of liquid in the container (fermentation liquid volume + inoculation quantity) (L), Mis the pretreated cornstalks (g), and Q is the ethanol yield (g/g).
RESULTS AND DISCUSSION
Process Optimization of Ethanol Production from Cornstalks
Table 1 shows that there are five major influencing factors in ethanol preparation from cornstalks: fermentation temperature, addition of Fe2+, inoculation quantity, cellulase dosage, and inoculation proportion. Through orthogonal experiments and mean value analysis, the optimum conditions were as follows: an inoculation proportion of 2:1, a fermentation temperature of 32 C, an inoculation quantity of 20%, an addition of Fe2+ in the amount of 4 mg/g (substrate), and a cellulase dosage of 30 U/g (substrate). An analysis of variance showed that at F0.05, the effect of these five factors on ethanol production from CS reached a significant level. The verification test was performed under the optimum conditions. The ethanol yield was 0.335 g/g (mean).
Table 1. Results of SSF Orthogonal Experiments in Addition of Fe2+to Fermentation Broth
The experiments were performed in duplicate and the test results were expressed as mean ± SD; the value of K1, K2, K3, K4, and R were calculated from the mean.
Table 2 illustrates that four major influencing factors in the preparation of ethanol from CS were important: fermentation temperature, inoculation quantity, cellulase dosage, and inoculation proportion. Through an orthogonal experiment and mean value analysis, the optimal conditions of the control groups were obtained as follows: the inoculation proportion was 2:1, fermentation temperature was 32 C, the inoculation quantity was 20%, and the cellulase dosage was 20 U/g (substrate). The replicated verification experiment was conducted under the optimized conditions, and the ethanol yield was 0.257 g/g.
Table 3 shows that the hemicellulose content of CS increased from 32.7% to 54.9%, lignin content decreased from 16.8% to 11.2% from liquefaction pretreatment, and therefore the binding sites between cellulase and the substrate increased, which were beneficial to fermentation. Compared with the control group, the ethanol yield in the group with added Fe2+ increased 30.4% under optimum conditions. The Fe2+ is an essential trace element for microbial growth, helping to improve the activity of cellulase and yeast (Izmirlioglu and Demirci 2016). The ethanol yield in the group with the addition of Fe2+ increased 30.4%, compared with the control group. These results showed that the ethanol yield from CS was improved via the addition of appropriate amounts of Fe2+, which was economically feasible.
Table 2. Results of SSF of Control Orthogonal Experiments
The experiments were performed in duplicate and the test results were expressed as mean ± SD, the value of K1, K2, K3, K4, and R were calculated from the mean.
Table 3. The Composition of Cornstalks (Percentage of Total Dry Weight)
Effects of Adding Fe2+ on Ethanol Yield
Based on the results of the optimization process, the effect of different Fe2+ concentrations (4 mg/g, 6 mg/g, 8 mg/g, and 10 mg/g) on ethanol fermentation was investigated. The experimental results are shown in Table 4. Figure 1 shows that ethanol yield was the greatest at an Fe2+ addition of 4 mg/g after 72 h of fermentation, which reached 0.335 mg/g (mean).
Table 4. Ethanol Yield at Different Times under Different Addition of Fe2+ (g/g)
The experiments were performed in duplicate and the test results were expressed as mean ± SD
A comparison of the ethanol yield in the liquefaction of CS at 72 h of fermentation with different quantities of Fe2+ added (4 mg/g, 6 mg/g, 8 mg/g, and 10 mg/g) is given in Fig. 1. Therefore, the authors concluded that low concentration additions of Fe2+ (at 4 mg/g) improved early ethanol yield from CS; however, higher concentration additions of Fe2+ (at 6 mg/g, 8 mg/g, and 10 mg/g) decreased the ethanol yield from CS. Published results showed that the microorganism has a partial inhibition of the biochemical reaction by adding Fe2+ under anaerobic conditions (Lü and Zhou 2015). In this work, there is essentially no chance that the Fe(II) would spontaneously form Fe(III) under the anaerobic conditions. One possible reason is that the Fe2+ is in the intermediate valence state, which can release and absorb the electron (Xu and Etcheverry 2008). This can hinder the normal life activities of microorganisms as an electron acceptor in the complex biochemical reaction process (Li et al. 2010). A high content of Fe2+ can lead to the loss of activity of yeast and cellulase (Huang et al. 2011).
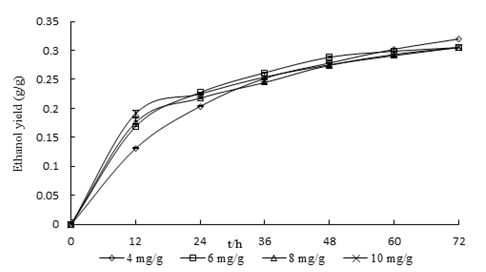
Fig. 1. Effect of the level of addition of FeSO4·7H2O on ethanol yield; the experiments were performed in duplicate and the test results were expressed as mean ± SD
Kinetic Analysis Based on Addition of Fe2+
Two-constant kinetics model
The kinetic analysis of a heterogeneous reaction was beneficial for improving the product yield. Different interface reactions had different features; thus, the kinetic equation also varies (Chen et al.2018). Kinetic modeling is important in designing and controlling bioprocess efficiency, and kinetics analysis improves product yield (Xu et al. 2008; Chen et al. 2014). Although the fractal kinetics model is the most widely used, it has several insufficiencies, which limits its applicable conditions (Yao et al. 2011; Zhang et al. 2012; Nguyen et al. 2015).
As shown in Fig. 1, under different Fe2+ additions, the ethanol yield gradually increased with increased time. The ethanol concentration was proportional to the ethanol yield, i.e., ethanol concentration increased with increased time. Because the curve was similar to that of the Langmuir adsorption isotherm, the researchers assumed that Eq. 3 could be used to describe the relationship between Q and t,
Eq. 3 can also be expressed as,
where Q is the ethanol concentration (g/g), a is the capacity for ethanol production by fermentation (mg/g), b is the rate constant (h-1), and t is fermentation time (h). The experimental data from Fig. 1 were fitted into Eq. 4 by one-dimensional nonlinear regression for each addition of Fe2+. Values of a and b determined from the fit were shown in Table 5. The regressive results were also listed in Table 5. The reliability of the relative coefficient with different additions of Fe2+ exceeded 96.7%.
Table 5. Calculation Results from the Two-constant Experiential Model
The values were calculated from the mean
It is visible from Table 5 that the catalytic kinetics of Fe2+ in the process of preparing ethanol was well-described by a two-constant experiential model with different additions of Fe2+. This process is actually a chemical reaction. Table 5 states clearly that parameter “a” decreased with an increased initial addition of Fe2+. Moreover, it was found that the capacity for ethanol production in fermentation increased with increased parameter α; in other words, ethanol yield reached the highest level with the addition of 4 mg/g of Fe2+. Table 5 shows that parameter “b” increased with the increased addition of Fe2+. Parameter “b”, representing rate constant, reached the fastest level with the addition of 10 mg/g of Fe2+. The peak rate of ethanol fermentation was achieved with the addition of 10 mg/g of Fe2+. Taking into account the maximum yield, the addition of 4 mg/g of Fe2+ was selected as the optimal process factor. Thus, the two-constant Langmuir isotherm model can adequately describe the kinetics of Fe2+ in ethanol production from CS.
Fractal-like kinetics model
As is known, the rate constant of reaction is independent of time in classical chemical kinetics. However, recent studies have shown that the rate of reaction is not proportional to the integral power of reaction time t. The cited previous studies reported that the kinetics of most heterogeneous reactions did not tally with the classical kinetics law, and the rate constant, k, is correlated with the reaction time (Clément et al. 1994; Xu et al. 2008; Zhang et al. 2012). The relation can be expressed as the following mathematical equation,
where h is the constant that measures the degree of local heterogeneity and k1 is a constant that is not related to time.
Considering the first-order kinetic integral equation, the rate coefficient k at t (t1, t2, t3, etc., were different fermentation moments) can be calculated by using the change of ethanol yield from t1 to t2and the k at t3 can be obtained by using the change of ethanol yield from t2 to t3 in the dynamic state. The others may be deduced by analogy. The k values at t (t1, t2, t3, etc., were different fermentation moments) could be calculated using by Eq. 6 (Liu et al. 2002):
The remaining k values are listed in Table 6.
As can be seen from Table 6, k decreased as the fermentation time increased with different additions of Fe2+. The fermentation process is fractal-like, and these k values appear to vary irregularly with the addition of Fe2+. The rate coefficient in this paper, which was a two-variable function of time and the addition of Fe2+, was different from the rate constant in classical kinetics. The authors obtained the ethanol yield from CS at different times and conditions with the addition of Fe2+. They found that ethanol yield steadily decreased with increased addition of Fe2+ at the fermentation time of 72 h. Thus, these k values might increase or decrease as the addition of Fe2+ increases, as shown in this paper.
Table 6. Reaction Rate Coefficient k at Different Additions of Fe2+ in Reaction Time
The values were calculated from the mean
To explore the quantitative relationship between the rate coefficient and time, Eq. 7 can also be expressed as follows:
The experimental data is regressed by using Eq. 5, the logk versuslogt was described with Eq. 7, and the result is listed in Table 7. Thus, the reliability of the relative coefficient with various additions of Fe2+exceeded 96.7%. This indicated that Eq. 7 was adapted to describe the quantitative relationship between the fermentation rate coefficient of CS and time in a static state. Namely, the fermentation kinetics of CS with different additions of Fe2+ was fractal-like.
Table 7. Modeling Results for logk and logt
The values were calculated from the mean
There was a decreasing tendency for parameter k1 and h while the addition of Fe2+ increased (except 6 g/g). Further, Eq. 5 showed kwas concerned with h. Therefore, the authors concluded that parameter h was relative to the fractal dimension (Xu et al. 2008). Because h was larger than 1, the fractal dimension and the order of reaction cannot be obtained. However, it was confirmed that the process of fermentation from CS with different additions of Fe2+ was fractal-like (Yao et al. 2011; Zhang et al. 2012; Chen et al. 2014; Nguyen et al. 2015).
In this work, we only considered the effect of Fe(II) on ethanol production from CS under anaerobic conditions. We didn’t consider the likelihood that a portion of the Fe(II) added to the system in the present work was converted to Fe(III) as a result of air oxidation. Moreover, Potassium Dichromate-DNS Colorimetric (He et al. 2013) was used in the experiment, some of the Fe(II) may be converted to Fe(III). Fe3+ is promoter in enhanced ethanol yield (Lü and Zhou 2011, 2015, 2017). Therefore, the results of ethanol yield determination may be smaller. We suggest future work in which the ratio of Fe(II) to Fe(III) is controlled, redox tests are carried out, and oxygen is excluded.
CONCLUSIONS
- The optimal conditions of ethanol production from CS with Fe2+ promoter were an inoculation proportion (ratio of P. tannophilus to S. cerevisiae) of 2:1, fermentation temperature of 32 C, inoculation quantity of 20%, an additional amount of Fe2+ 4 mg/g (substrate), and the cellulase dosage of 30 U/g (substrate). Compared with the control group without the addition of Fe2+, ethanol yield with the group containing Fe2+increased 30.4%. The enhancement was mainly observed in the earliest set of tests (12 h).
- Fe(II) addition at an optimal level shortened the time needed to achieve ethanol yields above 0.335 g/g. The time course of conversion of CS to ethanol under different conditions of added Fe2+ was matched with the fractal-like kinetic model. It was also clear that the relationship between the rate constant and fermentation time could be described using the Langmuir isotherm model and inverse power function.
- The good fit of the experimental data confirmed the applicability of the kinetic model to describe the complex characteristics of ethanol production from CS with Fe2+catalysis. This kinetic model provides a new way to analyze the complex kinetics of ethanol production from CS with Fe2+catalysis.
ACKNOWLEDGMENTS
The authors are grateful for the financial support of the Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ1601337), the China Scholarship Council (201508505060), the National Natural Science Foundation of China (No.51404049), the Research Foundation of Chongqing University of Science & Technology (CK2015B14), and the Chongqing Administration of Work Safety (CQAWS2013Y-004).
REFERENCES CITED
ASTM E1721-01 (2015). “Standard test method for determination of acid-insoluble residue in biomass,” ASTM International, West Conshohocken, PA.
ASTM E1755-01 (2015). “Standard test method for ash in biomass,” ASTM International, West Conshohocken, PA.
ASTM E1756-08 (2015). “Standard test method for determination of total solids in biomass,” ASTM International, West Conshohocken, PA.
Chen, K., Jing, X. F., and Liao, H. (2018). “Kinetic models and effects of Mn(II) ion on ethanol production from cornstalks,” BioResources 13(1), 954-966. DOI: 10.15276/biores.13.1.954-966
Chen, K., Xu, L. J., Bi, Z. M., and Fu, Z. L. (2013). “Kinetics analysis of the enzymatic hydrolysis of cellulose from straw stalk,” Chinese J. Geochem. 32(1), 41-46. DOI: 10.1007/s11631-013-0605-7
Chen, K., Xu, L. J., Yang, R., Bi, Z. M., and Fu, Z. L. (2014). “Kinetics of fuel ethanol production by simultaneous saccharification and fermentation of straw stalk,” Chemistry and Industry of Forest Products 34(1), 13-18. DOI: 10.3969/j.issn.0253-2417.2014.01.003
Clément, E., Kopelman, R., and Sander, L. (1994). “The diffusion-limited reaction A + A →0 in the steady state: Influence of correlations in the source,” Chem. Phys. 180(2-3), 337-341. DOI: 10.1016/0301-0104(93)E0413-P
He, C., Zhang, D. Z., Zhang, J., Yang, X., Su, Y., Zhou, P. P., and Yu, L. J. (2013). “Potassium dichromate-DNS colorimetric determination of the content of ethanol in the fermentation broth,” Life Science Research 17(1), 1-4.
Huang, H. J., Yuan, X. Z., Zeng, G. M., Wang, J. Y., Li, H., Zhou, C. F., Pei, X. K., You, Q., and Chen, L. (2011). “Thermochemical liquefaction characteristics of microalgae in sub- and supercritical ethanol,” Fuel Process. Technol. 92(1), 147-153. DOI: 10.1016/j.fuproc.2010.09.018
Izmirlioglu, G., and Demirci, A. (2016). “Improved simultaneous saccharification and fermentation of bioethanol from industrial potato waste with co-cultures of Aspergillus niger and Saccharomyces cerevisiae by medium optimization,” Fuel 185, 684-691. DOI: 10.1016/j.fuel.2016.08.035
Jain, A., Balasubramanian, R., and Srinivasan, M. P. (2016). “Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review,” Chem. Eng. J. 283, 789-805. DOI: 10.1016/j.cej.2015.08.014
Li, H., Yuan, X. Z., Zeng, G. M., Huang, D. L., Huang, H. J., Tong, J. Y., You, Q., Zhang, J. C., and Zhou, M. (2010). “The formation of bio-oil from sludge by deoxy-liquefaction in supercritical ethanol,” Bioresource Technol. 101(8), 2860-2866. DOI: 10.1016/j.biortech.2009.10.084
Li, S. C., Zhang, P. P., Gu, S. B., Liu, H. X., Liu, Y., and Liu S. N. (2013). “Screening of lipid high producing mutant from Rhodotorula glutinis by low ion implantation and study on optimization of fermentation medium,” Indian J. Microbiol. 53(3), 343-351. DOI: 10.1007/s12088-013-0361-8
Liu, C. L., Xu, L. J., and Xian X. F. (2002). “Fractal-like kinetic characteristics of rock salt dissolution in water,” Colloid. Surface. A201(1-3), 231-235. DOI: 10.1016/S0927-7757(01)01021-4
Liu, L., Sun, J. S., Cai, C. Y., Wang, S. H., Pei, H. S., and Zhang, J. S. (2009a). “Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation,” Bioresource Technol.100(23), 5865-5871. DOI: 10.1016/j.biortech.2009.06.048
Liu, L., Sun, J. S., Li, M., Wang, S. H., Pei, H. S., and Zhang, J. S. (2009b). “Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment,” Bioresource Technol. 100(23), 5853-5858. DOI: 10.1016/j.biortech.2009.06.040
Lü, J. L., and Zhou, P. J. (2011). “Optimization of microwave-assisted FeCl3 pretreatment conditions of rice straw and utilization of Trichoderma viride and Bacillus pumilus for production of reducing sugars,” Bioresource Technol. 102(13), 6966-6971. DOI: 10.1016/j.biortech.2011.04.044
Lü, J. L., and Zhou, P. J. (2015). “Ethanol production from microwave-assisted FeCl3 pretreated rice straw,” Energ. Source. Part A 37(21), 2367-2374. DOI: 10.1080/15567036.2011.649335
Lü, J. L., and Zhou, P. J. (2017). “Ethanol production from microwave-assisted FeCl3 pretreated rice straw using free and immobilized cells of Trichoderma viride and Saccharomyces cerevisiae,” J. Energ. Eng. 143(2), 04016043. DOI: 10.1061/(ASCE)EY.1943-7897.0000396
Mei, Y. Y., Che, Q. F., Yang, Q., Draper, C., Yang, H. P., Zhang, S. H., and Chen, H. P. (2016). “Torrefaction of different parts from a corn stalk and its effect on the characterization of products,” Ind. Crop. Prod. 92, 26-33. DOI: 10.1016/j.indcrop.2016.07.021
Monavari, S., Galbe, M., and Zacchi, G. (2011). “The influence of ferrous sulfate utilization on the sugar yields from dilute acid pretreatment of softwood for bioethanol production,” Bioresource Technol. 102(2), 1103-1108. DOI: 10.1016/j.biortech.2010.08.077
Nguyen, Q. A., and Tucker, M. P. (2002). “Dilute acid/metal salt hydrolysis of lignocellulosics,” U.S. Patent No. 6423145B1.
Nguyen, T. Y., Cai, C. M., Kumar, R., and Wyman C. E. (2015). “Co-solvent pretreatment reduces costly enzyme requirements for high sugar and ethanol yields from lignocellulosic biomass,” ChemSusChem 8(10), 1716-1725. DOI: 10.1002/cssc.201403045
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Crocker, D. (2012). Determination of Structural Carbohydrates and Lignin in Biomass (NREL/TP-510-42618), National Renewable Energy Laboratory, Golden, CO.
Wong, Y. C., and Fikri, A. M. (2014). “Synthesis of bio-ethanol from corn stalk by fermentation process,” Oriental Journal of Chemistry 30(2), 637-642. DOI: 10.13005/ojc/300232
Xu, C. B., and Etcheverry, T. (2008). “Hydro-liquefaction of woody biomass in sub- and super-critical ethanol with iron-based catalysts,” Fuel 87(3), 335-345. DOI: 10.1016/j.fuel.2007.05.013
Xu, L. J., Zhou, Z. G., Liu, C. L., and Xian, X. F. (2008). “Fractal-like adsorption kinetics of Pb2+ in rocks,” Chinese J. Geochem.27(2), 126-129. DOI: 10.1007/s11631-008-0126-y
Yan, Y. J., Li, T. C., Ren, Z. W., and Li, G. Z. (1996). “A study on catalytic hydrolysis of peat,” Bioresource Technol. 57(3), 269-273. DOI: 10.1016/S0960-8524(96)00103-4
Yao, M. J., Wang, Z. L., Wu, Z. Q., and Qi, H. S. (2011). “Evaluating kinetics of enzymatic saccharification of lignocellulose by fractal kinetic analysis,” Biotechnol. Bioproc. E. 16(6), 1240-1247. DOI: 10.1007/s12257-011-0283-4
Zhang, B. Z., Zhang, S. P., Zhao, S. T., Xu, Q. L., and Yan, Y. J. (2014). “Pretreatment of sweet sorghum stalk using dilute acid,” Energ. Source. Part A 36(16), 1835-1842. DOI: 10.1080/15567036.2011.596899
Zhang, Y., Xu, J. L., Qi, W., Yuan, Z. H., Zhuang, X. S., Liu, Y., and He, M. C. (2012). “A fractal-like kinetic equation to investigate temperature effect on cellulose hydrolysis by free and immobilized cellulase,” Appl. Biochem. Biotech. 168(1), 144-153. DOI: 10.1007/s12010-011-9362-4
Zhao, J., Zhang, H. M., Zheng, R. P., Lin, Z. X., and Huang, H. (2011). “The enhancement of pretreatment and enzymatic hydrolysis of corn stover by FeSO4 pretreatment,” Biochem. Eng. J. 56(3), 158-164. DOI: 10.1016/j.bej.2011.06.002
Article submitted: March 20, 2018; Peer review completed: May 1, 2018; Revised version received: May 9, 2018; Accepted: May 10, 2018; Published: May 15, 2018.
DOI: 10.15376/biores.13.3.4973-4985
