Abstract
Coconut shell, a natural biopolymer, is available in high amounts as waste in many countries. It could potentially be a crucial renewable source of raw materials for the carbon fiber industry. In this study, a series of aprotic ionic liquids, [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl, were used in the dissolution and regeneration process of coconut shell. The results indicate that the dissolution of coconut shell (up to 70 mg of coconut shell per g of solvent) can occur in aprotic ionic liquids under a nitrogen atmosphere at 110 °C (6 h) and 150 °C (2 h). The extraction efficiency was greatly influenced by temperature, time, particle size, and types of cations and anions in the ionic liquids. At 150 °C, 10% regenerated lignin was obtained in [Emim][Ace], which was higher compared with [Emim]Cl, [Bmim][Ace], and [Bmim]Cl. The recyclability of the ionic liquids during the dissolution process (up to four times) was also scrutinized. The structure and properties of the untreated coconut shell and regenerated lignin were characterized by Fourier transform infra-red (FTIR) spectroscopy, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), X-ray diffraction (XRD) analysis, and proton nuclear magnetic resonance (1H NMR).
Download PDF
Full Article
Lignin Extraction from Coconut Shell Using Aprotic Ionic Liquids
Siti Mastura Zakaria,a,b,*Azila Idris,a,b and Yatimah Alias a,b
Coconut shell, a natural biopolymer, is available in high amounts as waste in many countries. It could potentially be a crucial renewable source of raw materials for the carbon fiber industry. In this study, a series of aprotic ionic liquids, [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl, were used in the dissolution and regeneration process of coconut shell. The results indicate that the dissolution of coconut shell (up to 70 mg of coconut shell per g of solvent) can occur in aprotic ionic liquids under a nitrogen atmosphere at 110 °C (6 h) and 150 °C (2 h). The extraction efficiency was greatly influenced by temperature, time, particle size, and types of cations and anions in the ionic liquids. At 150 °C, 10% regenerated lignin was obtained in [Emim][Ace], which was higher compared with [Emim]Cl, [Bmim][Ace], and [Bmim]Cl. The recyclability of the ionic liquids during the dissolution process (up to four times) was also scrutinized. The structure and properties of the untreated coconut shell and regenerated lignin were characterized by Fourier transform infra-red (FTIR) spectroscopy, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), X-ray diffraction (XRD) analysis, and proton nuclear magnetic resonance (1H NMR).
Keywords: Ionic liquids; Biomass; Lignocellulosics; Lignin; Coconut shell
Contact information: a: Department of Chemistry, Faculty of Science, University of Malaya, 50603, Kuala Lumpur, Malaysia; b: University Malaya Centre for Ionic Liquids (UMCiL), University of Malaya, 50603 Kuala Lumpur, Malaysia; *Corresponding author: azila_idris@um.edu.my
INTRODUCTION
The accumulation of greenhouse gases resulting from over-dependence on non-renewable fossil fuel, has caused an increase in global warming (Xie and Gathergood 2013). To counteract this problem, researchers have considered utilizing waste biomass materials and converting them into biorefinery products. Biomass includes all organic matter produced by photosynthesis (Sriram and Shahidehpour 2005). Lignocellulosic feedstock biorefinery products are derived from agricultural crops or waste biomass, such as wood chips, maize, and corn (Kamm and Kamm 2004; Cherubinin 2010; Chandra et al. 2012). Biomass is a great and important source of renewable energy in agriculture-based countries because of the abundant supply and low cost (Stöcker 2008). This resource could be used in a more efficient manner as a preliminary material in the chemical industry.
Coconut palm, Cocos nucifera L., is a source of income, especially in developing countries (Sivapragasam 2008). It is primarily a plantation crop in Brazil, the Philippines, India, Indonesia, Malaysia, and Sri Lanka (Kumar 2011). In this study, coconut shell was chosen as the biomass because it is not currently used commercially. Huge amounts of coconut shell waste are discarded, which is detrimental to the environment because of its poor biodegradability (Goh et al. 2010; FAO 2012; Kanojia and Jain 2017). The main components of coconut shell are cellulose, lignin, and hemicelluloses (Rodrigues and Pinto 2007). In a previous study, powdered coconut shell was used for the biosorption of heavy metals such as cadmium, chromium, and arsenic, which can then be extracted by ultrasound to obtain high amounts of phenolic compounds (Pino et al. 2006). Coconut shell can also be used as an effective material precursor in water treatment and removal of impurities, and it produces high-quality activated charcoal (Cobb et al. 2012; Ewansiha et al. 2012).
Lignocellulosic biomass is mostly composed of three chemical fractions or precursors, which are cellulose (a glucose polymer), hemicellulose (a sugar polymer predominantly containing pentoses), and lignin (a polymer of phenols) (Sivapragasam 2008; Xie and Gathergood 2013). Lignin is an aromatic renewable resource and the second largest component of biopolymers. It has potential to be converted into desirable high-value phenolic products that can replace products derived from petroleum and fossil fuels (Ji et al. 2012). Unlike cellulose, lignin is an amorphous, polyphenolic polymer arising from the polymerization of phenylpropanoid monomers, including coniferyl, sinapyl, and p-coumaryl alcohols (Mathiasson and Kubát 1994; Pandey 1999; Kubo and Kadla 2005; Pu et al. 2007; Sivapragasam 2008; Ouyang et al. 2009; Cesarino et al. 2012; Baker and Rials 2013). Lignin is difficult to dissolve or extract because of the strong lignocellulosic structure, and it is contained primarily in the cell wall of polysaccharides (Cesarino et al. 2012). The heterogeneous molecular structure of lignin and the ineffectual modification and depolymerization methods cause the applications of lignin to be focused only on low-value products. It has been used as a dispersant (Ouyang et al. 2009), binder (Mathiasson and Kubát 1994), emulsifying agent (Chatel and Rogers 2013), and also in pharmaceutical processes, such as surface coatings and nanoglues (nanoparticles) (Lievonen 2015). Recently, lignin has attracted interest for its conversion into carbon fiber (Kubo and Kadla 2005; Baker and Rials 2013).
To utilize this biopolymer (lignin) and convert it into high-value products, there is a need to find alternative solvents that efficiently dissolve lignocellulosic biomass. The traditional lignin separation processes, such as kraft pulping, sulfite pulping, and soda pulping, are known to use harsh solvents and harsh conditions that contribute to environmental pollution (Wegener 1992; Tan et al. 2009; Ji et al. 2012; Xie and Gathergood 2013; Espinoza-Acosta et al. 2014). Extracting lignin from biomass using this ionic liquid has several advantages, including reaction at low pressure, no emission of toxic or odourous gases, capability of recycling the ionic liquid, no requirement for prolonged drying of material, and the ability to produce lignin with particular functional groups (Tan et al. 2009; Rashid et al. 2016). Thus, effective pretreatment of biomass is necessary to reduce the recalcitrance of the lignocellulosic structure (Pielhop et al. 2016). According to Tan et al. (2009), the glass transition temperature of lignin is around 150 °C; thus, high temperatures and high pressures are needed during the pretreatment process. Therefore, the aim of this study was to develop and investigate the use of aprotic ionic liquids for the purpose of dissolving and regenerating of coconut shell. The ionic liquids were based on imidazolium-salt of various anion, where the study were conducted under variety of conditions including various particle sizes, temperature, and duration time.
This research focuses on regenerated lignin from coconut shell using aprotic ionic liquids (AILs). ILs are generally defined as salts with a melting point below 100 °C, and they contain organic cations and organic/inorganic anions (Welton 1999; Sheldon et al. 2002; Wasserscheid and Welton 2007). ILs are considered to be green solvents compared with inorganic acids (sulfuric acid, hydrochloric acid, and nitric acid) because ILs have unique characteristics and they are suitable for use in safer and cleaner industries (Sriram and Shahidehpour 2005). Some distinctive features include a negligible vapor pressure, non-flammability, a low melting point, and they are found in liquid form at ambient atmosphere (Baranyai et al. 2004; Dorn et al. 2008). In addition, ILs also possess an excellent capacity to dissolve organic biomass (da Costa Lopes et al. 2013; Xie and Gathergood 2013). Previously, ILs have been used as the solvent media to dissolve biomass such as rice husk, Norway spruce sawdust, corn stover, and bamboo (Kilpeläinen et al. 2007; Ang et al. 2012; Yang et al. 2013; Mood et al. 2014). It has been demonstrated that ILs can dissolve biomass partially or completely, depending on the type of biomass (Sun et al. 2009; Brandt et al. 2011; Mora-Pale et al. 2011). The reaction process can be done at low or high temperatures (thermally stable) because ILs have wide liquidus ranges (Ignat’ev et al. 2005; Bejan et al. 2010). The liquidus range is defined as the temperature span between the freezing point and boiling point of a liquid (Wilkes 2002). Their tunable properties make ILs capable of being widely used in different fields of applications, such as the pharmaceutical, electrochemistry, catalysis, energy, and nanotechnology fields (Pârvulescu and Hardacre 2007; Ohno 2011; Armand et al. 2009; Wishart 2009; Dupont and Scholten 2010; Marrucho et al. 2014).
ILs can be divided into two groups, aprotic and protic ionic liquids (King et al. 2009). AILs are salts consisting solely of cations, which are not protonated, and anions (Freemantle 2010). Most AILs are prepared by the combination of alkylated organic cations (imidazolium, pyridinium, etc.) and various anions (chloride, bromide, dicyanamide, etc.) that are typically formed through ion exchange (King et al. 2009). The introduction of AILs into biomass is important to the sustainable chemical industry because they provide a variety of valuable chemical feedstocks. Recently, imidazolium-based ILs with short side alkyl chains have been extensively used for the dissolution and delignification of biomass (Pu et al. 2007; King et al. 2009).Among the ILs explored, imidazolium chloride was discovered to be more suitable for cellulose dissolution, whereas imidazolium acetate was considered to be fit for lignin dissolution (Kilpeläinen et al. 2007; Vitz et al. 2009; Zakrzewska et al. 2010; Rashid et al. 2016).
EXPERIMENTAL
Materials
The ILs 1-ethyl-3-methylimidazolium acetate ([Emim][Ace]), 1-ethyl-3-methyl-imidazolium chloride ([Emim]Cl), 1-butyl-3-methylimidazolium acetate ([Bmim][Ace]), 1-butyl-3-methylimidazolium chloride ([Bmim]Cl), 1-ethyl-3-methylimidazolium diethyl phosphate ([Emim][DEP]), 1-butyl-3-methylimidazolium octyl sulfate ([Bmim][OS]), 1-ethyl-3-methylimidazolium ethyl sulfate ([Emim][ESO4]), and 1-butyl-3-methylimida-zolium bromide ([Bmim]Br) were purchased from Sigma Aldrich (Missouri, United States) and dried under vacuum prior to use to remove any water content.
The coconut shell was purchased at a market in Kuala Lumpur, Malaysia, initially washed with water to remove the impurities, and then dried in a vacuum oven at 65 °C for 24 h. The dried coconut shell was manually broken into small pieces before undergoing pulverization, which was followed by sieving to obtain different mesh sizes. Then, the sieved coconut was filtered into different particle sizes (10-65 µm, 65-125 µm, 125-250 µm, and 250-500 µm) using a Laboratory Test Sieve (Endocotts Ltd, London, England).
Pretreatment of Biomass in Ionic Liquid: Dissolution and Extraction
The dissolution of the coconut shell biomass in the ILs was carried out, followed by the fractionation of cellulose and lignin according to the method reported by Sun et al. (2009) with minor modifications. The dissolution of the coconut shell in the ILs was investigated with various particle sizes. The dissolution process was carried out in glass vials, which were placed on a heating block while stirring under a nitrogen (N2) atmosphere. A temperature range of room temperature, 80, 90, 100, 110, and 150 °C was used in this study because the different transition temperature range has been found to increase the solubility in the ILs and production of chemical compositions (Tan et al. 2009).
The brownish solution formed was gradually added to a mixture of acetone/water (6:4) and rapidly stirred until regenerated cellulose-rich material was precipitated. Then, the material was centrifuged (4000 rpm, 10 min) and dried in an oven at 60 °C after being washed three times with distilled water. The supernatant was transferred to a beaker, and the acetone was allowed to evaporate at room temperature. The precipitated lignin was filtered by vacuum filtration using 0.8-µm nylon filter paper because of the smaller particle size of lignin. Then, the regenerated lignin was dried in a vacuum oven at 60 °C. The experiments were replicated (forth times).
Solubility Test
Solubility was screened in a series of ionic liquids in glass vials,which were fitted into a heating block under N2 atmosphere. To quantify the solubility, the sequential addition of 10 mg of coconut shell was added into 1 g of IL and stirred until they were completely dissolved in the ionic liquid. The coconut shell that dissolved completely in ionic liquids resulted in a vicious solution. The limiting solubility was determined through the sequential addition of coconut shell until the dissolution became too vicious for rapid dissolution, where coconut shell were no longer can dissolve in ionic liquids. Complete dissolution was confirmed by using a Motic microscope (Hong Kong,China) and laser beam to detect any presence of small particles. The solubility experiments were run at 110 °C for 6 h.
Recycling of Ionic Liquids
The acetone and water in ILs were removed using a rotary evaporator at 60 °C. Then, the ILs were further dried under vacuum before being employed for the next dissolution to obtain a moisture content less than 1.0 wt.%. The moisture content in the ILs was measured using Karl Fischer titration.
Characterizations
Fourier transform infra-red (FTIR) spectroscopy of the coconut shell, kraft lignin, and regenerated lignins was performed with a Perkin-Elmer Spectrum RX1 FT-IR spectrophotometer (Waltham, United States) and KBr pellets made up of 1% of the sample. The FTIR was performed in the wavenumber range of 800 to 4000 cm-1. The spectra were recorded at a resolution of 4 cm-1 with 32 scans. The spectra were baseline-corrected.
Powder X-ray diffraction (PXRD) analysis was performed to measure the coconut shell crystallinity. The PXRD analysis was done by calculating the area under the peaks of the XRD spectra obtained by a Bruker D8 Discover X-ray diffractometer (Madison, United States) using Cu-Kα-1 as the radiation source. For each experiment, approximately 1 g to 2 mg of finely ground sample was placed randomly on a locally designed flat brass sample holder fitted with an O-ring sealed Mylar sheet, which provided an airtight atmosphere. The sample was scanned and recorded with a 2θ of 5° to 50° and with a step size of 0.02°.
Thermogravimetric analysis (TGA) of the coconut shell and regenerated lignin was carried out using a Perkin-Elmer Pyris 400 TGA (Waltham, United States). Samples with an average weight of 5 mg were placed in a ceramic pan and heated from 40 to 900 °C at a rate of 20 °C/min in a flowing nitrogen atmosphere. The instrument was calibrated using nickel.
Differential scanning calorimetry (DSC) was conducted on a TA Instruments DSC Q200 (New Castle, Delaware) with 5 to 10 mg of sample in closed aluminum pans, at a temperature ramp rate of 10 °C/min. The untreated coconut shell and regenerated lignin samples were heated from room temperature to 200 °C. The transition temperature was reported using the maximum peak of the thermal transition, while the glass transition temperature (Tg) was defined as the midpoint of the temperature range at which the change in heat capacity occurred.
The Proton Nuclear Magnetic Resonance (1H NMR) spectra were obtained in deuterated DMSO and were recorded using a Bruker Advance 400 (Rheinstetten, Germany) at 400 MHz referenced to water at 3.67 ppm.
RESULTS AND DISCUSSION
The characterization of the coconut shell was done according to several TAPPI standard methods. The moisture content (5.66 ± 0.03 wt.%) was determined after drying the samples at 105 °C for 4 h (TAPPI T264 cm-97, 1997). The chemical composition, given on an oven-dry weight basis, was as follows: 1.7 ± 0.5% ash ((TAPPI T211 om-93, 2002), 36.8 ± 0.7% aqueous NaOH soluble matter (TAPPI T212 om-98, 1998), 18.4 ± 0.4% lignin (TAPPI T222 om-98, 1998), 23.8 ± 1% hemicellulose (Wise et al. 1946), and 32.6 ± 0.2% cellulose (Rowell 1984).
Stability of Aprotic Ionic Liquids
The thermal properties and decomposition of the AILs, [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl, were determined by TGA for the useful temperature range.
Fig. 1. TGA curves of the fresh AILs
The plot of the weight percent loss temperature in Fig. 1 represents the thermal decomposition of the four AILs. Temperatures below 150 °C corresponded to the drying period where water evaporation occurred. The decomposition temperatures of the fresh AILs occurred between 210 and 300 °C, and the maximum decomposition temperature was 350 °C. A small weight loss was observed for [Emim]Cl between 100 and 150 °C because of water loss.
The results (Fig. 1, Fig. 2, and Table 1) clearly show that [Bmim]Cl and [Emim]Cl were more thermally stable, both of which started to decompose at 260 °C. Meanwhile, [Bmim][Ace] and [Emim][Ace] were stable up to 210 and 220 °C, respectively. Thus, the temperatures 110 and 150 °C were chosen in this study to extract lignin from the coconut shell biomass.
Table 1. Decomposition Temperatures of the Fresh Ionic Liquids
T10%: Temperature at 10% weight loss
Dissolution of Coconut Shell in Ionic Liquids
Ionic liquids are known for their ability to dissolve large quantities of biomass (Swatloski et al. 2002; Hart et al. 2015; Teh et al. 2015), but coconut shell has not previously been explored. Hence, a range of aprotic ionic liquids were investigated to determine for their ability to dissolve the coconut shell. Initially, the dissolutions of coconut shell were attempted at room temperature, 80, 90 and 100 °C, however, no dissolution was observed. This could have been due to the highly crystalline structure of the lignocellulosic biomass (Stöcker 2008). At 110 °C, the coconut shell was completely dissolved in the ILs. This could have been due to the fact that the network of hydrogen bonds was destabilized during the dissolution of the coconut shell (Kilpeläinen et al. 2007; Xu et al. 2010).
Table 2 shows the solubility testing of the coconut shells in common ILs with the sequential addition of 10 mg of coconut shell in 1 g of IL for 6 h. The solubility results showed low dissolution of coconut shell in [Emim][DEP], [Bmim][OS], [Emim][ESO4], and [Bmim]Br, which was likely due to the low viscosities and thermal stabilities of those ILs (Swatloski et al. 2002).
Although, Brandt et al. (2013) reported the solubility of lignin on the ionic liquid was based on the sulfate anion. However, the highest dissolution of coconut shell was found in [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl, which had up to 70 mg of coconut shell in 1 g of IL. Therefore, they were selected to determine the extraction efficiency of lignin from coconut shell.
In this study, the parameters that affected the regeneration of lignin from the coconut shell biomass were investigated, which are time, temperature, particle size of biomass, types of cations and anions in the ILs, and efficiency of the recycled ILs.
Table 2. Solubility Testing of Coconut Shell in Common Ionic Liquids
[Emim] = 1-ethyl-3-methylimidazolium; [Bmim] = 1-butyl-3-methylimidazolium; [DEP] = diethyl phosphate; [OS] = octyl sulfate; [ESO4] = ethyl sulfate; Br = bromide; [Ace] = acetate; Cl = chloride
Effect of Time and Temperature
There are several factors that need to be explored to improve the solubilization of coconut shell, including time and temperature. The pretreatment process of 70 mg of coconut shell in 1 g of IL was carried out at different temperatures (110 and 150 °C) and incubation times until the coconut shell (with particle size of 10-65 µm) was completely dissolved in the ILs. High temperatures and long incubation times influence the yield of regenerated materials from biomass (Espinoza-Acosta et al. 2014). Tan et al. (2009) reported that the delignification of biomass can be performed by increasing the temperature above the Tg of lignin (170 to 190 °C). The production of industrial lignin (kraft lignin, lignosulphonate lignin, and soda lignin) commonly involves a high degree of delignification in pretreatment (Xie and Gathergood 2013; Fisher and Fong 2014). Table 3 shows the percentage of the regenerated lignin from ILs at 110 (6 h) and 150 °C (2 h).
Table 3. Percentage of Regenerated Lignin from Ionic Liquids at Various Temperatures and Times
At 150 °C, the percentage of regenerated lignin was higher than when the temperature was at 110 °C. High temperatures (120 to 180 °C) were used previously in the delignification of hardwood and softwood biomass in molecular organic solvents (Kiran and Balkan 1994; Nagle et al. 2002). Those studies showed that the percentage of lignin was increased by increasing the temperature above the Tg of lignin, which improved the breaking of the cellulose-lignin bonds. Li et al. (2011) also stated that the yield of regenerated cellulose and lignin increased when the temperature increased from 160 to 190 °C. This indicated that a high temperature accelerates the separation of cellulose and increases the precipitation of free lignin.
Effect of Particle Size
In order to increase the dissolution of biomass, milling or grinding is an important step to obtain smaller particle size. Small particle size of solutes provides a larger surface area, resulting in a higher rate of dissolution compared to larger particle sizes of solutes. This was due to the larger surface area, which allowed the IL to easily penetrate and break the lignocellulosic network (Sun et al. 2009). Table 4 shows the dissolution time with different particle sizes of [Emim][Ace]. The coconut shell started to dissolve after 30 min, when the color changed to black. The color indicated the presence of the soluble lignin fraction in the ILs (Sun and Cheng, 2002; Kilpeläinen et al. 2007). It was crucial to choose the right particle size for dissolving the biomass in ILs. It was indicated that the smaller biomass particle sizes (10 to 65 µm) are easier to dissolve in ionic liquids. Leskinen et al. (2013) indicated that the preparation of starting materials strong influence the dissolution of biomass in ionic liquids because the particle sizes and milling types affect the dissolution of sawdust. In addition, Padmanabhan et al. (2011) reported that the reduction of particle size of Miscanthus also reduced the dissolution time.
Table 4. Rate of Dissolution [Emim][Ace] with Different Particle Sizes at 110 °C
Effect of the Cations and Anions in the Ionic Liquids on the Dissolution Process
Swelling and dissolution of the biomass depend on the types of cations and anions in the ILs (George et al. 2011). The results showed that [Bmim][Ace] and [Emim][Ace] produced higher percentages of regenerated lignin compared to [Bmim]Cl and [Emim]Cl. Imidazolium-based ILs have high polarities because of their ionic characteristics, resulting in enhanced biopolymer dissolving capacities (Pinkert et al. 2009). Acetate-based ILs have been found to have low viscosities, low melting points, low toxicity, and are less corrosive than chloride-based ILs (Fendt et al. 2010). Previous studies also used acetate ions as a choice for the IL anion to dissolve lignocellulosic biomass (Swatloski et al. 2002; Stöcker 2008; Cobb et al. 2011; Li et al. 2011). The strong hydrogen bond acceptance of the acetate anion enabled the destruction of the hydrogen network of the polymer chain in the coconut shell. The high hydrogen basicity of acetate anion allows it to attack free hydroxyl groups and cleave intra and intermolecular hydrogen bonds, which lead to increase the dissolution of biomass (Sun et al. 2009; Brandt et al. 2012; Luo et al. 2013; Zhang et al. 2014). Anions influence the degree of lignin structural modifications, which leads to the cleavage of different linkages within lignin, and also controls the solubility of lignin (George et al. 2011; Sun et al. 2011). The interaction of ILs with terminal hydroxyl groups of lignin, resulting disruption of lignin-cellulose network of biomass, thus increase the release of the regenerated lignin. Lateef et al. (2009) also reported the disruption of the internal network within lignin molecule would affect the dissolution of lignin. Although, chloride anion showed good lignin extraction, but it has high liquid viscosity and high melting temperature compared to acetate anion. Therefore, acetate-based ILs are recognized as the best ILs for lignin dissolution. Moreover, ILs containing large, non-coordinating anions, such as PF6, OS, and ESO4, were unsuitable as solvents for lignin (Pu et al. 2007). The [Emim]-based ILs produced higher percentages of lignin compared with the [Bmim]-based ILs. This was due to the shorter alkyl side chain length on the imidazolium cation, which decreases the viscosity and toxicity of the ILs (Pinkert et al. 2009; Yu et al. 2009; Pinkert et al. 2011). [Emim][Ace] has a lower viscosity than [Bmim][Ace], which led to an increase in the ion mobility during the pretreatment process. According to Teh et al. (2015), [Emim][Ace] also was shown to have a high enough solubility to dissolve macadamia nut shells.
Fig. 2. The percentage of regenerated lignin in ILs with various particle sizes of coconut shell at 110 °C
Characterization of Regenerated Lignin
FTIR analysis
To further understand the structural differences in the untreated coconut shell and regenerated lignins from the AILs, FTIR measurements were performed. Figure 3 displays the absorption spectra of the untreated coconut shell, kraft lignin (standard), and regenerated lignins from the ILs. The absorption bands of all of the regenerated lignins were found to be similar to the commercial kraft lignin (standard) and were in agreement with the literature (Sun et al. 2009; Tan et al. 2009; Pinkert et al. 2011; Cesarino et al. 2012; Financie et al. 2016). The FTIR analysis of the spectra confirmed the standard lignin and regenerated lignin from [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl were similar to the data from previous studies (Tan et al. 2009; Hsu et al. 2010). It also showed the presence of functional groups in lignin, as reported in previous studies, such as hydroxyl, methyl, carbonyl, methoxyl, and carboxyl groups. The spectra showed a broad absorption band at 3500 to 3200 cm-1 that represents the stretching hydroxyl groups in phenolic and aliphatic structure. There was also C-H stretching in the methyl and methylene groups (2936 to 2938 cm-1), C-H stretching in the methoxy groups (2842 to 2849 cm-1), C=O stretching (1706 to 1653 cm-1), C=C stretching (1680 to 1640 cm-1), and C-O stretching (1300 to 1000cm-1) (Pinkert et al. 2011). The regenerated lignin from AILs displayed a significant increase absorption for -OH groups (~3300 cm-1) stretching and increase in C-H groups (~2850 cm-1) stretching in methoxy groups compared to kraft lignin. From the spectra, typical lignin structures were identified: aromatic skeletal vibrations (1597 to 1456 cm-1), syringyl ring breathing with C-O stretching (1120 cm-1), C-H in plane deformation in the guaiacyl ring (1113 cm-1), C-H in-plane deformation in the guaiacyl ring and C-O deformation in the primary alcohol (1033 cm-1), and aromatic C-H out-of-plane deformation (823 cm-1) (Prado et al. 2016). The bands located at 1270, 1033 cm-1 are corresponding to guaiacyl units of lignin, while 1250 and 1120 cm-1 can be attributed to syringyl units of lignin (Boeriu et al. 2004; Qu et al. 2015). The regenerated lignin from [Emim]Cl showed enhancement of absorption at 1250 and 1170 cm-1 , indicating the increase of syringyl units compared to the others.
Fig. 3. FTIR spectra of (a) untreated coconut shell, (b) kraft lignin, and regenerated lignin from the ILs (c) [Bmim][Ace], (d) [Bmim]Cl, (e) [Emim][Ace], and (f) [Emim]Cl
XRD analysis
The untreated coconut shell and regenerated lignin from the ILs were characterized by XRD to study the crystallinity of the materials. The XRD results are presented in Fig. 4. Two peaks for the crystal structure in the untreated coconut shell were observed, which typically happens in biomass (Darji et al. 2013). The peaks appeared at 2θ values of 13° and 18.9°. The XRD patterns were compared with the raw material and regenerated lignin from [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl. The spectra of the regenerated lignin showed a disappearance of the peaks at approximately 13° and 18.9° when compared with the untreated coconut shell. The dissolution and regeneration process reduced the crystalline structure of the regenerated materials (lignin). The formation of amorphous materials was observed.
Fig. 4. XRD spectra of (a) untreated coconut shell and regenerated lignin from the AILs,
(b) [Bmim][Ace], (c) [Bmim]Cl, (d) [Emim][Ace], and (e) [Emim]Cl
Thermal stability and phase behavior
The thermal decomposition of the untreated coconut shell and regenerated lignin from AILs were studied by TGA. The thermal stability of all of the samples were evaluated by their weight loss as the temperature increased from 50 to 900 °C. The TGA curves showed the thermal decomposition of the untreated coconut shell and regenerated lignin from [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl after the dissolution process and are displayed in Fig. 5. The thermal stability of the untreated coconut shell showed a higher stability compared with the regenerated lignin from the ILs, as it started to decompose at 250 °C. This was due to the high crystallinity of the untreated coconut shell, which contained high amounts of hydrogen bonds and led to an increase in the thermal decomposition temperature (Kim et al. 2010). In contrast, the regenerated lignin from [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl showed lower thermal decomposition temperatures (190 °C) compared with the untreated coconut shell. These materials have lower crystallinities compared with the untreated biomass. This corresponded to a noticeable drop in weight for all of the samples, which was due to the liberation of volatile hydrocarbons by thermal decomposition of the lignocellulosic biomass (Yang and Wu 2009). The regenerated lignin from AILs also displayed lower thermal decomposition compared with Kraft lignin, which around 260 °C. This might be due to the cross-linked network of hydrogen bonding in the regenerated lignin from AILs were disrupted, which led to lower stabilities. Previous studies also reported that the acid-insoluble lignin from alkaline extraction of bagasse started to decompose at 186 °C (Sun et al. 2003; Tan et al. 2009). Heat and chemical reactions also influence the decomposition rate, product yields, and composition of lignin (Burhenne et al. 2013). The thermal analysis was important for understanding the properties of the regenerated materials to determine if they can meet the standards for industrial applications.
Fig. 5. TGA plots of untreated coconut shell, Kraft lignin and regenerated lignin from the AILs
DSC analysis
The phase behavior of the kraft lignin and regenerated lignin from the ILs were studied using DSC.
Fig. 6. DSC curves of untreated coconut shell and regenerated lignin from the AILs [Bmim][Ace], [Bmim]Cl, [Emim][Ace], and [Emim]Cl
The samples were subjected to three consecutive heating and cooling cycles to obtain reproducible results, and the third cycles are reported. DSC can be used to measure the Tg of a polymer, where changes in heat capacity occur. Additionally, the Tg of the regenerated lignin was measured to understand the behavior of the lignin, so that it can be used in current industrial applications, especially in making carbon fiber (Kubo and Kadla 2004). The Tg of the kraft lignin and regenerated lignin from different ILs are presented in Fig. 6. The kraft lignin showed a lower Tg (115 °C) than the regenerated lignin from the ILs (165 and 185 °C). This could have been due to the interaction of the intermolecular hydrogen bonding and decomposition temperature during the pretreatment process (Popescu et al. 2006). Previous literature reported the Tg of lignin to be between 80 and 180 °C (Popescu et al. 2006; Tejado et al. 2007). Meanwhile, Tan et al. (2009) reported that the Tg of dried lignin from [Emim] alkylbenzenesulfonate was 144 °C.
Characterization of Fresh and Recycled Ionic Liquids
One of the challenges of ionic liquid pretreatment is that the ILs are expensive; however, many researchers have demonstrated the reusability and the stability of ionic liquids, allowing them to be reused as many as four times (Lee et al. 2009; Nguyen et al. 2010). In this study, the ILs [Bmim]Cl, [Bmim][Ace], [Emim][Ace], and [Emim]Cl were recycled up to four times. Reliable results were shown for the fresh ILs. Sometimes, there are impurities in recycled imidazolium-based ILs that affect the dissolution results (Badgujar and Bhanage 2015). Therefore, it was necessary to maintain similar purities or stabilities for the recycled ILs.
Fig. 7. 1H NMR of fresh and recycled [Bmim][Ace]
Additionally, Karl Fischer coulometry was used to evaluate the water content in the ILs. NMR was used to confirm the similarities in terms of the properties and characteristics between the fresh and recycled ILs. To determine how efficient the recycled ILs were, the regenerated lignin from the recycled ILs was characterized with FTIR-KBR.
Nuclear magnetic resonance of fresh and recycled ionic liquids
1H NMR analysis was used to determine any changes to the properties of the ILs before and after recycling several times. There were no noticeable differences between the spectra of the fresh and recycled ILs. No other peaks or impurities were detected, except for a water solvent peak detected in the NMR spectra (chemical shift 3.53 ppm). This showed that the properties of the recycled ILs remained the same compared with the fresh ILs. Although water was detected in the NMR, the value was less than 1%, as detected by KFC, which was acceptable for the dissolution process. Figure 7 shows the 1H NMR spectra of the fresh and recycled [Bmim][Ace]. The 1H NMR spectra for [Bmim]Cl, [Emim][Ace], and [Emim]Cl are presented in the Supplementary Information.
CONCLUSIONS
- Lignin extraction from coconut shell in ILs was dependent on the temperature and the cations and anions of the ILs to disrupt the lignocellulosic network.
- [Emim][Ace] and [Bmim][Ace] showed better properties for regenerating lignin compared with [Emim]Cl and [Bmim]Cl.
- The anions (acetate) of the ILs played an important role in regenerating lignin because of the cleavage of different linkages within the biomass.
- The regenerated lignin successfully extracted from the coconut shell from [Emim][Ace], [Emim]Cl, [Bmim][Ace], [Bmim]Cl and so did the regenerated lignin from the recycled ILs. ILs can also be recycled up to four times, as no impurities were observed from the 1H NMR spectra. Hence, the IL dissolution pretreatment of coconut shell can extract up to 10% of lignin.
ACKNOWLEDGMENTS
This research was supported by High Impact Research MoE Grant UM.C/625/1/HIR/MoE/SC/04 from the Ministry of Education Malaysia, UMRG Program RP012A-14SUS, Postgraduate Research Grant PG223-2016A, and University Malaya Centre for Ionic Liquids (UMCiL).
REFERENCES CITED
Ang, T. N., Ngoh, G. C., Chua, A. S. M., and Lee, M. G. (2012). “Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses,” Biotechnol. Biofuels 5(1), 67. DOI: 10.1186/1754-6834-5-67
Armand, M., Endres, F., MacFarlane, D. R., Ohno, H., and Scrosati, B. (2009). “Ionic-liquid materials for the electrochemical challenges of the future,” Nat. Mater. 8(8), 621-629. DOI: 10.1038/nmat2448
Badgujar, K. C., and Bhanage, B. M. (2015). “Factors governing dissolution process of lignocellulosic biomass in ionic liquid: Current status, overview and challenges,” Bioresour. Technol. 178, 2-18. DOI: 10.1016/j.biortech.2014.09.138
Baker, D. A., and Rials, T. G. (2013). “Recent advances in low-cost carbon fiber manufacture from lignin,” J. Appl. Polym. Sci. 130(2), 713-728. DOI: 10.1002/app.39273
Baranyai, K. J., Deacon, G. B., MacFarlane, D. R., Pringle, J. M., and Scott, J. L. (2004). “Thermal degradation of ionic liquids at elevated temperatures,” Aust. J. Chem. 57(2), 145-147. DOI: 10.1071/CH03221
Bejan, D., Ignat’ev, N., and Willner, H. (2010). “New ionic liquids with the bis[bis(pentafluoroethyl)phosphinyl]imide anion, [(C2F5)2P(O)]2N−—Synthesis and characterization,” J. Fluorine Chem. 131(3), 325-332. DOI: 10.1016/j.jfluchem.2009.11.004
Brandt, A., Ray, M. J., To, T. Q., Leak, D. J., Murphy, R. J., and Welton, T. (2011). “Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid–water mixtures,” Green Chem. 13(9), 2489-2499. DOI: 10.1039/C1GC15374A
Brandt, A., Erickson, J. K., Hallett, J. P., Murphy, R. J., Potthast, A., Ray, M. J., Rosenau, T., Schrems, M., and Welton, T. (2012). “Soaking of pine wood chips with ionic liquids for reduced energy input during grinding,” Green Chem. 14, 1079-1085. DOI: 10.1039/C2GC15663F
Brandt, A., Grasvik, J., Hallet, J. P., and Welton, T. (2013). “Deconstruction of lignocellulosic biomass with ionic liquids,” Green.Chem. 15, 550-583. DOI: 10.1039/C2GC36364J
Burhenne, L., Messmer, J., Aicher, T., and Laborie, M. P. (2013). “The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis,” J. Anal. Appl. Pyrol. 101, 177-184. DOI: 10.1016/j.jaap.2013.01.012
Boeriu, C. G., Bravo, D., Gosselink, R. J., and van Dam, J. E. (2004). “Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy,” Ind. Crop. Prod. 20(2), 205-218. DOI: 10.1016/j.indcrop.2004.04.022
Cesarino, I., Araújo, P., Domingues Júnior, A. P., and Mazzafera, P. (2012). “An overview of lignin metabolism and its effect on biomass recalcitrance,” Braz. J. Botany 35(4), 303-311. DOI: 10.1590/S0100-84042012000400003
Chandra, R., Takeuchi, H., and Hasegawa, T. (2012). “Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production,” Renew. Sust. Energ. Rev. 16(3), 1462-1476. DOI: 10.1016/j.rser.2011.11.035
Chatel, G., and Rogers, R. D. (2013). “Review: Oxidation of lignin using ionic liquids — An innovative strategy to produce renewable chemicals,” ACS Sust. Chem. Eng. 2(3), 322-339. DOI: 10.1021/sc4004086
Cherubini, F. (2010). “The biorefinery concept: Using biomass instead of oil for producing energy and chemicals,” Energ. Convers. Manage. 51(7), 1412-1421. DOI: 10.1016/j.enconman.2010.01.015
Cobb, A., Warms, M., Maurer, E. P., and Chiesa, S. (2012). “Low-tech coconut shell activated charcoal production,” Int. J. Serv. Learn. Eng. 7(1), 93-104.
da Costa Lopes, A. M., João, K. G., Rubik, D. F., Bogel-Łukasik, E., Duarte, L. C., Andreaus, J., and Bogel-Łukasik, R. (2013). “Pre-treatment of lignocellulosic biomass using ionic liquids: Wheat straw fractionation,” Bioresour. Technol. 142, 198-208. DOI:10.1016/j.biortech.2013.05.032
Darji, D., Alias, Y., and Som, F. M. (2013). “Dissolution of biomass from rubberwood with 1-butyl-3-methyl imidazolium acetate ionic liquid,” J. Rubber Res. 16(3), 169-178.
Dorn, S., Wendler, F., Meister, F., and Heinze, T. (2008). “Interactions of ionic liquids with polysaccharides–7: Thermal stability of cellulose in ionic liquids and N-Methylmorpholine-N-oxide,” Macromol. Mater. Eng. 293(11), 907-913. DOI: 10.1002/mame.200800153
Dupont, J., and Scholten, J. D. (2010). “On the structural and surface properties of transition-metal nanoparticles in ionic liquids,” Chem. Soc. Rev. 39(5), 1780-1804. DOI: 10.1039/B822551F
Espinoza-Acosta, J. L., Torres-Chávez, P. I., Carvajal-Millán, E., Ramírez-Wong, B., Bello-Pérez, L. A., and Montaño-Leyva, B. (2014). “Ionic liquids and organic solvents for recovering lignin from lignocellulosic biomass,” BioResources 9(2), 3660-3687. DOI: 10.15376/biores.9.2.3660-3687
Ewansiha, C. J., Ebhoaye, J. E., Asia, I. O., Ekebafe, L. O., and Ehigie, C. (2012). “Proximate and mineral composition of coconut (Cocos nucifera) shell,” Int. J. Pure Appl. Sci. Technol. 13(1), 53-56.
FAO. Food and Agriculture Organization of the United Nations (2012) Economic and Social Department, The Statistical Division. http://faostat.fao.org/site/291/default.aspx. Accessed 23 Sep 2012
Fendt, S., Padmanabhan, S., Blanch, H. W., and Prausnitz, J. M. (2010). “Viscosities of acetate or chloride-based ionic liquids and some of their mixtures with water or other common solvents,” J. Chem. Eng. Data 56(1), 31-34. DOI: 10.1021/je1007235
Financie, R., Moniruzzaman, M., and Uemura, Y. (2016). “Enhanced enzymatic delignification of oil palm biomass with ionic liquid pretreatment,” Biochem. Eng. J. 110, 1-7. DOI: 10.1016/j.bej.2016.02.008
Fisher, A. B., and Fong, S. S. (2014). “Lignin biodegradation and industrial implications,” AIMS Bioeng. 1(2), 92-112. DOI: 10.3934/bioeng.2014.2.92
Freemantle, M. (2010). An Introduction to Ionic Liquids, The Royal Society of Chemistry, Cambridge, UK.
George, A., Tran, K., Morgan, T. J., Benke, P. I., Berrueco, C., Lorente, E., Wu, B. C., Keasling, J. D., Simmons, B. A., and Holmes, B. M. (2011). “The effect of ionic liquid cation and anion combinations on the macromolecular structure of lignins,” Green Chem. 13(12), 3375-3385. DOI: 10.1039/C1GC15543A
Goh, C. S., Tan, K. T., Lee, K. T., and Bhatia, S. (2010). Bio-ethanol from lignocellulose: Status, perspectives and challenges in Malaysia. Bioresour. Technol. 101(13), 4834-4841. DOI: 10.1016/j.biortech.2009.08.080
Hart, W. E., Harper, J. B., and Aldous, L. (2015). “The effect of changing the components of an ionic liquid upon the solubility of lignin,” Green Chem. 17(1), 214-218. DOI: 10.1039/C4GC01888E
Hsu, T. C., Guo, G. L., Chen, W. H., and Hwang, W. S. (2010). “Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis,” Bioresour. Technol. 101(13), 4907-4913. DOI: 10.1016/j.biortech.2009.10.009
Ignat’ev, N. V., Welz-Biermann, U., Kucheryna, A., Bissky, G., and Willner, H. (2005). “New ionic liquids with tris(perfluoroalkyl)trifluorophosphate (FAP) anions,” J. Fluorine Chem. 126(8), 1150-1159. DOI: 10.1016/j.jfluchem.2005.04.017
Ji, W., Ding, Z., Liu, J., Song, Q., Xia, X., Gao, H., Wang, H., and Gu, W. (2012). “Mechanism of lignin dissolution and regeneration in ionic liquid,” Energ. Fuel. 26(10), 6393-6403. DOI: 10.1021/ef301231a
Kanojia, A., and Jain, S. K. (2017). “Performance of coconut shell as coarse aggregate in concrete,” Constr. Build Mater. 140, 150-156. DOI: 10.1016/j.conbuildmat.2017.02.066
Kamm, B., and Kamm, M. (2004). “Principles of biorefineries,” Appl. Microbiol. Biot. 64(2), 137-145. DOI: 10.1007/s00253-003-1537-7
Kilpeläinen, I., Xie, H., King, A., Granstrom, M., Heikkinen, S., and Argyropoulos, D. S. (2007). “Dissolution of wood in ionic liquids,” J. Agr. Food Chem. 55(22), 9142-9148. DOI: 10.1021/jf071692e
Kim, U. J., Eom, S. H., and Wada, M. (2010). “Thermal decomposition of native cellulose: Influence on crystallite size,” Polym. Degrad. Stabil. 95(5), 778-781. DOI: 10.1016/j.polymdegradstab.2010.02.009
King, A. W. T., Zoia, L., Filpponen, I., Olszewska, A., Xie, H., Kilpeläinen, I., and Argyropoulos, D. S. (2009). “In situ determination of lignin phenolics and wood solubility in imidazolium chlorides using 31P NMR,” J. Agr. Food Chem. 57(18), 8236-8243. DOI: 10.1021/jf901095w
Kiran, E., and Balkan, H. (1994). “High-pressure extraction and delignification of red spruce with binary and ternary mixtures of acetic acid, water, and supercritical carbon dioxide,” J. Supercrit. Fluid. 7(2), 75-86. DOI: 10.1016/0896-8446(94)90043-4
Kubo, S., and Kadla, J. F. (2004). “Poly(ethylene oxide)/organosolv lignin blends: Relationship between thermal properties, chemical structure, and blend behavior,” Macromolecules 37(18), 6904-6911. DOI: 10.1021/ma0490552
Kubo, S., and Kadla, J. F. (2005). “Lignin-based carbon fibers: Effect of synthetic polymer blending on fiber properties,” J. Polym. Environ. 13(2), 97-105. DOI: 10.1007/s10924-005-2941-0
Kumar, S. N. (2011). “Variability in coconut (Cocos nucifera L.) germplasm and hybrids for fatty acid profile of oil,” J. Agr. Food Chem. 59(24), 13050-13058. DOI: 10.1021/jf203182d
Lateef, H., Grimes, S., Kewcharoenwong, P., and Feinberg, B. J. (2009). Chem. Technol. Biotechnol. 84, 1818-1827. DOI: 10.1002/jctb.2251
Lee, S. H., Doherty, T. V., Linhardt, R. J., and Dordick, J. S. (2009). “Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis,” Biotechnol. Bioeng. 102(5), 1368-1376. DOI: 10.1002/bit.22179
Li, W., Sun, N., Stoner, B., Jiang, X., Lu, X., and Rogers, R. D. (2011). “Rapid dissolution of lignocellulosic biomass in ionic liquids using temperatures above the glass transition of lignin,” Green Chem. 13(8), 2038-2047. DOI: 10.1039/C1GC15522A
Lievonen, M. (2015). Preparation and Characterization of Lignin Nanoparticles, Master’s Thesis, Aalto University, Helsinki, Finland.
Leskinen, T., King, A. W. T., Kilpelainen, I., and Argyropoulos, D. S. (2013). “Fractionation of lignocellulosic materials using ionic liquids: Part 2. effect of particle size on the mechanisms of fractionation,” Ind. Eng. Chem. Res. 52(11), 3958–3966. DOI: 10.1021/ie302896n
Luo, J., Cai, M., and Gu, T. (2013). “Pretreatment of lignocellulosic biomass using green ionic liquids,” in: Green Biomass Pretreatment for Biofuels Production, Springer, Netherlands. DOI: 10.1007/978-94-007-6052-3_6
Marrucho, I. M., Branco, L. C., and Rebelo, L. P. N. (2014). “Ionic liquids in pharmaceutical applications,” Ann. Rev. Chem. Biomol. Eng. 5, 527-546. DOI: 10.1146/annurev-chembioeng-060713-040024
Mathiasson, A., and Kubát, D. G. (1994). “Lignin as binder in particle boards using high frequency heating,” Holz Roh. Werkst. 52(1), 9-18. DOI: 10.1007/BF02615010
Mood, S. H., Golfeshan, A. H., Tabatabaei, M., Abbasalizadeh, S., Ardjmand, M., and Jouzani, G. S. (2014). “Comparison of different ionic liquids pretreatment for corn stover enzymatic saccharification,” Prep. Biochem. Biotech. 44(5), 451-463. DOI: 10.1080/10826068.2013.833112
Mora-Pale, M., Meli, L., Doherty, T. V., Linhardt, R. J., and Dordick, J. S. (2011). “Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass,” Biotechnol. Bioeng. 108(6), 1229-1245. DOI: 10.1002/bit.23108
Nagle, N. J., Elander, R. T., Newman, M. M., Rohrback, B. T., Ruiz, R. O., and Torget, R. W. (2002). “Efficacy of a hot washing process for pretreated yellow poplar to enhance bioethanol production,” Biotechnol. Progr. 18(4), 734-738. DOI: 10.1021/bp0155078
Nguyen, T. A. D., Kim, K. R., Han, S. J., Cho, H. Y., Kim, J. W., Park, S. M., Park, J. C., and Sim, S. J. (2010). “Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars,” Bioresour. Technol. 101(19), 7432-7438. DOI: 10.1016/j.biortech.2010.04.053
Ohno, H. (2011). Electrochemical Aspects of Ionic Liquids, John Wiley & Sons, Inc., Hoboken, NJ.
Ouyang, X., Ke, L., Qiu, X., Guo, Y., and Pang, Y. (2009). “Sulfonation of alkali lignin and its potential use in dispersant for cement,” J. Disper. Sci. Technol. 30(1), 1-6. DOI: 10.1080/01932690802473560
Padmanabhan, S., Kim, M., Blanch, H. W., and Prausnitz, J. M. (2011). “Solubility and rate of dissolution for Miscanthus in hydrophilic ionic liquids,” Fluid Phase Equilib. 309, 89-96. DOI: 10.1016/j.fluid.2011.06.034
Pandey, K. K. (1999). “A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy,” J. Appl. Polym. Sci. 71(12), 1969-1975. DOI: 10.1002/(SICI)1097-4628(19990321)71:12<1969::AID-APP6>3.0.CO;2-D
Pârvulescu, V. I., and Hardacre, C. (2007). “Catalysis in ionic liquids,” Chem. Rev. 107(6), 2615-2665. DOI: 10.1021/cr050948h
Pielhop, T., Larrazábal, G. O., and von Rohr, P. R. (2016). “Autohydrolysis pretreatment of softwood – Enhancement by phenolic additives and the effects of other compounds,” Green Chem. 18(19), 5239-5247. DOI: 10.1039/C6GC01447J
Pinkert, A., Marsh, K. N., Pang, S., and Staiger, M. P. (2009). “Ionic liquids and their interaction with cellulose,” Chem. Rev. 109(12), 6712-6728. DOI: 10.1021/cr9001947
Pinkert, A., Goeke, D. F., Marsh, K. N., and Pang, S. (2011). “Extracting wood lignin without dissolving or degrading cellulose: Investigations on the use of food additive-derived ionic liquids,” Green Chem. 13(11), 3124-3136. DOI: 10.1039/C1GC15671C
Pino, G. H., de Mesquita, L. M. S., Torem, M. L., and Pinto, G. A. S. (2006). “Biosorption of heavy metals by powder of green coconut shell,” Separ. Sci. Technol. 41(14), 3141-3153. DOI: 10.1080/01496390600851640
Popescu, C., Vasile, C., Popescu, M., Singurel, G., Popa, V. I., and Munteanu, B. S. (2006). “Analytical methods for lignin characterization. II. Spectroscopic studies,” Cell. Chem. Technol. 40(8), 597-621.
Prado, R., Erdocia, X., and Labidi, J. (2016). “Study of the influence of reutilization ionic liquid on lignin extraction,” J. Clean. Prod. 111, 125-132. DOI: 10.1016/j.jclepro.2015.04.003
Pu, Y., Jiang, N., and Ragauskas, A. J. (2007). “Ionic liquid as a green solvent for lignin,” J. Wood Chem. Technol. 27(1), 23-33. DOI: 10.1080/02773810701282330
Qu, Y., Luo, H., Li, H., and Xu, J. (2015). “Comparison on structural modification of industrial lignin by wet ball milling and ionic liquid pretreatment,” Biotechnol. Rep. 6, 1-7. DOI: 10.1016/j.btre.2014.12.011
Rashid, T., Kait, C. F., Regupathi, I., and Murugesan, T. (2016). “Dissolution of kraft lignin using protic ionic liquids and characterization,” Industrial Crops and Products 84, 284-293. DOI: 10.1016/j.indcrop.2016.02.017
Rodrigues, S., and Pinto, G. (2007). “Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder,” J. Food Eng. 80(3), 869-872. DOI: 10.1016/j.jfoodeng.2006.08.009
Rowell, R. M. (1984). The Chemistry of Solid Wood, American Chemical Society, Washington, D.C.
Sheldon, R. A., Lau, R. M., Sorgedrager, M. J., van Rantwijk, F., and Seddon, K. R. (2002). “Biocatalysis in ionic liquids,” Green Chem. 4(2), 147-151. DOI: 10.1039/B110008B
Sivapragasam, A. (2008). “Coconut in Malaysia – Current developments and potential for re-vitalization,” in: 2nd International Plantation Industry Conference and Exhibition (IPICEX 2008), Shah Alam, Malaysia, pp. 18-21.
Sriram, N., and Shahidehpour, M. (2005). “Renewable biomass energy,” in: IEEE Power Engineering Society General Meeting, Chicago, IL, pp. 1-6.
Stöcker, M. (2008). “Biofuels and biomass-to-liquid fuels in the biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials,” Angew. Chem. Int. Edit. 47(48), 9200-9211. DOI: 10.1002/anie.200801476
Sun, Y., and Cheng, J. (2002). “Hydrolysis of lignocellulosic materials for ethanol production: A review,” Bioresour. Technol. 83(1), 1–11. DOI: 10.1016/S0960-8524(01)00212-7
Sun, J. X., Sun, X. F., Sun, R. C., Fowler, P., and Baird, M. S. (2003). “Inhomogeneities in the chemical structure of sugarcane bagasse lignin,” J. Agr. Food Chem. 51(23), 6719-6725. DOI: 10.1021/jf034633j
Sun, N., Rahman, M., Qin, Y., Maxim, M. L., Rodríguez, H., and Rogers, R. D. (2009). “Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate,” Green Chem. 11(5), 646-655. DOI: 10.1039/B822702K
Sun, N., Rodríguez, H., Rahman, M., and Rogers, R. D. (2011). “Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass?,” Chem. Commun. 47(5), 1405-1421. DOI: 10.1039/C0CC03990J
Swatloski, R. P., Spear, S. K., Holbrey, J. D., and Rogers, R. D. (2002). “Dissolution of cellulose with ionic liquids,” J. Am. Chem. Soc. 124(18), 4974-4975. DOI: 10.1021/ja025790m
Tan, S. S., MacFarlane, D. R., Upfal, J., Edye, L. A., Doherty, W. O., Patti, A. F., Pringle, J. M., and Scott, J. L. (2009). “Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzene sulfonate ionic liquid,” Green Chem. 11(3), 339-345. DOI: 10.1039/B815310H
TAPPI T 211 om-02 (2002). “Ash in wood, pulp, paper and paperboard: Combustion at 525 °C,” TAPPI Test Method, Atlanta.
TAPPI T264 cm-97 (1997). “Preparation of wood for chemical analysis,” TAPPI, Atlanta
TAPPI T222 om-98 (1998). “Acid insoluble lignin in wood and pulp,” TAPPI, Atlanta.
TAPPI T212 om-98 (1998). “One percent sodium hydroxide solubility of wood and pulp,” TAPPI, Atlanta.
Teh, W. X., Hossain, M. M., To, T. Q., and Aldous, L. (2015). “Pretreatment of macadamia nut shells with ionic liquids facilitates both mechanical cracking and enzymatic hydrolysis,” ACS Sust. Chem. Eng. 3(5), 992-999. DOI: 10.1021/acssuschemeng.5b00126
Tejado, A., Peña, C., Labidi, J., Echeverria, J. M., and Mondragon, I. (2007). “Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis,” Bioresour. Technol. 98(8), 1655-1663. DOI: 10.1016/j.biortech.2006.05.042
Vitz, J., Erdmenger, T., Haensch, C., and Schubert, U. S. (2009). “Extended dissolution studies of cellulose in imidazolium based ionic liquids,” Green Chem. 11(3), 417-424. DOI: 10.1039/B818061J
Wasserscheid, P., and Welton, T. (2007). Ionic Liquids in Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
Wise, L. E., Murphy, M., and D’Adieco, A. A. (1946). “A chlorite holocellulose, its fractionation and bearing on summative wood analysis and studies on the hemi- celluloses,” Pap. Trade J. 122, 35-43.
Wegener, G. (1992). “Pulping innovations in Germany,” Ind. Crop. Prod. 1(2-4), 113-117. DOI: 10.1016/0926-6690(92)90008-J
Welton, T. (1999). “Room-temperature ionic liquids. Solvents for synthesis and catalysis,” Chem. Rev. 99(8), 2071-2084. DOI: 10.1021/cr980032t
Wilkes, J. S. (2002). “A short history of ionic liquids—From molten salts to neoteric solvents,” Green Chem. 4(2), 73-80. DOI: 10.1039/B110838G
Wishart, J. F. (2009). “Energy applications of ionic liquids,” Energ. Environ. Sci. 2(9), 956-961. DOI: 10.1039/B906273D
Xie, H., and Gathergood, N. (2013). The Role of Green Chemistry in Biomass Processing and Conversion, John Wiley and Sons, Hoboken, NJ.
Xu, A., Wang, J., and Wang, H. (2010). “Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems,” Green Chem. 12(2), 268-275. DOI: 10.1039/B916882F
Yang, D., Zhong, L. X., Yuan, T. Q., Peng, X. W., and Sun, R. C. (2013). “Studies on the structural characterization of lignin, hemicelluloses and cellulose fractionated by ionic liquid followed by alkaline extraction from bamboo,” Ind. Crop. Prod. 43, 141-149. DOI: 10.1016/j.indcrop.2012.07.024
Yang, Q., and Wu, S. (2009). “Thermogravimetric characteristics of wheat straw lignin,” Cell. Chem. Technol. 43(4), 133-139.
Zakrzewska, M. E., Bogel-Łukasik, E., and Bogel-Łukasik, R. (2010). “Solubility of carbohydrates in ionic liquids,” Energ. Fuel. 24(2), 737-745. DOI: 10.1021/ef901215m
Zhang, J., Wang, Y., Zhang, L., Zhang, R., Liu, G., and Cheng, G. (2014). “Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD,” Bioresource Technol 151, 402-405. DOI: 10.1016/j.biortech.2013.10.009
Article submitted: February 20, 2017; Peer review completed: April 17, 2017; Revised version received and accepted: May 15, 2017; Published: June 26, 2017.
DOI: 10.15376/biores.12.3.5749-5774
APPENDIX – SUPPLEMENTARY INFORMATION
Nuclear Magnetic Resonance
1H NMR spectra for [Bmim]Cl, [Emim][Ace], and [Emim]Cl are presented here.
Fig. S1. 1H NMR of [Bmim]Cl, fresh and recycled up to four times
Fig. S2. 1H NMR of [Emim][Ace], fresh and recycled up to four times
Fig. S3. 1H NMR of [Emim]Cl, fresh and recycled up to four times
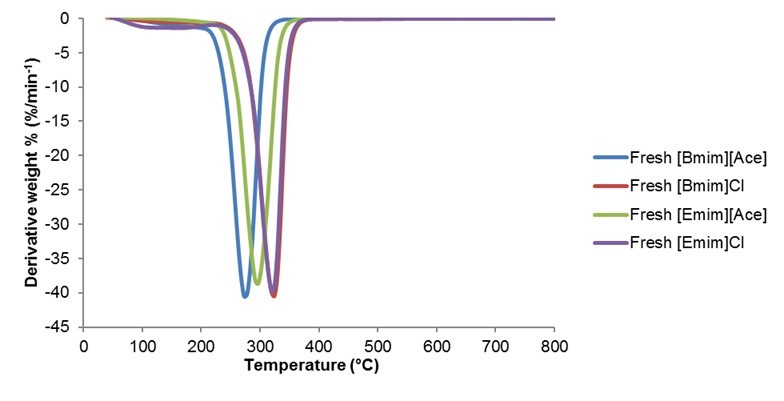
Fig. S4. DTG curves for ionic liquids.
