Abstract
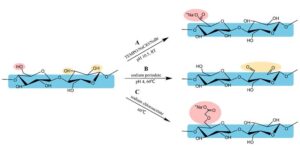
Cotton fibers were modified by TEMPO oxidation, sodium periodate oxidation, and sodium chloroacetate etherification to obtain carboxylated cellulose fibers with similar carboxyl content (about 70 mmol/100 g). The characteristics of carboxylated cellulose fibers were analyzed by comparing the morphology, chemical structure, crystallinity, carboxyl content, yield, water retention value, degree of polymerization (DP), and cost. The results showed that etherification and oxidation are both important ways to introduce carboxyl groups into the molecular structure of cellulose. When the carboxyl group with similar content is introduced into cellulose, the three modification methods will encourage a certain degree of cellulose degradation. TEMPO oxidation and sodium periodate oxidation will degrade cellulose more obviously, whereas chloroacetate etherification can obtain a higher yield, DP, and lower cost.
Download PDF
Full Article
Modification Methods’ Effects on the Characteristics of Carboxylated Cellulose Fibers: Carboxyl Group Introduction Method versus Physical Properties
Jian Wang,a,* Yubo Wang,a Zetan Liu,b Xinyi Shao,a Yuxuan Lin,a Wenbao Song,a Dehua Xu,a Yifei Gao,a and Jialan Han a
Cotton fibers were modified by TEMPO oxidation, sodium periodate oxidation, and sodium chloroacetate etherification to obtain carboxylated cellulose fibers with similar carboxyl content (about 70 mmol/100 g). The characteristics of carboxylated cellulose fibers were analyzed by comparing the morphology, chemical structure, crystallinity, carboxyl content, yield, water retention value, degree of polymerization (DP), and cost. The results showed that etherification and oxidation are both important ways to introduce carboxyl groups into the molecular structure of cellulose. When the carboxyl group with similar content is introduced into cellulose, the three modification methods will encourage a certain degree of cellulose degradation. TEMPO oxidation and sodium periodate oxidation will degrade cellulose more obviously, whereas chloroacetate etherification can obtain a higher yield, DP, and lower cost.
DOI: 10.15376/biores.19.1.1590-1601
Keywords: Carboxyl group; TEMPO; Periodate; Etherification; Degree of polymerization
Contact information: a: College of Bioresources Chemical and Materials Engineering, Shaanxi University of Science & Technology, Xi’an 710021, China; b: Gold East Paper (jiangsu) Co., Ltd., Zhenjiang 212000, China; *Corresponding author: zzwangjian@sust.edu.cn
GRAPHICAL ABSTRACT

INTRODUCTION
Cellulose, the most abundant renewable natural resource on earth, has numerous applications because of its fascinating properties (Praskalo et al. 2009). Natural cellulose typically presents itself in the form of fibers with porous structure and is composed of microfibrils 10 nm to 30 nm in width (Zhang et al. 2016). Those microfibrils three-dimensionally connect with each other and form the multi-hierarchical structure of natural cellulose fibers (Yang and Berglund 2021). It has been effectively utilized in the papermaking industry because of its good water filtration performance, non-woven construction, and other characteristics (Nikolic et al. 2017). To further take advantage of the multi-hierarchical fibrous structure of cellulose, microcrystalline cellulose and nanocellulose have been developed and used in many fields (Halim et al. 2019; Kwon et al. 2020; Shao et al. 2020; Nascimento et al. 2021; Lorente et al. 2023). In recent years, based on the multi-hierarchical fibrous structure of cellulose, carboxylated cellulose fibers have been used as adsorption material and achieved good benefits (Abou-Zeid et al. 2018; Fiol et al. 2019; Wang et al. 2019a; Wang et al. 2020; He et al. 2022). This utilization of natural cellulose requires the introduction of ionic groups into the molecular structure of cellulose and redesign of the molecular structure of cellulose on the basis of retaining the fiber morphology (Toshikj et al. 2018). Carboxyl groups are commonly introduced as anions to modify cellulose fibers because of their higher sequestration capacity and lower cost (Phan et al. 2021).
Etherification and oxidation are both important ways to introduce carboxyl groups into cellulose macromolecules. The etherification of cellulose is generally achieved by the reaction of the hydroxyl group on the anhydroglucose units with the etherifying reagent under alkaline conditions (Shao et al. 2021). In this reaction, chloroacetic acid with strong permeability is often used as an etherifying agent and NaOH as a catalyst (Hedlund and Germgård 2006). This is an important way to prepare anionic water-soluble cellulose derivatives (carboxymethylcellulose) with a high degree of substitution (DS) (Casaburi et al. 2018). Robles Barros et al. (2020) etherified soybean hulls to produce CMC with a DS of 1.45. Santos et al. (2015) prepared CMC with a DS of 1.46 by using brewer’s spent grain. As a polyhydroxy compound, cellulose is easily oxidized by oxidants. According to the different oxidation reaction positions of cellulose, it can be divided into nonselective oxidation and selective oxidation (Yu et al. 2021). Nonselective oxidation not only causes the primary and secondary hydroxyl groups on the cellulose unit to react at the same time, but it also breaks the ether bond of the cellulose chain, resulting in the fragmentation of cellulose fibers (Li et al. 2016). Compared to nonselective oxidation, selective oxidizing agents have lower oxidation capacity and only convert the target hydroxyl group to carboxyl groups. More importantly, the fibrous morphology of cellulose can be retained. Those methods have been used to prepare the cellulose nanofibers (CNF). For example, Ono et al. (2021) obtained cellulose nanofibrils with a large number of carboxyl groups (1.62 to 1.63 mmol/g) using dried cotton linters and ramie cellulose. Xu et al. (2022) oxidized Eucalyptus globulus bleached kraft pulp fibers by TEMPO oxidation to prepare cellulose nanofibrils with a high carboxyl group content of 1.7 mmol/g. Periodate was used as a catalyst to convert dialdehyde groups into dicarboxylic groups to produce carboxylated cellulose fiber nanocrystals with a plentiful carboxyl group (1.28 mmol/g) (Wang et al. 2023).
The abovementioned findings show that etherification and oxidation are both important ways to introduce carboxyl groups into the molecular structure of cellulose. However, more study is required to investigate the properties of cellulose fiber when many carboxyl groups are introduced into its molecular structure. In this study, carboxylated cellulose fibers with same level of carboxyl groups were prepared by three modes, and the characteristics of those fibers were comparatively studied to provide a theoretical foundation for the preparation of functional cellulose-based antibacterial materials with high mechanical properties and washability.
EXPERIMENTAL
Materials
The cellulose was obtained from the absorbent cotton pulp board (CPF, ∼90 wt% solids content), purchased from Shandong Silver Eagle Chemical Fiber Co., Ltd. (Shandong, China). The sodium chloroacetate (MCA), sodium chlorite (NaClO2), sodium bicarbonate (NaHCO3), sodium periodate (NaIO4), and sodium hypochlorite (NaClO) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Sodium bromide (NaBr), potassium bromide (KBr), acetic acid (CH3COOH), hydrochloric acid (HCl), absolute ethanol (CH3CH2OH), and sodium hydroxide (NaOH) were procured from Sinopharm Group Chemical Reagent Co., Ltd. (Tianjin, China). The 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) was purchased from Bide Pharmatech Co., Ltd. (Shanghai, China). All chemicals used were reagent grade and used as received. The deionized water (DW) was used throughout the work.
Preparation of Carboxylated Cellulose Fibers
In a preliminary experiment, carboxylated cellulose fibers with the same content of carboxyl group (about 70 mmol/100 g) were prepared by controlling the reaction time of the TEMPO oxidation system, the amount of sodium periodate, and the etherification reaction time, respectively. The specific preparation methods are as follows:
Preparation of TEMPO-oxidized cotton fibers (TOF)
The TEMPO oxidation was performed according to a well-established literature method (Saito and Isogai 2004). Briefly, the dried cotton pulp (10 g), TEMPO (0.025 g), NaBr (0.25 g), and DW (300 mL) were mixed in a three-necked flask and stirred for 15 min at room temperature to ensure a good dispersion of all the components. Subsequently, a specified amount of NaClO solution was slowly added dropwise to the cellulose suspension. Then, the pH of the mixture was adjusted to 10 with NaOH (0.4 M). The oxidation was performed for 60 min at room temperature. After the designated time of stirring, the oxidation reaction was terminated by quenching with ethanol (12 mL). Then, water was used to wash oxidized fibers and rinsed several times with DW until the filtrate solution was neutral. Finally, the fibers were lyophilized for 24 h.
Preparation of sodium periodate-oxidized cotton fibers (POF)
Periodate oxidation of cotton pulp was performed as follows (Nikolic et al. 2010): Dried cotton pulp (10 g) was dispersed in a three necked flask containing 500 mL of acetic acid buffer (pH 4.0) and NaIO4 (4 g). The mixture was stirred at 60 ℃ for 30 min in the dark to assure a good dispersion of all the components. Then, the cellulose obtained in the previous step was filtered, washed, and reacted in a three necked flask containing 500 mL acetic acid (1 M), and NaClO2 (9.05 g), for 12 h at room temperature. Subsequently, the oxidized fibers were washed thoroughly with DW until the filtrate solution was neutral. Finally, the fibers were freeze-dried for 24 h.
Preparation of sodium chloroacetate-etherified cotton fibers (CEF)
The synthesis of CMC consists of two stages (dos Santos et al. 2015): Alkalization and etherification. In the alkalization stage, the dried cotton pulp (10 g), NaOH (10 g), and DW (12 mL) were mixed and impregnated at 40 ℃ for 2 h to activate cellulose. In the etherification stage, the swollen fibers obtained in the previous step were transferred into a beaker that contained an MCA solution (15 g of MCA/26 g of H2O) at 80 °C for 4 h. After reaction, the fibers were washed with HCl (0.1 M) to remove residual alkali, and then with DW to wash the fibers until the filtrate solution was neutral. Finally, the fibers were freeze-dried for 24 h.
Characterization of the Carboxylated Fibers
Production yield
The yield was measured by the gravimetric analysis and calculated according to the following Eq. 1,
(1)
where W1 is the dry weight (g) of the original cotton fibers and W2 is the dry weight (g) of the modified fibers.
Detection of carboxyl group content
The carboxylic acid content in each sample was measured based on the standard method of TAPPI T237 cm-22 (2007) with appropriate modifications. More precisely, the dosage of NaHCO3-NaCl solution was adjusted from 50 mL to 100 mL, and the sample was washed with saturated CO2 deionized water to eliminate the interference of sulfonic acid groups in the sample. The carboxyl content of the fibers can be calculated according to the following Eq. 2,
(2)
where A (mL) is the volume of HCl (0.010 N) consumed in a titration of the pulp filtrate; B (mL) is the volume of HCl (0.010 N) consumed in a titration of the NaHCO3-NaCl solution; C (g) is the weight of water in the pulp pad (i.e., the weight of wet pad minus oven dry weight of test specimen); N is the concentration of HCl standard solution; and W (g) is the weight of dried fibers.
Water retention value (WRV)
The WRV can indicate the degree of fiber swelling and the magnitude of the bonding force between fibers. The WRV was determined according to the method of SCAN-C 62:00 (2000). Firstly, a certain amount of dry samples was weighed and swelled in distilled water for 30 min. Next, the samples filtered through a sand core funnel were wrapped in gauze and centrifuged for 10 min at the speed of 8000 r/min to remove excess water. The WRV can be calculated according to Eq. 3,
(3)
where m0 is the weight of the sample after centrifugation (g) and m1 is the weight of the sample after drying (g).
Degree of polymerization (DP)
The DP of cellulose was determined based on the viscosity method according to ISO 5351 (2010). A known amount of the cellulose sample was dissolved in fresh copper ethylenediamine (CED). The intrinsic viscosity of this solution at 25 ℃ was measured with a pulp viscometer (P41430, Frank-PTI, Germany). The intrinsic viscosity is converted to the value of DP according to the following Eq. 4 (Sihtola 1963):
(4)
Fourier transform infrared spectroscopy (FT-IR)
The chemical compositions of the samples were tested using a FT-IR spectrometer (VERTEX 70, Bruker, Germany). All the samples were mixed with KBr, then ground and pressed into pellets. The scanned range used was 4000 to 500 cm-1.
Scanning electron microscopy (SEM)
The surface microstructure of the samples was characterized by SEM (VEGA 3 SBH, TESCAN, Czech Republic) at an accelerating voltage of 3.0 kV.
X-ray diffraction (XRD)
The crystallinity of fiber samples was tested by XRD (D8 Advance, Bruker, Germany) in the range 2θ of 10° to 40° with increments of 0.02° min-1. The relative crystallinity index (CrI) can be calculated according to the Eq. 5 (Segal et al. 1962),
(5)
where I002 is the maximum diffraction intensity of crystalline from plane (002) at 2θ = 22.5° for cellulose I, and 2θ = 21.7° for cellulose II, while Iam is the intensity of amorphous scatter at 2θ = 18° for cellulose I, and 2θ = 16° for cellulose II (French 2013).
RESULTS AND DISCUSSION
Effect of Modified Methods on Physical Properties of Cotton Fibers
The physical properties of the carboxylated cellulose fibers prepared by different modification methods were compared, as shown in Table 1.
Table 1. Determination of Physicochemical Properties of Modified Cotton
From Table 1, although the carboxyl group contents of the three fibers achieved roughly the same level (about 70 mmol/100 g), the physical properties of the fibers were significantly different due to the different modification principles. In the process of oxidation, some cellulose was oxidized and degraded into smaller units, resulting in a decrease in fiber yield. When carboxyl groups were introduced into the molecular structure of cellulose through etherification, part of modified cellulose with a high degree of substitution was dissolved in water and lost. The hydrophilic ability of carboxyl group is significantly higher than that of hydroxyl group. Therefore, when the approximate content of carboxyl group was introduced into the molecular structure of cellulose, all the water absorption capacity of modified celluloses increased from 159% to about 300%. There was no significant difference in the water retention value of the three kinds of modified fibers.
It is noteworthy that there were obvious differences in DP of three kinds of modified fibers. The modified fibers obtained by etherification have a higher DP than that of modified fibers obtained by oxidation (916 vs. 732 and 102). Under the same carboxyl content level, the TEMPO oxidation had the lowest DP. There were more transverse cracks appearing on the surfaces of the fibers because of the corrosiveness of the TEMPO oxidant, which led to the oxidant easily infiltrating into the fiber and reacting with fibrils, causing the depolymerization of cellulose. Meanwhile, the polysaccharide containing glucuronic acid will undergo a certain degree of β elimination reaction, which leads to the breaking of molecular chains and the decrease of the DP. Besides, the oxidation of the hydroxyl group at the C2 and C3 positions of the glucose unit in cellulose to form an aldehyde or dicarboxylic structure by NaClO may also be one of the reasons for the depolymerization of cellulose (Mishra et al. 2011). The C2-C3 bond of the glycosidic ring was selectively broken during periodate oxidation, forming aldehyde groups on it. During this process, part of the 1–4 β-glycoside bonds of cellulose had been cleaved, which resulted in the decrease of cellulose DP.
Moreover, the DP of cellulose was still decreased in process of etherification due to the breaking of 1–4 β-glycoside bonds under strongly alkaline circumstances. Compared with oxidation degradation of cellulose, the degree of alkaline degradation was lower, such that a higher DP was obtained for the modified fibers.
Infrared Analysis of Modified Cellulose
The three kinds of carboxylated cellulose fibers that were prepared by different modified methods were then analyzed by FT-IR spectroscopy. According to Fig. 1, representing the samples before and after modification, all the fibers exhibited peaks around 3460, 2900, and 1050 cm-1. Among them, the signal at 3460 cm-1 is attributed to the -OH stretching vibration (Chen et al. 2011); moreover, the broad peak at approximately 2900 cm-1 is assigned to the stretching of C-H (Jiang et al. 2017); the bands at 1050 cm-1 corresponded to C-O-C stretching of the cellulose skeleton (Kambli et al. 2017). The peaks at 3460, 2900, and 1050 cm-1 are considered to be the characteristic peaks of cellulose.
Fig. 1. FT-IR spectra of CPF, TOF, POF, and CEF
The results showed that all the modification methods did not destroy the inherent structure of the cellulose molecule. Compared with the spectrum of CPF, the modified fibers presented the peaks at 1750 and 1650 cm-1, which correspond to C=O stretching frequencies of carboxyl groups in the acidic form and the C=O stretching vibration of carbonyl as the carboxylate, respectively (Calvini et al. 2006; Liu et al. 2015). This study demonstrated that the carboxyl groups were successfully introduced on fibers after modification. Compared with CPF, it is worth noting that the absorption peaks of the C-O-C of three carboxylated fibers showed different degrees of weakening at 1050 cm-1, indicating that the breakage of cellulose macromolecular skeleton and the occurrence of ring-opening reaction. Meanwhile, the stretching vibration peak of hydroxyl group moved to the high wave number of three carboxylated fibers. For CEF, the native cellulose Ⅰ was transformed into cellulose II under high alkaline conditions (Fig. 2). Compared with cellulose I, cellulose II has shorter and denser hydrogen bonds, which causes the vibration frequency to increase and the peak of the hydroxyl group absorption to move towards higher wave numbers. For TOF and POF, similarly, the stretching vibration peak of hydroxyl group moved to the high wave number due to the high bonding energy of hydrogen bonds formed from the carboxyl groups (Peresin et al. 2010).
Crystal Structure of Cellulose after Modification
The CPF and the three kinds of modified fibers were subjected to X-ray diffraction scanning, as shown in Fig. 2. From the XRD patterns of cotton fibers, it can be observed that three diffraction peaks appeared at around 14.9 °, 16.7 °, and 22.8 °, corresponding to (11 ̅0), (110), and (200) crystallographic planes, respectively, which indicated the typical characteristic peaks of cellulose Ι (French 2013). In the XRD patterns of TOF and POF, there were peaks found still at 2θ of 14.9°, 16.7°, and 22.8°, which established that the two modification methods did not change the crystal type of cellulose fibers. However, in the XRD pattern of CEF, the new diffraction spectrum showed a wide peak at 2θ = 20.5°, which indicated that natural cellulose I had been mercerized into cellulose II during carboxymethylation of CPF because of the high concentration of NaOH (Hashem et al. 2013).
Regarding crystal structure, it seems remarkable that even after mercerization to the cellulose II form, the CEF treatment still maintained a high molecular mass. There are plenty of articles reporting losses of DP associated with the regeneration of cellulose by various means. After alkaline treatment of fibers, cellulose would cause intra- and inter-granular swellings, resulting in irreversible changes in the crystal structure of cellulose. The CrI values of CPF, POF, TOF, and CEF were 83.2%, 82.8%, 82.8%, and 50.7% respectively. The introduction of carboxyl group could increase the charge between cellulose molecules, so the hydrogen bonds between and within CEF molecules were broken, which disrupted the crystalline region of CEF. The fiber network of the crystal-type cellulose II is tighter and more stable in thermodynamics, which is conducive to improving the application of fiber materials in other fields.
Fig. 2. X-ray diffraction patterns of CPF, TOF, POF, and CEF
Surface Morphology of Cotton Fibers
In Fig. 3a, the native cotton fibers appeared flat, and the surfaces of fibers were relatively complete and smooth. However, the structure of carboxylated fiber became more compact after drying due to the improvement of swelling capacity of cellulose fibers after carboxylation. After TEMPO oxidation, more transverse cracks appeared on the fibers’ surface and part of areas experienced collapse due to the corrosion of TEMPO agent (Fig. 3b). For sodium periodate oxidation, a large number of transverse and longitudinal cracks appeared on the fibers’ surface (Fig. 3c), which may be caused by the degradation and erosion of sodium periodate on the fiber. Compared to cotton fibers, the fibers etherified with chloroacetic acid exhibited a cylindrical structure (Fig. 3d). There were axial periodic spiral lines on the surface of the fiber, and some fibers showed collapsed holes on the surface. An explanation for this phenomenon is that sodium hydroxide solution penetrates into the cell, the fibers in the S1 layer of the secondary wall undergo transverse swelling, combined with longitudinal shrinkage, which leads to the depressed surface of the fibers. At the same time, the periodic growth of fine fibers and fiber axes in the S1 layer limit the uniform swelling of the inner layers, leading to the appearance of spiral concave lines (Moigne et al. 2010).
Fig. 3. SEM micrographs of (a) CPF; (b) TOF; (c) POF; and (d) CEF
Costs of Modified Fiber
Table 2. Raw Materials Consumption
Calculations from Table 2 are as follows, the costs of CEF, POF, and TOF are approximately 9000 CNY/ton, 17000 CNY/ton, and 11000 CNY/ton (The CNY means the legal tender of China), respectively. Among them, CEF has a relatively obvious price advantage, which will be conducive to its promotion.
CONCLUSIONS
- Carboxyl groups were introduced into the cellulose molecular chain by sodium periodate oxidation, TEMPO oxidation, and etherification modification, respectively. Carboxylated cellulose fibers with the same level of carboxyl content (about 70mmol/100g) were successfully obtained by controlling the modification conditions.
- Fourier transform infrared (FT-IR) results showed that etherification and oxidation are both important ways to introduce carboxyl groups into the molecular structure of cellulose. In addition, the water retention value (WRV) of carboxylated fibers was doubled due to the hydrophilicity of carboxyl groups. Based on the scanning electron microscopy (SEM) analysis, there were some cracks and subsidence holes appeared on the surface of fiber.
- The modification methods had a significant effect on the degree of polymerization (DP) of the modified fiber. Briefly, the modified fiber obtained by etherification modification had higher yield and DP, while the modified fiber obtained by TEMPO oxidation can only obtain lower yield and lowest DP because of the presence of oxidants and β eliminating reactions.
- The cost of modified fiber obtained by etherification modification was the lowest among the three types of modified fibers.
- The properties of carboxylated cellulose fiber prepared by three different modification methods were evaluated to provide a possibility for the preparation of a kind of cellulose-based functional material with high molecular weight.
ACKNOWLEDGMENTS
The financial support provided by the Foundation of High-level Foreign Experts Project (No. GDT20186100425) is gratefully acknowledged.
REFERENCES CITED
Abou-Zeid, R. E., Dacrory, S., Ali, K. A., and Kamel, S. (2018). “Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution,” International Journal of Biological Macromolecules 119, 207-214. DOI: 10.1016/j.ijbiomac.2018.07.127
Calvini, P., Gorassini, A., Luciano, G., and Franceschi, E. J. V. S. (2006). “FTIR and WAXS analysis of periodate oxycellulose: Evidence for a cluster mechanism of oxidation,” Vibrational Spectroscopy 40(2), 177-183. DOI: 10.1016/j.vibspec.2005.08.004
Casaburi, A., Montoya Rojo, Ú., Cerrutti, P., Vázquez, A., and Foresti, M. L. (2018). “Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose,” Food Hydrocolloids 75, 147-156. DOI: 10.1016/j.foodhyd.2017.09.002
Chen, B., Chen, Z., and Lv, S. J. B. T. (2011). “A novel magnetic biochar efficiently sorbs organic pollutants and phosphate,” Bioresource Technology 102(2), 716-723. DOI: 10.1016/j.biortech.2010.08.067
dos Santos, D. M., Bukzem Ade, L., Ascheri, D. P., Signini, R., and de Aquino, G. L. (2015). “Microwave-assisted carboxymethylation of cellulose extracted from brewer’s spent grain,” Carbohydrate Polymers 131(20), 125-133. DOI: 10.1016/j.carbpol.2015.05.051
Fiol, N., Vásquez, M. G., Pereira, M., Tarrés, Q., and Delgado-Aguilar, M. J. C. (2019). “TEMPO-oxidized cellulose nanofibers as potential Cu(II) adsorbent for wastewater treatment,” Cellulose 26, 903-916. DOI: 10.1007/s10570-018-2106-7
French, A. D. (2013). “Idealized powder diffraction patterns for cellulose polymorphs,” Cellulose 21, 885-896. DOI: 10.1007/s10570-013-0030-4
Halim, A., Xu, Y., Lin, K.-H., Kobayashi, M., Kajiyama, M., and Enomae, T. (2019). “Fabrication of cellulose nanofiber-deposited cellulose sponge as an oil-water separation membrane,” Separation and Purification Technology 224, 322-331. DOI: 10.1016/j.seppur.2019.05.005
Hashem, M., Sharaf, S., Abd El-Hady, M. M., and Hebeish, A. J. C. P. (2013). “Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethyl-cellulolse-hydrogel-ZnO-nanocomposites,” Carbohydrate Polymers 95(1), 421-427. DOI: 10.1016/j.carbpol.2013.03.013
He, J., Sun, Z., Chen, Y., Xu, B., Li, J., and Qian, L. (2022). “Grafting cellulose nanocrystals with phosphazene-containing compound for simultaneously enhancing the flame retardancy and mechanical properties of polylactic acid,” Cellulose 29, 6143-6160. DOI: 10.1007/s10570-022-04647-x
Hedlund, A., and Germgård, U. (2006). “Some aspects on the kinetics of etherification in the preparation of CMC,” Cellulose 14, 161-169. DOI: 10.1007/s10570-006-9096-6
ISO 5351 (2010). “Pulps — Determination of limiting viscosity number in cupri-ethylenediamine (CED) solution,” International Organization for Standardization, Geneva, Switzerland.
Kambli, N. D., Mageshwaran, V., Patil, P. G., Saxena, S., and Deshmukh, R. R. J. C. (2017). “Synthesis and characterization of microcrystalline cellulose powder from corn husk fibres using bio-chemical route,” Cellulose 24, 5355-5369. DOI: 10.1007/s10570-017-1522-4
Kwon, G., Lee, K., Kim, D., Jeon, Y., Kim, U. J., and You, J. (2020). “Cellulose nanocrystal-coated TEMPO-oxidized cellulose nanofiber films for high performance all-cellulose nanocomposites,” Journal of Hazardous Materials 398(5), article ID 123100. DOI: 10.1016/j.jhazmat.2020.123100
Li, Z., Meng, C., Zhou, J., Li, Z., Ding, J., Liu, F., and Yu, C. (2016). “Characterization and control of oxidized cellulose in ramie fibers during oxidative degumming,” Textile Research Journal 87(15), 1828-1840. DOI: 10.1177/0040517516659380
Liu, L., Gao, Z. Y., Su, X. P., Chen, X., Jiang, L., and Yao, J. M. J. A. S. C. (2015). “Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent,” ACS Sustainable Chemistry & Engineering 3(3), 432-442. DOI: 10.1021/sc500848m
Lorente, A., Huertas-Alonso, A. J., Salgado-Ramos, M., Gonzalez-Serrano, D. J., Sanchez-Verdu, M. P., Cabanas, B., Hadidi, M., and Moreno, A. (2023). “Microwave radiation-assisted synthesis of levulinic acid from microcrystalline cellulose: Application to a melon rind residue,” International Journal of Biological Macromolecules 237(15), article ID 124149. DOI: 10.1016/j.ijbiomac.2023.124149
Mishra, S. P., Thirree, J., Manent, A. S., Chabot, B., and Daneault, C. J. B. (2011). “Ultrasoued-catalyzed TEMPO-mediated oxidation of native cellulose for the production of nanocellulose: Effect of process variables,” BioResources 6, 121-143. DOI: 10.15376/biores.6.1.121-143
Moigne, N. L., Bikard, J., and Navard, P. J. C. (2010). “Rotation and contraction of native and regenerated cellulose fibers upon swelling and dissolution: The role of morphological and stress unbalances,” Cellulose 17, 507-519. DOI: 10.1007/s10570-009-9395-9
Nascimento, E. S., Barros, M. O., Cerqueira, M. A., Lima, H. L., Borges, M.d. F., Pastrana, L. M., Gama, F. M., Rosa, M. F., Azeredo, H. M. C., and Gonçalves, C. (2021). “All-cellulose nanocomposite films based on bacterial cellulose nanofibrils and nanocrystals,” Food Packaging and Shelf Life 29, article ID 100715. DOI: 10.1016/j.fpsl.2021.100715
Nikolic, T., Kostic, M., Praskalo, J., Pejic, B., Petronijevic, Z., and Skundric, P. (2010). “Sodium periodate oxidized cotton yarn as carrier for immobilization of trypsin,” Carbohydrate Polymers 82(3), 976-981. DOI: 10.1016/j.carbpol.2010.06.028
Nikolic, T., Korica, M., Milanovic, J., Kramar, A., Petronijevic, Z., and Kostic, M. (2017). “TEMPO-oxidized cotton as a substrate for trypsin immobilization: Impact of functional groups on proteolytic activity and stability,” Cellulose 24, 1863-1875. DOI: 10.1007/s10570-017-1221-1
Ono, Y., Takeuchi, M., Zhou, Y., and Isogai, A. (2021). “TEMPO/NaBr/NaClO and NaBr/NaClO oxidations of cotton linters and ramie cellulose samples,” Cellulose 28, 6035-6049. DOI: 10.1007/s10570-021-03944-1
Peresin, M. S., Habibi, Y., Zoppe, J. O., Peresin, M. S., and Habibi, Y. (2010). “Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization,” Biomacromolecules 11(3), 674-681. DOI: 10.1021/bm901254n
Phan, D. N., Khan, M. Q., Nguyen, N. T., Phan, T. T., Ullah, A., Khatri, M., Kien, N. N., and Kim, I. S. (2021). “A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes,” Carbohyd. Polym. 252(15), article ID 117175. DOI: 10.1016/j.carbpol.2020.117175
Praskalo, J., Kostic, M., Potthast, A., Popov, G., Pejic, B., and Skundric, P. (2009). “Sorption properties of TEMPO-oxidized natural and man-made cellulose fibers,” Carbohydrate Polymers 77(4), 791-798. DOI: 10.1016/j.carbpol.2009.02.028
Robles Barros, P. J., Ramirez Ascheri, D. P., Siqueira Santos, M. L., Morais, C. C., Ramirez Ascheri, J. L., Signini, R., Dos Santos, D. M., de Campos, A. J., and Alessandro Devilla, I. (2020). “Soybean hulls: Optimization of the pulping and bleaching processes and carboxymethyl cellulose synthesis,” International Journal of Biological Macromolecules 144(1), 208-218. DOI: 10.1016/j.ijbiomac.2019.12.074
Saito, T., and Isogai, A. J. B. (2004). “TEMPO-mediated oxidation of native cellulose. the effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions,” Biomacromolecules 5(5), 1983-1989. DOI: 10.1021/bm0497769
SCAN-C 62:00 (2000). “Water retention value,” Scandinavian, Pulp, Paper and Board Testing Committee, Stockholm, Sweden.
Segal, L., Creely, J. J., Martin, A. E., and Conrad, C. M. (1959). “An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer,” Textile Research Journal 29(10), 786-794. DOI: 10.1177/004051755902901003
Shao, X., Wang, J., Liu, Z., Hu, N., Liu, M., Duan, C., Zhang, R., and Quan, C. (2021). “Cellulose based cation-exchange fiber as filtration material for the rapid removal of methylene blue from wastewater,” Cellulose 28, 9355-9367. DOI: 10.1007/s10570-021-04103-2
Shao, X., Wang, J., Liu, Z., Hu, N., Liu, M., and Xu, Y., (2020). “Preparation and characterization of porous microcrystalline cellulose from corncob,” Industrial Crops and Products 151(1), article ID 112457. DOI: 10.1016/j.indcrop.2020.112457
Sihtola, H. (1963). “Comparison and conversion of viscosity and DP values determined by different methods,” Paper ja Puu 45, 225-323.
TAPPI T237 cm-22 (2007). “Carboxyl content of pulp test method,” TAPPI Press, Atlanta, GA, USA.
Toshikj, E., Tarbuk, A., Grgić, K., Mangovska, B., and Jordanov, I. (2018). “Influence of different oxidizing systems on cellulose oxidation level: Introduced groups versus degradation model,” Cellulose 26, 777-794. DOI: 10.1007/s10570-018-2133-4
Wang, B., Zhao, X., Duan, C., Li, J., Zeng, J., Xu, J., Gao, W., and Chen, K. (2023). “Novel carboxylated cellulose nanocrystals synthesized by co-oxidation of sodium periodate/Fenton as a green solid emulsifier for oil-in-water Pickering emulsion,” Journal of Colloid Interface Science 630(15), 604-617. DOI: 10.1016/j.jcis.2022.09.152
Wang, D., Peng, H., Wu, Y., Zhang, L., Li, M., Liu, M., Zhu, Y., Tian, A., and Fu, S. (2020). “Bioinspired lamellar barriers for significantly improving the flame-retardant properties of nanocellulose composites,” ACS Sustainable Chemistry and Engineering 8(11), 4331-4336. DOI: 10.1021/acssuschemeng.9b07745
Wang, J., Hu, N., Liu, M., Sun, J., and Xu, Y. (2019a). “A novel core–shell structured biosorbent derived from chemi-mechanical pulp for heavy metal ion removal,” Cellulose 26, 8789-8799. DOI: 10.1007/s10570-019-02693-6
Xu, H., Sanchez-Salvador, J. L., Balea, A., Blanco, A., and Negro, C. (2022). “Optimization of reagent consumption in TEMPO-mediated oxidation of Eucalyptus cellulose to obtain cellulose nanofibers,” Cellulose 29, 6611-6627. DOI: 10.1007/s10570-022-04672-w
Yang, X., and Berglund, L. A. (2021). “Structural and ecofriendly holocellulose materials from wood: Microscale fibers and nanoscale fibrils,” Advanced Materials 33(28), article ID e2001118. DOI: 10.1002/adma.202001118
Yu, H., Zheng, L., Zhang, T., Ren, J., Cheng, W., Zhang, L., and Meng, P. (2021). “Adsorption behavior of Cd (II) on TEMPO-oxidized cellulose in inorganic/ organic complex systems,” Environmental Research 195, article ID 110848. DOI: 10.1016/j.envres.2021.110848
Zhang, C., Li, P., Zhang, Y., Lu, F., Li, W., Kang, H., Xiang, J.-F., Huang, Y., and Liu, R. (2016). “Hierarchical porous structures in cellulose: NMR relaxometry approach,” Polymer 98(19), 237-243. DOI: 10.1016/j.polymer.2016.06.036
Article submitted: November 29, 2023; Peer review completed: January 3, 2024; Revised version received and accepted: January 15, 2024; Published: January 22, 2024.
DOI: 10.15376/biores.19.1.1590-1601
