Abstract
The present work deals with the use of multiple-step procedures to obtain valuable sub-products, including nanocellulose, from rice husk. Each sub-product was characterized after every step by analyzing the chemical composition (mainly based on thermogravimetric analysis, Fourier transformed infrared spectra, and X-ray diffraction) and morphology (using visual observations and scanning electron microscopy). The results clearly showed that the selected procedure gave the possibility to separate silica in the first step and then to purify the resultant material, leading to nanocellulose production. All acquired sub-products can be used as additives and fillers in a very wide range of applications. The obtained results will be useful both from technological and academic points of view, mainly for people working in the field of biocomposites. The final material could give added value to a raw biomass material source such as rice husk.
Download PDF
Full Article
NANOCELLULOSE FROM RICE HUSK FOLLOWING ALKALINE TREATMENT TO REMOVE SILICA
Leandro Ludueña, Diana Fasce, Vera. A. Alvarez, and Pablo M. Stefani *
The present work deals with the use of multiple-step procedures to obtain valuable sub-products, including nanocellulose, from rice husk. Each sub-product was characterized after every step by analyzing the chemical composition (mainly based on thermogravimetric analysis, Fourier transformed infrared spectra, and X-ray diffraction) and morphology (using visual observations and scanning electron microscopy). The results clearly showed that the selected procedure gave the possibility to separate silica in the first step and then to purify the resultant material, leading to nanocellulose production. All acquired sub-products can be used as additives and fillers in a very wide range of applications. The obtained results will be useful both from technological and academic points of view, mainly for people working in the field of biocomposites. The final material could give added value to a raw biomass material source such as rice husk.
Keywords: Rice husk; Chemical treatment; Cellulose; Thermal properties; Morphology; Nanocellulose
Contact information: Research Institute of Material Science and Technology (INTEMA), Engineering Faculty, National University of Mar del Plata, Argentina, Juan B. Justo 4302 (B7608FDQ) Mar del Plata, Argentina; *Corresponding author: pmstefan@fi.mdp.edu.ar
INTRODUCTION
Rice husk (RH) is a raw biomass material with high potential for manufacturing value-added products. The global rice production for 2006/07 was 420 million tons (FAO). In the course of producing the rice, approximately 80 million tons of husk residues were produced. In Asia and South America most of the rice husk is used as bedding material for animals or burned for energy generation, while the industrial applications of this material are still limited (Garcia et al. 2007; Leiva et al. 2007; Park et al. 2003, 2004; Ruseckaite et al. 2007; Stefani et al. 2005). Therefore, it will be useful to consider the use of this waste for producing value-added products, providing a clear positive effect on the environment.
The main components of rice husk are cellulose (25 to 35%), hemicelluloses (18 to 21%), lignin (26 to 31%), silica (15 to 17%), solubles (2 to 5%), and moisture ca. 7.5% (Gerardi et al. 1998; Leiva et al. 2007; Mansaray and Ghaly 1998; Stefani et al. 2005). Some of these ingredients can be recovered for further applications by suitably combining chemical and thermal treatments. Many previous studies have been focused mainly on the production of amorphous silica from rice husk; such silica can be used for multiple applications. Amorphous silica can be obtained from direct or after pre-treatment combustion of rice husk at temperatures lower than 600 ºC (Stefani et al. 2006; Sun and Gong 2001). Calcination is the most used method to obtain rice husk sub-products, nevertheless only amorphous silica can be recovered in this case, resulting in the loss of the entire organic cellulose-rich phase. Cellulose is found in the cell wall of lignocellulosic materials as microfibrils embedded in the non-cellulosic matrix, which is mainly formed by hemicellulose and lignin (Ruseckaite et al. 2007; Stefani et al. 2005; Zuluaga et al. 2009). Several routes have been used to isolate microfibrils from natural resources such as sisal (Moran et al. 2008), hemp (Wang et al. 2007), lemon and maize (Rondeau-Mouro et al. 2003), and potato (Dufresne et al. 2000). In addition, those cellulose microfibrils are composed of nanocrystalline domains and amorphous regions (Moran et al. 2008). A controlled acid hydrolysis could separate both regions, permitting isolation of crystalline domains with high elastic modulus (Samir et al. 2004). These nanofibers have been shown to be useful as new reinforcing agents in the production of nanocomposites (Faria et al. 2006; Zuluaga et al. 2007).
In addition, untreated or treated RH has been proposed as a possible substitute for wood in the production of panels (Ajiwe et al. 1998; Ciannamea et al. 2010; Gerardi et al. 1998; Lee et al. 2003; Leiva et al. 2007; Ndazi et al. 2007). These panels have competitive properties with respect to wood-based boards, with an additional advantage because rice husk has constant average dimensions, avoiding the need for grinding operations during board preparation. However, the high silica content causes a key problem during RH-based panel’s production (Lee et al. 2003). Previous reported studies indicate that silica content in wood higher than 0.5% causes excessive tool wear during particleboard production (Torelli and Čufar 1995). In addition, silica reduces the adhesion capacity of some adhesives (Ciannamea et al. 2010; Lee et al. 2003; Leiva et al. 2007). Therefore, separation of silica and cellulose from rice husk by means of chemical treatment is a key aspect to be considered in order to obtain cellulose and, after that, to produce cellulose-based nanofibers. In this sense Daifullah et al. (2004) have proposed a simple method derived from combined chemical treatments to separate the inorganic components present in RH without calcinations. Silica from rice husk can be removed by a combination of alkaline (KOH) treatment followed by precipitation with acid (HCl). After that nanocellulose can be produced by selecting an adequate chemical treatment route (from previous studies carried out on other vegetal sources).
In the present work rice husk was submitted to sequential chemical treatments in order to obtain nanocellulose, evaluating the effects of each treatment step on the chemical structure and morphology of original material by means of several techniques, mainly spectroscopic (FTIR), thermogravimetric analysis (TGA), and scanning electron microscopy (SEM).
EXPERIMENTAL
Materials
The rice husk (RH) used in the present work consisted of residues from the rice industries of Entre Ríos (Argentina). RH was extensively washed with distilled water to remove dust and other impurities. This operation was performed several times at room temperature and under vigorous stirring. After successive washings, RH was dried to equilibrium moisture (about 7 wt %) in an air-circulated oven at 100 ± 2 ºC. This material was stored in hermetic plastic containers in order to prevent microbial attack (i.e. fungi).
The reagents used for the chemical treatments were: sulfuric acid (H2SO4, Cicarelli, Argentina); hydrochloric acid (HCl, Cicarelli, Argentina); sodium hydroxide (NaOH, Anedra, Argentina); potassium hydroxide (KOH, Anedra, Argentina); sodium chlorite (NaClO2, FlukaChemie, Germany); sodium bisulphate (Na2SO4, Barker, USA); and buffer solution pH 4 (Anedra, Argentina).
Methods
RH treatments
The washed and dried rice husk samples (C0) were submitted to different types of chemical treatments: Rice husk (C0) was stirred with 3% (w/v) KOH at weight ratio of 1:12 and boiled for 30 min; then the mixture was left overnight. The filtrate was washed twice with doubly distilled water, and 10% (v/v) HCl was added (100 mL). The formed precipitate of silica was separated from organic residue (C1) (Daifullah et al. 2004). After that, the lignocellulosic residue was treated with 0.7 % (w/v) sodium chlorite at a ratio of 1:50 g solid/ ml liquor at pH 4 and kept boiling for 2 h. The remaining solid was first treated with 5% (w/v) sodium bisulphite solution at room temperature for 1 h using a solid to liquor ratio of 1 g/50 ml and then washed with distilled water and dried at 100 ± 2 ºC in an air-circulated oven until constant weight was reached (C2). After that, the sample was treated with 17.5 % (w/v) sodium hydroxide (NaOH) solution, at room temperature for 8 hours using a solid to liquor ratio of 1 g/50 mL, washed, and dried again as in the previous step (C3). Figure 1 presents a scheme of the chemical treatments carried out on rice husk.
Fig. 1. Scheme of different steps of chemical treatments carried out on rice husk
Nanocellulose production
The nanocellulose (NC) samples were prepared by the acid hydrolysis of the obtained cellulose-based product (C3). The procedure was carried out with 60 wt% sulphuric acid solution, H2SO4, for 30 minutes under continuous stirring. After that, several washes with distilled water were done in order to dilute the acid concentration until the pH was neutral. The final solution was centrifuged, and finally a liophilizator was used for drying.
Scanning Electron Microscopy (SEM)
SEM observations of the internal and external faces of untreated and treated rice husks were performed with a JEOL JSM-6460 LV. (Tokyo, Japan) microscope at acceleration voltage of 15kV. Prior to the observation, the surfaces were sputter-coated with a gold layer of about 100 Å thickness to avoid charging under the electron beam.
Thermogravimetric analysis (TG)
Thermogravimetric analysis (TG) was used as a tool to determine the initial relative composition and the effect of chemical treatments on the composition of RH and NF. TG measurements were carried out in a thermo-balance Shimadzu TGA-50 (Japan). The samples (between 5 to 7 mg) were heated from 25 to 750 ºC at a heating rate of 10 ºC/min under nitrogen and air atmospheres (20 mL/min).
Fourier transformed infrared (FTIR)
FTIR spectra of treated and untreated rice husk were obtained in transmission mode. Samples were ground in a ball mill, and the powders were pressed with KBr and analyzed in a Mattson Genesis II spectrometer at a resolution of 2 cm-1; 16 scans were performed over each sample.
X-Ray diffraction
X-Ray patterns were obtained from a PW1710 diffractometer equipped with an X-Ray generator (λ = 0.179 nm) in the range of 2 from 5 to 60º at 1º/min. From the obtained patterns, it is possible to estimate the crystallinity index of cellulose based materials as follows (Mwaikambo and Ansell 2002),
(1)
where I002 is the intensity of the peak 2 = 26º (which is separated in two peaks at 26º and 24º as cellulose is transformed from cellulose I to cellulose II) and represents the crystalline part of the material. Iam is the intensity of the peak 2 = 18º and represents the amorphous part.
The relative quantity of cellulose II respect to cellulose I was calculated by relating the I24 and I26 intensities (of the peaks centered at 2 = 24° and 26º respectively), whose represent the crystalline part of the material.
Atomic Force Microscopy (AFM)
AFM was performed with a 5500 Scanning Probe Microscopy from Agilent Technologies operating in the tapping mode in air. Samples were prepared by dispersing 1 mg of nanofibers in 20 mL of dimethylformamide in an ultrasonic bath for 30 min. A droplet of the resulting solution was cast onto a microscopy slide and dried in vacuum oven at 70 ºC for 1 h. The AFM images were collected from dispersed nanocellulose in tapping mode.
RESULTS AND DISCUSSION
Characterization of Untreated Rice Husk (C0)
SEM micrographs of outer and inner surfaces of rice husk are shown in Fig. 2a-b.
Fig. 2. Morphology of original rice husk: SEM micrographs of outer (a) and inner (b) surface, respectively
As shown in Fig. 2a, the inner epidermis of rice husk has a smooth surface, while the outer surface of rice husk (Fig. 2b) is highly roughened and shows ridged structures. On the other hand, the external epidermal cells are arranged in linear ridges, which are punctuated with prominent domes (Park et al. 2003; Ruseckaite et al. 2007). The silica is mainly localized in the tips of the domes, whereas a lower amount of silica can be found in other regions of the rice husk. In addition, Park et al. (2003) have demonstrated, from field-emission SEM (FE-SEM) and energy dispersive X-ray micro-analysis (EDXA) experiments, that silica appears to be present throughout the outer surface of rice husk (domes and their shoulder). The high silica content on outer epidermis provides strength and stiffness to the husk (Park et al. 2003; Ruseckaite et al. 2007).
Thermogravimetric analysis (TG) was used as a tool to determine the presence or absence of different organic components of rice husk and to evaluate the amount of inorganic material. The TG curves (in nitrogen and air atmospheres) of original rice husk (C0) are shown on Fig. 3.
Fig. 3. TG and DTG curves of rice husk (C0) in nitrogen (solid line) and air (dashed line) atmosphere
The complex mix of organic materials (mainly cellulose, hemicellulose, lignin, and waxes) suffers a variety of physical and chemical changes when they are submitted to the heating program. Thus, TG curves show several decomposition steps. In nitrogen atmosphere, the main peak appears at around 360 ºC and is due to the thermal decomposition of -cellulose (Ciannamea et al. 2010; Mansaray and Ghaly 1998; Ndazi et al. 2008; Stefani et al. 2005). In addition, a shoulder at lower temperatures (around 300 ºC) was observed; this can be attributed to the thermal decomposition of hemicellulose and the glycosidic links of cellulose (Ciannamea et al. 2010; Mansaray and Ghaly 1998; Ndazi et al. 2008; Stefani et al. 2005). The difference in these decomposition temperatures is due to the hemicellulose and amorphous cellulose, which degrade more easily than crystalline cellulose. The wider lignin peak covers the range from 200 ºC to 500 ºC with a maximum at 350 ºC and appears superimposed by the peaks of the other two components (Ciannamea et al. 2010; Mansaray and Ghaly 1998; Ndazi et al. 2007; Stefani et al. 2005). The residue at 700 ºC in nitrogen atmosphere is composed of carbonaceous products and silica from the original sample, and accounts for about 44 % of the total weight, in agreement with previous results obtained for the same rice variety (Ruseckaite et al. 2007; Stefani et al. 2005). On the other hand, the thermal decomposition in air displayed a third stage between 366 and 410 ºC, which was attributed to the oxidation of the degradation products from the second stage (Mansaray and Ghaly 1999). The residue at 700 ºC was almost exclusively comprised of silica and accounted for 24% of the total weight, in agreement with previous results of different rice varieties (Liou et al. 2004; Mansaray and Ghaly 1999).
Figure 4 shows the FTIR spectra of original rice husk (C0). The main characteristic peaks associated with organic and inorganic components are labeled in the spectra.
Fig. 4. FTIR spectra of rice husk (C0)
As it was explained in the introduction, the organic part of the rice husk is mainly composed of cellulose, hemicellulose, lignin, and waxes, which most likely consist of alkene, esters, aromatics, ketones, and alcohols, with different oxygen-containing functional groups, e.g., OH stretching (3650 to 3200 cm-1) and C=O stretching hemi-celluloses (1732 cm-1) (Demirbas et al. 2000; De Rosa et al. 2010; Sócrates et al. 1994). Besides, some characteristic peaks of cellulose in RH can be observed at 1640 cm-1 (OH bending of adsorbed water), 1420 cm-1(CH2 strain), 1375 and 1270 cm-1 (CH bending) (O’Connor et al. 1958; Liang et al. 1959; Zuluaga et al. 2009). On the other hand, the vibrations of the aromatic rings can be seen at 1606 and 1515 cm-1 (aromatic ring vibrations), which can be only related to lignins (Zuluaga et al. 2009). The inorganic part is characterized by three bands associated to Si-O-Si bonds (situated at frequencies of 1100-1070, 799, and 465 cm-1) (Sócrates et al. 1994). The previously mentioned bands will be used to evaluate the effectiveness of each chemical treatment on the preferential removal of different components.
Characterization of treated rice husk (C1, C2, and C3)
Figure 5 shows the outer and inner surfaces of rice husk after different chemical treatments (C1 to C3).
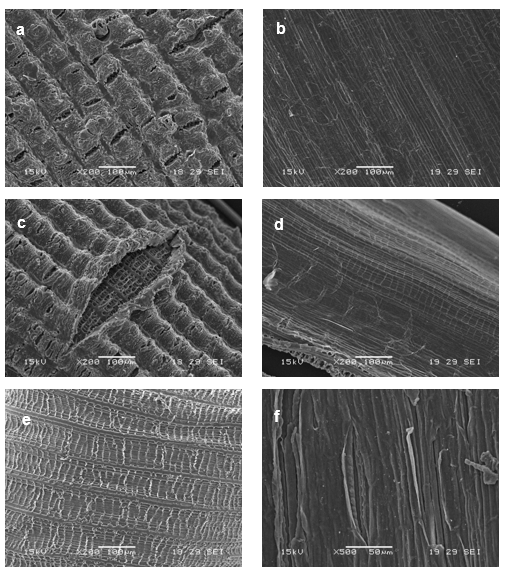
Fig. 5. SEM micrographs of treated rice husk (a-b) C1, (c-d) C2 and (e-f) C3 (outer and inner surfaces respectively)
The outer surface micrographs show domes broken after the first treatment step (KOH + HCl), indicating that at least part of inorganic fraction (mainly silica) was removed. The organic part was also affected; the decrease of the 1732 cm-1 peak (Fig. 6a) can be related to the contribution of KOH + HCl to the elimination of waxes and/or hemicellulose. DTG curves (Fig. 6b) showed one degradation peak for C1 (remember that two peaks were present in original rice husk: C0), which in turns confirmed the elimination of hemicellulose (Alvarez and Vazquez 2004, Ndazi et al. 2008). After the first treatment (C1) the temperature of maximum rate loss associated with -cellulose was reduced by about 9 °C, which could be probably associated to partial removal of cementing materials (Ndazi et al. 2008; Ciannamea et al. 2010). The color of this sample (C1) remained dark, which was related to the presence of lignin, which as also verified by the 1515 cm-1 FTIR peak. The residual mass (TGA in air see Table 1) and the disappearance of the 465 and 799 cm-1 FTIR peaks are unambiguous suggestions of the effectiveness of the selected treatment to remove the inorganic components (Sócrates et al. 1994). Another change that took place at this stage was related to a partial transformation (around 10%) of cellulose from type I to type II (see Fig. 7 and Table 1).
Table 1. Characteristics of Original and Treated Rice Husk
The subsequent treatment (with NaClO2, C2) produced an increase in the degree of crystallinity or crystalline length of cellulose (remember that the hemicellulose was already removed in the previous step), together with a higher transformation from cellulose I to cellulose II (around 50%) (Fig. 7). The previous statement was also inferred from the shoulder of the cellulose peak that appears in the DTGA curve (Fig. 6b). There were slight changes in intensity at 1606 and 1515 cm-1 after treatment, suggesting that the sample still contained aromatic residues associated with the partial extraction/removal of lignin; whereas the 1732 cm-1 peak (Fig. 6a) was reduced but it was still present due to some residual waxes. In addition, the residual silica is clearly removed from the outer surface (Fig. 5c) exposing the inner structure as “cat eyes”.
The last treatment with NaOH (C3) caused the complete removal of the outer surface (Fig. 5e), the lightness of the sample seems to show that the resultant sub-product could be mainly cellulose but with lower thermal stability than the original one (Fig. 6b), since all cellulose was transformed to type II as it was confirmed by RXD (Fig. 7). The FTIR spectrum showed the complete disappearance of the 1732 cm-1 absorbance, indicating that neither hemicelluloses nor waxes were present in this material. Even after this last treatment the peaks at 1606 and 1515 cm-1 appeared in the FTIR spectra, but with a reduced intensity, indicating the presence of un-extracted aromatic components.
Characterization of nanocellulose (NC)
Sample C3 was submitted to acid hydrolysis in order to produce nanocellulose (NC). Figure 8 shows the AFM image and diameter distribution of the obtained NC. The average diameter of the obtained NC was 12.4 ± 4.6 nm (Fig. 7 b). Interestingly, the diameter of the nanocellulose from rice husk was smaller than from other lignocellulosic sources such as cotton (Ludueña et. al. 2010) and sisal (Moran et. al. 2008), for which the average fiber diameter have been reported as 200 80 nm and 30.9 12.5 nm, respectively, and with smaller size distribution than NC from pineapple leaf fiber, for which the diameters ranges were 5 to 60 nm (Cherian et. al. 2010).
Fig. 6. (a) FTIR spectra and (b) DTG curves of original and treated rice husk (C0 to C3) and nanocellulose (NC)
Fig. 7. XRD of original rice husk (C0) and samples with different treatment (C1, C2, C3, and NC)
Fig. 8. (a) AFM image of NF and (b) diameter distribution of NF
Figure 6 also includes the FTIR spectra of NC. Characteristic cellulose peaks were observed at 1375 and 1270 cm-1 (CH bending). The presence of small peaks at 1606 and 1515 cm-1indicate that aromatic residues were present even in the NC. The temperature of maximum rate loss of NC was quite similar to the C3 sample and lower than original rice husk, despite the fact that the cellulose fraction was isolated and then a higher thermal stability was expected. However, it is possible that sulphate groups introduced during acid hydrolysis could decrease the thermal stability of cellulose crystallites, as has been reported in the literature (Roman and Winter 2004). In addition, RX results (Fig. 7) indicate that the last treatment did not affect the structure of the obtained cellulose, which was type II but with higher Ic (76%).
CONCLUSIONS
- Sub-products of rice husk obtained from several chemical treatments were obtained and characterized.
- The employed sequence of treatments made it possible to obtain nanocellulose from the organic waste.
- All results give the impression that obtained cellulose-based materials could be used in the future mainly as fillers or additives in several engineering applications, providing added value to the abundant and inexpensive RH.
- In the first step (KOH + HCl), the inorganic part (silica) started to be affected (which continued in the subsequent processes); cellulose was partially transformed to type II, and the elimination of hemicellulose took place, whereas lignin and waxes remained in the material after this step.
- During the subsequent treatment (with NaClO2) both initiated processes (extraction of silica and transformation of cellulose I to cellulose II) were emphasized. The last treatment (with NaOH) produced the complete removal of the outer surface and all results seem to shown that the final sub-product could be cellulose II without hemicellulose, waxes, or silica.
ACKNOWLEDGMENTS
The authors want to thank to National Research Council, Argentina (CONICET), contract grant number PIP 112-200801-0183 and ANPCYT, contract grant number: PICT-2006-01560, for their financial support. Authors are also thankful to Mr. E. Britos del Pino and Mr. O. Casemayor for his technical assistance.
REFERENCES CITED
Ajiwe, V. I. E., Okeke, C. A., Ekwuozor, S. C., and Uba, I. C. (1998). “A pilot plant for the production of ceiling boards from rice husks,” Bioresour. Technol. 66, 41-43.
Alvarez, V. A., and Vazquez, A. (2004). “Thermal degradation of cellulose derivatives starch blends and sisal fibres biocomposites,” Polym. Degrad. Stab. 84, 13-21.
Ciannamea, E. M., Stefani, P. M., Ruseckaite, R. A. (2010). “Medium-density particleboards from modified rice husks and soybean protein concentrate-based adhesives,” Biores. Technol.101, 818-825.
Cherian, B. M., Lopes Leão, A., Ferreira de Souza, S., Thomas, S., Pothan, L. A., and Kottaisamy, M. (2010) “Isolation of nanocellulose from pineapple leaf fibres by steam explosion,” Carbohydrate Polymers 81, 720-725.
Daifullah, A. A. M., Awwad, N. S., and Reefy, S. A. (2004). “Purification of wet phosphoric acid from ferric ions using modified rice husk,” Chem. Eng. Process. 43, 193-201.
Demirbas, A. (2000). “Mechanisms of liquefaction and pyrolysis reactions of biomass,” Energy. Convers. Manage. 41, 633-646.
De Rosa, I. M., Kenny, J. M., Puglia, D., Santulli, C., and Sarasini, F. (2010). “Morphological, thermal and mechanical characterization of okra (Abelmoschus esculentus) fibres as potential reinforcement in polymer composites,” Compos. Sci. Technol. 70, 116-122.
Dufresne, A., Dupeyre, D., and Vignon, M. R. (2000). “Cellulose microfibrils from potato tuber cells: Processing and characterization of starch-cellulose microfibril composites,” J. App. Polym. Sci. 76, 2080-2092.
Faria, H., Cordeiro, N., Belgacem, M. N., and Dufresne, A. (2006). “Dwarf Cavendish as a source of natural fibers in poly(propylene)-based composites,” Macromol. Mater. Eng. 291, 16-26.
Garcia, D., López, J., Balart, R., Ruseckaite, R. A., and Stefani, P. M. (2007). “Composites based on sintering rice husk–waste tire rubber mixtures,” Mater. Des. 28, 2234-2238.
Gerardi, V., Minelli, F., and Viggiano, D. (1998). “Steam treated rice industry residues as an alternative feedstock for the wood based particleboard industry in Italy,” Biomass. Bioenergy14, 295-299.
Lee, Y. K., Kim, S., Yang, H. S., and Kim, H. J. (2003). “Mechanical properties of rice husk flour-wood particleboards by urea-formaldehyde resin,” Mokchae Konghak 31, 42-49.
Leiva, P., Ciannamea, E. M., Ruseckaite, R. A., and Stefani, P. M. (2007). “Medium-density particleboards from rice husks and soybean protein concentrate,” J. Appl. Polym. Sci. 106, 1301-1306.
Liang, C. Y., and Marchessault, R. H. (1959). “Infrared spectra of crystalline polysac-charides. I. Hydrogen bonds in native celluloses,” Journal of Polymer Science 37, 385-395.
Liou, T. H. (2004). “Preparation and characterization of nano-structured silica from rice husk,” Mater. Sci. Eng. A 364, 313-323.
Ludueña, L., Vazquez, A., and Alvarez, V. A. (2010). “Effect of lignocellulosic filler type and content on the behavior of the polycaprolactone based composites” Carbohydrate Polymers, submitted to publication (CARBPOL-D-10-01014R1).
Mansaray, G., and Ghaly, A. E. (1998). “Thermal degradation of rice husks in nitrogen atmosphere,” Bioresour. Technol. 65, 13-20.
Mansaray, K. G., and Ghaly, A. E. (1999). “Determination of kinetic parameters of rice husks in oxygen using thermogravimetric analysis,” Biomass. Bioenergy. 17, 19-31.
Moran, J. I., Alvarez, V. A, Cyras, V. P., and Vázquez, A. (2008). “Extraction of cellulose and preparation of nanocellulose from sisal fibers,” Cellulose 15, 149-159.
Mwaikambo, L.Y., and Ansell, M. P. (2002). “Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization,” J. App. Polym. Sci. 84, 2222-2234.
Ndazi, B. S., Karlsson, S., Tesha, J. V., and Nyanumwa, C. W. (2007). “Chemical and physical modifications of rice husks for use as composite panels,” Compos. Part. A 38, 925-935.
Ndazi, B. S., Nyahumwa, C., and Tesha, J. (2008). “Chemical and thermal stability of rice husks against alkali treatment,” BioResources 3(4), 1267-1277.
O’Connor, R. T., DuPré, E. F., and Mitcham, D. (1958). “Applications of infrared absorption spectroscopy to investigations of cotton and modified cottons. Part I. Physical and crystalline modifications and oxidations,” Textile Research Journal 28, 382-392.
Park, B. D., Wi, S. G., Lee, K. H., Singh, A. P., Yoon, T. H., and Kim, Y. S. (2003). “Characterization of anatomical features and silica distribution in rice husk using microscopic and micro-analytical techniques,” Biomass. Bioenergy. 25, 319-327.
Park, B. D. and Wi, S. G., Lee, K. H., Singh, A. P., Yoon, T. H., and Kim, Y. S. (2004). “X-ray photoelectron spectroscopy of rice husk surface modified with maleated polypropylene and silane,” Biomass. Bioenergy. 27, 353-363.
Roman, M. and Winter, W.T. (2004) “Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose,” Biomacromolecules 5, 1671-1677
Rondeau-Mouro, C., Bouchet, B., Pontoire, B., Robert, P., Mazoyer, J., and Buléon, A. (2003). “Structural features and potential texturising properties of lemon and maize cellulose microfibrils,” Carbohydr. Polym. 53, 241-252.
Ruseckaite, R. A., Ciannamea, E. M., Leiva, P., and Stefani, P. M. (2007). “Particleboards based on rice husk,” In: Zaikov, G. E., and Jimenez, A. (eds), Polymer and Biopolymer Analysis and Characterization, Nova Science Publishing Inc., New York, pp. 1-12.
Samir, M., Alloin, F., Paillet, M., and Dufresne, A. (2004). “Tangling effect in fibrillated cellulose reinforced nanocomposites,” Macromolecules 37, 4313-4316.
Sócrates, G. (1994). Infrared Characteristic Group Frequencies, John Wiley and Sons, New York.
Stefani, P. M., Garcia, D., Lopez, J., and Jimenez, A. (2005). “Thermogravimetric analysis of composites obtained from sintering of rice husk–scrap tire mixtures,” J. Therm. Anal. Calorim. 81, 315-320.
Stefani, P. M., Cyras, V. A., Tejeira, A., and Vazquez, A. (2006). “Mechanical properties and thermal stability of rice husk ash filled epoxy foams,” J. App. Polym. Sci. 99, 2957-2965.
Stefani, P. M., Pérez, C. J., Alvarez, V. A., and Vázquez, A. (2008). “Microcellulose fibers-filled epoxy foams,” J. Appl. Polym. Sci. 109, 1009-1013.
Sun, L., and Gong, K. (2001). “Silicon-based materials from rice husks and their applications” Ind. Eng. Chem. Res. 40, 5861-5877.
Torelli, N., and Čufar, K. (1995). “Mexican tropical hardwoods. Comparative study of ash and silica content,” Holz. Roh. Werkst. 53, 61-62.
Wang, B., Sain, M., and Oksman, K. (2007). “Study of structural morphology of hemp fiber from the micro to the nanoscale,” Appl. Compos. Mater. 14, 89-103.
Zuluaga, R., Putaux, J. L., Restrepo, A., Mondragon, I., and Gañán, P. (2007). “Cellulose microfibrils from banana farming residues: Isolation and characterization,” Cellulose. 14, 585-592.
Zuluaga, R., Putaux, J. L., Cruz, J., Vélez, J., Mondragon, I., and Gañán, P. (2009). “Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features,” Carbohydr. Polym. 76, 51-59.
Article submitted: November 18, 2010; Peer review completed: January 15, 2011; Revised version received and accepted: March 10, 2011; Published: March 11, 2011.
