Abstract
Download PDF
Full Article
Optimization Protocol for the Microwave-Assisted Extraction of Antioxidant Components from Pinus elliottii Needles Using Response Surface Methodology
Hui Ouyang,a,* Kun Hou,a Lishu Wang,b and Wanxi Peng b,*
Response surface methodology (RSM) based on a Box–Behnken rotatable design was used to determine the optimum conditions for the microwave-assisted extraction of antioxidant compounds from Pinus elliottii needles. Four process variables were evaluated at three levels (29 experimental conditions): ethanol (50, 70, and 90%), solvent:solute ratio (25:1, 20:1, and 15:1), extraction temperature (60, 70, and 80 °C), and ultrasonic power (100, 150, and 200 W). Using RSM, a quadratic polynomial equation was obtained by multiple regression analysis to predict the optimized extraction protocol. The radical scavenging capacity was determined by O2−, ·OH, and DPPH methods. For the microwave-assisted extraction of antioxidant compounds from Pinus elliottii needles, the optimum process used ethanol at 72%, a solvent:solute ratio of 21:1 mL/g, an extraction temperature of 67 °C, and an ultrasonic power of 200 W. The results indicated good correlation between total polyphenols content and O2−, ·OH, and DPPH radical scavenging activities.
Keywords: Total phenolics; Optimization; Response surface methodology; Box–Behnken rotatable design; Antioxidant compounds; O2− scavenging activity; ·OH scavenging activity; DPPH scavenging activity
Contact information: a: College of Chemistry and Chemical Engineering, and Key Laboratory of Hunan Forest Products and Chemical Industry Engineering, Jishou University, 416000 Jishou, PR China; b: School of Materials Science and Engineering, Central South University of Forestry and Technology, 410004 Changsha, PR China; *Corresponding authors: oyhmail@163.com; pengwanxi@163.com
INTRODUCTION
Pine is widely utilized in Chinese drinks and herbal medicine and is recommended in Ayurvedic medicine for treating rheumatism, diabetes, obesity, gonorrhea, chronic bronchitis, cancer, and stomach and cardiovascular diseases (Kim and Chung 2000; Singh et al. 2011, 2015; Zeng et al. 2011, 2012b; Zhang et al. 2015, 2016). Pine needles is a recognized health food material and is widely used as a flavoring agent in foods and beverages (Kim and Chung 2000; Ratola et al. 2010; Zeng et al. 2012a; van Drooge et al. 2014; Mahajan et al. 2016). The biological properties of pine needle extracts have been the subject of recent interest in academia and food industries because of their potential as antioxidants and radical scavengers (Zeng et al. 2011, 2012a, 2012b).
Pinus elliottii, commonly called slash pine, is native to the Southeastern USA (Caetano da Silva et al. 2014; Chaudhary et al. 2014; Parker et al. 2014; Susaeta et al. 2014; Zhai et al. 2015; Nunes et al. 2016). It is common throughout the southeastern United States, Taiwan, and South China (Chaudhary et al. 2014). The Pinus elliottii plant has a long history of traditional and ethnobotanical applications in diverse cultures (Chaudhary et al. 2014; Nunes et al. 2016).
Microwave-assisted extraction has been successfully used in recent years for the extraction of functional components from different plant matrices. By the use of ultrasonic mechanical crushing and cavitation, as an alternative to traditional extraction procedures, one can considerably reduce not only the extraction time, but also solvent consumption and energy requirements. Importantly, it also has been shown to result in high extraction efficiency compared to conventional techniques (Xu et al. 2015a,2015b; Pinela et al. 2016).
Antioxidant activity can be measured indirectly on the basis of its effects on model systems. Most methods are based on a radical generating system (Valavanidis et al. 2004; Zhang et al. 2014; Xu et al. 2015a,2015b; Chen et al. 2016; Rashidinejad et al. 2016; Santos et al. 2016). Radical-based methods such as superoxide anion radical scavenging activity (O2−), hydroxy radical scavenging activity (·OH), and 1,1-diphenyl-2-picrylhydrazyl scavenging activity (DPPH) assays have been proposed to evaluate the antioxidant capacity of green tea (Zhao et al. 2008; Balabani et al. 2011; Mandawad et al. 2013; Anissi et al. 2014; Zhu et al. 2015; Gramza-Michałowska et al. 2016).
Response surface methodology (RSM) is a mathematical and statistical technique that is used to study and optimize multivariable systems by finding the true relationship between the response and the set of independent variables. The optimization of analytical procedures has been carried out by using multivariate statistic techniques to simultaneously optimize the levels of these variables to attain the best system performance.
To date, the scavenging activities of polyphenols from Pinus elliottii needles against superoxide anion radicals, hydroxy radicals, and DPPH radicals have not been determined. Therefore, the objectives of this study were to optimize the conditions (ethanol/water ratio, solvent/solute ratio, extraction temperature, and ultrasonic power) for the extraction of antioxidant compounds from Pinus elliottii needles, to determine the total polyphenols content, and to determine the active composition of polyphenols in Pinus elliottii needles. The polyphenols composition and antioxidant capacity were related in an attempt to establish a correlation between the total polyphenols content and the total antioxidant activity.
EXPERIMENTAL
Materials
Collection of sample
Pinus elliottii needles were collected from Miluo, Yueyang, Hunan Province, China, in 2015. Samples were collected, washed, dried in a hot air oven at 50 to 60 °C, and converted to powder in a grinder. These samples were stored in airtight polythene bags at 4 °C for the extraction process.
Chemicals and reagents
DPPH, gallic acid, and Folin-Ciocalteu reagent were obtained from Sinopharm Chemical Company (Beijing, China). All other chemicals and solvents used were of analytical grade from Hunan Chemical Reagent Factory (Changsha, China) and were used as received or dried by standard procedures, unless stated otherwise.
Methods
Optimization of parameters for extraction of antioxidant components
A protocol for the extraction of antioxidant components from Pinus elliottii needles was established by response surface methodology (RSM), which was employed to determine the best combination of variables for the optimum extraction yield and antioxidant activity. RSM was used to analyze the influence of four extraction process variables on the yield of antioxidant components: ethanol/water ratio, solvent/solute ratio, extraction temperature, and ultrasonic power.
Protocol requirements were considered when choosing the factorial levels. According to previous work (Xu et al. 2015b; Pinela et al. 2016), the ethanol/water ratio, solvent/solute ratio, extraction temperature, and ultrasonic power were varied in the ranges 90:10, 70:30, 50:50; 25:1, 20:1, and 15:1 (mL:g); 60, 70, and 80 °C; 100, 150, and 200 W, and extraction time 60 min, respectively. The response variable used to build the model corresponded to the yield of antioxidant components obtained in each experiment. The regression equation and analysis of variance (ANOVA) were obtained using Design Expert 8.0.6 software (Stat-Ease Inc., Minneapolis, USA). ANOVA was used to summarize the results obtained under all the experimental conditions. A confidence interval of 95% was set to test the significance of the factors and their interaction. The F statistic test was used to evaluate whether the regression model was adequate to describe the observed data. The variability of the optimization parameter was analyzed by R2 statistics. In addition, the normal probability plots of the residuals and the plots of the residuals versus the predicted response were utilized to evaluate the adequacy of the model.
Preparation of antioxidant extracts
Antioxidant extracts were prepared using an orbital shaker and all of the combinations suggested by RSM. Ground samples in 500 mL conical flasks were suspended in solvent, and the flasks were placed on an orbital shaker for the optimum time. The extracts were separated from solids by filtration (Whatman No. 1) and concentrated under reduced pressure using a rotary evaporator. Viscous extracts were stored at 4 °C until testing and analysis (Hussain et al. 2012; Monroy et al. 2016).
Determination of total polyphenols content (TPC)
Following the method reported earlier (Hussain et al. 2012), TPCs from all isolated extracts were assessed using the Folin-Ciocalteu reagent with spectrophotometric determination. Crude extract (50 mg) was mixed with 7.5 mL of Milli-Q-water and 0.5 mL of Folin-Ciocalteu phenol reagent. The mixture was kept at room temperature for 10 min before 1.5 mL of 20% (w/v) sodium carbonate solution was added. The mixture was heated in a water bath at 40 °C for 20 min and then cooled in an ice bath. The absorbance was measured at 755 nm using a UV-Visible spectrophotometer (Bio Tek Instrument, Winooski, VT, USA). A standard curve based on gallic acid was used for the conversion of absorbance to polyphenols concentration in gallic acid equivalents.
Superoxide anion radical scavenging assay
Superoxide anion radical (O2−) scavenging activity was analyzed by electron spin resonance (ESR) spectroscopy, which used the 5,5-dimethyl-1-pyrroline N-oxide (DMPO) spin adduct generated by the hypoxanthine and xanthine oxidase reaction (Nakai et al. 2003). ESR spectra were recorded with a JEOL JES-FR30 spectrometer (Tokyo, Japan), which used an aqueous quartz flat cell (JEOL LC-12 ESR cuvette; internal size 60 × 10 × 0.31 mm; effective volume of 160 μL). The sampling procedure used a 100 mM sodium phosphate buffer solution (pH 7.4) as the solvent. A total of 50 μL of 2.0 mM hypoxanthine solution, 35 μL of 5.5 mM diethylenetriamine-N,N,N′,N″,N″-pentaacetic acid (DTPA), 50 μL of the sample solution, 15 μL of DMPO, and 50 μL of xanthine oxidase (0.4 unit/mL) were added to a test tube. After rapid stirring, 200 μL of the mixture was taken into the flat cell. ESR spectrum recording started 60 s after the addition of xanthine oxidase, with a recording rate of 5 mT/min. After recording, the signal intensity of the lowest field peak in the spectrum was normalized as the relative height against the standard signal intensity of the manganese oxide marker. The absolute concentration of DMPO–O2− was finally determined by the double integration of the ESR spectrum. Ethanol (100%) was used as the blank. The percent scavenging was calculated according to Eq. 1,
Scavenging (%) = 100 × (Sblank − Ssample/Sblank) (1)
where Sblank is the peak area of the DMPO–O2− solution and Ssample is the peak area of the extract solution. The extract concentration that provided 50% scavenging (IC50) was calculated from the plot of inhibition percentage against extract concentration.
Hydroxy radical scavenging assay
Hydroxy radical (·OH) scavenging activity was analyzed in the same way as superoxide anion radical scavenging activity, which used the DMPO spin adduct generated by the Fenton reaction (Nakai et al. 2003). The absolute concentration of DMPO–·OH was determined by the double integration of the ESR spectrum. Ethanol (100%) was the blank. The percent scavenging was calculated according to Eq. 1, where Sblank is the peak area of the DMPO–·OH solution. IC50 against the hydroxyl radical was calculated from the plot of inhibition percentage against extract concentration.
The sampling procedure used a 100 mM sodium phosphate buffer solution (pH 7.4) as the solvent. 75 μL of 200 μM FeSO4 and 200 μM DTPA solution, 50 μL of sample solution, and 20 μL of 0.879 M DMPO were added to a test tube. A 20 μL aliquot of 10 mM H2O2 was added to the tube and the mixture was stirred for 10 s before being transferred to the flat cell. The ESR spectrum was recorded with acquisition starting 60 s after the addition of H2O2, where Sblank is the peak area of the DMPO–·OH solution.
DPPH radical scavenging assay
ESR analyzed DPPH scavenging activity with a recording rate of 5 mT/min. After recording, the signal intensity of the lowest field peak was normalized as the relative height against the standard signal intensity of the manganese oxide marker. The absolute concentration of DPPH was finally determined by the double integration of the ESR spectrum. Ethanol (100%) was used as the blank. The percent scavenging was calculated according to Eq. 1, where Sblank was the peak area of the DPPH solution. IC50 against the hydroxyl radical was calculated from the plot of inhibition percentage against extract concentration.
A total of 100 μL of 60 μM DPPH in 50% CH3CN and 100 μL of the sample in 50% CH3CN were added to a test tube. After stirring for 10 s, the reaction mixture was added to the flat cell. ESR spectrum recording began 60 s after the addition of DPPH, with a recording rate of 5 mT/min. Afterward, the signal intensity of the lowest field peak was normalized as the relative height against the standard signal intensity of the manganese oxide marker. Ethanol (100%) was used as the blank. The percent scavenging was calculated according to Eq. 1. The IC50 value was calculated as described above.
Statistical analysis
Extraction conditions for Pinus elliottii needles were analyzed individually in triplicate. RSM was employed with a Box-Behnken rotatable design to investigate the effect of different solvent concentrations, solvent/solute ratio, temperature of extraction, and ultrasonic power. The experimental plan was designed and the results obtained were analyzed using Design Expert 8.0.6 software (Stat-Ease Inc., Minneapolis, USA). This was done to build and evaluate models and plot contours and three-dimensional (3D) response surface curves.
RESULTS AND DISCUSSION
Optimization
In this study, four operational variables (solvent concentrations, solvent/solute ratio, temperature of extraction, and ultrasonic power) were optimized for maximum antioxidant extract yield, i.e. total phenolics content (TPC) by using RSM.
Table 1. Experimental Design for the Extraction of Antioxidants from Pinus elliottii Needles Using Response Surface Analysis
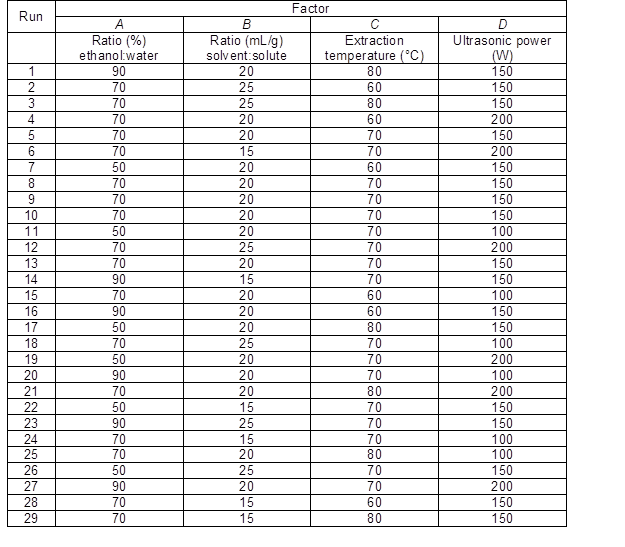
Table 1 shows the template of the Box-Behnken rotatable design. Initially, influential factors including solvent concentration, particle size, liquid/solid ratio, and extraction time were investigated separately to determine the extraction yield.
Table 2 shows the experimental and predicted data in terms of TPC and radical scavenging capacity. By using RSM, the TPC ranged from 1.14 to 4.93 g/100 g (Table 2). Normal probability plots of the residuals and the plots of the residuals versus fitted values were used for the adequacy of the model (Fig. 1a and b). The maximum TPC was obtained at ethanol concentration of 72%, solvent:solute ratio of 21:1, extraction temperature of 67 °C, and ultrasonic power of 200 W.
Table 2. Total Polyphenols Content and Free Radical Scavenging Activity of Pinus elliottii Needles
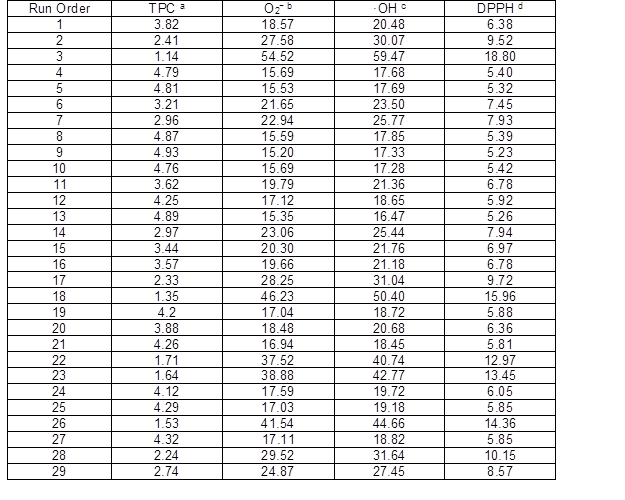
* a Total phenolic content (g/100 g of dry plant material, measured as gallic acid equivalent), b O2− scavenging IC50 (mg/mL), c ·OH scavenging IC50 (mg/mL), d DPPH scavenging IC50 (mg/mL)
Among the 29 runs (Table 2), run 9 produced the highest total polyphenols content (4.93 g/100 g) and run 3 produced the lowest (1.14 g/100 g). For O2−, ·OH, and DPPH, runs 9 and 13 both produced the highest radical scavenging capacity IC50 (15.20 mg/mL TPC for O2−, 16.47 mg/mL TPC for ·OH, and 5.23 mg/mL TPC for DPPH). Run 3 for O2−, ·OH, and DPPH produced the lowest radical scavenging IC50 (54.52 mg/mL TPC for O2−, 59.47 mg/mL TPC for ·OH, and 18.80 mg/mL TPC for DPPH).
Fitting the data with various models, ANOVA showed that the TPC and radical scavenging capacity could be described using quadratic polynomial models. A large F-value implied that the models were significant at a 95% confidence level (Table 3).
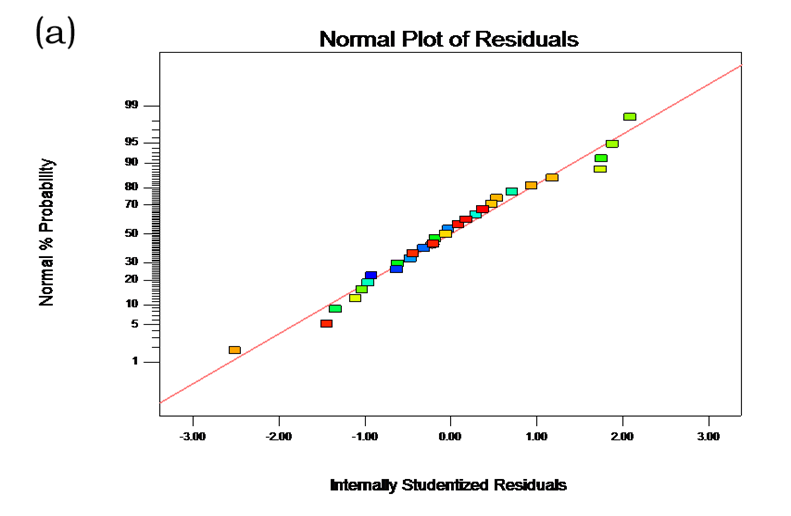
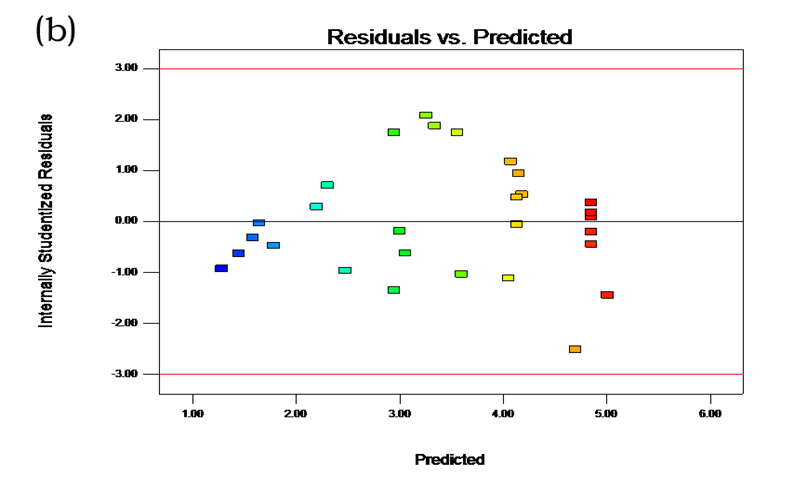
Fig. 1. (a) Normal plot of the residuals for TPC; (b) Residuals vs. predicted for TPC
All models were highly significant because the “Prob > F” relation was less than 0.0001. The lack-of-fit p value was larger than 0.05; it was not significant relative to the pure error. Furthermore, the low values of pure error indicated good reproducibility of the data. “R2” and “Adj R2” also revealed excellent correlations between the independent variables. Table 4 demonstrates the relationship between independent variables, target content, and radical scavenging capacity in second-order polynomial models.
Table 3. Analysis of Variance for Response Surface Quadratic Model
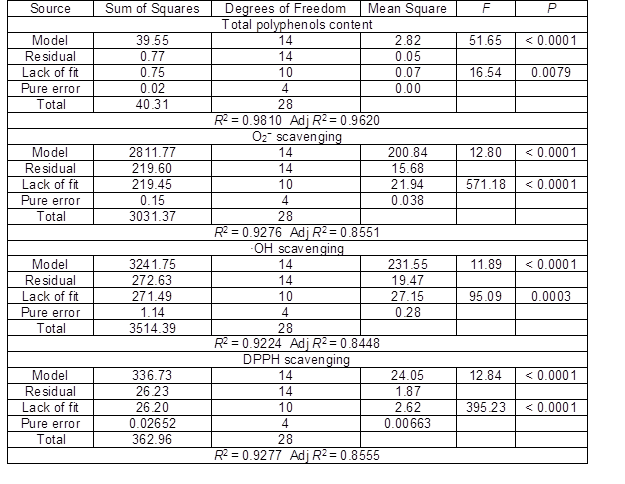
Table 4. Second-Order Polynomial Equations for Investigated Response Variables
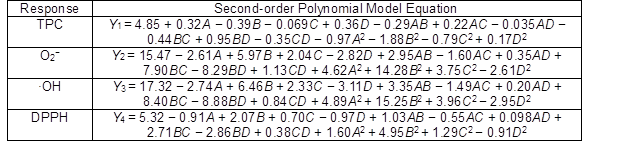
The effects of independent factors and their interactions on TPC, O2− IC50 value, ·OH IC50 value, and DPPH IC50 value were calculated by response surface plots. Figure 2 shows the response surfaces for the interactions of independent variables on the extraction efficiency of TPC (a-1, b-1, c-1, d-1, e-1, and f-1), O2− scavenging activity (a-2, b-2, c-2, d-2, e-2, and f-2), ·OH scavenging activity (a-3, b-3, c-3, d-3, e-3, and f-3), and DPPH scavenging activity (a-4, b-4, c-4, d-4, e-4, and f-4). The main activity was attributed to curcuminoids because the TPC was positively associated with radical scavenging capacity (Fig. 3), but the effects of other bioactive compounds could not be excluded.
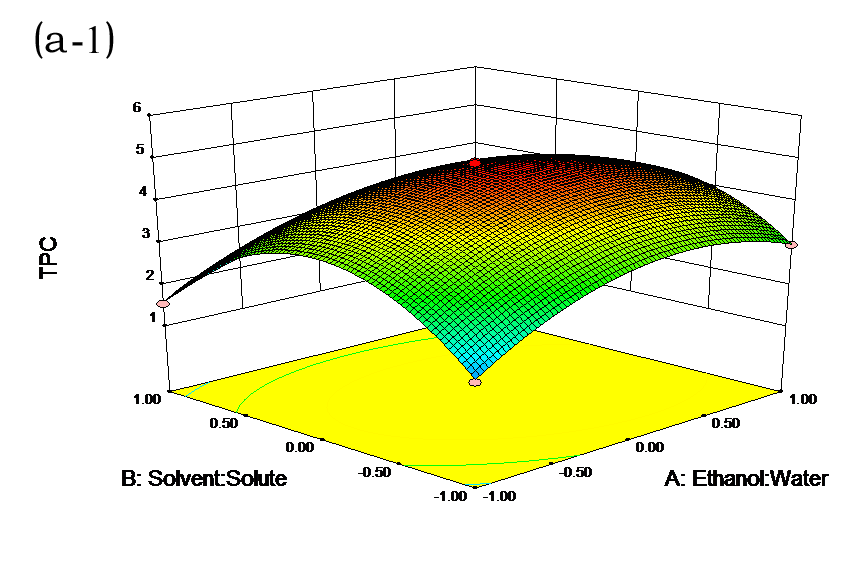
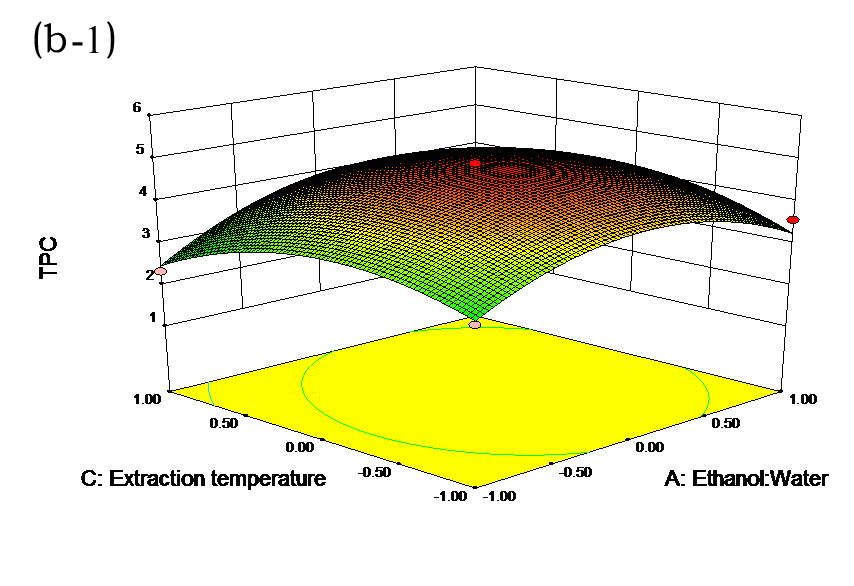
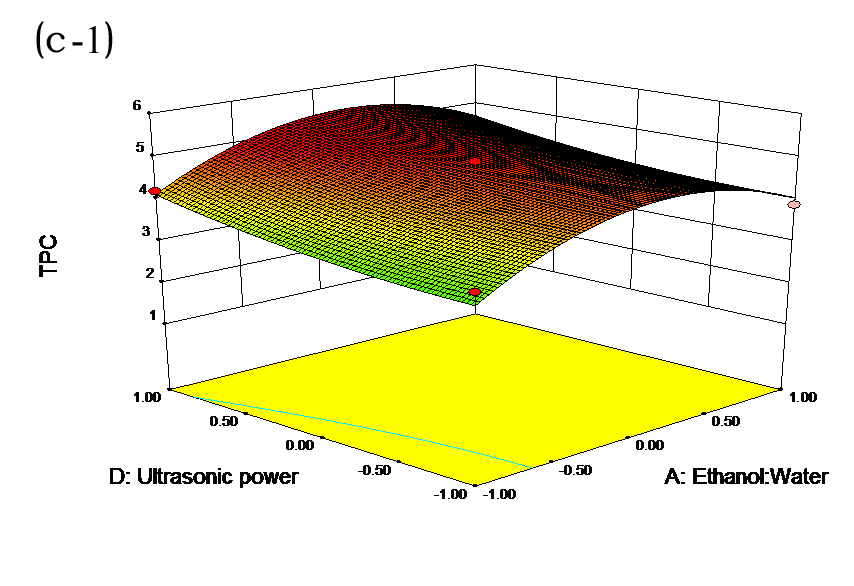
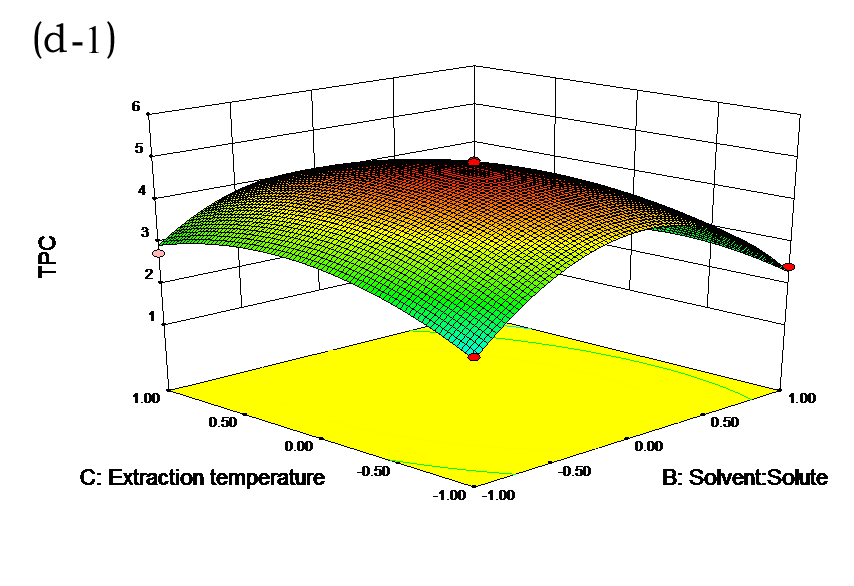
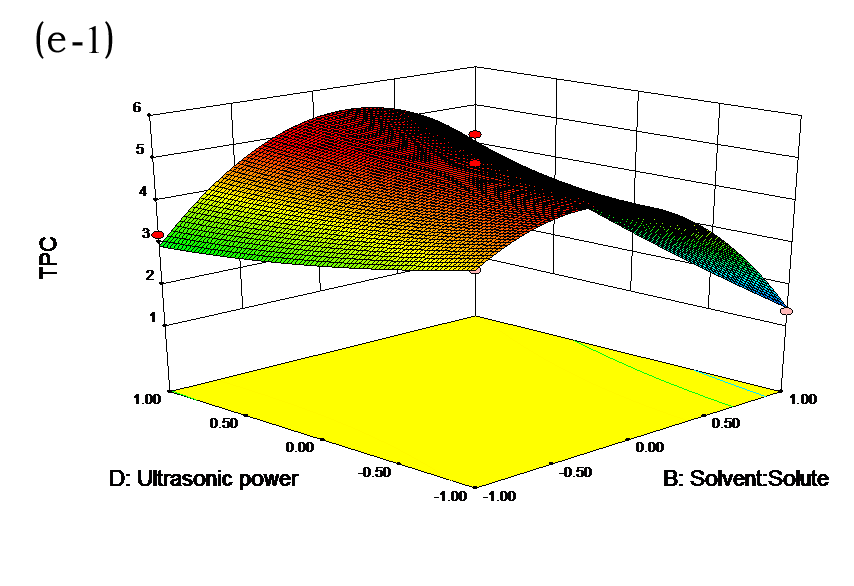
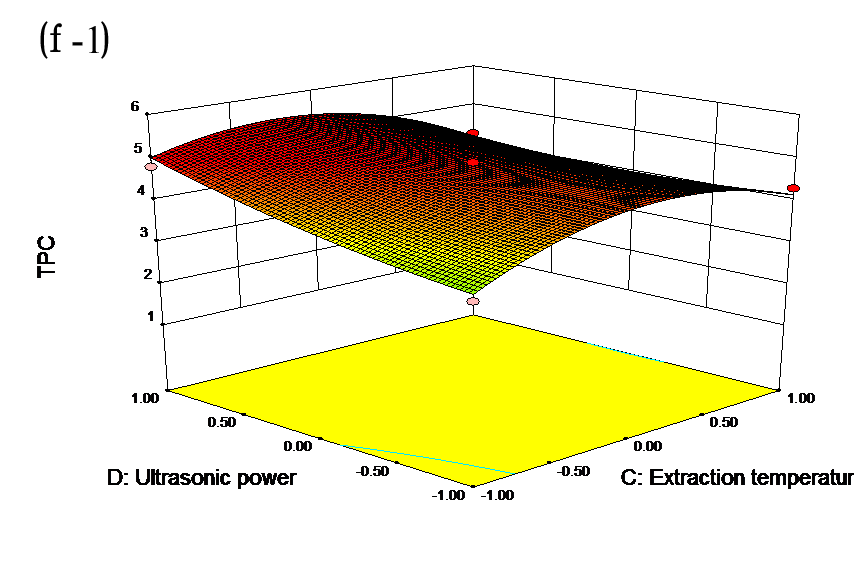
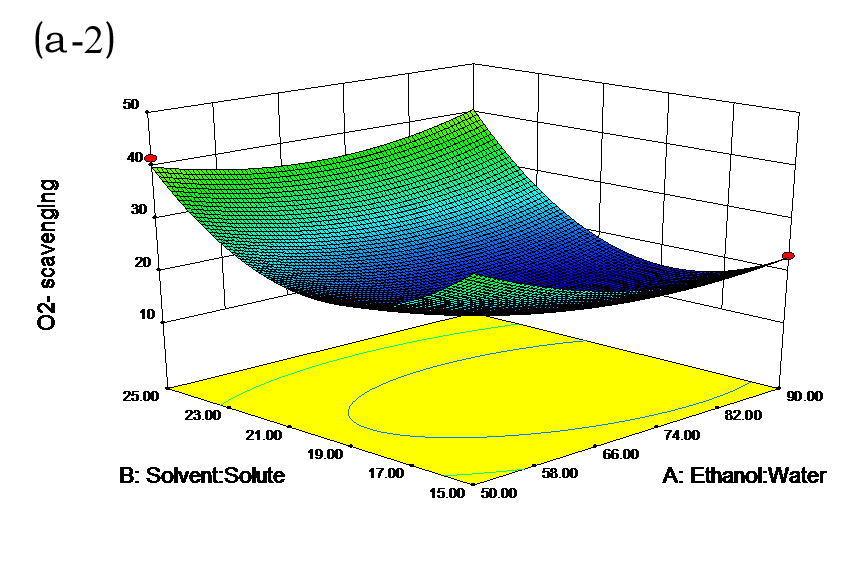
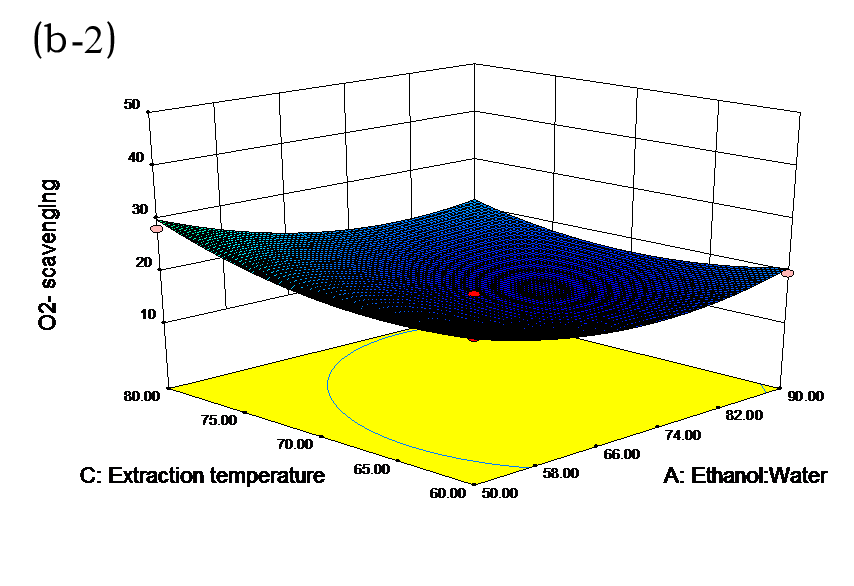
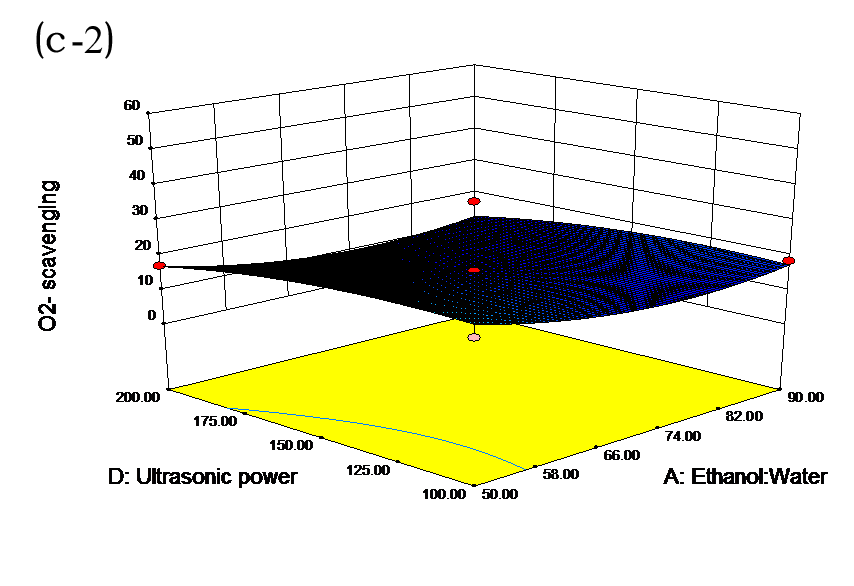
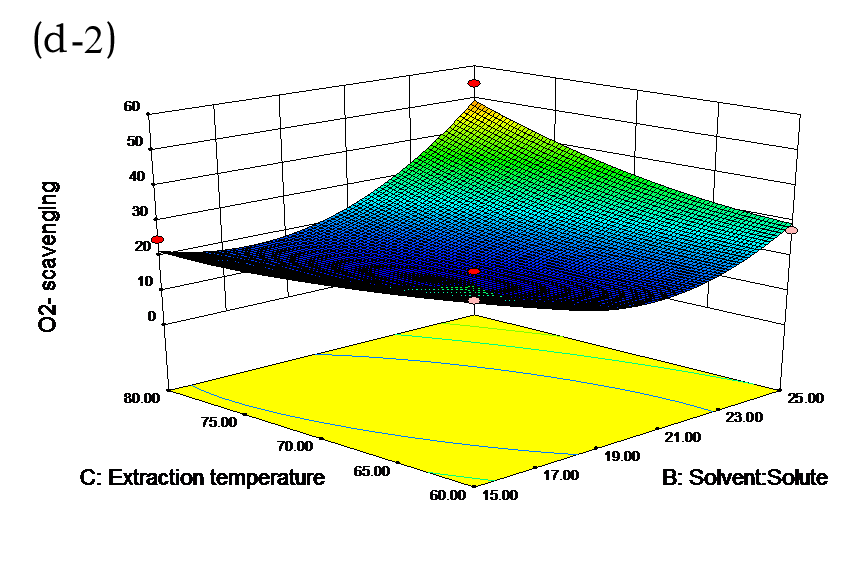
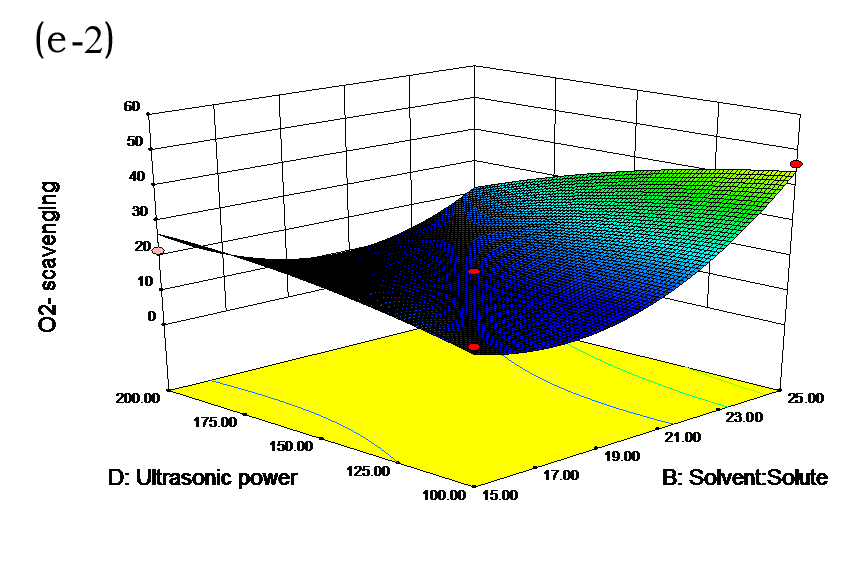
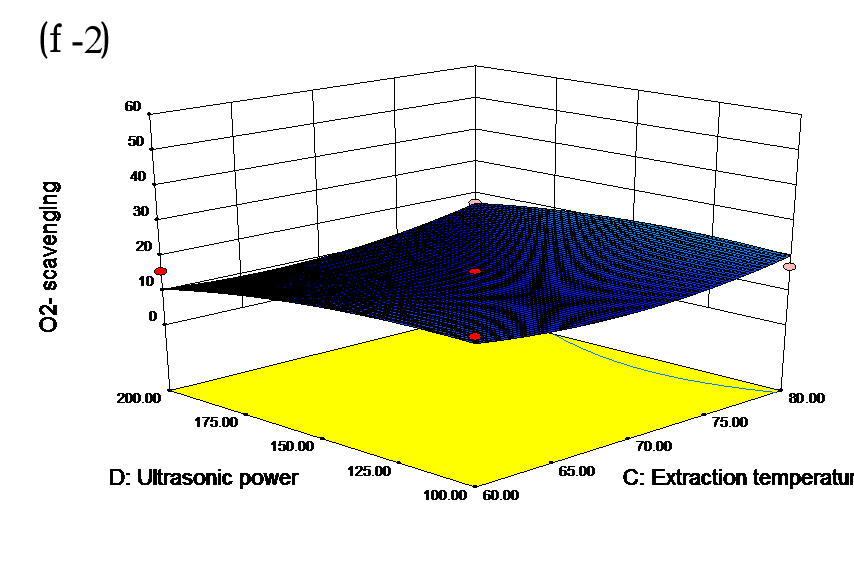
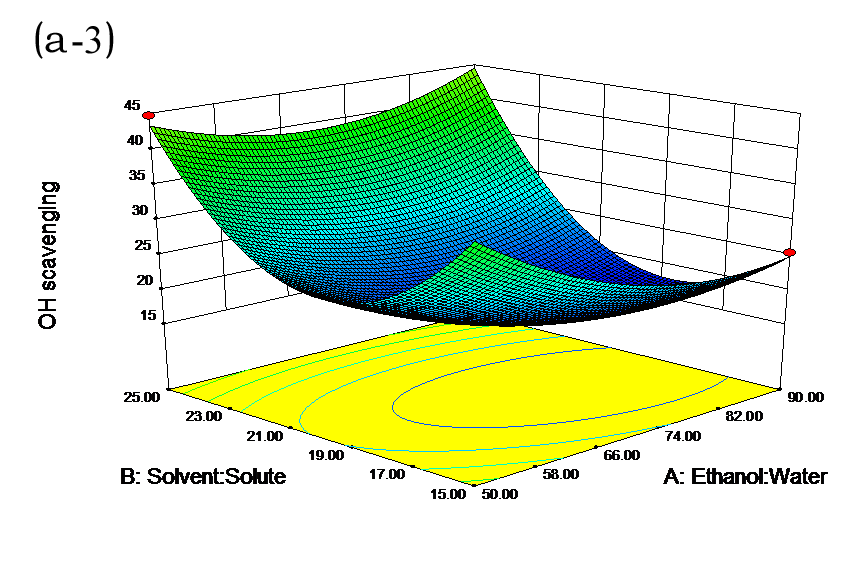
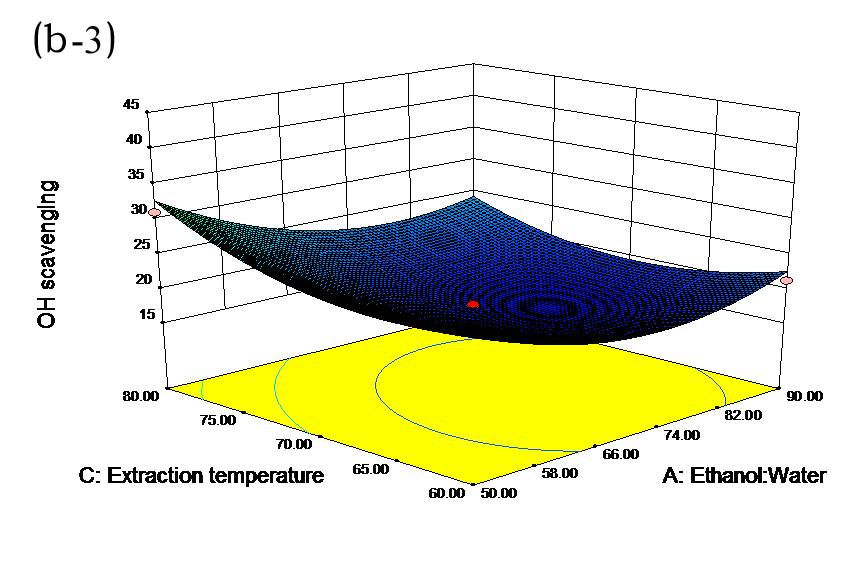
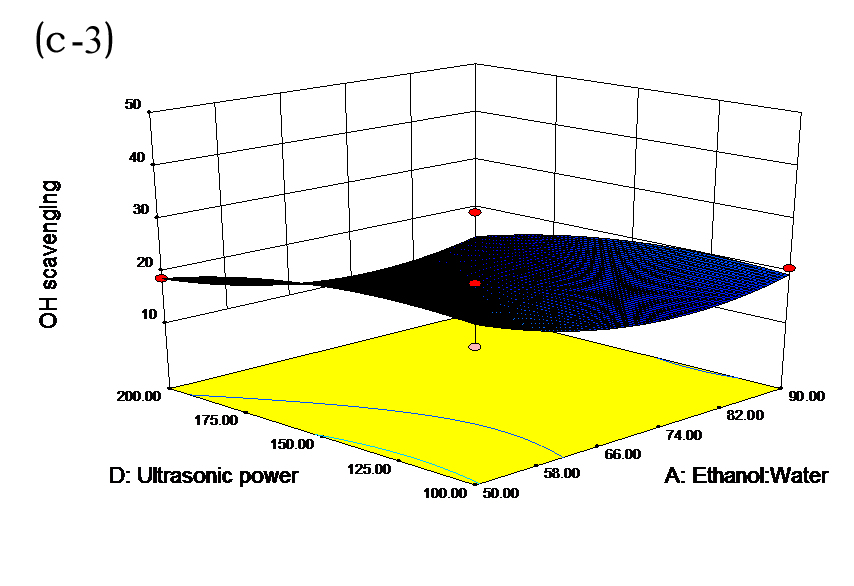
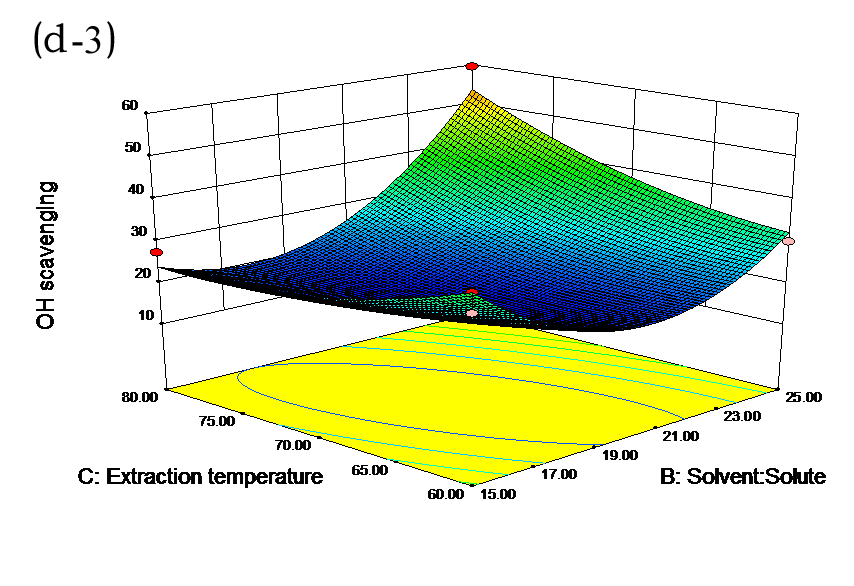
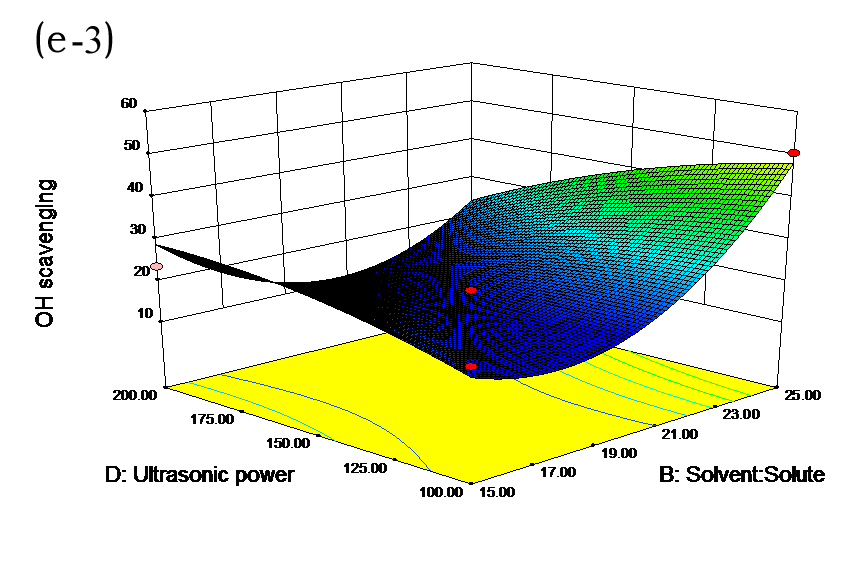
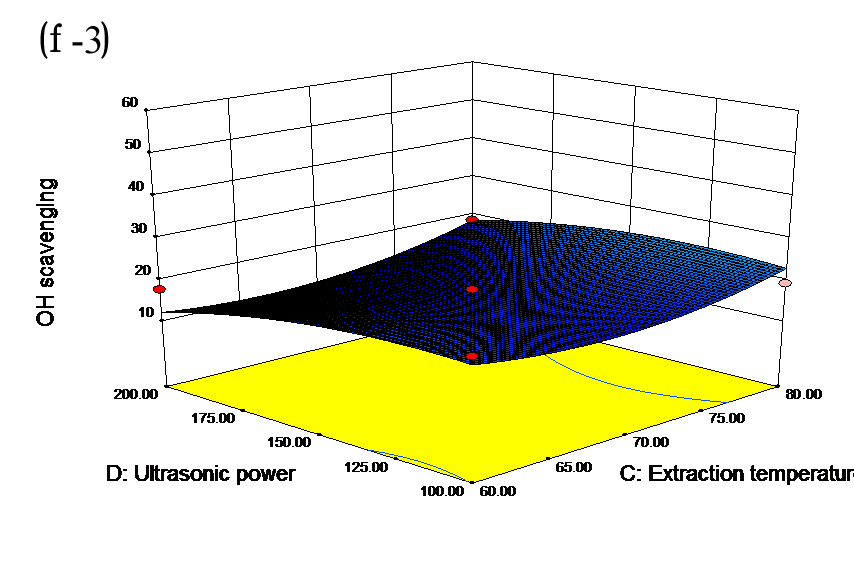
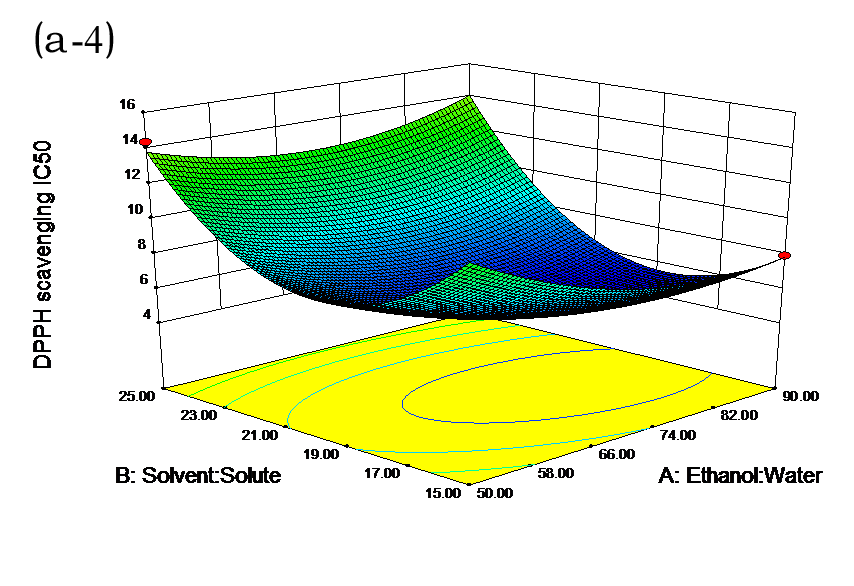
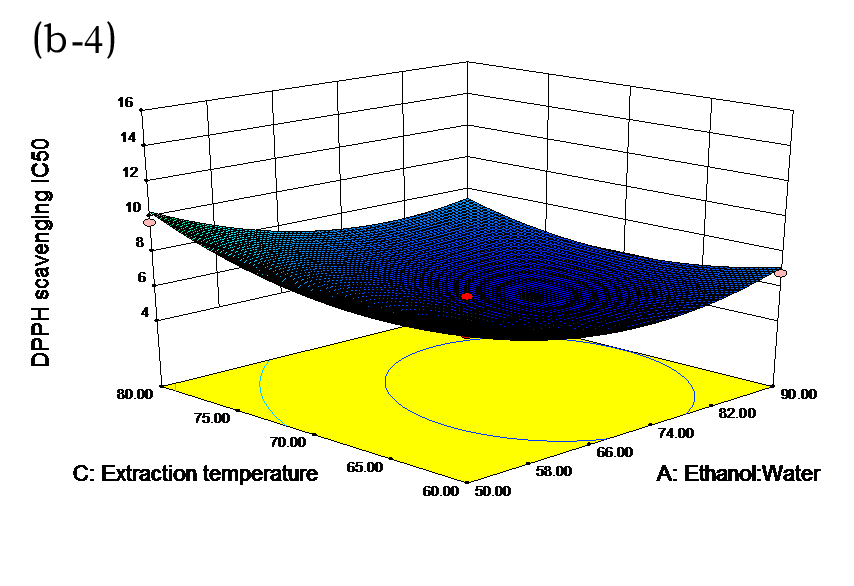
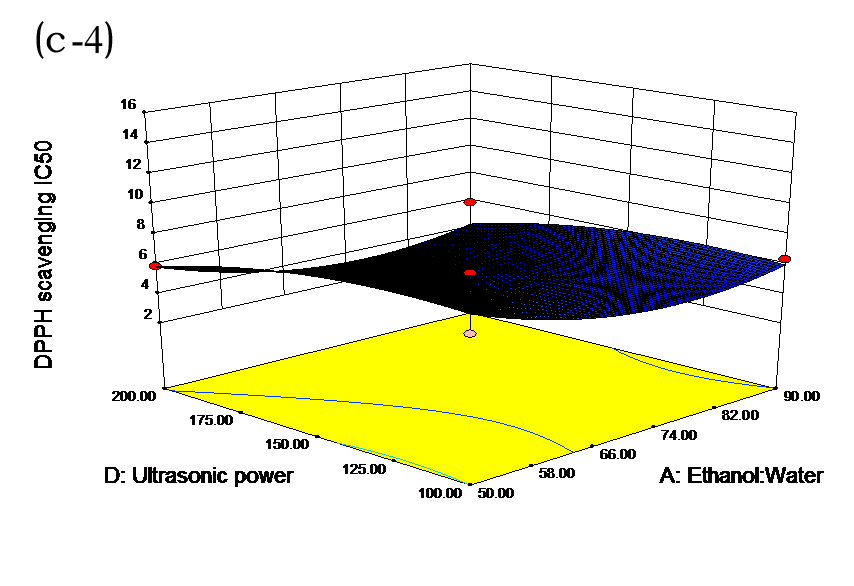
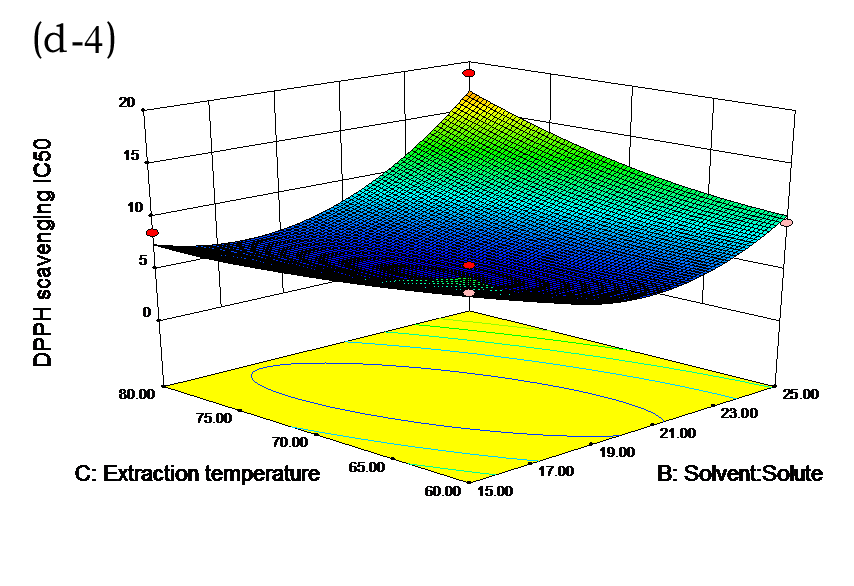
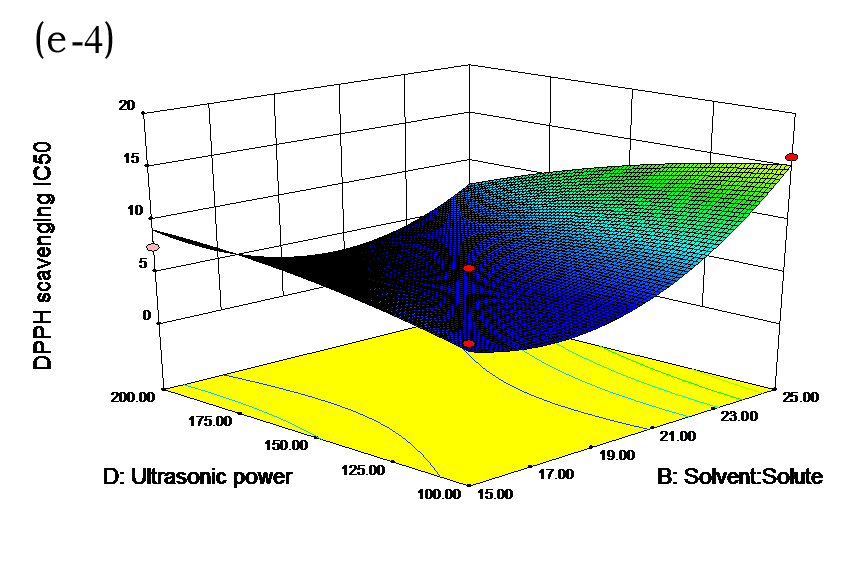
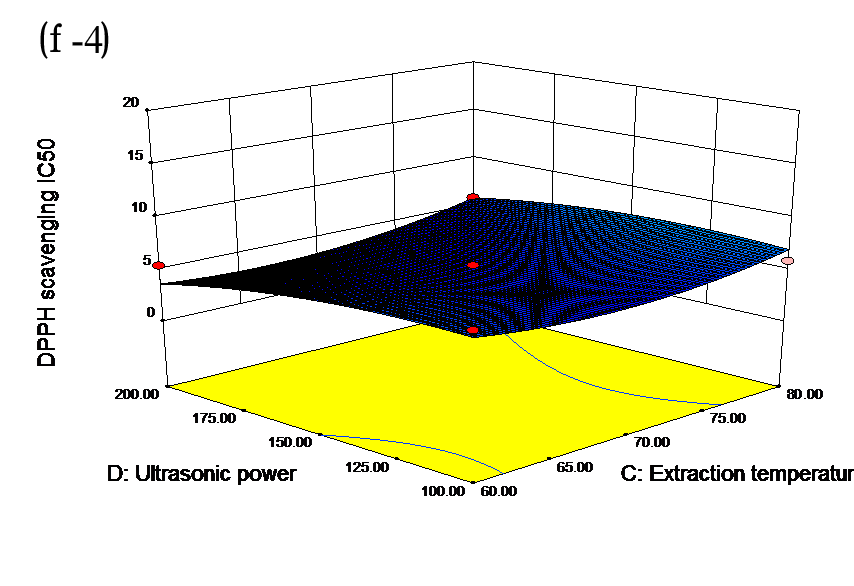
Fig. 2. Response surfaces for the interactions of independent variables on the extraction efficiency of TPC (a-1, b-1, c-1, d-1, e-1, and f-1), O2− scavenging activity (a-2, b-2, c-2, d-2, e-2, and f-2), ·OH scavenging activity (a-3, b-3, c-3, d-3, e-3, and f-3), and DPPH scavenging activity (a-4, b-4, c-4, d-4, e-4, and f-4)
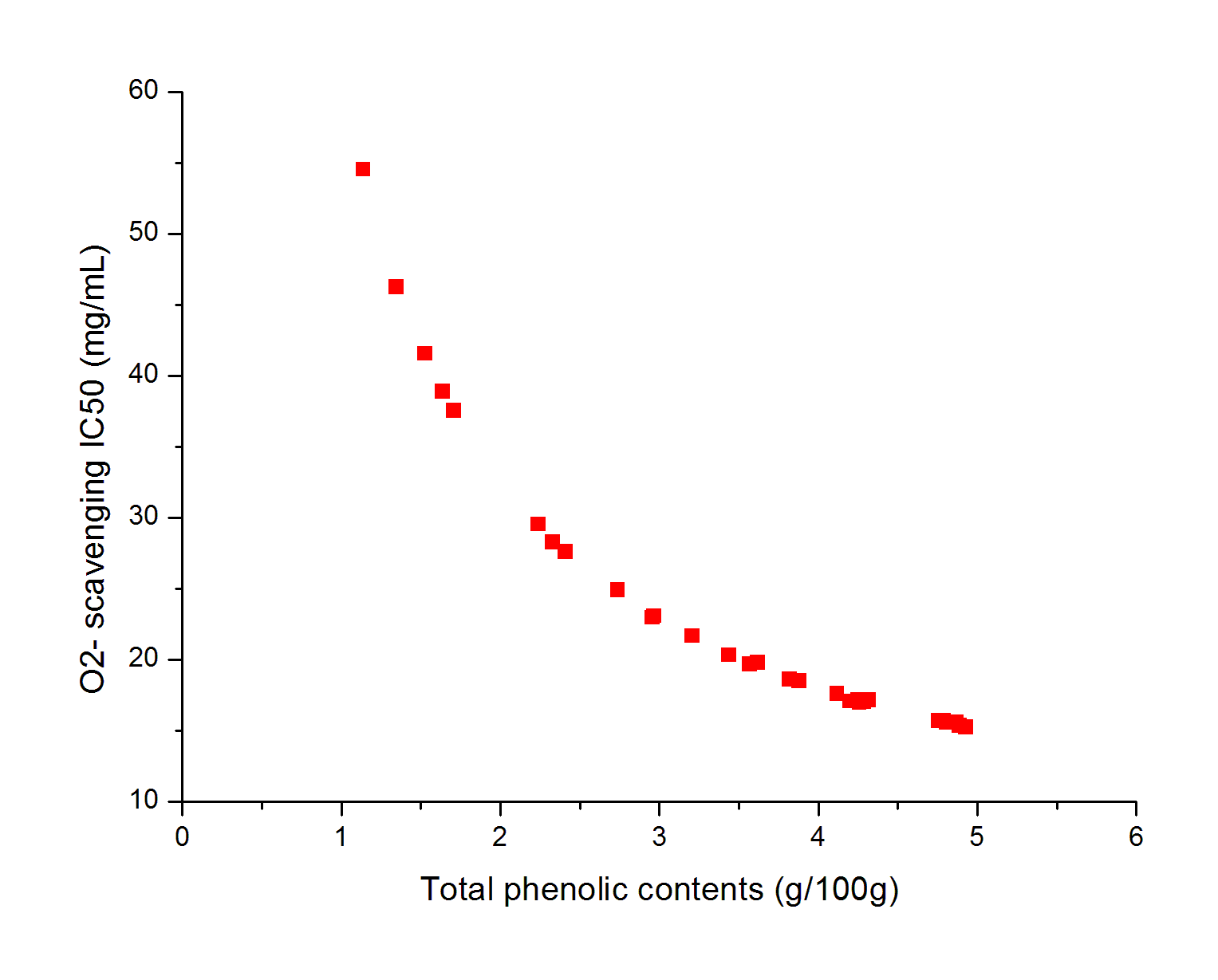
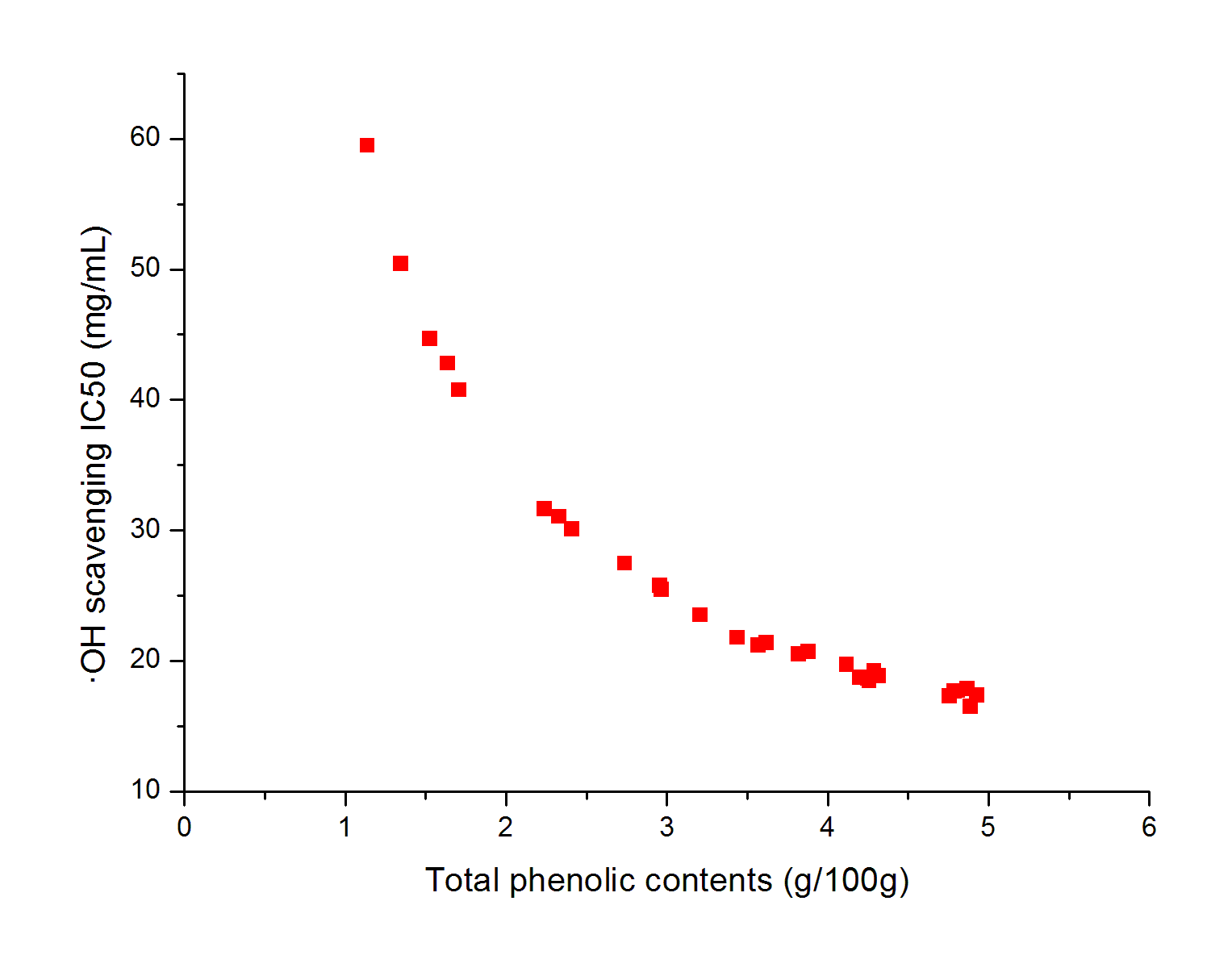
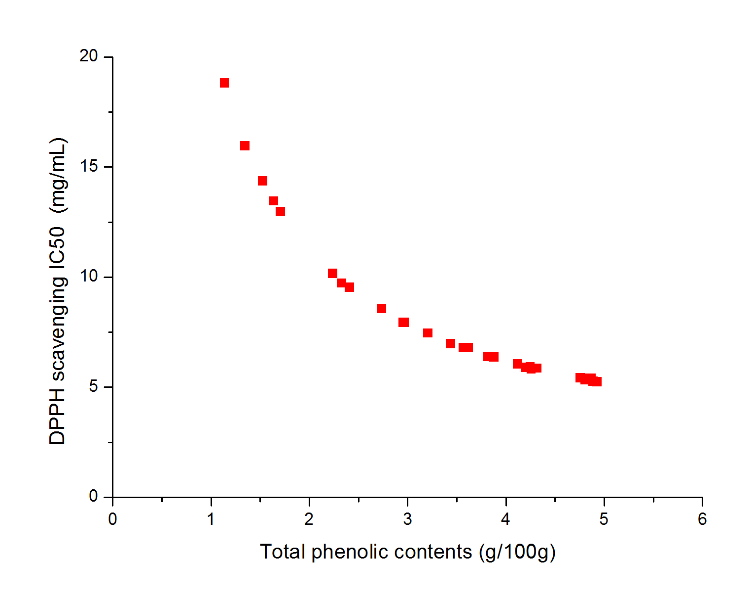
Fig. 3. Linear regression plots between total polyphenols content (TPC) and IC50 values for O2− scavenging, ·OH scavenging, and DPPH radical scavenging
Verification Tests
The optimum microwave-assisted extraction conditions for the response variables from TPC obtained by RSM are presented in Table 5. The verification tests were conducted under the optimum conditions (an ethanol:water ratio of 72:28, a solvent:solute ratio of 21:1, an extraction temperature of 67 °C, and an ultrasonic power of 200 W). The actual extraction efficiency was 5.11 g/100 g TPC, with IC50 values of 54.52 mg/mL for O2−, 59.47 mg/mL for ·OH, and 18.80 mg/mL for DPPH. Under these conditions, the highest TPC and radical scavenging capacity were obtained. These experimental results matched the predicted results, which indicated the polynomial models gave good correlations.
Table 5. Predicted and Experimental Values of Responses under Optimum Conditions
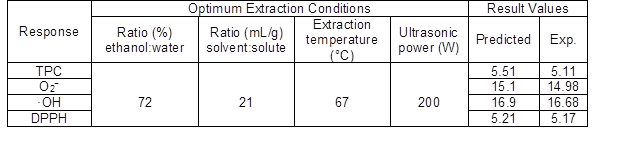
Exp., Experimental
CONCLUSIONS
- RSM was successfully employed to optimize the microwave-assisted extraction of phenolic antioxidants from Pinus elliottii
- A second-order polynomial model gave a satisfactory description of the experimental data. A set of optimized conditions for the maximum extraction of antioxidant extracts was determined.
- The results indicated good correlation between total polyphenols content and O2−, ·OH, and DPPH radical scavenging activities.
- The results of this study should prove useful in the development of the industrial extraction processes, including further studies to establish the optimal number of steps in large-scale extraction systems.
ACKNOWLEDGEMENTS
This project was supported by the National Natural Science Foundation of China (Project No. 31560192; Youth Science Funds, Project No. 31100421), the Natural Science Foundation of Hunan Province (Project No. 13JJB011), and the Science and Technology Project of the Key Laboratory for Ecotourism of Hunan Province (Project No. JSSTLY1516).
REFERENCES CITED
Anissi, J., E., El Hassouni, M., Ouardaoui, A., and Sendide, K. (2014). “A comparative study of the antioxidant scavenging activity of green tea, black tea and coffee extracts: A kinetic approach,” Food Chemistry 150, 438-447. DOI: 10.1016/j.foodchem.2013.11.009
Balabani, A., Hadjipavlou-Litina, D. J., Litinas, K. E., Mainou, M., Tsironi, C.-C., and Vronteli, A. (2011). “Synthesis and biological evaluation of (2,5-dihydro-1H-pyrrol-1-yl)-2H-chromen-2-ones as free radical scavengers,” European Journal of Medicinal Chemistry 46(12), 5894-5901. DOI: 10.1016/j.ejmech.2011.09.053
Caetano da Silva, S. D., Mendes de Souza, M. G., Oliveira Cardoso, M. J., da Silva Moraes, T., Ambrósio, S. R., Sola Veneziani, R. C., and Martins, C. H. G. (2014). “Antibacterial activity of Pinus elliottii against anaerobic bacteria present in primary endodontic infections,” Anaerobe 30, 146-152. DOI: 10.1016/j.anaerobe.2014.09.013
Chaudhary, A. K., Ahmad, S., and Mazumder, A. (2014). “Protective effect of Cedrus deodara and Pinus roxburghii on experimentally induced gastric ulcers in rat,” International Journal of Pharmacy and Pharmaceutical Sciences 6(4), 587-591
Chen, Z., Yu, L., Wang, X., Gu, Z., and Beta, T. (2016). “Changes of phenolic profiles and antioxidant activity in canary seed (Phalaris canariensis L.) during germination,” Food Chemistry 194, 608-618. DOI: 10.1016/j.foodchem.2015.08.060
Gramza-Michałowska, A., Kobus-Cisowska, J., Kmiecik, D., Korczak, J., Helak, B., Dziedzic, K., and Górecka, D. (2016). “Antioxidative potential, nutritional value and sensory profiles of confectionery fortified with green and yellow tea leaves (Camellia sinensis),” Food Chemistry 211, 448-454. DOI: 10.1016/j.foodchem.2016.05.048
Hussain, A. I., Chatha, S. A., Noor, S., Khan, Z. A., Arshad, M. U., Rathore, H. A., and Sattar, M. Z. (2012). “Effect of extraction techniques and solvent systems on the extraction of antioxidant components from peanut (Arachis hypogaea L.) hulls,” Food Analytical Methods 5(4), 890-896.
Kim, K.-Y., and Chung, H.-J. (2000). “Flavor compounds of pine sprout tea and pine needle tea,” Journal of Agricultural and Food Chemistry 48(4), 1269-1272.
Mahajan, D., Bhat, Z. F., and Kumar, S. (2016). “Pine needles (Cedrus deodara (Roxb.) Loud.) extract as a novel preservative in cheese,” Food Packaging and Shelf Life 7, 20-25. DOI: 10.1016/j.fpsl.2016.01.001
Mandawad, G. G., Dawane, B. S., Beedkar, S. D., Khobragade, C. N., and Yemul, O. S. (2013). “Trisubstituted thiophene analogues of 1-thiazolyl-2-pyrazoline, super oxidase inhibitors and free radical scavengers,” Bioorganic & Medicinal Chemistry 21(1), 365-372. DOI: 10.1016/j.bmc.2012.09.060
Monroy, Y. M., Rodrigues, R. A. F., Sartoratto, A., and Cabral, F. A. (2016). “Optimization of the extraction of phenolic compounds from purple corn cob (Zea mays L.) by sequential extraction using supercritical carbon dioxide, ethanol and water as solvents,” The Journal of Supercritical Fluids 116, 10-19. DOI: 10.1016/j.supflu.2016.04.011
Nakai, M., Harada, M., Nakahara, K., Akimoto, K., Shibata, H., Miki, W., and Kiso, Y. (2003). “Novel antioxidative metabolites in rat liver with ingested sesamin,” Journal of Agricultural and Food Chemistry 51(6), 1666-1670.10.1021/jf0258961
Nunes, S., Santos, C., Moutinho-Pereira, J., Correia, C., Oliveira, H., Ferreira de Oliveira, J. M., Pereira, V. T., Almeida, T., Marum, L., and Dias, M. C. (2016). “Physiological characterization and true-to-typeness evaluation of in vitro and ex vitro seedlings of Pinus elliottii: A contribution to breeding programs,” Plant Physiology and Biochemistry 107, 222-227. DOI: 10.1016/j.plaphy.2016.05.039
Parker, K. C., Jensen, C., and Parker, A. J. (2014). “The growth response of slash pine (Pinus elliottii) to climate in the Georgia coastal plain,” Dendrochronologia 32(2), 127-136. DOI: 10.1016/j.dendro.2014.03.003
Pinela, J., Prieto, M.A., Barreiro, M.F., Carvalho, A.M., Oliveira, M.B.P.P., Vázquez, J.A., Ferreira, I.C.F.R. (2016). “Optimization of microwave-assisted extraction of hydrophilic and lipophilic antioxidants from a surplus tomato crop by response surface methodology,” Food and Bioproducts Processing, 98, 283-298. DOI: 10.1016/j.fbp.2016.02.002
Rashidinejad, A., Birch, E. J., and Everett, D. W. (2016). “Antioxidant activity and recovery of green tea catechins in full-fat cheese following gastrointestinal simulated digestion,” Journal of Food Composition and Analysis 48, 13-24. DOI: 10.1016/j.jfca.2016.02.004
Ratola, N., Amigo, J. M., and Alves, A. (2010). “Comprehensive assessment of pine needles as bioindicators of PAHs using multivariate analysis. The importance of temporal trends,” Chemosphere 81(11), 1517-1525. DOI: 10.1016/j.chemosphere.2010.08.031
Santos, K. A., Klein, E. J., Gazim, Z. C., Gonçalves, J. E., Cardozo-Filho, L., Corazza, M. L., and da Silva, E. A. (2016). “Wood and industrial residue of candeia (Eremanthus erythropappus): Supercritical CO2 oil extraction, composition, antioxidant activity and mathematical modeling,” The Journal of Supercritical Fluids 114, 1-8. DOI: 10.1016/j.supflu.2016.02.015
Singh, L. K., Chaudhary, G., Majumder, C., and Ghosh, S. (2011). “Utilization of hemicellulosic fraction of lignocellulosic biomaterial for bioethanol production,” Advances in Applied Science Research 2(5), 508-521.
Singh, P., Tripathi, K., Yadav, R., and Yadav, K. (2015). “Devadaru (Cedrus deodara (Roxb.) Loud.): A critical review on the medicinal plant,” International Journal of Ayurveda and Pharma Research 2(1), 1-10.
Susaeta, A., Peter, G. F., Hodges, A. W., and Carter, D. R. (2014). “Oleoresin tapping of planted slash pine (Pinus elliottii Engelm. var. elliottii) adds value and management flexibility to landowners in the southern United States,” Biomass and Bioenergy 68, 55-61. DOI: 10.1016/j.biombioe.2014.06.003
Valavanidis, A., Nisiotou, C., Papageorgiou, Y., Kremli, I., Satravelas, N., Zinieris, N., and Zygalaki, H. (2004). “Comparison of the radical scavenging potential of polar and lipidic fractions of olive oil and other vegetable oils under normal conditions and after thermal treatment,” Journal of Agricultural and Food Chemistry 52(8), 2358-2365. DOI: 10.1021/jf030491h
van Drooge, B. L., Garriga, G., and Grimalt, J. O. (2014). “Polycyclic aromatic hydrocarbons in pine needles (Pinus halepensis) along a spatial gradient between a traffic intensive urban area (Barcelona) and a nearby natural park,” Atmospheric Pollution Research 5(3), 398-403. DOI: 10.5094/APR.2014.046
Xu, J.-K., Li, M.-F., and Sun, R.-C. (2015a). “Identifying the impact of ultrasound-assisted extraction on polysaccharides and natural antioxidants from Eucommia ulmoides Oliver,” Process Biochemistry 50(3), 473-481. DOI: 10.1016/j.procbio.2014.12.021
Xu, J., Wang, W., Liang, H., Zhang, Q., Li, Q. (2015b). “Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology,” Industrial Crops and Products, 76, 487-493. DOI: 10.1016/j.indcrop.2015.07.025
Zeng, W. C., Jia, L. R., Zhang, Y., Cen, J. Q., Chen, X., Gao, H., Feng, S., and Huang, Y. N. (2011). “Antibrowning and antimicrobial activities of the water soluble extract from pine needles of Cedrus deodara,” Journal of food science 76(2), C318-C323
Zeng, W. C., He, Q., Sun, Q., Zhong, K., and Gao, H. (2012a). “Antibacterial activity of water-soluble extract from pine needles of Cedrus deodara,” International Journal of Food Microbiology 153(1–2), 78-84. DOI: 10.1016/j.ijfoodmicro.2011.10.019
Zeng, W. C., Zhang, Z., Gao, H., Jia, L. R., and He, Q. (2012b). “Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara),” Journal of food science 77(7), C824-C829
Zhai, L., Jokela, E. J., Gezan, S. A., and Vogel, J. G. (2015). “Family, environment and silviculture effects in pure- and mixed-family stands of loblolly (Pinus taeda L.) and slash (P. elliottii Engelm. var. elliotttii) pine,” Forest Ecology and Management 337, 28-40. DOI: 10.1016/j.foreco.2014.10.030
Zhang, Q., Zhu, M., Zhang, J., and Su, Y. (2014). “Improved on-line high performance liquid chromatography method for detection of antioxidants in Eucommia ulmoides Oliver flower,” Journal of Bioscience and Bioengineering 118(1), 45-49. DOI: 10.1016/j.jbiosc.2013.12.009
Zhang, Y., Zhang, H., Wang, F., Yang, D., Ding, K., and Fan, J. (2015). “The ethanol extract of Eucommia ulmoides Oliv. leaves inhibits disaccharidase and glucose transport in Caco-2 cells,” Journal of Ethnopharmacology 163, 99-105. DOI: 10.1016/j.jep.2015.01.015
Zhang, Z., Yang, T., Mi, N., Wang, Y., Li, G., Wang, L., and Xie, Y. (2016). “Antifungal activity of monoterpenes against wood white-rot fungi,” International Biodeterioration & Biodegradation 106, 157-160. DOI: 10.1016/j.ibiod.2015.10.018
Zhao, G.-R., Zhang, H.-M., Ye, T.-X., Xiang, Z.-J., Yuan, Y.-J., Guo, Z.-X., and Zhao, L.-B. (2008). “Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B,” Food and Chemical Toxicology 46(1), 73-81. DOI: 10.1016/j.fct.2007.06.034
Zhu, C.-H., Lei, Z.-L., and Luo, Y.-P. (2015). “Studies on antioxidative activities of methanol extract from Murraya paniculata,” Food Science and Human Wellness 4(3), 108-114. DOI: 10.1016/j.fshw.2015.07.001
Article submitted: August 1, 2016; Peer review completed: October 2, 2016; Revised version received and accepted: November 14, 2016; Published: November 22, 2016.
DOI: 10.15376/biores.12.1.478-494
