Abstract
Download PDF
Full Article
Localized Wood Surface Modification, Part I: Method Characterization
Diego Elustondo,a Olena Myronycheva,a Bror Sundqvist,b and Olov Karlsson a
This study assesses the potential of an open process for treatment of European Scots pine (Pinus sylvestris) with chemicals that could potentially make the surfaces stronger, more dimensionally stable, or more durable, depending on the treatment solution. The method provides an intermediate solution between full volume impregnation by pressure treatment and superficial surface treatment by dipping. Figuratively speaking, the process creates the equivalent of a layer of coating applied below the wood surfaces rather than above. Two different techniques were compared, namely, heating-and-cooling (H&C) and compression-and-expansion (C&E). Taking into account that commercial suppliers recommend 0.15 to 0.25 L/m2 of coating in sawn wood and 0.1 to 0.15 L/m2 in planed wood surfaces, then this study demonstrates that the H&C method can impregnate an equivalent amount of solution under the surfaces in less than 15 min using treatment temperatures below 150 °C.
Keywords: Scots pine; Wood surface modification; Heating-and-cooling; Compression-and-expansion
Contact information: a: Luleå University of Technology, Division of Wood Science and Engineering, Campus Skellefteå, Forskargatan 1, 93187 Skellefteå, Sweden; b: SP Technical Research Institute of Sweden, Laboratorgränd 2, SE-931 77 Skellefteå, Sweden;
* Corresponding author: diego.elustondo@ltu.se
INTRODUCTION
Wood is a biodegradable material. Thus, surfaces that are exposed to the external environment (such as in cladding, decking, flooring, and furniture) experience a slow degradation that is caused by a combination of moisture, temperature, sunlight, mechanical abrasion, and biological attack (Williams 2005). This problem is exacerbated in wood that was obtained from the periphery of the logs – such as the so called “side boards” in Sweden – since sapwood has normally lower durability (Homan and Jorissen 2004). Coating is the most widely used method for protecting wood surfaces from weathering, but the time that the material can be used between maintenance cycles (service life) is limited. Typical wood cladding used in residential and low rise buildings must be re-painted and re-stained every five to seven years (Athena 2002).
This problem is leading society slowly but consistently towards a future in which a renewable material such as wood will be replaced by other much more energy-intensive industrial alternatives that may irreversibly change the planet for future generations. One possible solution is to modify the entire volume of wood by impregnation with chemicals that can make wood stronger, more stable, or more durable, depending on the application. Examples include acetylation, furfurylation, and impregnation with oils and resins (Homan and Jorissen 2004). Acetylation (impregnation with acetic anhydride) replaces the moisture-accessible hydroxyls groups in the wood with hydrophobic acetyl groups (Hill 2006). Furfurylation (impregnation with furfuryl alcohol) creates a highly branched and cross-linked polymer bonded to the wood cell walls (Westin et al. 2003; Lande 2008). Impregnation with oils is an effective method to control the absorption of moisture in wood (Alireza 2011), while impregnation with resins refers to the application of solutions that polymerize inside the wood (Nurmi 1998; Gsöls et al. 2003). It is well known, for instance, that impregnation with phenol formaldehyde resins improve wood durability, water resistance, and dimensional stability of wood (Takahashi and Imamura 1990; Ryu et al. 1991; Deka and Saikia 2000; Hill 2006; Gabrielli and Kamke 2010).
The most widely used method for impregnating the entire volume of wood with chemicals is pressure treatment (Hunt and Garratt 1967; Ormrod and Van Dalfsen 1993; Richardson 2003). Pressure treatment is applied inside pressure chambers, typically steel cylinders (Kollmann and Côté 1984), in which the wood is first exposed to vacuum to remove the air and then to pressures in the order of 8.5 to 12 atm to force the penetration of chemicals (Forest Products Laboratory 2010). However, fully modified wood has not yet found a large number of applications, probably because of the high volume of chemicals and long processing times required to impregnate the entire volume of wood. This is reflected in the cost. It was estimated that the approximate market prices for fully acetylated wood, furfurylated wood, and wood impregnated with sodium-silicate were 10 to 4 times higher than untreated pine (Sandberg et al. 2013).
Another option for impregnating chemicals is by submerging the wood into the treatment solution and letting the chemicals penetrate by diffusion (Tamblyn 1985). This is customarily called dipping. Preservatives that can be dissolved in water or oils can be potentially absorbed by dipping. Reported treatment times have ranged from seconds or minutes (Kollmann and Côté 1984) to hours or even days (Blew 1948). In this study the objective is to test a third option that provides an intermediate solution between pressure treatment and dipping. Two different techniques were compared. These are referred to in this study as heating-and-cooling (H&C) and compression-and-expansion (C&E) methods. The H&C method consists of increasing the temperature of the wood and subsequently submerging it into treatment solution at room temperature (Crawford 1907). The C&E method consists in compressing the wood with a press and then allowing it to expand while submerged in treatment solution (Fry 1973; Inoue et al. 2008). In principle, both methods have the capacity to induce internal vacuum by reducing the density of the gases. The H&C method reduces the gas temperature at constant volume and the C&E method increases the gas volume at constant temperature. In this study the C&E method was only implemented to assess its potential in comparison with the H&C method, but the effect of the process parameters was not investigated further. The methods were implemented with European Scots pine (Pinus sylvestris) side boards which predominantly contain sapwood.
EXPERIMENTAL
Materials
The wood for this study was European Scots pine (Pinus sylvestris) supplied by Norra Skogsägarna sawmill in Kåge, Sweden. The boards were 19 mm thick in radial direction by 75 mm wide in the tangential direction by 5 m long in the longitudinal direction, and they contained mainly sapwood (referred as side boards in the industry). The boards thickness was reduced to approximately 18 mm with a planer, cut into 200 mm long sections free of knots and bark, and then end-sealed with commercial glue that can stand up to 300 °C (Silikon 300 °C, Sika Norge AS, Skjetten, Norway). Twelve samples were cut from each board, thus producing 12 sets of 6 matched samples.
Before the H&C tests, samples were oven-dried at 103 °C until constant weight, in order to eliminate moisture content as a variable of the study. Three liquids were tested as examples of treatment solutions, namely: water, vegetable oil, and biodiesel. The vegetable oil was rapeseed oil bought in a local store (Coop Trading A/S, Izegem, Beligum), and the biodiesel was rapeseed oil methyl ester (RME) supplied by a local company (Ecobränsle i Karlshamn AB, Karlshamn, Sweden). The properties of these particular liquids were not measured in this study, but there are European standards for rapeseed oil and biodiesel that were used for comparison (Bernat et al. 2011). Water properties are also available in the literature (Venard and Street 1975). Table 1 reports the density and kinematic viscosity of water, rapeseed oil, and biodiesel according to the cited literature. The purpose of selecting those liquids was to assess the potential of the H&C method in terms of solution uptake and processing times. In Part II of this study, the H&C method will be implemented with resins to investigate the possibility of creating a permanent layer of protection below the wood surfaces.
Table 1. Density and Kinematic Viscosity of Water, Rapeseed Oil, and Biodiesel
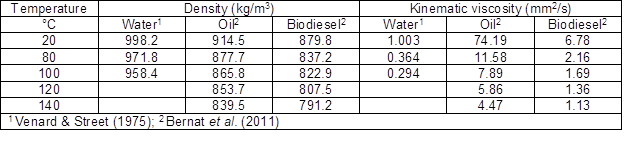
Methods
Four temperatures were tested for the heating-and-cooling (H&C) method, namely, 20, 90, 120, and 150 °C, where the 20 ºC test was representative of the dipping method with both wood and liquid at room temperature (Fig. 1).
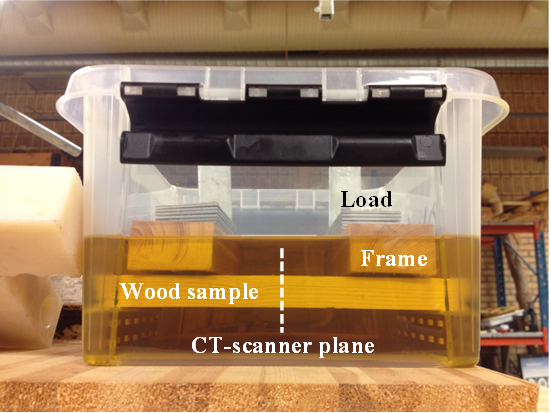
Fig. 1. Set of three hot samples submerged in cold treatment solution
The H&C process was monitored in real time with a fourth generation Siemens SOMATOM Emotion computed tomography scanner (CT-scanner) available at Luleå University of Technology. The procedure consisted in warming up the samples in an oven for approximately 4 h to guarantee uniform temperature, and then submerging the samples in the treatment solution that was approximately at 20 ºC. The H&C process was performed inside 30 cm x 23 cm plastic boxes with enough capacity for accommodating three samples. Sets of three samples were fastened to a wood frame and metal loads were used to hold the samples submerged in the treatment solution. The metal loads were placed over the ends of the samples so that the center of the samples was unobstructed for CT-scanning.
The plastic box was inside the CT-scanner field of view when the samples were submerged (Fig. 2), but it took approximately 30 s to start the first scan. Despite this delay, the first CT-scan image was used as reference to calculate weight gain as function of the treatment time. The weight gain measured with the CT-scanner was representative of a 10 mm slice in the center of the samples (Based on the CT-scanner parameters). The weight gain was not confirmed with a balance for the entire sample because the wood frame was covering part of the surfaces close to the sample ends. Each experiment was performed two times, thus resulting in 6 replicates per treatment.

Fig. 2. Set of three samples positioned inside the CT-scanner field of view
The weight gain based on the CT-scanner measurements was calculated from the CT-images by using ImageJ image analysis software. The procedure consisted of selecting a polygon around the cross section image of each sample and then calculating the total mass contained inside that polygon by multiplying area and average density. The average density of the first CT-image was also used to determine the initial wood density. The measured initial density was approximately 480 kg/m3 with a standard deviation of 43 kg/m3. Because the samples were first oven-dried and subsequently warmed up in an oven for approximately 4 h, it was assumed that the initial moisture content was close to 0%.
For the compression-and-expansion (C&E) tests, a laboratory press was used with a plate size of approximately 140 mm x 140 mm. The original 18 mm thick by 75 mm wide by 200 mm long samples were trimmed to a length of approximately 180 mm to fit into the press compartment, and no end-sealing was applied. It was speculated that end-sealing could have blocked the air from flowing out of the wood during compression. The force applied by the press was set to the maximum of 100 kN, that for 75 mm wide samples represented approximately 9.5 MPa. The liquid solution at room temperature was placed in approximately 16 cm x 23 cm aluminum pans, and the samples were maintained under the liquid with metal loads placed over the samples ends (that protruded 20 mm from the back and front of the press plate). Two aluminum bars were also placed submerged at the right and left sides of the wood samples to stop the press at exactly 12 mm thickness. Once the 12 mm thickness was reached, the press was programed to remain in that position for 10 s and then release the pressure. After releasing the pressure, the sample thickness recovered quickly (spring back), but it was allowed to remain submerged a longer time so that the total immersion time was 3 min for all experiments. Reference samples were also submerged 3 min in the solution but without any compression. The total weight gain by the C&E method was measured with a balance after removing the excess liquid from the surfaces by hand with a paper towel (Tork “Basic Papper”, brown, cat. no. 150109).
The comparison between H&C and C&E was included in this study to show that under the present experimental conditions neither of the methods resulted in considerably higher solution uptake than the other. Based on this comparison, the H&C method was selected for further characterization. In order to be conservative, the comparison probably overestimated the C&E uptake per unit of area. It was assumed that all solution absorption due to spring back occurred through the top wood area that was compressed by the press plate, thus meaning that absorption due to spring back through the bottom wood surface in contact with the container floor was assumed negligible. Based on this criterion, the absorption area was calculated as the wood width multiplied by the plate length = 75·140 mm2.
Theory
The basic principle behind the proposed method is the theory of ideal gases. The theory of ideal gases postulates that the pressure of a gas is equal to R·n·T/V, where R is the ideal gas constant, n is the number of gas molecules, T is the absolute temperature, and V is the gas volume. Consequently, if the temperature reduces because the wood is cooled down or the volume increases because the wood springs back, then there will be a proportional reduction of pressure inside the wood that can produce suction of an external solution. Based on this theory, solution uptake in the H&C method is expected to be directly proportionally to the treatment temperature and reduce gradually with time as the wood cools down.
RESULTS AND DISCUSSION
Figure 3 shows an example of the H&C process implemented with Scots pine side boards initially at 120 °C and submerged in rapeseed oil methyl ester (RME) at approximately 20 °C. The figure shows the initial CT-scanned density distribution of a wood sample (top image) and subsequently the differences with other CT-scanned density distributions taken at 5, 10, 15, and 29 min during the H&C process. The lighter areas represent the penetration front, where it can be observed that for scots pine side boards the penetration front developed relatively uniformly below the wood surfaces, with some areas of course absorbing more RME than others. The core of the samples also showed a higher tendency to absorb solution, arguably because of internal tensions developed during the process. It is speculated that internal stresses may have been caused by swelling of the external wood surfaces in contact with the treatment solution, but this could not be detected with the CT-Scanner. The CT-scanner showed however that internal checks developed in some pieces when water was used as model of treatment solution, but internal checks were never observed during rapeseed oil or RME absorption.
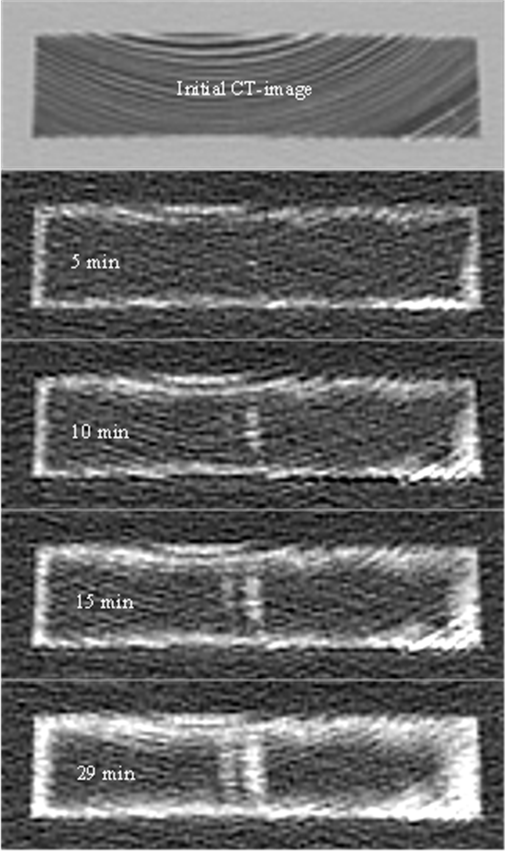
Fig. 3. Initial CT-image and density differences after 5, 10, 15, and 29 min of RME absorption
The fact that some wood areas absorb more liquid than others may be a disadvantage or an advantage if more protection is desired in areas with higher liquid permeability. This also makes the process more difficult to predict. Figure 4 is presented as an example of the variability observed among individual samples for the particular case of RME absorption. Similar variability among individual samples was also observed for rapeseed oil and water absorption. In the figure the solution uptake was converted to L/m2, which is a customary unit for commercial coatings. The conversion from grams to liter was based on the average densities at 20 °C reported in Table 1, while the sample external area was calculated as 2·10·(75+18) mm2, based on the sample width (75 mm), thickness (18 mm), and 10 mm length in the longitudinal direction set by the CT-scanner parameters.
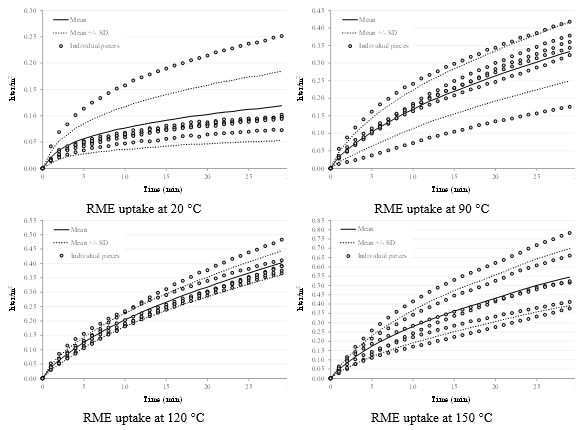
Fig. 4. Experimental average and standard deviation of six matched samples H&C treated in RME at 20, 90, 120, and 150 °C
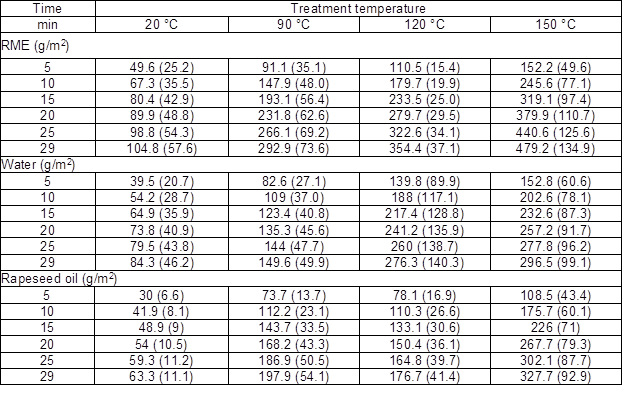
The average solution uptake measured in g/m2 and the standard deviation for selected impregnation times are reported in Table 2.
Each average and standard deviation represents six matched samples, with the exception of the rapeseed oil at 120 ºC where only three matched samples were included. The first set of three samples was discarded because the oil level reduced below the wood surface during the H&C treatment.
It was evident from the data that variability between samples was high, but there were general trends that could be described mathematically. It was observed that for a fixed treatment temperature, the solution uptake rate reduced gradually with the time, while for a fixed treatment time, the solution uptake rate increases gradually with the temperature. For this study the observed solution uptake trends were described as the product of two power functions of temperature and time,
Uptake = (a ∙ ( T – T0 )m + b ) ∙ tn (1)
where T is the treatment temperature (°C), T0 is the room temperature (20 °C), t is the treatment time (min), a is a parameter quantifying the solution uptake due to temperature difference, b is a parameter quantifying the solution uptake due to diffusion at room temperature, m is a power factor describing the solution uptake rate as function of temperature, and n is a power factor describing the solution uptake rate as function of the time.
Table 2. Average Solution Uptake and Standard Deviation (in parentheses) for Selected Treatment Times
The parameters for Eq. 1 were determined by numerical fitting of the experimental data based on the minimum mean square error method. The rapeseed oil absorption data at 120 °C were not used because it was contradictorily lower than the rapeseed oil data at 90 °C, likely because only three samples were used. One sample for RME at 20 °C and one sample for water at 120 °C were also excluded because they were conspicuously higher than the rest.
The numerical results of the data fitting are reported in Table 3, and the comparison between the mathematical description and the experimental data is shown in figures 5, 6, and 7 for respectively RME, water and rapeseed oil.

Fig. 5. Experimental data and mathematical description of RME absorption (after removing the data for one sample at 20 °C)

Fig. 6. Experimental data and mathematical description of water absorption (after removing the data for one sample at 120 °C)
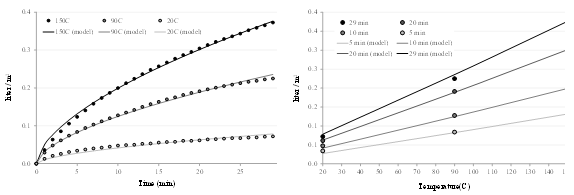
Fig. 7. Experimental data and mathematical description of rapeseed oil absorption (after removing the data for all samples at 120 °C)
Table 3. Results of Fitting the H&C Absorption Data with Eq. 1 from 0 to 29 min and 20 to 150 ºC
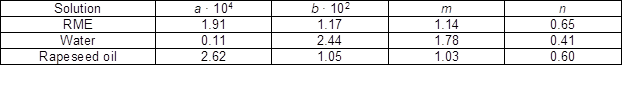
Table 3 indicates that the power factor m is always higher than 1, meaning that the effect of the temperature is increasingly important as the treatment temperature increases, while the power factor n is always lower than 1, meaning that H&C process becomes slower as time proceeds. Table 3 also shows that the fitting parameters are similar between RME and rapeseed oil, which is reasonable as the biodiesel used in this study was derived from rapeseed oil. Parameters are different for water. The power factor m is close to 1 in RME and rapeseed oil, meaning that the solution uptake rate increases proportionally to the increase of the treatment temperature, but it is much higher in water, approximately 1.8. This is explained by the formation of internal checks.
Figure 8 shows the CT-images of three samples that were exposed to H&C water absorption. This example shows that wood treated with water tended to develop internal checks, which in turn were filled with water. This was equivalent to the pattern observed in Fig. 3, where the center of the pieces tended to absorb higher amounts of solution, but in the case of water it resulted in the development of internal checks at some point of the treatment.

Fig. 8. CT-images of water absorption at 120 °C showing internal checks filled with water after 29 min of treatment
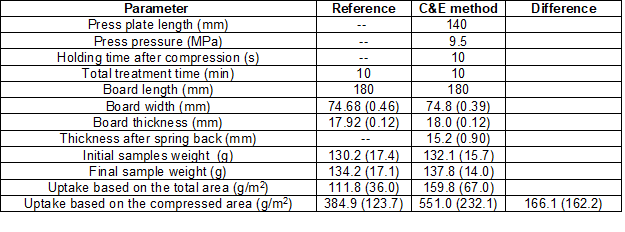
The C&E method was tested with six samples and their matched references for only one treatment solution (RME). The results are reported in Table 4. The solution uptake based on the total area accounts for the diffusion effect through all the surfaces, included the unsealed ends, while the solution uptake through the compressed 140 mm x 75 mm area accounts for the C&E effect. Assuming that the C&E contribution is the difference between compressed and reference samples, then the average solution uptake due to C&E method was 166.1 g/m2. This was equivalent to approximately 12 min at 90 ºC, 9 min at 120 ºC, and 6 min at 150 ºC with the H&C method. The uptake results in the C&E method were in the same order of magnitude as the uptake results in the H&C method with reasonable treatment times; thus the C&E method was not further investigated. The main disadvantage of the C&E method, in the opinion of the authors, was that the samples only recovered half of the initial thickness by immediate spring back, leaving the other half to likely recover in service when the wood is exposed to moisture.
Table 4. Average Results and Standard Deviation (in parentheses) of the C&E Method Implemented with RME
CONCLUSIONS
- Based on Scots pine side boards as model for treated material, and water, rapeseed oil, and rapeseed oil methyl ester (RME) as models for treatment solutions, this study demonstrates that the heating-and-cooling (H&C) method can be used as an intermediate technology between full volume impregnation and superficial surface coating for depositing a layer of protective chemicals below the external surfaces of wood materials.
- It was found that absorption rate increases proportionally to the increase of treatment temperature, but the relationship is not linear as predicted by the theory of ideal gases. The absorption rate is increasingly higher when the temperature increases, thus suggesting the influence of other possible factors such as a reduction of the solution viscosity or an increase in the wood diffusion coefficient.
ACKNOWLEDGMENTS
This project was funded by BioInnovation in partnership with VINNOVA, Formas, Energimyndigheten, and the industry members of TräCentrum Norr. A special thanks to Sveaskog for directly supporting the project and Frans and Carl Kempe Foundation 1984 for supporting a Ph.D. student involved in the project activities. Thanks very much as well to Norra Skogsägarna for providing wood for the project.
REFERENCES CITED
Alireza, S. M. (2011). Effect of Wood Constituents Oxidation of Unsaturated Fatty Acids, Licentiate thesis, KTH Royal Institute of Technology, Stockholm, Sweden.
Athena (2002). Maintenance, Repair and Replacement for Building Envelope Materials, AthenaTM Sustainable Materials Institute (www.athenasmi.org/wp-content/uploads/2011/10/2_Maintenance_Repair_And_Replacement.pdf).
Bernat, E., Jordi-Roger, R., Grau, B., Antoni, R., and Rita, P. (2011). “Temperature dependence of density and viscosity of vegetable oils,” Biomass & Bioenergy 42(2012),164-171. DOI: 10.1016/j.biombioe.2012.03.007
Blew, J. O. (1948). Treating Wood in Pentachlorophenol Solutions by the Cold-Soaking Method, Report No. R1445, Forest Products Laboratory, Madison, WI.
Crawford, C. G. (1907). The Open-Tank Method for the Treatment of Timber (Circular 101), US Forest Service, Washington, DC.
Deka, M., and Saikia, C. N. (2000). “Chemical modification of wood with thermosetting resin: effect on dimensional stability and strength property,” Bioresour. Technol. 73(2), 179-181. DOI: 10.1016/S0960-8524(99)00167-4
Fry, H. J. (1973). “Compression impregnation of wood veneers,” US Patent # 3950577A.
Forest Products Laboratory (2010). Wood Handbook—Wood as an Engineering Material, General Technical Report FPL-GTR-190, U.S. Department of Agriculture, Forest Products Laboratory, Madison, WI.
Gabrielli, C. P., and Kamke, F. A. (2010). “Phenol–formaldehyde impregnation of densified wood for improved dimensional stability,” Wood Sci. Technol. 44(1), 95-104. DOI: 10.1007/s00226-009-0253-6
Gsöls, I., Rätzsch, M., and Ladner, C. (2003). “Interactions between wood and melamine resins effect on dimensional stability properties and fungal attack,” in: Proceedings of the First European Conference on Wood Modification, Ghent, Belgium, pp. 221-225.
Hill, C. A. S. (2006). Wood Modification: Chemical, Thermal and Other Processes, C. V. Stevens (ed.), John Wiley & Sons, UK.
Homan, W. J., and Jorissen, A. J. M. (2004). “Wood modification developments,” HERON 49(4), 361-386.
Hunt, G. M., and Garratt, G. A. (1967). Wood Preservation, 3rd Ed., McGraw-Hill, New York, NY.
Inoue, M., Adachi, K., Tsunoda, K., Rowell, R. M., and Kawai, S. (2008). “A new procedure for treating wood,” Wood Material Science & Engineering 3(1-2), 46-54. DOI: 10.1080/17480270802605495
Kollmann, F. F. P., and Côté, W. A. (1984). Principles of Wood Science and Technology. Volume I: Solid Wood, Springer-Verlag, Berlin, Germany.
Lande, S. (2008). Furfurylation of Wood – Wood Modification by the Use of Furfuryl Alcohol, Ph.D dissertation, Norwegian University of Life Science, Ås, Norway.
Nurmi, A. (1998). Natural Resins as a Potential Wood Protecting Agent (Final Report), EC’s fourth framework programme, FAIR CT95-0089, 1995-1998.
Ormrod, D. J., and Van Dalfsen, B. (1993). Wood Preservation on the Farm (Final Report), Ministry of Agriculture, Fisheries and Food, British Columbia, Canada.
Richardson, B. A. (2003). Wood Preservation (2nd Ed.), Chapman & Hall, London, UK.
Ryu, J. Y., Takahashi, M., Imamura, Y., and Sato, T. (1991). “Biological resistance of phenol-resin treated wood,” Mokuzai Gakkaishi 37(9), 852-858.
Sandberg, K., Pousette, A., Karlsson, O., Sundqvist, B. (2013). Fasader i Trä för Flervåningsbyggnader Jämförelse Mellan Material och Behandlingsmetoder (SP Rapport 2013:21), SP Technical Research Institute of Sweden, Skellefteå, Sweden.
Takahashi, M., and Imamura, Y. (1990). Biological Resistance of Phenol-Resin Treated Wood, Doc. No. IRG/WP 3602, International Research Group on Wood Preservation, Stockholm, Sweden.
Tamblyn, N.E. (1985). “Treatment of wood by diffusion,” in: Preservation of Timber in the Tropics, W. P. K. Findlay (ed.), Springer, Amsterdam, The Netherlands, pp. 121-140. DOI: 10.1007/978-94-017-2752-5_6
Tikkurila (2016). Commercial Information from Paint Supplier, Vantaa, Finland, (www.tikkurila.sk/pdfexport/index.phtml%3Fs%3D80.pdf).
Venard, J. K., and Street, R. L. (1975). Elementary Fluid Mechanics (5th ed.), Wiley, New York, NY.
Westin, M., Lande, S., and Schneider, M. (2003). “Furfurylation of wood – Process, properties and commercial production,” in: Proceedings of the First European Conference on Wood Modification, Ghent, Belgium, pp. 289-306.
Williams, R. S. (2005). “Weathering of wood,” in: Handbook of Wood Chemistry and Wood Composites, R.M. Rowell (ed.), CRC Press, Boca Raton, FL, pp. 139-185.
Article submitted: June 25, 2016; Peer review completed: October 6, 2016; Revised version received and accepted: October 30, 2016; Published: November 14, 2016.
DOI: 10.15376/biores.12.1.283-295.
