Abstract
In recent decades, the scientific community has become interested in improving the extraction of bioactive compounds from plants through new extraction techniques. Microwave-assisted extraction (MAE) is an innovative and effective method to extract compounds from different plants of agronomic, medicinal, nutritional, or cosmetic interest. This technique is considered environmentally friendly, as it uses non-toxic solvents. In addition, its energy consumption is lower than conventional extraction techniques. Likewise, this extraction avoids the degradation of the compounds, making it a feasible method. For microwave extraction to be efficient, the following parameters must be considered: solvent characteristics, volume, exposure time, temperature, size and characteristics of plant material, power (MHz), and type of equipment. This review focuses on the interactions of various factors involved to achieve a successful extraction process. The optimization and importance of microwave extraction technology in the research of plant bioactive compounds are discussed in this review article.
Download PDF
Full Article
Microwave-assisted Extraction of Functional Compounds from Plants: A Review
Herminia López-Salazar,* Brenda Hildeliza Camacho-Díaz, Martha Lucia Arenas Ocampo, and Antonio Ruperto Jiménez-Aparicio
In recent decades, the scientific community has become interested in improving the extraction of bioactive compounds from plants through new extraction techniques. Microwave-assisted extraction (MAE) is an innovative and effective method to extract compounds from different plants of agronomic, medicinal, nutritional, or cosmetic interest. This technique is considered environmentally friendly, as it uses non-toxic solvents. In addition, its energy consumption is lower than conventional extraction techniques. Likewise, this extraction avoids the degradation of the compounds, making it a feasible method. For microwave extraction to be efficient, the following parameters must be considered: solvent characteristics, volume, exposure time, temperature, size and characteristics of plant material, power (MHz), and type of equipment. This review focuses on the interactions of various factors involved to achieve a successful extraction process. The optimization and importance of microwave extraction technology in the research of plant bioactive compounds are discussed in this review article.
DOI: 10.15376/biores.18.3.Lopez-Salazar
Keywords: Microwave energy; Extraction mechanism; Natural products
Contact information: Department of Biotechnology, Centro de Desarrollo de Productos Bióticos, Instituto Politécnico Nacional, P.O. Box 24, Yautepec 62730, Morelos, Mexico;
* Corresponding author: herminia784@gmail.com
GRAPHICAL ABSTRACT

INTRODUCTION
The first step in obtaining a plant extract is to choose the extraction method. To obtain active compounds, it is necessary to extract them with different solvents for qualitative and quantitative analysis to ensure their use in the food sector in agricultural pharmacology and phytotherapy. Among the conventional extraction methods, maceration, water percolation, and Soxhlet extraction have been used. These methods have some disadvantages, such as long extraction times, use of toxic solvents, and thermal decomposition of heat-labile compounds (De Castro and García-Ayuso 1998; Jadhav et al. 2009).
In general, the new methods have numerous benefits compared to the conventional ones, such as quicker extraction, greater output, and reduced environmental impact. However, each technique has its pros and cons, and the selection of a suitable method is determined by the characteristics of the compounds to be extracted and the intended use. There are various innovative approaches available for the extraction of natural compounds, which include:
a) Supercritical fluid extraction (SFE): This is a green technique for extracting compounds from plants that employ supercritical fluids, such as carbon dioxide, as a solvent. SFE offers several advantages over conventional extraction techniques because of the physicochemical properties of supercritical solvents, which include improved transport characteristics that result in quicker extraction rates. The high-quality extracts generated by SFE make it a hopeful method in the domains of food, pharmaceuticals, and cosmetics. (Da Silva et al. 2016).
b) Ultrasound-assisted extraction (UAE): This is a fast, cost-effective, and scalable technique for extracting compounds from plant resources. The process involves using ultrasound waves, which enhance the mass transport of bio-active constituents from the plant material to the solvent media through cavitation, mechanical agitation, and thermology. UAE is a simple and easy technique that consumes less solvent, has a shorter extraction time, and operates at lower process temperatures, resulting in higher extraction efficiency. Therefore, UAE has potential applications in the chemical and food industries (Chakraborty et al. 2020).
c) Pressurized liquid extraction (PLE): Pressurized liquid extraction (PLE) is an automated method of extracting compounds from various materials using traditional solvents. PLE operates under constant pressure and controlled parameters, including temperature, extraction time, and number of cycles. As the final extract is collected, it is automatically filtered, simplifying the process, and making it more efficient. PLE is an environmentally friendly technique that can utilize water and ethanol to recover polar to medium polarity compounds. This aspect contributes to its eco-friendly properties, making it an attractive option for industries seeking sustainable and cost-effective extraction methods (Xynos et al. 2014).
d) Enzyme-assisted extraction (EAE): This is a method that utilizes hydrolytic enzymes to dismantle cell walls and other constituents, facilitating the more efficient retrieval of metabolites from plant material. This technique offers environmental sustainability and cost-effectiveness, presenting a promising advancement over traditional and contemporary extraction approaches. EAE exhibits the potential in enhancing the recuperation of diverse bioactive metabolites, encompassing polyphenols, carotenoids, polysaccharides, terpenes, and essential oils (Łubek-Nguyen et al. 2022).
e) Microwave-assisted extraction (MAE): This is a technique that utilizes the power of microwaves to stimulate the movement of liquids’ molecules, allowing for efficient extraction of target components. Compared to traditional extraction methods, MAE offers benefits such as shorter extraction times, reduced solvent costs, and increased automation. MAE heats both the solvent and the material evenly, resulting in a greater yield of extracted compounds (Sun et al. 2020).
Microwave energy used in organic synthesis was first mentioned in 1986 (Gedye et al. 1986; Giguere et al. 1986). Subsequently, Ganzler et al. (1990) used this energy for the extraction of biological matrices. Microwave-assisted extraction (MAE) is based on the direct impact of electromagnetic radiation on a material that can absorb electromagnetic energy and convert it into heat (Xie et al. 2010). Such energy brings many advantages to the extraction process compared to traditional extraction techniques, such as less solvent and energy consumption, a short analysis time, and a higher yield (Longares-Patrón and Canizares-Macias 2006).
It is possible to obtain active compounds through MAE, regardless of the type of plant material used. Among these compounds are essential oils (Abd El-Gaber et al. 2018), flavonoids (Xu et al. 2021), terpenes, phenols (Mustapa et al. 2015), alkaloids (Du et al. 2010), and glucosides (López-Salazar et al. 2019). Rodríguez-Padrón et al. (2020) obtained phenolic compounds with antioxidant properties from green walnut shell residues through microwave extraction.
In a study conducted by Rodsamran and Sothornvit in 2019, a comparative analysis was conducted. They compared conventional and microwave heating methods for extracting pectin from lime peel waste, using different acid extractants and peel-to-extractant ratios. The results indicated that microwave extraction produced pectin with a higher equivalent weight and degree of esterification, as well as a lighter color. Citric acid was identified as a suitable extractant for microwave extraction, without compromising the quality of the pectin. The viscosity and viscoelastic properties of the pectin solution improved with increasing solid concentration. Although microwave extraction had a lower pectin yield, it was regarded as a significant energy-saving technique with reduced extraction time. The authors suggested exploring longer irradiation times to improve pectin yield. In another study conducted by the same authors.
Currently, studies are comparing the new extraction methods, such as the one reported by Rodsamran and Sothornvit (2019b). That work compared the efficiency of MAE and UAE for extracting phenolic compounds from lime peel waste. The aim was to optimize the extraction conditions for both methods. The study utilized response surface methodology to predict the optimal extraction conditions based on total phenolic content, antioxidant activity, and half-maximal inhibitory concentration. Results showed that UAE was more effective than MAE in extracting total phenolics with high antioxidant activity and saved 33% of extraction time.
Another study by Aourach et al. (2021) aimed to compare UAE and MAE methods for extracting phenolic compounds from Santolina chamaecyparissus L. (S. chamae-cyparissus). The researchers optimized extraction factors and identified five major phenolic compounds using Ultra-High-Performance Liquid Chromatography—Quadrupole Time of Flight—Mass Spectrometry (UHPLC-QToF-MS): chlorogenic acid, quercetin 3-O-galactoside, quercetin 3-O-glucoside, isoorientin, and cynarin. The findings demonstrated that MAE was a more efficient method than UAE for extracting phenolic compounds from S. chamaecyparissus.
MAE and UAE are effective for extracting phenolic compounds from various matrices. The choice of extraction method for phenolic compounds can vary depending on factors such as sample type, chemical composition, and equipment availability. While some studies indicate that MAE may be faster and require less solvent compared to UAE, both methods can be equally efficient in terms of yield and quality of phenolic extracts. Ultimately, the choice of extraction method should depend on the specific nature of the sample and extraction objectives. It is recommended to perform optimization tests for each specific sample to determine the optimal extraction method. Both methods can be effective for extracting phenolic compounds and should be chosen based on the nature of the sample and specific extraction objectives (Aourach et al. 2021).
At present, there are two main methods for conducting MAE. The first uses controlled temperature and pressure, and a closed container is used. The second model uses an open container, and the extraction temperature depends on the solvent to be used (boiling point), all within atmospheric pressure. The plant sample undergoes dielectric heating, due to electromagnetic radiation between 300 MHz and 300 GHz, caused by the frictional resistance of the ion flows and the continuous rotation of the dipole. Both phenomena produce an increase in thermal energy, leading to efficient extraction (Veggi et al. 2012). The characteristics of the plant material and the solvent influence the efficiency of the extraction. Other advantages offered by MAE are the extraction of volatile compounds known as solvent extraction and that of non-volatiles called dry extraction (Belwal et al. 2017).
This review provides information on MAE theory, MAE techniques, extraction procedures, and factors that influence the performance of a successful extraction.
THEORETICAL MECHANISM OF THE MAE PROCESS
The interconnection of the magnetic and electric fields of microwave energy with materials results in heating, which is produced by magnetic and dielectric losses.
Microwaves are electromagnetic waves with a frequency of 300 MHz (radio radiation) to 300 GHz. Two frequencies are used in research: 2.45 GHz for laboratory equipment and 915 MHz for industrial equipment (Woodhouse 2017). Two phenomena known as ionic conduction and dipole rotation are involved in producing microwave heating. The first is when the charge carriers develop an electrophoretic migration, among which are ions and electrons, which are subjected to the effect of the electric field of microwaves. The heating is the result of “friction” that is produced by the migration between the medium and the moving ions. Dipole rotation occurs when dipolar molecules try to adapt to the alternating electric field. This phenomenon occurs in a microwave environment. Collisions occur between the dipoles and the surrounding molecules, resulting in the generation of heat (Zhang et al. 2011). Both phenomena take place at the same time. As a result, a change from microwave energy to thermal energy is generated.
In certain extractions, it is advantageous to use a water-based solution of specific organic solvents because the inclusion of water enhances the solvent’s ability to penetrate the sample matrix, resulting in improved heating efficiency (Alfaro et al. 2003). Due to this reason, when the plant material is dry, it is crucial to add enough water to rehydrate it and allow for the use of microwave heating. This rehydration process is utilized in Solvent-Free Microwave-Assisted Extraction (SFMAE), which is also referred to as in-situ microwave-generated hydrodistillation (MGH) (Vinatoru et al. 2017). Despite being a low-cost and safe option, one disadvantage is that water fosters the development of mold and bacteria, can cause hydrolysis or the breakdown of plant metabolites, and requires elevated temperatures for evaporation (Belwal et al. 2018).
MECHANISM FOR OBTAINING BIOACTIVE COMPOUNDS FROM PLANT MATERIAL USING MICROWAVES
Secondary metabolites of plant origin are obtained by MAE because microwave energy has a rapid application in the matrix of the plant material. The energy is assimilated by the extract and the plant material, which is absorbed by the substances present in the plant material, having a greater affinity for polar molecules, for example, water. The internal temperature of the vegetable matrix increases, which causes overheating, which then causes vaporization. This phenomenon causes the rupture of plasma membranes and cell walls (Rostagno et al. 2010).
The secondary metabolites are distributed in the plant in different places. Some are found in the cytoplasm or the cell walls. Favorable location of the secondary metabolites will facilitate the transfer of secondary metabolites to the solvent and vice versa from the solvent to the plant material; this allows for a more successful extraction (Mandal et al. 2007).
The difference between MAE and conventional extraction methods (Soxhlet extraction and heat flow extraction) lies in the fact that conventional methods rely on a permeation and solubilization process to obtain the intracellular compounds from the plant material (Gao et al. 2007). Microwave heating heats the entire volume of the sample of plant material from the inside. This is unlike what happens with conventional heating, in which the heat comes from the outside and where contact with a hot surface is required to conduct heat. In addition, the water contained in the plant cells in situ is also heated with microwave radiation (Li et al. 2013).
To understand the effect of microwave energy in obtaining secondary metabolites from plant material, microscopic analyzes have been performed. These tools have been used to demonstrate the structural changes produced in plant samples after the application of microwaves.
An example of this is the work carried out with the Erigeron breviscapus plant (Gao et al. 2007), where a comparative analysis of samples processed and not treated by microwaves was carried out to isolate scutellarin. SEM was used to carry out this analysis, demonstrating that the samples treated with microwaves presented damage to the integrity of the E. breviscapus tissue, causing the release of compounds of interest in the extract.
In 2020, López conducted a comparative analysis using SEM on samples of Agave angustifolia Haw plant stem with different sizes (1 cm and 0.074 cm), with and without the application of microwave energy. The samples with a diameter of 1 cm that did not receive microwave energy (Figs. 1a and 1b) showed the characteristic bundles of individual fibers glued together, which are typical of this species. However, in the samples that did receive microwave treatment (Figs. 1c and 1d), there was an apparent change in morphology, although not significant. Using a different plant sample size of 0.074 mm, they performed the same comparative analysis without and with microwave application. Figures 2a and 2b (without microwaves) show the complete morphology of the plant sample, while in Figs. 2c and 2d (with microwaves), the lignin structure is seen to be broken, and an individual fiber can be observed, indicating significant structural damage.
Fig. 1. SEM micrographs of morphological changes in Agave angustifolia Haw stems with microwave treatment. Plant fragments 1 cm in diameter, cross-sectional view, (a) and (b) show the untreated samples, and (c) and (d) show the samples treated with microwaves. (López 2020). White arrows point to tissue destruction.
Fig. 2. SEM micrographs of morphological changes in Agave angustifolia Haw stems with microwave treatment. Plant fragments size 0.074 mm, longitudinal view, (a) and (b) show the untreated samples, and (c) and (d) show the samples treated with microwaves (López 2020). White arrows indicate tissue destruction.
Another study demonstrated the effect of MAE on Epimedium koreanum leaves by electron microscopy. This work found that the number of chloroplasts had decreased and there was also a color change from colorless to green in the extract. With these results, they concluded that MAE could damage plant tissues and/or cells (including organelles) (Zhang et al. 2011). Olalere et al. (2021) evaluated the yield of total Cola nitida phenols obtained by electromagnetic-based microwave reflux extraction. Plant material after extraction was analyzed to find changes in morphology. They found a breakdown of the fatty and lignocellulosic structures. Field emission scanning electron microscopy (FESEM) evidenced the loss of continuity of the cellulose membrane of the treated plant material, causing an opening, which helped the components of interest (phenolics) to detach from the cellulosic skeleton.
THE DEVELOPMENT OF MAE TECHNIQUES
In the immediate past, some microwave extraction methods have been improved. These improvements are intended to overcome possible limitations of MAE, such as oxidation of certain secondary metabolites and thermal decomposition, which in past practices can lead to a decrease in extraction yield. MAE subtypes have been developed and are described below.
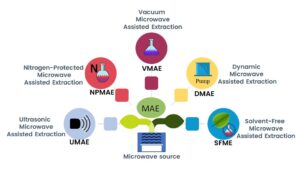
Nitrogen-Protected Microwave-Assisted Extraction (NPMAE)
NPMAE is a technique that deals with preventing the oxidation of secondary metabolites during the extraction process by using nitrogen to pressurize the extraction vessel. This method was applied to obtain ascorbic acid from different types of peppers (cayenne, green, yellow) and guava. Considering its performance, this NPAMAE technique is better than standard MAE and Soxhlet. This difference may be due to the protection caused by nitrogen. (Yu et al. 2009).
Vacuum Microwave-Assisted Extraction (VMAE)
VMAE has the characteristic of improving the mass transfer mechanism because it favors the propagation process of secondary metabolites in the solvent through suction pressure. The pressurized vacuum process reduces the risk of oxidation and thermal degradation because this causes the boiling points of the solvents used to be lower. Research papers report an increase in the yield of vitamin C from guava, soybean, tea leaves, and green pepper by applying VMAE compared to standard MAE (Xiao et al. 2009).
VMAE entails performing microwave-assisted extraction within a vacuum environment, leveraging the reduced boiling point of the extraction solvent under vacuum conditions as compared to normal atmospheric pressure. This enables extraction to take place at lower temperatures, thereby preventing the degradation of heat-sensitive compounds. Maintaining the solvent in a boiling state and promoting reflux at lower temperatures enhances the blending of the sample with the solvent and facilitates the extraction of compounds. Additionally, the removal of air from the extraction system reduces the oxidation of thermosensitive compounds due to minimal oxygen presence. Consequently, VMAE combines the benefits of MAE with its suitability for extracting thermosensitive compounds at lower temperatures and with reduced oxygen exposure (Wang et al. 2008).
In the cases where NPMAE and VMAE are used, additional nitrogen sources and a vacuum pump are included. The vacuum pump is used to create a vacuum pressure in VMAE and is also employed to remove oxygen before pressurizing the vessel with nitrogen in NPMAE. Furthermore, a reflux system is installed to prevent excessive pressure accumulation during the extraction process. In certain NPMAE scenarios, the inert gas is pressurized directly into the extraction vessel that contains the sample-solvent mixture and subsequently placed inside the closed microwave cavity (Saha et al. 2018).
Ultrasonic Microwave-Assisted Extraction (UMAE)
UMAE is another type of microwave extraction. This technique improves the mass transfer process during extraction. It is characterized by an additional ultrasonic wave emitted by UMAE that serves to intensify the mass transfer process, by the combination of ultrasonic waves and microwaves. Therefore, the rupture of the plant cells is conducted by a stronger energy, which causes the exit of the secondary metabolites to the solvent (Chen et al. 2010). This extraction method has been applied to extract lycopene from tomatoes. (Zhang and Liu 2008), and vegetable oil (Cravotto et al. 2008).
In 2022, Lasunon and Sengkhamparn conducted a study that revealed the benefits of microwave heating at high power in terms of increasing pectin yield and galacturonic acid (GalA) contents. The researchers also found that combining UAE with MAE significantly enhanced pectin yield and GalA content. The UMAE, which is a combined extraction method of UAE and MAE, was shown to be an efficient and effective approach for producing a higher pectin yield compared to the individual methods.
The researchers evaluated various UMAE conditions but excluded pH and solid-liquid ratio as factors. The results indicated that MAE outperformed UAE in terms of both pectin yield and extraction time. The extraction techniques differ in that MAE uses electric field-induced heating to vibrate water molecules, increasing temperature and pressure, which leads to cell destruction and the release of pectin.
On the other hand, UAE generates gas microbubbles through sonication, inducing cavitation effects and cell disruption. It is important to note that UAE disrupts the cell matrix more slowly than microwave heating. Therefore, the combination of UAE and MAE resulted in a higher pectin yield and GalA content compared to performing only UAE or MAE, indicating that UAE synergized the effect of MAE. Notably, the extraction techniques did not affect the structure of pectin.
Dynamic Microwave-Assisted Extraction (DMAE)
DMAE has emerged as a highly effective method for extraction processes. The dynamic approach in DMAE provides numerous advantages, particularly in terms of how the solute is distributed within the extraction media. By continuously introducing fresh solvent into the extraction vessel, the overall efficiency of the process is enhanced. The rate of desorption does not have to be significantly greater than the rate of adsorption to effectively remove the targeted solute. Ericsson and Colmsjö (2003) developed a DMAE apparatus that utilized a microwave oven as the heat source while employing an HPLC detector to continuously monitor the extraction process.
Gao et al. in 2006 introduced an automated, continuous, and rapid extraction technique for analyzing flavonoids in dried cell cultures of Saussurea medusa Maxim using a newly designed DMAE system. In a specific study focused on extracting flavonoids from dried cell cultures of S. medusa, the researchers developed an automated, continuous, and rapid extraction method using a newly designed DMAE system. They optimized several factors that influence the extraction process, including microwave irradiation power, liquid-to-solid ratio, solvent flow rate, and irradiation time. Under the optimal conditions (1200W of radiation power, 50:1 liquid-to-solid ratio, and 50 mL/s solvent flow rate), the yield of flavonoids reached 4.97% within a 60-minute timeframe. This dynamic microwave-assisted extraction approach highlighted significant advantages, such as a shorter extraction duration and higher efficiency, without causing degradation of the target components when compared to dynamic solvent extraction without microwave assistance.
Chen et al. (2008) discovered that DMAE yielded higher extraction efficiencies of flavonoids in Herba epimedii compared to ultrasonic extraction (UE), heat reflux extraction (RE), and Soxhlet extraction (SOX). Moreover, DMAE notably decreased the extraction duration. The extraction yield achieved with DMAE resembled that of another method known as PMAE (pressurized microwave-assisted extraction). Importantly, the flavonoids in H. epimedii exhibited resistance to decomposition when the extraction time was extended using DMAE.
DMAE offers the advantage of transferring the extracted analytes from the sample matrix by continuously flowing a fresh solvent out of the extraction vessel. Chen et al. (2008), constructed an extraction apparatus in their laboratory for their investigations. The diagram illustrates the schematic representation of the DMAE system. It demonstrates the setup and components used in the extraction process (Fig. 3).
The setup employed a microwave resonance cavity, developed in their previous study (Chen et al. 2007), as the coupling device for microwave energy. A microwave source with a maximum power output of 100 W was employed, while a peristaltic pump was responsible for delivering the extraction solvent into the extraction vessel made of polytetrafluoroethylene (PTFE). To filter the extract and maintain the solvent in a liquid state, an online filter referred to as the ending fitting and a restrictor were positioned at the outlet of the vessel.
Fig. 3. Diagram of DMAE system
The study established that DMAE exhibited superior extraction yields and reduced extraction times for flavonoids in H. epimedii compared to alternative extraction methods. DMAE facilitated the efficient transfer of analytes while preserving the stability of the desired compounds. The experimental setup provides valuable information on the practical application of DMAE for the extraction of bioactive compounds.
Overall, DMAE has demonstrated its potential as an efficient extraction technique because it has shown higher extraction efficiency and lower degradation of target compounds, making it a valuable tool for various extraction processes, including the extraction of flavonoids from plant materials.
Solvent-Free Microwave-Assisted Extraction (SFMAE)
This type of microwave extraction is widely used to obtain essential oils, but it is also possible to use water when the objective is to obtain certain compounds. This type of method has certain advantages, including the reduction of extraction time compared to conventional techniques. Another benefit observed in the quality of the oils obtained is that oxidation and hydrolysis are avoided, since they may be caused when conventional techniques are used. For this reason, this type of technique is considered the first option for obtaining essential oils (Wang et al. 2006). The polar substances are rapidly heated by microwaves, and the reduced water content helps prevent the thermal and chemical breakdown of compounds through processes such as hydrolysis, transesterification, or oxidation (Flórez et al. 2015).
INSTRUMENTATION AND EQUIPMENT SETUP OF MAE
There are different varieties of laboratory equipment related to MAE, and these are designed to facilitate extraction and meet different specific needs of the extraction process. This type of equipment offers the advantage of being able to optimize the extraction processes as well as being used for analytical applications. MAE can be performed using the modified domestic microwave oven, but it has the disadvantage of being less efficient compared to commercial MAE equipment because it does not offer an automated system (pressure and temperature control) (Chan et al. 2011). Various commercial microwave extraction systems are illustrated in Table 1.
Table 1. Commercially Available Microwave Systems
FACTORS THAT AFFECT OR BENEFIT THE PERFORMANCE OF THE MAE
MAE is influenced by several factors, so it is important to use a combination of variables that has a positive impact on MAE. The components that can affect the MAE are the microwave power, temperature and extraction time, cycles, type of solvent, the solid-liquid ratio, as well as the characteristics and size of the plant material. Therefore, it is important to recognize the interaction and impact of these elements in MAE development.
Effect of Solvent Nature
One of the important factors for the success of MAE is the proper choice of solvent. The dielectric constant of the solvent or its mixture is one of the important factors to be well selected to have an effective and selective MAE (Kharlamova 2019).
The dielectric constant is a material property that signifies its capacity to store electrical energy within an electric field. Within the context of MAE, the dielectric constant plays a crucial role. In MAE, microwaves are employed to selectively heat a sample, aiding the release of targeted compounds. Materials possessing a high dielectric constant exhibit an enhanced capability to absorb microwave energy and convert it into heat (Vinatoru et al. 2017).
As a result, when a sample with a high dielectric constant is subjected to microwaves, its molecules interact with the microwave’s electric field, rapidly vibrating and rotating and generating heat within the sample. The dielectric constant establishes a material’s ability to absorb microwave energy and convert it into heat, thereby facilitating the more efficient extraction of desired compounds during MAE. The efficiency of microwave heating depends on the material’s dissipation factor, which is interconnected with the dielectric constant (Singh et al. 2014).
Additionally, the dielectric constant also influences the selectivity of heating, limiting the process to materials with specific dielectric constants. In summary, the dielectric constant is a significant property in MAE as it determines a material’s capacity to absorb microwave energy and convert it into heat, thus enabling efficient extraction of desired compounds (Singh et al. 2014).
The selection of solvents plays a vital role in compound extraction and should be in line with the chemical properties of the target compounds. Polar solvents, such as ethanol, methanol, and ethyl acetate, are commonly utilized for extracting hydrophilic components from plants, while non-polar solvents including hexane, ether, and petroleum ether are preferred for extracting lipophilic secondary metabolites. Although water is inexpensive and non-toxic, it promotes the growth of mold and bacteria, can lead to hydrolysis or decomposition of plant metabolites, and requires high temperatures for evaporation (Proestos and Komatis 2008).
Ethanol and methanol are effective solvents for alkaloids, flavonoids, terpenes, glycosides, and coloring compounds, but they are unable to dissolve polysaccharides, tannins, gums, and waxes. When choosing a solvent for the extraction process, it is important to carefully evaluate various factors, including the solvent’s ability to dissolve the desired compounds, its boiling point, reactivity, viscosity, recovery rate, vapor pressure, safety profile, toxicity, and cost. The choice of solvent depends not only on the nature of the compounds being extracted but also on the extraction process and its intended purpose (Proestos and Komatis 2008).
In microwave-assisted extraction (MAE), it is crucial to consider factors such as the solubility of target molecules and the dielectric characteristics of the solvent. Non-polar solvents, characterized by low dielectric constants, are not suitable for microwave extraction, whereas polar solvents are preferred due to their high dielectric constants, which facilitate microwave absorption. However, the “broken cell wall theory” supports the use of microwave-transparent non-polar solvents in MAE (Proestos and Komatis, 2008).
Despite these differing opinions, both polar and non-polar solvents have been successfully employed in microwave extraction. Direct heating of the plant matrix using microwaves can extract thermo-sensitive compounds into surrounding non-polar solvents at lower temperatures compared to polar solvents. In other cases, polar solvents brought to their boiling points can efficiently extract compounds that are not affected by high temperatures. The selectivity of extraction can be adjusted by using solvent mixtures, and rehydrating dried plant tissues enhances their susceptibility to dielectric effects, resulting in improved yields (Belwal et al. 2008).
For some extractions, it is advisable to choose aqueous solutions of organic solvents. This is because the presence of water improves the penetration of the solvent into the plant material. This contributes to more efficient heating (Alfaro et al. 2003). The organic solvents methanol, ethanol, and acetone have been used and have proven to be effective for this type of extraction (Casazza et al. 2010).
Ethanol is the most used solvent; in addition to being a good microwave absorber, it has the advantage of being able to extract different secondary metabolites (Luo et al. 2021). Another way of working and being able to modulate the selectivity of the extraction, different mixtures of solvents can be used. Among these mixtures are water, ethanol (Mesa et al. 2021), and methanol (Belwal et al. 2020).
The selection of a solvent for MAE is not determined by conventional extraction methods, as solvents effective in traditional techniques may not be suitable. For example, diethyl ether, commonly used for solubilizing steroids from the Saxifragaceae family, is unsuitable for MAE. However, adding a modifier can enhance solvent performance. Water was added as a modifier to diethyl ether in order to improve the microwave heating efficiency during the extraction of steroids from Rodgersia aesculifolia Batal (Lu et al. 2007).
In some cases, where a poor microwave absorber like hexane is used, ethanol or water can be added to enhance extraction efficiency, as observed in the extraction of ginger of Zingiber officinale, using MAE (Alfaro et al. 2003). The controlled addition of strongly absorbing substances effectively modifies the dielectric properties of the matrix. These substances, which do not affect the quality of the extract, are incorporated without requiring any extra steps for extract recovery. As a result, this modification enables a significantly higher yield to be obtained in considerably shorter extraction times compared to conventional methods. Another study conducted by Mandal et al. (2008) involved the MAE of curcumin from Curcuma longa, where they found that adding methanol improves the extraction when acetone is used as a solvent.
MAE is recognized as a green extraction technique. Green extraction is a fascinating concept that aims to generate safe and high-quality extracts while minimizing the use of solvents, time, and energy, all in an environmentally friendly manner (Belwal et al. 2020).
Effect of Solid to Liquid Ratio
To obtain adequate heating, the solid-liquid relationship must be considered. It is known that if there is a larger amount of solvent, the heating produced by the microwaves is affected. This is because the solvent would absorb the microwave radiation. Excessive absorption of microwaves by the solvent used for extraction can hinder the passage of an adequate amount of microwaves to reach the plant matrix beyond the solvent layer. Consequently, simultaneous heating of the plant matrix, which plays a crucial role in breaking down the cell wall and releasing the desired analytes, may not occur effectively (Mandal and Mandal 2010). If there is an insufficient amount of solvent in the plant material, that will cause a decrease in the movement of secondary metabolites in the cell matrix, thus forming a mass transfer barrier (Amarni and Kadi 2010). It is necessary to use an adequate amount of solvent to completely submerge the plant material in the solvent while undergoing microwave irradiation. However, increasing the ratio of solvent to material would not lead to improved extraction efficiency, as it could result in uneven distribution and inadequate exposure to microwaves (Xu et al. 2017).
Ruan and Li (2007) mentioned that there is an interactive effect on MAE by considering the container size and solvent ratio. The combination of these elements affects the extraction, and it is more noticeable in the closed MAE. The internal pressure will be higher when using the same proportion of solvent if a smaller container is used instead of a larger one. This would make the extraction faster, but weak compounds would be affected. In addition, the nature of the solvent must be considered when choosing the solvent-to-plant matrix ratio to allow mobility of the extracted compounds and provide adequate heating of the plant material (Chan et al. 2011).
Microwave Power
To destroy the matrix of the plant material in such a way that the secondary metabolite diffuses and can dissolve in the solvent, adequate microwave power is necessary to generate heating and produce the force required for such destruction. Therefore, an increase in power has the potential to reduce the time and improve the extraction yield (Alfaro et al. 2003).
A high level of microwave power could lead to a decrease in extraction yield due to the degradation of heat-sensitive compounds. Typically, the extraction yield shows a proportional increase with higher microwave power, reaching a limit beyond which the increase becomes insignificant or starts to decline. Microwave power plays a crucial role by providing localized heating within the sample and acting as a driving force for MAE to break down the plant matrix, allowing the analyte to diffuse and dissolve in the solvent (Chan et al. 2011).
The exposure time of secondary metabolites is reduced when high potency is used. But it is important to keep in mind that high power can also cause a decrease in yield, as compounds are more at risk of thermal degradation (Bellumori et al. 2016; Belwal et al. 2017). Using low power, more irradiation time will be needed to obtain compounds. With the above, it can be mentioned that radiation exposure time and microwave power produce opposite effects (Kwon et al. 2003).
Extraction Temperature
Extraction temperature and microwave power are interrelated. When the microwave power increases, the extraction temperature also increases. A high extraction temperature is beneficial for extraction as it increases solubility (Kharlamova and Praliyev 2018). Increasing the temperature causes the potency of the solvent to increase due to the decrease in viscosity and surface tension (Xiao et al. 2008). A more reasonable approach to microwave use would be to apply low and medium power with longer exposure.
Kharlamova and Praliyev (2018) refer to two situations: the first, extraction at higher temperatures, produces more rapid destruction of the cell wall, with this the secondary metabolites are integrated into the solvent, and the second situation can induce the rupture of the wall cell when using low power; this will allow selective extraction. They mention that the recommended temperature range should be from 30 to 60-140 °C. One recommendation is to choose the temperature considering the stability of the compound of interest (Xiao et al. 2008; Tsubaki et al. 2010).
Extraction Time and Cycle
To avoid the risk of thermal degradation and oxidation, the extraction time of the MAE is an important factor that must be controlled (Wang et al. 2009). Different times usually range from minutes to seconds (Mustapa et al. 2015; López-Salazar et al. 2019).
Cyclic extraction is used to avoid thermal degradation of the compounds. This cycle is carried out by adding a new solvent to the residual plant matrix to continue the extraction, thus ending the extraction. The total number of cycles must be individualized based on each plant (characteristics) and compound of interest. It is important to keep this in mind, and to properly implement this cycling procedure, to save solvent and save extraction time (Chen et al. 2007; Yan et al. 2010).
Characteristics of the Plant Matrix
Different plants have been used to obtain various bioactive compounds (phenols, sterols, flavonoids, and essential oils, among others). It is recommended, before starting an extraction process, to know how the cellular structure of the plant is formed. Because secondary metabolites have specific sites (tissues, cells, or organelles) where their biosynthesis, transport, and accumulation take place.
Some compounds, for example, essential oils, are localized in special cellular structures, either in plant tissues or on the plant surface (Svoboda et al. 2000). Another example is sterile glycosides (SG) and sterile acyl glycosides (ASG) that accumulate in microdomains (lipid rafts) located in the plant plasma membrane (PM) (Ferrer et al. 2017).
Other compounds are stored in the central vacuoles of the guard and epidermal cells, including the subepidermal cells of shoots and leaves (Lattanzio et al. 2006). It is also important to know the type of union that exists between the secondary metabolites and others that are in the plant. An example of this is polyphenols that are usually covalently bound to the cell wall of plants, similarly, there are also types of secondary metabolites in waxes or on the external surfaces of plant organs bound by glycosides (Lattanzio et al. 2006; Ferrer et al. 2017).
Zill et al. (2011) found that the solvent-free microwave hydro diffusion and gravity (MHG) technique is favored over the traditional method for extracting phenolic compounds from onions. The reason behind this preference is that microwaves cause the formation of vacuoles and disruptions in the cell walls, ultimately enhancing the effectiveness of the MHG technique for extraction.
The microscopic features of tissues processed with MHG were compared to those of the control group. The control tissues exhibited a typical leaf structure, consisting of a well-defined mesophyll surrounded by layers of epidermal cells. In all tissue samples, the cells were fully differentiated and densely packed, with intercellular spaces limited to the cell angles. As the cells progressed from the outer to the inner layers, their cytological characteristics revealed a prominent vacuole occupying a significant portion of the cell volume, while the cytoplasm formed a thin layer pressed against the cell wall.
On the other hand, the sections from MHG-treated scales showed noticeable structural alterations, particularly in the cell walls and vacuoles. The cell walls of the epidermal, sub-epidermal, and mesophyll cells underwent extensive breakdown, as evidenced by the changed appearance of the polysaccharides comprising the cell wall and the presence of fibrillar material instead of the middle lamella. These changes resulted in the loss of cell cohesion and disrupted cell structure. Additionally, the vacuoles throughout the scale tissues appeared compressed, with irregular contours indicating a release of their contents.
The observed modifications in cell walls and vacuoles caused by MHG provide supporting evidence for the reported effectiveness of this technique. It is important to note that flavonoids, such as quercetin, are known to accumulate in vacuoles. Hence, the MHG-induced alterations in cell walls and vacuoles, leading to eventual cell plasmolysis, likely facilitate the extraction of flavonoids and contribute to the method’s effectiveness.
In conventional extraction, plant materials are taken up dry but are known to retain small traces of moisture. Such moisture serves for microwave heating to occur. Then, the presence of water, which can be either naturally present in the plant sample or added, can enhance heating by increasing the polarity of the extractor. Another advantage of the presence of water is that it causes the plant matrix to swell (Li et al. 2013).
Particle Size and Shape
The particle size of the plant material should be very small (between 100 μm and 2 mm) to increase the contact surface of the sample with the solvent (Wang and Weller 2006). Then, when the size of the plant material is smaller, it will provide more surface area for the solvent to penetrate, cause cell rupture and enhance mass transfer, thus achieving successful extraction (Vilkhu et al. 2008).
Vinatoru et al. (2017) mention the importance of the shape of the plant material. When it has an irregular shape and after being suspended in the solvent, its shape becomes round and becomes smooth. The plant material is then surrounded by a stagnant layer formed by the used solvent, and transport may be slowed by the need for the secondary metabolites to diffuse through that layer.
Effect of Stirring
When agitation is incorporated into the MAE, it positively enhances this process. This is because it directly influences mass transfer. The active compounds, when concentrated in a single region, create a mass transfer barrier, which can be lowered by continuous agitation, which has a benefit in extraction performance. The extraction rate benefits by being accelerated by stirring, by increasing the release and dissolution of the compounds of interest (Sousa et al. 2010; Liazid et al. 2011).
OPTIMUM OPERATING CONDITIONS
The optimization of MAE conditions is a laborious task (increase in the number of runs, among others). Because of this, multivariate analysis has been considered for optimization. Different models and statistical designs have been used such as Central composite design (CCD), the differential evolution technique (DET), neural networks (NN), the response surface method (RSM), multiple-component analysis (MCA), and Box-Behnken design (BBD), which have been used in botanical extraction. Various extraction optimization models used these statistical models (Tables 2 and 3).
Table 2. Some Examples of MAE Optimization Models
Table 3. Some Examples of MAE Optimization Models
Abbreviations: Central composite design (CCD); Differential evolution technique (DET); Neural networks (NN); Response surface method (RSM); Multiple-component analysis (MCA); Box-Behnken design (BBD); Polyethylene glycol 8000 (PEG 8000)
COMPARISON BETWEEN MAE AND OTHER EXTRACTION TECHNIQUES
MAE has several advantages over traditional extraction methods. One of the advantages is reduced extraction time and solvent volume savings. Better yields of secondary metabolites are also observed. Another great advantage is that it is an automated method, which makes it reproducible (Olalere and Gan 2021). Different works compare MAE with conventional extraction methods, reporting that MAE is a suitable and reliable method for obtaining bioactive compounds.
In 2015, Mustapa et al. conducted a study comparing MAE, SFE, and Soxhlet extraction methods for extracting Clinacanthus nutans Lindau. The results showed that MAE was more efficient than Soxhlet in terms of extraction time, yield, and content of polar polyphenols. MAE required only 13 g of solvent per feed, compared to 21 g in Soxhlet. Additionally, the extraction time for MAE was significantly shorter (15 s) compared to Soxhlet (480 min), and the energy consumption was also reduced. These findings suggest that microwave-assisted extraction is a favorable method for extracting C. nutans.
López et al. (2019) obtained a higher yield of β-sitosterol glucoside of “pineapple” of Agave angustifolia obtained by MAE with the application of a catalyst (KOH), in less extraction time (5 s), concerning the maceration technique of 48 h.
In another study, MAE was developed to extract flavonoids from Radix Astragali rapidly (Xiao et al. 2008). The optimization of the extraction protocol involved studying factors such as microwave power, ethanol concentration, extraction temperature, and solvent-to-material ratio. The highest yield of flavonoids was achieved through dual extraction with 90% ethanol at a 25 mL/g material ratio, a temperature of 110°C, and a duration of 25 minutes. The developed protocol maintained the integrity of flavonoids. MAE exhibited an optimal yield of 1.190 ± 0.042 mg/g, which was comparable to Soxhlet extraction with methanol for 4 hours (1.292 ± 0.033 mg/g), and higher than ultrasound-assisted extraction with methanol for 2× 30 minutes and heat reflux extraction with 90% ethanol for 2× 2 hours (Xiao et al. 2008).
CONCLUSIONS
Currently, microwave-assisted extraction (MAE) is considered a viable extraction method. Different types of MAE have led to improved yields in the extraction of secondary plant metabolites, including nitrogen protected (NPMAE), vacuum (VMAE), ultrasonic (UMAE), dynamic (DMAE), and solvent-free (SFMAE) versions of MAE. For this reason, the future of MAEs in research is very promising. Moreover, with the advent of new funding and research programs, technologies that promise to be environmentally friendly are set to increase in the coming years. Therefore, MAE seems to have become the extraction method of choice.
REFERENCES CITED
Abd El-Gaber, A. S., El Gendy, A. N. G., Elkhateeb, A., Saleh, I. A., and El-Seedi, H. R. (2018). “Microwave extraction of essential oil from Anastatica hierochuntica (L): Comparison with conventional hydro-distillation and steam distillation,” Journal of Essential Oil Bearing Plants 21(4), 1003-1010. DOI: 10.1080/0972060X.2018.1504695
Alfaro, M. J., Bélanger, J. M., Padilla, F. C., and Paré, J. J. (2003). “Influence of solvent, matrix dielectric properties, and applied power on the liquid-phase microwave-assisted processes (MAP™) extraction of ginger (Zingiber officinale),” Food Research International 36(5), 499-504. DOI: 10.1016/S0963-9969(02)00198-9
Amarni, F., and Kadi, H. (2010). “Kinetics study of microwave-assisted solvent extraction of oil from olive cake using hexane: Comparison with the conventional extraction,” Innovative Food Science and Emerging Technologies 11(2), 322-327. DOI: 10.1016/j.ifset.2010.01.002
Aourach, M., González-de-Peredo, A. V., Vázquez-Espinosa, M., Essalmani, H., Palma, M., and Barbero, G. F. (2021). “Optimization and comparison of ultrasound and microwave-assisted extraction of phenolic compounds from cotton-lavender (Santolina chamaecyparissus L.),” Agronomy 11(1), 84. DOI: 10.3390/agronomy11010084
Araújo, R. G., Rodriguez-Jasso, R. M., Ruiz, H. A., Govea-Salas, M., Pintado, M. E., and Aguilar, C. N. (2020). “Process optimization of microwave-assisted extraction of bioactive molecules from avocado seeds,” Industrial Crops and Products 154, article 112623. DOI: 10.1016/j.indcrop.2020.112623
Behere, M., Patil, S. S., and Rathod, V. K. (2021). “Rapid extraction of watermelon seed proteins using microwave and its functional properties,” Preparative Biochemistry and Biotechnology 51(3), 252-259. DOI: 10.1080/10826068.2020.1808792
Bellumori, M., Innocenti, M., Binello, A., Boffa, L., Mulinacci, N., and Cravotto, G. (2016). “Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound-and microwave-assisted extraction procedures,” Comptes Rendus Chimie 19(6), 699-706. DOI: 10.1016/j.crci.2015.12.013
Belwal, T., Bhatt, I. D., Rawal, R. S., and Pande, V. (2017). “Microwave-assisted extraction (MAE) conditions using polynomial design for improving antioxidant phytochemicals in Berberis asiatica Roxb. Ex DC. leaves,” Industrial Crops and Products 95, 393-403. DOI: 10.1016/j.indcrop.2016.10.049
Belwal, T., Ezzat, S. M., Rastrelli, L., Bhatt, I. D., Daglia, M., Baldi, A., and Atanasov, A. G. (2018). “A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies,” TrAC Trends in Analytical Chemistry 100, 82-102. DOI: 10.1016/j.trac.2017.12.018
Belwal, T., Pandey, A., Bhatt, I. D., and Rawal, R. S. (2020). “Optimized microwave assisted extraction (MAE) of alkaloids and polyphenols from Berberis roots using multiple-component analysis,” Scientific Reports 10(1), 1-10. DOI: 10.1038/s41598-020-57585-8
Casazza, A. A., Aliakbarian, B., Mantegna, S., Cravotto, G., and Perego, P. (2010). “Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques,” Journal of Food Engineering 100(1), 50-55. DOI: 10.1016/j.jfoodeng.2010.03.026
Chakraborty, S., Uppaluri, R., and Das, C. (2020). “Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from bitter gourd using response surface methodology (RSM),” Food and Bioproducts Processing, 120, 114-122. DOI: 10.1016/j.fbp.2020.01.003
Chan, C. H., Yusoff, R., Ngoh, G. C., and Kung, F. W. L. (2011). “Microwave-assisted extractions of active ingredients from plants,” Journal of Chromatography A 1218(37), 6213-6225. DOI: 10.1016/j.chroma.2011.07.040
Chen, L., Ding, L., Yu, A., Yang, R., Wang, X., Li, J., and Zhang, H. (2007). “Continuous determination of total flavonoids in Platycladus orientalis (L.) Franco by dynamic microwave-assisted extraction coupled with on-line derivatization and ultraviolet–visible detection,” Analytica chimica acta, 596(1), 164-170. DOI: 10.1016/j.aca.2007.05.063
Chen, L., Jin, H., Ding, L., Zhang, H., Li, J., Qu, C., and Zhang, H. (2008). “Dynamic microwave-assisted extraction of flavonoids from Herba Epimedii,” Separation and Purification Technology, 59(1), 50-57. DOI: 10.1016/j.seppur.2007.05.025
Chen, Y., Xie, M. Y., and Gong, X. F. (2007). “Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum,” Journal of Food Engineering 81(1), 162-170. DOI: 10.1016/j.jfoodeng.2006.10.018
Chen, Y., Gu, X., Huang, S. Q., Li, J., Wang, X., and Tang, J. (2010). “Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities,” International Journal of Biological Macromolecules 46(4), 429-435. DOI: 10.1016/j.ijbiomac.2010.02.003
Chenni, M., El Abed, D., Neggaz, S., Rakotomanomana, N., Fernandez, X., and Chemat, F. (2020). “Solvent free microwave extraction followed by encapsulation of O. basilicum L. essential oil for insecticide purpose,” Journal of Stored Products Research 86, article 101575. DOI: 10.1016/j.jspr.2020.101575
Cravotto, G., Boffa, L., Mantegna, S., Perego, P., Avogadro, M., and Cintas, P. (2008). “Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves,” Ultrasonics Sonochemistry 15(5), 898-902. DOI: 10.1016/j.ultsonch.2007.10.009
De Castro, M. L., and Garcıa-Ayuso, L. E. (1998). “Soxhlet extraction of solid materials: an outdated technique with a promising innovative future,” Analytica Chimica Acta 369(1-2), 1-10. DOI: 10.1016/S0003-2670(98)00233-5
Da Silva, R. P., Rocha-Santos, T. A., and Duarte, A. C. (2016). “Supercritical fluid extraction of bioactive compounds,” TrAC Trends in Analytical Chemistry 76, 40-51. DOI: 10.1016/j.trac.2015.11.013
Du, F., Xiao, X., Xu, P., and Li, G. (2010). “Ionic liquid-based microwave-assisted extraction and HPLC analysis of dehydrocavidine in Corydalis saxicola Bunting,” Acta Chromatographica 22, 459-471. DOI: 10.1556/AChrom.22.2010.3.9
Ericsson, M., and Colmsjö, A. (2003). “Dynamic microwave-assisted extraction coupled on-line with solid-phase extraction and large-volume injection gas chromatography: determination of organophosphate esters in air samples,” Analytical Chemistry 75(7), 1713-1719. DOI: 10.1021/ac026287v
Ferrer, A., Altabella, T., Arró, M., and Boronat, A. (2017). “Emerging roles for conjugated sterols in plants,” Progress in Lipid Research 67, 27-37. DOI: 10.1016/j.plipres.2017.06.002
Flórez, N., Conde, E., and Domínguez, H. (2015). “Microwave assisted water extraction of plant compounds,” Journal of Chemical Technology & Biotechnology 90(4), 590-607. DOI: 10.1002/jctb.4519
Ganzler, K., Szinai, I., and Salgo, A. (1990). “Effective sample preparation method for extracting biologically active compounds from different matrices by a microwave technique,” Journal of Chromatography A 520, 257-262. DOI: 10.1016/0021-9673(90)85109-9
Gao, M., Song, B. Z., and Liu, C. Z. (2006). “Dynamic microwave-assisted extraction of flavonoids from Saussurea medusa Maxim cultured cells,” Biochemical Engineering Journal 32(2), 79-83. DOI: 10.1016/j.bej.2006.09.004
Gao, M., Huang, W., RoyChowdhury, M., and Liu, C. (2007). “Microwave-assisted extraction of scutellarin from Erigeron breviscapus Hand-Mazz and its determination by high-performance liquid chromatography,” Analytica Chimica Acta 591(2), 161-166. DOI: 10.1016/j.aca.2007.04.004
Gedye, R., Smith, F., Westaway, K., Ali, H., Baldisera, L., Laberge, L., and Rousell, J. (1986). “The use of microwave ovens for rapid organic synthesis,” Tetrahedron Letters 27(3), 279-282. DOI: 10.1016/S0040-4039(00)83996-9
Giguere, R. J., Bray, T. L., Duncan, S. M., and Majetich, G. (1986). “Application of commercial microwave ovens to organic synthesis,” Tetrahedron Letters 27(41), 4945-4948. DOI: 10.1016/S0040-4039(00)85103-5
Jadhav, D., Rekha, B. N., Gogate, P. R., and Rathod, V. K. (2009). “Extraction of vanillin from vanilla pods: A comparison study of conventional Soxhlet and ultrasound-assisted extraction,” Journal of Food Engineering 93(4), 421-426. DOI: 10.1016/j.jfoodeng.2009.02.007
Kharlamova, Т. V., and Praliyev, K. D. (2018). “Microwave radiation, its influence on solutions and use for extraction of components of plant material: 1-st Report. The systems of microwave-assisted extraction and their application for extraction of natural compounds,” Chemical Journal of Kazakhstan.
Kharlamova, Т. V. (2019). “Microwave radiation, its influence for solutions and use for extractions of components of plant materials: 2nd Report. The interaction of microwave radiation with plant material and factors affecting the process of extraction of natural compounds,” Chemical Journal of Kazakhstan.
Kwon, J. H., Lee, G. D., Bélanger, J. M., and Jocelyn Paré, J. R. (2003). “Effect of ethanol concentration on the efficiency of extraction of ginseng saponins when using a microwave‐assisted process (MAP™),” International Journal of Food Science and Technology 38(5), 615-622. DOI: 10.1046/j.1365-2621.2003.00688.x
Lasunon, P., and Sengkhamparn, N. (2022). “Effect of ultrasound-assisted, microwave-assisted and ultrasound-microwave-assisted extraction on pectin extraction from industrial tomato waste,” Molecules 27(4), article 1157. DOI: 10.3390/molecules27041157
Lattanzio, V., Lattanzio, V. M., and Cardinali, A. (2006). “Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects,” Phytochemistry: Advances in Research 661(2), 23-67.
Li, Y., Fabiano-Tixier, A. S., Vian, M. A., and Chemat, F. (2013). “Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry,” TrAC Trends in Analytical Chemistry 47, 1-11. DOI: 10.1016/j.trac.2013.02.007
Liazid, A., Guerrero, R. F., Cantos, E., Palma, M., and Barroso, C. G. (2011). “Microwave assisted extraction of anthocyanins from grape skins,” Food Chemistry 124(3), 1238-1243. DOI: 10.1016/j.foodchem.2010.07.053
Longares-Patrón, A., and Canizares-Macias, M. P. (2006). “Focused microwaves-assisted extraction and simultaneous spectrophotometric determination of vanillin and p-hydroxybenzaldehyde from Vanilla fragans,” Talanta 69(4), 882-887. DOI: 10.1016/j.talanta.2005.11.030
López-Salazar, H., Camacho-Díaz, B. H., Ávila-Reyes, S. V., Pérez-García, M. D., González-Cortazar, M., Arenas Ocampo, M. L., and Jiménez-Aparicio, A. R. (2019). “Identification and quantification of β-Sitosterol β-d-Glucoside of an ethanolic extract obtained by microwave-assisted extraction from Agave angustifolia Haw,” Molecules 24(21), article 3926. DOI: 10.3390/molecules24213926
López-Salazar, H. (2020). Actividad Antiinflamatoria de un Extracto Estandarizado de Glucósido de β-sitosterol de Agave angustifolia Haw Obtenido Por Extracción Asistida Por Microondas, Ph.D. Dissertation, Instituto Politécnico Nacional, Centro de Desarrollo de Productos Bióticos, México.
Lu, Y., Yue, X. F., Zhang, Z. Q., Li, X. X., and Wang, K. (2007). “Analysis of Rodgersia aesculifolia Batal. rhizomes by microwave-assisted solvent extraction and GC–MS,” Chromatographia 66(5), 443-446. DOI: 10.1365/s10337-007-0335-2
Łubek-Nguyen, A., Ziemichód, W., and Olech, M. (2022). “Application of enzyme-assisted extraction for the recovery of natural bioactive compounds for nutraceutical and pharmaceutical applications,” Applied Sciences 12(7), 3232. DOI: 10.3390/app12073232
Luo, M., Zhou, D. D., Shang, A., Gan, R. Y., and Li, H. B. (2021). “Influences of microwave-assisted extraction parameters on antioxidant activity of the extract from Akebia trifoliata peels,” Foods 10(6), article 1432. DOI: 10.3390/foods10061432
Mandal, V., Mohan, Y., and Hemalatha, S. (2007). “Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research,” Pharmacognosy Reviews 1(1), 7-18.
Mandal, V., Mohan, Y., and Hemalatha, S. (2008). “Microwave assisted extraction of curcumin by sample–solvent dual heating mechanism using Taguchi L9 orthogonal design,” Journal of Pharmaceutical and Biomedical Analysis 46(2), 322-327. DOI: 10.1016/j.jpba.2007.10.020
Mandal, V., and Mandal, S. C. (2010). “Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: Oleanolic acid,” Biochemical Engineering Journal 50(1-2), 63-70. DOI: 10.1016/j.bej.2010.03.005
Mesa, S. T., Flórez-Méndez, J., López, J., and Bustos, R. (2021). “Optimization of conventional solid-liquid extraction and microwave-assisted extraction of polyphenols and antioxidant compounds of blueberry (Vaccinium corymbosum) pomace through response surface methodology,” Journal of Berry Research 11(4), 649-668. DOI: 10.3233/JBR-210007
Mustapa, A. N., Martin, A., Mato, R. B., and Cocero, M. J. (2015). “Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction,” Industrial Crops and Products 74, 83-94. DOI: 10.1016/j.indcrop.2015.04.035
Olalere, O. A., Gan, C. Y., Akintomiwa, O. E., Adeyi, O., and Adeyi, A. (2021a). “Optimisation of microwave‐assisted extraction and functional elucidation of bioactive compounds from Cola nitida pod,” Phytochemical Analysis 32(5), 850-858. DOI: 10.1002/pca.3030
Olalere, O. A., and Gan, C. Y. (2021b). “Microwave-assisted extraction of phenolic compounds from Euphorbia hirta leaf and characterization of its morphology and thermal stability,” Separation Science and Technology 56(11), 1853-1865. DOI: 10.1080/01496395.2020.1795678
Proestos, C., and Komaitis, M. (2008). “Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds,” LWT-Food Science and Technology 41(4), 652-659. DOI: 10.1016/j.lwt.2007.04.013
Rodsamran, P., and Sothornvit, R. (2019a). “Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method,” Food Chemistry 278, 364-372. DOI: 10.1016/j.foodchem.2018.11.067
Rodsamran, P., and Sothornvit, R. (2019b). “Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions,” Food Bioscience 28, 66-73. DOI: 10.1016/j.fbio.2019.01.017
Rostagno, M. A., D’Arrigo, M., and Martínez, J. A. (2010). “Combinatory and hyphenated sample preparation for the determination of bioactive compounds in foods,” TrAC Trends in Analytical Chemistry 29(6), 553-561. DOI: 10.1016/j.trac.2010.02.015
Ruan, G. H., and Li, G. K. (2007). “The study on the chromatographic fingerprint of Fructus xanthii by microwave assisted extraction coupled with GC–MS,” Journal of Chromatography B 850(1-2), 241-248. DOI: 10.1016/j.jchromb.2006.11.036
Saha, S., Singh, A. K., Keshari, A. K., Raj, V., Rai, A., and Maity, S. (2018). “Modern extraction techniques for drugs and medicinal agents,” in: Ingredients Extraction by Physicochemical Methods in Food, Academic Press, pp. 65-106. DOI: 10.1016/B978-0-12-811521-3.00002-8
Singh, A., Nair, G. R., Liplap, P., Gariepy, Y., Orsat, V., and Raghavan, V. (2014). “Effect of dielectric properties of a solvent-water mixture used in microwave-assisted extraction of antioxidants from potato peels,” Antioxidants 3(1), 99-113. DOI: 10.3390/antiox3010099
Sousa, A. M., Alves, V. D., Morais, S., Delerue-Matos, C., and Gonçalves, M. P. (2010). “Agar extraction from integrated multitrophic aquacultured Gracilaria vermiculophylla: Evaluation of a microwave-assisted process using response surface methodology,” Bioresource Technology 101(9), 3258-3267. DOI: 10.1016/j.biortech.2009.12.061
Svoboda, K. P., Svoboda, T. G., and Syred, A. D. (2000). Secretory Structures of Aromatic and Medicinal Plants: A Review and Atlas of Micrographs, Microscopix Publications, Knighton, UK.
Tran, Q. T., Vu Thi, T. L., Do, T. L., Pham Thi, H. M., Hoang Thi, B., Chu, Q. T., and Thu Phung, H. T. (2020). “Optimization of microwave-assisted extraction process of Callicarpa candicans (Burm. f.) Hochr essential oil and its inhibitory properties against some bacteria and cancer cell lines,” Processes 8(2), 173. DOI: 10.3390/pr8020173
Tsubaki, S., Sakamoto, M., and Azuma, J. I. (2010). “Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions,” Food Chemistry 123(4), 1255-1258. DOI: 10.1016/j.foodchem.2010.05.088
Veggi, P. C., Martinez, J., and Meireles, M. A. A. (2012). “Fundamentals of microwave extraction,” in: Microwave-assisted Extraction for Bioactive Compounds, Springer, Boston, MA, pp. 15-52. DOI: 10.1007/978-1-4614-4830-3_2
Vilkhu, K., Mawson, R., Simons, L., and Bates, D. (2008). “Applications and opportunities for ultrasound assisted extraction in the food industry—A review,” Innovative Food Science and Emerging Technologies 9(2), 161-169. DOI: 10.1016/j.ifset.2007.04.014
Vinatoru, M., Mason, T. J., and Calinescu, I. (2017). “Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials,” TrAC Trends in Analytical Chemistry 97, 159-178. DOI: 10.1016/j.trac.2017.09.002
Wang, L., and Weller, C. L. (2006). “Recent advances in extraction of nutraceuticals from plants,” Trends in Food Science and Technology 17(6), 300-312. DOI: 10.1016/j.tifs.2005.12.004
Wang, Z., Ding, L., Li, T., Zhou, X., Wang, L., Zhang, H., and He, H. (2006). “Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L. and Zanthoxylum bungeanum Maxim,” Journal of Chromatography A 1102(1-2), 11-17. DOI: 10.1016/j.chroma.2005.10.032
Wang, J. X., Xiao, X. H., and Li, G. K. (2008). “Study of vacuum microwave-assisted extraction of polyphenolic compounds and pigment from Chinese herbs,” Journal of Chromatography A, 1198, 45-53. DOI: 10.1016/j.chroma.2008.05.045
Wang, J., Zhang, J., Wang, X., Zhao, B., Wu, Y., and Yao, J. (2009). “A comparison study on microwave-assisted extraction of Artemisia sphaerocephala polysaccharides with conventional method: Molecule structure and antioxidant activities evaluation,” International Journal of Biological Macromolecules 45(5), 483-492. DOI: 10.1016/j.ijbiomac.2009.09.004
Woodhouse, I. H. (2017). Introduction to Microwave Remote Sensing, CRC Press. DOI: 10.1201/9781315272573
Xiao, W., Han, L., and Shi, B. (2008). “Microwave-assisted extraction of flavonoids from Radix astragali,” Separation and Purification Technology 62(3), 614-618. DOI: 10.1016/j.seppur.2008.03.025
Xiao, X. H., Wang, J. X., Wang, G., Wang, J. Y., and Li, G. K. (2009). “Evaluation of vacuum microwave-assisted extraction technique for the extraction of antioxidants from plant samples,” Journal of Chromatography A 1216(51), 8867-8873. DOI: 10.1016/j.chroma.2009.10.087
Xu, D. P., Li, Y., Meng, X., Zhou, T., Zhou, Y., Zheng, J., and Li, H. B. (2017). “Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources,” International Journal of Molecular Sciences 18(1), 96. DOI: 10.3390/ijms18010096
Xu, J., Wu, J., Qi, J., Li, J., Liu, Y., Miao, Z., Qiu, G., and Jia, W. (2021). “Microwave-assisted extraction of flavonoids from Phyllostachys heterocycla leaves: Optimization, mechanism, and antioxidant activity in vitro,” BioResources 16(4), 8060-8081. DOI: 10.15376/biores.16.4.8060-8081
Xynos, N., Papaefstathiou, G., Gikas, E., Argyropoulou, A., Aligiannis, N., and Skaltsounis, A. L. (2014). “Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology,” Separation and Purification Technology 122, 323-330. DOI: 10.1016/j.seppur.2013.10.040
Yan, M. M., Liu, W., Fu, Y. J., Zu, Y. G., Chen, C. Y., and Luo, M. (2010). “Optimisation of the microwave-assisted extraction process for four main astragalosides in Radix astragali,” Food Chemistry 119(4), 1663-1670. DOI: 10.1016/j.foodchem.2009.09.021
Yu, L., Meng, Y., Wang, Z. L., Cao, L., Liu, C., Gao, M. Z., and Fu, Y. J. (2020). “Sustainable and efficient surfactant-based microwave-assisted extraction of target polyphenols and furanocoumarins from fig (Ficus carica L.) leaves,” Journal of Molecular Liquids 318, article 114196. DOI: 10.1016/j.molliq.2020.114196
Yu, Y., Chen, B., Chen, Y., Xie, M., Duan, H., Li, Y., and Duan, G. (2009). “Nitrogen‐protected microwave‐assisted extraction of ascorbic acid from fruit and vegetables,” Journal of Separation Science 32(23‐24), 4227-4233. DOI: 10.1002/jssc.200900487
Zhang, H. F., Yang, X. H., and Wang, Y. (2011). “Microwave assisted extraction of secondary metabolites from plants: Current status and future directions,” Trends in Food Science and Technology 22(12), 672-688. DOI: 10.1016/j.tifs.2011.07.003
Zhang, L.-F., and Liu, Z.-L. (2008). “Optimization and comparison of ultrasound/ microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes,” Ultrasonics Sonochemistry 15(5), 731-737. DOI: 10.1016/j.ultsonch.2007.12.001
Zill-E-Huma, Vian, M. A., Fabiano-Tixier, A. S., Elmaataoui, M., Dangles, O., and Chemat, F. (2011). “A remarkable influence of microwave extraction: Enhancement of antioxidant activity of extracted onion varieties,” Food Chemistry 127(4), 1472-1480. DOI: 10.1016/j.foodchem.2011.01.112
Article submitted: April 6, 2023; Peer review completed: May 7, 2023; Revised version received and accepted: June 8, 2023; Published: June 14, 2023.
DOI: 10.15376/biores.18.3. Lopez-Salazar
