Abstract
Forming an adhesive joint between two wet cellulose surfaces before a drying step is important when manufacturing paper, foams, aerogels, other novel materials from wood pulp fibers, and various types of nanocellulose. This paper reviews the literature with an emphasis on the role of adhesive polymers on wet cellulose adhesion. Linkages between the organization of adhesives between the bonded surfaces and the strength of joints are emphasized. Relevant adhesion results from the surface forces apparatus, colloidal probe atomic force microscopy, paper wet-web strength, and wet-peeling of laminated regenerated cellulose membranes are considered.
Download PDF
Full Article
Polymers that Strengthen Never-dried Joints between Wet Cellulose Surfaces – A Review
Robert H. Pelton,* Dong Yang, and Emil Gustafsson
Forming an adhesive joint between two wet cellulose surfaces before a drying step is important when manufacturing paper, foams, aerogels, other novel materials from wood pulp fibers, and various types of nanocellulose. This paper reviews the literature with an emphasis on the role of adhesive polymers on wet cellulose adhesion. Linkages between the organization of adhesives between the bonded surfaces and the strength of joints are emphasized. Relevant adhesion results from the surface forces apparatus, colloidal probe atomic force microscopy, paper wet-web strength, and wet-peeling of laminated regenerated cellulose membranes are considered.
Keywords: Adhesion; Cellulose; Nanocellulose; Papermaking; Wet-web strength
Contact information: Department of Chemical Engineering, McMaster University, Hamilton, Ontario, Canada, *Corresponding author: Peltonrh@mcmaster.ca
INTRODUCTION
Objects formed from cellulose fibers, cellulose nanocrystals (CNC), and cellulose nanofibrils (CNF) all share the same limitation. They are weak when first formed, until most of the water has been removed. The relevant property is the never-dried strength or green strength of wet cellulose joints between contacting surfaces in water. A low never-dried strength causes a number of manufacturing challenges. For example, 3D-printed CNC inks must have a sufficient yield stress to maintain their shape after printing (Siqueira et al. 2017). Similarly, templated ultralow-density CNF foams must be dried by expensive processes, such as critical point drying, freeze-drying (Cervin et al. 2012), freeze-casting (Wicklein et al. 2015), and solvent exchange (Jin et al. 2004), to prevent damage from capillary forces during drying. Finally, when wet paper is formed by filtration of dilute fiber suspensions, the never-dried paper strength, called the wet-web strength, can limit the speed and efficiency of the papermaking process (Pikulik 1997; Belle and Odermatt 2016). The unifying feature of these examples is the difficulty in forming strong joints when cellulose surfaces are pushed together in water.
The catastrophic loss of strength when many paper products are exposed to water is a common experience. In contrast, paper coffee filters and kitchen towels are sufficiently strong because the cellulose fiber network has been strengthened with heat curing polymers, called wet-strength resins (Wågberg and Björklund 1993; Epsy 1995). Although the chemistry varies, wet-strength resins all employ curing reactions that crosslink the resins and produce covalent grafts to wood pulp fiber surfaces, which strengthen the fiber-fiber joints. Most of the curing reactions require water removal and are promoted by the elevated temperatures associated with drying on paper machines. For nearly 100 years, these resins have produced paper products that are strong enough to survive exposure to water for short periods. In contrast, there are not any commercial polymeric products that have been applied to increase the strength of cellulose-cellulose joints as they are formed in water. Nevertheless, there are published reports that suggest such green strength polymers are possible.
This paper reviews the literature on never-dried cellulose adhesion with an emphasis on the roles of adhesive polymers. Most of the old literature focused on paper wet-web strength, whereas the recent literature has included many AFM-CP studies of model cellulose surfaces. The goal is to propose design rules for adhesive polymers that enhance the never-dried adhesion between wet cellulose surfaces. Although this is believed to be the first review of polymers for never-dried adhesion between cellulose surfaces, excellent texts and reviews in relating areas are acknowledged, which includes, but is not limited to the interactions of polyelectrolyte-coated surfaces (Claesson et al. 2005), adhesion (Kendall 2001), soft adhesives (de Gennes 1996), wet adhesion between once-dried cellulose surfaces (Lindström et al. 2005), and wet-web paper strength (Retulainen and Salminen 2009).
Contributions to Wet Adhesion
This section reviews the interactions that can contribute to the adhesion between cellulose surfaces in water. Van der Waals forces are always present and are attractive between like bodies in water. Colloidal probe atomic force microscopy (AFM-CP) has been used to measure the attractive van der Waals forces between cellulose surfaces in water (Notley et al. 2004). A combined Hamaker constant of 3.5 x 10-21 J has been reported, which is similar to silica or polystyrene interactions in water. In other words, van der Waals attractive forces are substantial. Nevertheless, wet adhesion forces between cellulose surfaces in water are weak. The usual explanations include electrostatic repulsion, surface roughness preventing close approach, and the presence of a hydrated surface layer causing steric repulsion. Whatever the reason, van der Waals forces are not sufficient to promote adhesion between wet cellulose surfaces.
Covalent crosslinking between strength-enhancing polymer chains and covalent grafting to cellulose surfaces are important contributors to never-dried wet adhesion. Alcohol and carboxyl groups, the main functional groups on cellulose surfaces, are not very reactive in water at low temperature. Therefore to achieve grafting to cellulose, strength polymers must have reactive groups. However, very reactive groups such as isocyanides also react with water. Most of the published work demonstrating never-dried wet adhesion involves aldehyde chemistry. The aldehyde functional group can be present either on cellulose, a result of oxidation, or as part of the adhesive polymer. Cationic aldehyde starch (CAS) and cationic glyoxalated polyacrylamide (GCPAM) are examples of commercial, water-soluble aldehyde containing polymers. All polymer abbreviations are defined in the Appendix.
Scheme 1 shows the covalent linkages formed when aldehydes react with alcohols and with other aldehydes. Hemiacetal and acetal formation are catalyzed by acid or base. However these linkages are more stable in acidic conditions (Espy 1995). The labile nature of hemiacetal linkages in water results in “temporary” wet strength when aldehyde chemistry is employed in wet-strength resins. Also shown in Scheme 1 is the aldol condensation, a crosslinking reaction between two aldehydes. The aldol product has been proposed by Young (1978) to contribute to wet strengthening.

Scheme 1. Aldehyde coupling by the aldol condensation reaction and aldehyde reactions with alcohols to form hemiacetal and acetal linkages
The reaction products of polyvinylamine with aldehyde groups on an oxidized cellulose surface are shown in Scheme 2. Although imine linkages (also called Schiff bases) are also hydrolytically unstable, the authors’ adhesion results suggest that polyvinylamine (PVAm) forms stable bonds with aldehyde groups in water, and these are probably aminal linkages (Yang et al. 2018b).
Scheme 2. Grafting reactions for PVAm on oxidize cellulose. Figure adapted from Yang et al. (2018b).
The final type of interaction that contributes to never-dried wet adhesion is polyelectrolyte complex formation. When a cationic water-soluble polymer solution is mixed with an anionic polymer, polyelectrolyte complexes (PECs) form and can be present as soluble complexes, colloidal hydrogel particles, or as a macroscopic precipitate. If the oppositely charged polymers are attached to surfaces (i.e. grafted), the resulting complexes form an adhesive junction between the two surfaces. Examples will be given in subsequent sections,
Measuring Wet Adhesion
The cellulose never-dried adhesion literature is dominated by results from four techniques: the surface forces apparatus (SFA), atomic force microscopy with colloidal probes (AFM-CP), wet-peeling, and tensile strength measurements of never-dried wet fiber webs. The first two provide measurements of both the work to push the surfaces into contact (lamination) and to pull off. In contrast, wet-peeling and wet tensile experiments yield macroscopic measurements of the work required to break bonds. Each technique will be briefly described.
Surface Forces Apparatus
The SFA technique was developed by Israelachvili (1991) and measures both the forces as the surfaces move into contact (i.e., the lamination forces) and the adhesive forces as the surfaces are separated. The substrates forming the joint are atomically smooth mica sheets that are coated with thin regenerated cellulose films. The adhesive polyelectrolytes are applied by adsorption application. Achieving thin, atomically smooth cellulose substrates is challenging and hence only a few publications have involved wet cellulose (Kontturi et al. 2006).
Colloidal Probe Atomic Force Microscopy
First reported by Ducker et al. (1992), AFM-CP has been applied by a number of researchers to measure cellulose-cellulose wet adhesion and friction. In this technique, a micron-sized cellulose sphere or a nanocellulose covered silica sphere, glued to an AFM cantilever, replaces the usual sharp tip. Atomic force microscopy measures the force to approach a planar cellulose film (the lamination step) and adhesion forces required to separate the surfaces after contact. Adsorption application is used to apply the adhesive. A cellulose sphere (i.e., colloidal probe) and planar cellulose substrate can be treated separately with polyelectrolyte.
It is challenging to relate AFM-CP pull-off forces, which are usually in the of order mN/m, to macroscopic wet-peel or paper wet-web strength. A direct comparison is given below, showing that the work of joint rupture, as determined by AFM-CP, is 1000 times less than the wet-peel work.
Wet-peeling
Following the lead of McLaren (1948), an adhesion measurement was developed whereby two wet regenerated cellulose membranes are treated with adhesive and pressed together (i.e., laminated) with a contact area of 5 cm × 2 cm. The wet-peel strength is determined as the force required to separate the laminated membranes by 90° peeling on a mechanical testing machine. Wet-peeling results were first published by Kurosu and Pelton (2004) and a review of the technique highlighting the important experimental aspects was recently published by Yang et al. (2018a). Using wet-peeling, it is possible to employ tests of all of the joint compositions. Furthermore, wet-peel experiments without a drying step result in never-dried wet adhesion measurements that are reproducible and sensitive to adhesive chemistry.
Paper Wet-web Strength
The papermaking technology literature usually presents wet-web tensile strengths plotted as a function of the paper solids content, and the resulting curves can be fitted to power laws (Shallhorn 2002). There is a substantial amount of literature that describes the influence of pulp types (Seth 1995), fillers, and other papermaking variables on the wet-web strength. These factors are not reviewed here because they were recently summarized in a review by Belle and Odermatt (2016).
There are no adhesive interactions between wood pulp fibers in water in the absence of added polymers because all fiber-fiber interactions (electrostatic and/or steric) are repulsive. In dilute aqueous suspension, flexible wood pulp fibers form flocs because of mechanical entanglement (Celzard et al. 2009). The classic explanation of wet-web strength invokes capillary forces pulling fibers into contact. Page (1993) modified his paper tensile strength model to account for capillary forces and was able to explain the influence of fiber length and coarseness on wet-web strength (Page 1993).
Tejado and van de Ven (2010) critically evaluated the classic theories of paper wet-web strength and proposed that wet-web strength is a combination of entanglement friction plus a fiber-fiber adhesion component at higher solids contents. Entanglement involves the work required to pull apart mechanically entwined, long thin fiber. Thus it depends upon fiber length, flexibility, and friction coefficient, whereas the entanglement contribution is not particularly sensitive to the solids content.
Figure 1 shows a more generic version of a figure by Tejado and van de Ven (2010). Capillary forces will disappear when the free water is gone, whereas entanglement forces may increase with solids content. The detailed nature of the non-adhesive contributions (entanglement vs. capillary forces vs. something else) remain an open issue. The focus of this review is the potential to increase the adhesive contribution with polymers, shifting the fiber-fiber adhesion curve towards the upper left of Fig. 1.
Fig. 1. Contributions to the wet-web strength
The interpretation of wet-web mechanical properties in the presence of polymeric adhesives is complicated by the fact that polyelectrolytes can enhance drainage, which results in a higher solids content and changes the sheet structure by fines deposition and fiber flocculation. Therefore, polymers that improve the wet-web strength do not necessarily influence fiber-fiber adhesion or friction. Trout (1951) emphasized the need to compare wet-web properties as a function of the solids content.
This review examines the literature that describes never-dried cellulose-cellulose joints. The never-dried cellulose adhesion literature is divided into two sections: results involving a single adhesive and those employing two or more adhesives, usually applied in multiple steps.
Wet Joints employing a Single Adhesive
Joint Structures – One Adhesive Polymer
An idealized adhesive joint consists of a layer of adhesive sandwiched between two substrate surfaces, forming a joint. In general, joint fabrication consists of three steps: 1) applying adhesive to one or both substrate surfaces, 2) lamination by forcing the surfaces into contact, and 3) curing and/or drying the joint. In the case of never-dried wet cellulose joints, only the adhesive application and lamination steps are relevant because the joints are not dried.
The following sections will show that never-dried adhesion is extremely sensitive to the quantity and distribution of adhesive in the cellulose-cellulose joints. There is a range of possible joint structures, along with a nomenclature scheme. In papermaking, the adhesive application is achieved by adsorption onto fibers in the wet-end of the paper machine, and fiber surfaces are usually coated with a saturated monolayer. When two coated fiber surfaces come together, they form an αα joint (Fig. 2). The αα joint is the most important type because it is the easiest to manufacture. The polymer content in αα joints is denoted as Γsat, and is in the range of 1-10 mg/m2 for PVAm on regenerated cellulose (Yang et al. 2018b). There are few published values for saturation coverage of adsorbed on wood pulp fibers expressed as mg/m2 because the required fiber specific surface areas (m2/g) are often unknown.
Fig. 2. Schematic illustration of the types of polymer-reinforced wet cellulose-cellulose joints assembled with one type of polymer; Γ is the quantity of adhesive polymer in the joint expressed as mg of dry polymer per m2 of joint.
Joint type α is prepared by coating one cellulose surface to give a saturated monolayer of polymer, followed by lamination with an untreated cellulose surface. Although α joints can be strong, they are not easily manufactured. This will be examined further in the final discussion.
Finally, type d-Γ joints, where Γ is the coverage of dry adhesive in the joint expressed as mg/m2, can be prepared by casting cellulose fiber or particles and suspending them in an adhesive polymer solution. Because the specific gravity of most dried polymers is close to 1, Γ is also equal to the average thickness in nm of the dried adhesive film in the joints. Type d-Γ joints can range from sub-monolayer adhesive coverages to a dry adhesive layer with a thickness of hundreds of nanometers. In a later section describing two or more adhesive applications, this joint structure classification will be discussed further.
Table 1 lists publications that report never-dried wet adhesion in the presence of an individual type of polyelectrolyte adhesive.
Table 1. Summary of the Never-dried Adhesion Studies Involving Cellulose and a Single Adhesive α and αα Joints
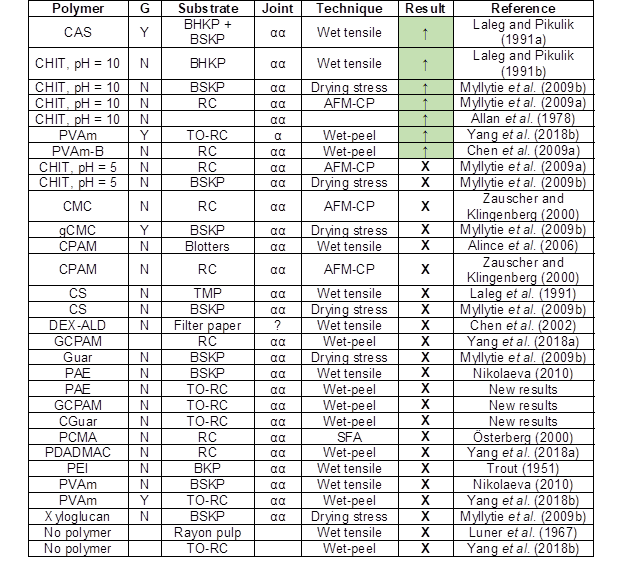
G – grafting; Y means that there is an expectation of polymer grafting to wet cellulose; N means that grafting is unlikely; ↑ – can increase never-dried wet adhesion; X – cannot increase never-dried wet adhesion; and see Table A1 for meaning of acronyms
The first column in Table 1 gives the type of strength enhancing polymer. The second column titled “G” indicates whether or not the adhesive polymer has the potential to form covalent grafts to the cellulose substrate surface in the presence of free water. The third column shows the cellulose substrate type. The next column describes the adhesive joint type (Fig. 2).
It is impossible to quantitatively compare results from such a wide range of experiments. Instead, it was simply stated if the polymer can or cannot increase never-dried wet adhesion. Only the first few entries in Table 1 correspond to polymers that cause never-dried wet adhesion. The ineffective polymers will be discussed first with a view of understanding the key mechanisms.
Ineffective Polymers
Most of the polymers listed in Table 1 are ineffective at enhancing the never-dried adhesion between wet cellulose surfaces, which raises the question: why are single polymers so ineffective? Österberg (2000) reported SFA measurements and showed that the approach of cellulose surfaces in water was repulsive when the surfaces were coated with an adsorbed layer of a linear quaternary ammonium polyelectrolyte, i.e., an αα joint. Only sub-monolayer polyelectrolyte coatings yielded adhesive interactions. Poptoshev et al. (2000) reported measurements between glass and cellulose surfaces in the presence of polyvinylamine (PVAm), a highly cationic linear polymer. They also reported that αα joints were repulsive in water with little adhesion upon retraction.
Zauscher and Klingenberg (2000) used AFM-CP to measure the approach and adhesion forces for cellulose surfaces coated with monolayers of adsorbed cationic polyacrylamide (CPAM) or carboxymethyl cellulose (CMC). The approach of cellulose surfaces saturated with either polyelectrolyte was repulsive in water. Only sub-monolayer coverages of CPAM gave some bridging adhesion. Adsorbed layers of CPAM or CMC also reduced the friction between wet cellulose surfaces (Zauscher and Klingenberg 2001). Reduced friction should decrease the entanglement contribution to the wet-web strength (Fig. 1).
Laine et al. (2000) described a process by which aqueous CMC in the presence of calcium ions at elevated temperatures was irreversibly deposited onto cellulose surfaces. The adsorbed amount of high-molecular weight CMC was 10 mg/m2 to 20 mg/m2, which corresponded to an 8-nm- to 15-nm-thick CMC film, assuming a specific surface area of bleached kraft pulp fibers of 1.37 m2/g (Lindström and O’Brien 1986). Liu et al. (2011) studied deposition onto model regenerated cellulose films at lower temperatures and reported that the dry CMC coverage was approximately 0.3 mg/m2 and that the adsorbed layer was swollen with a water content of 90% to 95%. In spite of subsequent publications (Kargl et al. 2012), the attachment mechanism is unknown. The literature suggests that CMC is firmly attached, so it was grouped with other cellulose grafted polymers and called grafted carboxymethyl cellulose (gCMC) (Laine et al. 2002). Although gCMC can provide spectacular improvements in the paper dry strength and once-dried wet strength when combined with polyamide-amine epichlorohydrin (PAE) (Laine et al. 2002), αα joints based on gCMC have no never-dried wet strength (Myllytie et al. 2009b).
An alternative to treating fibers with polymers is to modify the fiber surface properties to promote adhesion. The following discussion summarizes some of the major studies that link cellulose oxidation to the joint strength. While many studies showed an improved dry strength and once-dried wet strength, none improved the never-dried wet strength. The priming of cellulose surfaces by oxidation has a long history. The work of Luner et al. (1967) with regenerated cellulose is typical in that it showed that aldehyde groups increase the wet strength (after drying), whereas there was no evidence of an improved never-dried wet-web strength. They proposed that the enhanced once-dried wet strength with oxidized fiber-fiber joints was because of enhanced hydrogen bonding between the aldehydes and cellulose. This was an unusual suggestion because hemiacetal and acetal formation between aldehydes and alcohols is usually suggested as the mechanism for aldehyde wet strength resins. Aldol condensation reactions have also been proposed (Young 1978).
Inspired by Kitaoka et al. (1999) and the increase in nanocellulose publications, there have been reports describing TEMPO-mediated oxidation of cellulose, which generates a mixture of carboxyl and aldehyde groups on exposed surfaces (Saito and Isogai 2006). Saito and Isogai (2007) compared the once-dried wet strength of papers prepared from TEMPO-oxidized fibers with four different polymers, and came up with the following ranking: PVAm > PAE CPAM > PEI. Also, Saito and Isogai (2007) proposed specific covalent bond formation mechanisms between the various polymers and oxidized cellulose. However, none of these combinations have any wet-web strength benefits.
The never-dried wet-peel strength of TEMPO-oxidized regenerated cellulose is 2 N/m at a 55% laminate solids content (Yang et al. 2018b), which is the lowest value that can be measured. When the laminates were allowed to dry at room temperature and were then rewetted, the wet-peel force was approximately 10 N/m (Yang et al. 2018a), which is weak. Finally, the interest in nanocellulose materials has rejuvenated interest in oxidation. The recent work by Erlandsson et al. (2018) is a good example of this. They showed hemiacetal bonds formed when periodate oxidized CNF particles and were pushed together by freezing ice. In another example, Syverud et al. (2015) showed the TEMPO-oxidized CNF suspensions formed hydrogels in the presence of low molecular diamines forming shift base crosslinks. These two examples show routes to wet-adhesion without adhesive polymers.
In summary, both the nanoscale and macroscale measurements provide overwhelming evidence to support the conclusion that for αα joints formed between cellulose surfaces bearing adsorbed polyelectrolyte, work must be applied to force the surfaces together to overcome electrosteric repulsion and the cohesive interactions after contact are low because of limited interpenetration of adsorbed polymers. This is not a new conclusion, and a similar conclusion has been reached in the literature before (Alince et al. 2006). Nevertheless, some of the polymers in Table 1 were shown to be effective, and these will now be considered.
Effective Polymers
Cationic aldehyde starch
In a series of studies, paper machine trials were reported, in which 1% cationic aldehyde starch (CAS) improved the wet-web breaking length of a fine paper furnish by 18% with a 50% solids content (Solarek et al. 1987; Laleg and Pikulik 1991a, 1993a,b).
Figure 3 was adapted from the original publication of Laleg and Pikulik (1993a) and compares the never-dried wet tensile strength and solids contents for laboratory handsheets. All of the webs with solids contents below 45% were weak, whereas all of the webs with solids contents above 50% had a strength that increased with the solids content. Comparing the curves showed that the CAS resulted in stronger wet-webs. Laleg et al. (1991) proposed that the wet-web strength was improved by the formation of covalent acetal and hemiacetal linkages between the starch and fibers. This is a controversial explanation because these linkages are difficult to form in water. For example, it is shown below (Table 3) that there is no never-dried wet adhesion between oxidized cellulose membranes bearing aldehyde groups. On the other hand, once-dried joints formed with oxidized membranes give some wet adhesion (~10 N/m wet-peel), suggesting hemiacetal formation during drying Yang (2018). Although the mechanisms may be debatable, Laleg’s results have been validated by others. Retulainen and Salminen (2009) recently demonstrated that an experimental dialdehyde starch increased the wet-web strength, as measured by the extent of web stretching on a high-speed (1 m/s) laboratory web tension device.
Dextran aldehyde
The wet tensile strength properties of filter papers impregnated with dextran-aldehyde prepared by Chen et al. (2002) were similar to those of starch aldehyde produced by Laleg et al. (1991). No evidence of wet strength enhancement was observed, unless the filter papers were dried first. It was concluded that it is difficult, but not impossible, to form acetal and hemiacetal crosslinks with fiber surfaces, unless the water was removed.
Fig. 3. Influence of the CAS on the tensile strength of never-dried laboratory sheets made from a mixed bleached kraft pulp; data replotted from Laleg and Pikulik (1993a)
Chitosan
Allan et al. (1978) were among the earliest to suggest the use of chitosan strengthening of wet webs and wet paper. They established that chitosan was most effective when applied at a pH of 10 and conditions under which chitosan was not water soluble. They recognized that instead of an adsorbed monolayer from solution, phase-separated chitosan particles were depositing on the fiber surfaces. This was recognized as an advantage because it put more chitosan on the fiber surfaces without inducing fiber flocculation before sheet making.
Laleg and Pikulik (1991b) confirmed the earlier experimental results and proposed molecular-scale interactions leading to wet-web strength. In a nice example of the application of modern techniques, Myllytie et al. (2009a) used quartz crystal microbalance measurements to show that adsorbed chitosan layers shrank and became more elastic at a high pH. Using AFM-CP, they confirmed that chitosan-coated surfaces were repulsive. However, by forcing the surfaces together, a remarkable never-dried pull-off force was observed. The pull-off force of the joints increased with the compression load during their formation, and also with increasing pH. They suggested that a possible mechanism involved the presence of tacky adsorbed colloidal chitosan particles. The tacky nanoparticle mechanism is intriguing and might explain why some colloidal-sized polyelectrolyte complexes (PECs) can increase the wet-web strength (see the PEC Joints section below).
PVAm on oxidized cellulose
Kurosu and Pelton (2004) and DiFlavio et al. (2005) reported wet-peeling results using PVAm as an adhesive and TEMPO-oxidized cellulose as the substrate. As long as the joints were dried once, notable wet strength values were observed, even with room temperature drying at a 50% relative humidity. At the time, it was proposed that drying was necessary to promote PVAm chemical grafting to cellulose. However, results from a recent investigation suggested that PVAm forms imine and aminal linkages with aldehydes (see Scheme 2) present on oxidized cellulose in water, and thus drying is not necessary for grafting to occur (Yang et al. 2018b). Figure 4 compares the never-dried adhesion for three joint structures of PVAm on TEMPO-oxidized cellulose.
Fig. 4. Influence of the joint structure on the never-dried adhesion of TEMPO-oxidized regenerated cellulose membranes laminated with PVAm (340 kDa) (Yang et al. 2018b)
The differences are dramatic, as the results showed that α joints were strong in spite of having only 1.9 mg/m2 dry PVAm in the joints, αα joints with twice as much polymer were weak, and d-30 joints with a high polymer content (30 mg/m2) had an intermediate adhesion (Yang et al. 2018b). With α joints, the PVAm layer can simultaneously graft to both cellulose surfaces in water. The enhanced strength of α joints compared with αα joints has also been observed in SFA and AFM-CP experiments (Claesson et al. 2003). In contrast, αα joints were weak because electrosteric repulsion prevented attractive interactions between the adsorbed polymer layers. Finally, the thick d-30 joints resulted in some adhesion at higher solids contents.
The role of aldehyde groups in never-dried adhesion with PVAm is illustrated in Fig. 5. The reduction process converted aldehydes to alcohols, whereas the carboxyl groups remained unchanged. The removal of aldehydes resulted in lower wet-peel values. This supported the conclusion that primary amines condense with aldehydes giving aminal and imine linkages in water – see Scheme 2.
Fig. 5. Influence of aldehyde grafting sites on the never-dried wet adhesion with PVAm α laminates; the reduced membranes have carboxyl groups, but no aldehyde groups; new results from Yang (2018)
Polyvinylamine-g-phenylboronic acid
A decade ago the polymer polyvinylamine-g-phenylboronic acid (PVAm-B) was developed, which gives rise to never-dried wet adhesion between unmodified regenerated cellulose surfaces (Chen et al. 2009b). The structure of PVAm-B is presented in Fig. 6A. It was proposed that the never-dried wet adhesion resulted from covalent linkages between the borate and reducing end of the cellulose chains, as well as PVAm-B/PVAm-B cohesion because of electrostatic and possibly dative bond formation.
Figure 6A shows the 90° wet-peel delamination force for untreated membranes laminated with adsorbed PVAm-B, as a function of the lamination pH. To put these results in context, anything below 10 N/m is very weak, whereas 60 N/m is very high. The delamination force units of N/m are dimensionally equivalent to units of energy/area, which is the peeling work required to separate αα laminates. For example, the peeling work (pH = 10.5) of 55 N/m is equivalent to 55 J/m2. This high delamination force requires both high cellulose/PVAm-B adhesion and high PVAm-B/PVAm-B cohesion.
Figure 6A also shows that the maximum AFM-CP pull-off force is a function of the solution pH. The wet-peel delamination forces and AFM-CP maximum pull-off forces showed similar pH dependencies.
Figure 6B shows a sample retraction force curve from an AFM-CP experiment. The approach curve (not shown) was repulsive, which reflected the electrosteric repulsion of PVAm-B-coated surfaces in water. After reaching a compression force of 15 nN, the probe was retracted. The pull-off required the application of a maximum force of approximately -15 nN. For comparison with wet-peeling, the retraction force curve is roughly integrated in Fig. 6B to show that approximately 7.5 x 10-16 J were consumed when pulling the probe from the surface. It was further assumed that the contact area on the film between the sphere and cellulose film was 0.75 μm2, which corresponded to a sphere radius of 12 μm, embedded in a 10-nm-thick layer of PVAm-B. The corresponding pull-off work per bonded area was 0.001 N/m, which was four orders of magnitude smaller than the work from wet-peeling. This reflected the fundamental differences in the two test types. With AFM-CP, the lamination pressure is approximately 15 kPa, whereas it is 323 kPa for the wet-peel test. The joint age before testing (i.e., dwell time) and crack growth rates were different for the two types of experiments as well.
Fig. 6. Comparison of AFM-CP and wet-peel adhesion measurements for PVAm-B: (A) Influence of environmental pH on AFM-CP maximum pull-off force (data from Notley et al. 2009) and wet-peel force (data from Chen et al. 2006c); and (B) An example colloidal probe experiment at a pH of 10 (data from Notley et al. 2009)
Joints Employing Two or More Adhesive Types
Summarizing so far, with the exception of CAS and PVAm-B, no other single soluble polyelectrolyte treatment has been found that can increase never-dried wet cellulose adhesion of αα joints. Wet αα joints (Fig. 2) based on adsorbed polymers are weak for two reasons: 1) physically adsorbed polymers are not strongly attached, which results in weak adhesion, and 2) contacting water-soluble polymers do not adhere, which also results in weak cohesion. Covalent grafting of the α polymer to cellulose solves the weak adhesion problem; however, weak cohesion still remains. The following sections show that the treatment of cellulose surfaces with two or more polymers gives more complex joint structures that can display remarkable never-dried wet adhesion.
Joint Structures – Two Polymers
Figure 7 shows idealized assemblies and the variety of possible arrangements of two polymers in an adhesive joint. An αα joint structure is also shown for comparison. The dashed horizontal line shows the lamination plane, which is the dividing plane between the two surfaces pushed together to form the laminate. Except for joints based on PECs, the polymers must be applied to the cellulose substrates in two or more steps.
The αβα joint type is the simplest joint involving two or more polymers, with a monolayer of β polymer applied between two α-coated cellulose substrates. The dashed horizontal line depicts the interface between the surfaces pushed together to form the joint. These are idealized depictions. The literature on layer-by-layer (LbL) assemblies show evidence of intermixing and degrading of the layered structure (Decher and Schlenoff 2002). Although αβα joints are easy to prepare in the laboratory, there is no easy way to prepare them in papermaking or related processes.
Fig. 7. Idealized joint structure cross-sections that can be prepared with two polymer types; the dashed horizontal line shows the lamination plane, which is the dividing plane between the two surfaces pushed together to form the laminate
Type α(d-Γβ)α joints are closely related to αβα joints, because instead of an adsorbed monolayer of β polymer, there is a thick β layer with a dry coverage of Γβ (mg/m2). Type α(d-Γβ)α joints can be prepared in manufacturing processes by casting α-coated cellulose surfaces in a solution of β polymer and removing the water. Note that there is no clearly defined lamination plane with this type of joint.
The joint type αββα is prepared by contacting two cellulose surfaces, each bearing an αβ bilayer. It will be seen in a number of examples that these joints result in a notable never-dried strength. This was surprising because ββ repulsion was expected, resulting in weak cohesion, as was seen with αα joints (Table 1).
The LbL assembly gives two possible joint structures: (αβ)n(βα)n and (αβ)nαα(βα)n, where n is the number of pairs of adsorbed polymers. In the case of wood pulp fibers, the α polymer is typically a cationic polyelectrolyte that spontaneously adsorbs onto anionic fibers. In this case, (αβ)nαα(βα)n joints with two cationic layers in contact are frequently stronger than (αβ)n(βα)n joints with two anionic polymers in contact. Finally, with PECs, two interacting polymers are pre-mixed to form colloidal complexes. The net surface charge of colloidally stable PECs should be opposite to that of the target surfaces to drive adsorption. We are not aware of never-dried wet adhesion studies involving PECs. However tacky complexes could be very effective.
It will be discussed below that a commonly used α layer is gCMC. Because gCMC is an anionic polyelectrolyte, obvious β candidates are cationic polyelectrolytes that will form PECs with gCMC. Most examples of joints employing two polymer types employ electrostatically driven adsorption/complexation between sequentially applied polymers. However, multilayer polymer assemblies on surfaces in water can also be based on hydrogen bonding and ester formation between phenylboronic acid groups and polyols (Zhang et al. 2007).
AFM-CP and Wet-web Tensile Results
Most of the literature reporting never-dried wet cellulose adhesion involves either AFM-CP, wet-web strength measurements, or wet-peel results. The AFM-CP and wet-web results will be considered first, and the key contributions are listed in Table 2. The wet-peel results are presented separately because all of the tests were performed in the authors’ laboratory under standard conditions, facilitating quantitative comparisons (Yang et al. 2018).
αββα joints
Myllytie et al. (2009b) treated CMC-grafted fibers with chitosan at a high pH, which resulted in a high wet-web strength. The resulting joint structure was αββα, where the α polymer was gCMC and the β polymer was chitosan. In a related study, Wu and Farnood (2014) reported results for wet webs formed with CMC-grafted fibers that were then exposed to chitosan. Some of their data are shown in Fig. 8.
Fig. 8. Strength of the gCMC fiber webs prepared with and without treatment with chitosan after the web was formed; the once dried measurements were made on rewetted sheets after room temperature drying, whereas the never-dried measurements were made with wet sheets without drying (Wu and Farnood 2014).
The initial sheets prepared with gCMC had a low wet-web strength. Subsequent treatment with chitosan increased the wet-web strength. Interestingly, drying did not seem to strengthen the sheets compared with the never-dried results. By forming the paper web structure before chitosan deposition, fiber flocculation and the resulting poor paper formation that can occur from forming paper sheets with a suspension of adhesive fiber coatings were avoided.
Aarne et al. (2013) studied once-dried paper wet strength for bleached kraft pulp fibers, where the α layer was gCMC and the β polymer was one of a series of cationic ionenes and a PDADMAC. Some of the ionenes increased the once-dried strength, where gCMC alone and gCMC plus PDADMAC as the β polymer did not increase the once-dried wet strength. The authors stated that none of the polymer combinations resulted in a never-dried wet strength.
PEC joints
Papermakers routinely consecutively add oppositely charged polymers to a pulp suspension before the filtration step that forms wet paper. The motivation is usually to increase filler and fines retention (Moore 1976). For wet-strength papers, the cationic wet strength resin PAE is often used in combination with CMC, an anionic polymer. For the dry strength, there are many examples of improved strength (Lofton et al. 2005). In these examples, at least some of the polymers were present as colloidal-sized PECs that spontaneously formed when oppositely charged polymers were present in the water (Gernandt et al. 2003). However, in spite of the near ubiquitous presence of such complexes in papermills, there are few never-dried cellulose adhesion studies that involve PECs. The once-dried wet-peel adhesion of PVAm/CMC complexes has been studied (Feng et al. 2007). It was found that it was nearly impossible to produce PEC dispersions without excess anionic or cationic soluble polymer in solution. The non-complexed polymer can adsorb onto available surfaces, which competes with the complexes. Thus, fundamental measurements can be compromised. Some authors have used ultrafiltration to remove un-complexed polymer (Gärdlund et al. 2005). In contrast, with LbL surface treatments, which are described in the next section, it is easy in laboratory studies to remove excess polymer in solution by washing after each layer deposition step.
Salmi et al. (2007) described an AFM-CP investigation of PECs based on blends of cationic and anionic polyacrylamide. Their study appears to have been complicated by the presence of a large excess of soluble CPAM. Nevertheless, their study showed that PECs did increase the pull-off force and extension before failure. The adhesive properties were dependent on the polymer properties. A lower molecular weight and higher charge density cationic polymer resulted in stronger adhesion.
Discussed in the next section is the never-dried adhesion of joints formed between surfaces bearing polyelectrolyte multilayers. Ankerfors et al. (2009) showed that PECs do not completely cover surfaces, whereas LbL coatings do. Thick multilayers gave the highest dry strength, whereas PEC joints were stronger at low coverages. None of the experiments involved the never-dried strength.
To summarize, pre-formed PECs are easily prepared in continuous processes and are a relatively easy way to add more polymer in adhesive joints compared with treatments using an individual linear polymer. However, it is difficult to avoid the complications caused by excess individual polymer chains in solution.
Table 2. A Summary of Publications Describing Never-dried Adhesion Values for Joints Laminated with Two Or More Polymers
List of all of the results found with cellulose substrates; for other substrates, the list is not complete; G – grafting; Y means that there is an expectation of polymer grafting to cellulose; N means that grafting is unlikely; and see Table A1 for meaning of acronyms
LbL joints
Wågberg et al. (2002) pioneered LbL polymer assembly on pulp fiber surfaces for enhanced wet and dry paper strength. Most of the never-dried adhesion results were AFM-CP pull-off forces for LbL assemblies on silica and most of the early publications involved the polymer pair polyallylamine hydrochloride (PAH)/polyacrylic acid (PAA) (Notley et al. 2005; Lingström et al. 2007; Johansson et al. 2009a), with later works including PAH/ hyaluronic acid (HA) (Pettersson et al. 2014), cationic/anionic starches (Johansson et al. 2009b), and more recently innovative block copolymers (Träger et al. 2016).
The conclusions from this large body of works, specific to never-dried adhesion, include:
- The adhesion (pull-off force or work) increases with the number of layers. However, plots of pull-off force versus layer number are often not linear with the first five layers, and often they show low adhesion with a low or possibly negative slope (Notley et al. 2005; Johansson et al. 2009a; Pettersson et al. 2014). Never-dried adhesion is stronger when the last deposited layer is cationic. Therefore, plots of pull-off (and often dry adhesion) versus layer number have a saw tooth shape for the strength.
- The pull-off force increases with the total time the maximum pressure is applied while forming the joint (i.e., the lamination time) (Johansson et al. 2009b; Pettersson et al. 2014).
- Pull-off forces are higher for lower molecular weight PAH (Johansson et al. 2009b).
- Layer formation at higher ionic strengths results in a more swollen LbL assembly and stronger adhesion (Pettersson et al. 2014).
- Never-dried wet adhesion is sensitive to polyelectrolyte structures. For example, HA caused stronger joints than PAA (Pettersson et al. 2014; Marais et al. 2015). Träger et al. (2016) demonstrated that LbL assemblies based on oppositely charged block copolymer micelles resulted in pull-off forces an order of magnitude higher than obtained with non-micellar, random polyelectrolyte copolymers.
Virtually all of the above observations were obtained with silica substrates. For thick joints, for example formed by two surfaces each coated with seven or more polyelectrolyte layers, most of the delamination work is associated with energy dissipation within the polyelectrolyte multilayer. However, with thinner LbL assemblies, differences between the silica and cellulose substrates are likely to be notable.
Junka et al. (2014) compared pull-off forces for two types of LbL assemblies, chitosan/CMC and HECE/CMC, where HECE is a quaternary ammonium derivative of hydroxyethylcellulose ethoxylate. The substrate was a smooth film based on CNF. The chitosan resulted in higher pull-off forces than HECE. The pull-off forces decreased with an increasing number of layers up to four layers, which was in agreement with previously reported behaviors of PAH/PAA multilayers (Notley et al. 2005).
Wet-peel Results
Table 3 summarizes our never-dried adhesion results obtained over the years, where WP55 corresponds to the wet-peel values (N/m) interpolated for a laminate solids content of 55 wt.%. Four types of regenerated cellulose membrane substrates are listed: 1) untreated (RC), which is essentially pure cellulose, 2) TEMPO-oxidized (TO-RC), which has a mixture of aldehyde and carboxyl groups, 3) reduced TEMPO-oxidized (R-TO-RC), where the aldehydes are reduced back alcohols and the carboxyls remain (Saito and Isogai 2006), and 4) membranes bearing gCMC (Laine et al. 2000). Below each substrate type are two columns; one gives the joint structure and the other gives the corresponding WP55 values. Finally, the left-hand column gives the type of lamination adhesive, most of which were commercial polymers. No effort was made to choose the best-in-class of the various polymer types. For interpretation of the WP55 values, a value greater than 40 N/m is very high, 20 N/m is high, 10 N/m is weak, and less than 10 N/m is very weak.
None of the polymers in Table 3, down to and including PAE, promoted never-dried adhesion on any of the three substrates. It should be noted that PAE is one of the most common paper wet strength resins. However, it did not improve the never-dried adhesion because curing occurs at high temperatures in the dryer section of paper machines.
Glyoxylated cationic polyacrylamide (GCPAM) adsorbed onto CMC-grafted cellulose membranes resulted in the strongest never-dried adhesion values (Table 3). The GCPAM (Luredur Plus 555, BASF, Ludwigshafen, Germany) is a high-molecular weight CPAM that has been reacted with glyoxal. The GCPAM can interact with the gCMC surfaces by forming an electrostatically driven PEC. Additionally, the aldehyde functional groups on the glyoxal moieties can react with hydroxyls on CMC and cellulose, as well as crosslink with other GCPAM polymer segments.
Table 3. Influence of the Adhesive Type and Membrane Pretreatment on WP55
The applied adhesive solutions were in 1 mM NaCl at a pH of 7; a – Yang et al. (2018a); b – Yang (2018); c – Gustafsson et al. (2016); d – Yang et al. (2017); e – unpublished; f – Yang et al. (2018b); g – 500 kDa measurements by Dong Yang; and see Table A1 for meaning of acronyms
Figure 9 shows the wet-peel force versus solids contents for cellulose membranes treated with GCPAM.
Fig. 9. GCPAM can give high never-dried strength: (A) influence of the cellulose surface treatment on the GCPAM adhesion; and (B) comparison of GCPAM and PDADMAC on the gCMC-cellulose (Yang et al. 2018a)
Figure 9A shows that GCPAM resulted in a very high never-dried adhesion on the gCMC-cellulose, whereas the adhesion was low on the TEMPO-oxidized cellulose. These results suggested that the CMC/GCPAM complexation is an important contribution to the mechanism. Additionally, the ability of GCPAM to form covalent linkages to itself and carbohydrates in water is an important contribution to the overall adhesion. Any cationic water-soluble polymers should form complexes with CMC in water. However, the ability to form complexes is not synonymous with adhesion. The PDADMAC forms complexes with CMC (Hubbe 2005), but results in a poor never-dried adhesion for gCMC-cellulose, as is seen in Fig. 9B.
The joint structure is important. Table 3 compares the delamination force at a 55% solids content for CMC-grafted cellulose joints laminated with a single adsorbed PVAm layer (αβα, WP55 = 6 N/m), PVAm adsorbed on both surfaces before lamination (αββα, WP55 = 16 N/m), and a thick layer of PVAm (α(Γ-15)α, WP=32 N/m). More is better when PVAm is the β polymer. Further results for PVAm on CMC-grafted cellulose were recently published (Gustafsson et al. 2016). To conclude, the wet-peel results of this study indicated that CMC-grafted cellulose joints can have a notable never-dried strength when laminated with a second polymer. However, the properties of the cationic β polymer are important, including the charge density, molecular weight, and presence of reactive moieties.
Approaches Not Found
Most commercial adhesives and binders are based on soft, tacky latexes made of acrylate or styrene butadiene copolymers. In the paper industry, latex binders are used to fix mineral particles in paper coatings. No publications or data in the patent literature describing polymer dispersions (latex) that improve the never-dried adhesion between cellulose surfaces have been found. There are patents that describe latex binders for nonwoven materials, including glass fiber mats. The authors showed that crosslinked, water-swollen microgels are mediocre once-dried (Wen and Pelton 2012) or never-dried adhesives (Yang et al. 2017) because crosslinked microgels do not display viscous dissipation. However, tacky latex should be effective.
Also, systematic studies into amphoteric adhesives have not been found. The electrostatic component of the repulsion between surfaces pushed into contact would be attenuated by lowering the net charge density. There is some evidence that zwitterionic derivation of fibers causes enhanced wet adhesion (Delgado et al. 2004). The amphoteric hydrazide derivatized microgels used in this study showed a higher once-dried and never-dried wet adhesion on TEMPO-oxidized cellulose compared with similar anionic hydrazide microgels (Yang et al.2017).
Remarks
Why are αα joints weak, whereas some αββα and LbL joints are strong?
The literature summarized herein shows that, with the exception of CAS and PVAm-B or chitosan at a high pH, all other αα joints are weak, even in cases where the α polymer is covalently grafted to cellulose. Colloid science teaches that electrosteric repulsion between grafted polyelectrolytes is substantial and could prevent entanglement of grafted layers necessary for cohesion (Fleer et al. 1993). Therefore, weak αα joints seem easy to explain. However, the formation of αββα and LbL joints also involves bringing together two similar polymers; therefore, the same electrosteric repulsion should be operative. However, many of the combinations in Tables 2 and 3 show remarkable never-dried adhesion. For example, the highest WP55 value in Table 3 is for the αββα joint, where the α polymer is CMC grafted to cellulose and the β polymer is GCPAM. It is therefore proposed that the interactions of the gCMC layer grafted with the adsorbing GCPAM layer, before lamination, produce a PEC layer with an amphoteric property. The electrostatic and osmotic contributions to repulsion are less compared with two surfaces with a single adsorbed polymer (i.e., when forming an αα joint).
When is polymer grafting to cellulose necessary?
Conventional wet-strength resins, including PAE and aldehyde resins, form covalent grafts onto cellulose when the paper is dried at elevated temperatures. Is grafting required for never-dried wet strength? Assuming the irreversible attachment of gCMC is equivalent to grafting, the highest never-dried adhesion results in Table 3 corresponded to cellulose membranes surfaces with gCMC as the α layer. In contrast, PECs and LbL constructs with multiple pairs of layers can cause substantial adhesion without grafting. Therefore, the question is: when is grafting required? Figure 10 compares the once-dried wet adhesion for PVAm on oxidized versus non-oxidized cellulose (Yang et al. 2018b). The PVAm forms covalent grafts to TO-RC, whereas there is no reaction with unoxidized regenerated cellulose (RC). The X-axis in the plots shows the quantity of PVAm in the laminates, and the Y-axis shows the corresponding wet-peel adhesion. For thin adhesive layers up to 10 mg/m2, only the grafted laminates showed a notable wet-peel force. At a high PVAm coverage or thickness, the non-grafted RC had some strength that was associated with the work required to disrupt the viscoelastic PVAm layers. Therefore, it is proposed that grafting is essential for thin adhesive layers and preferable for thicker layers.
Fig. 10. Influence of PVAm grafting to cellulose on wet adhesion (Yang et al. 2018b)
Engineering Challenges
Can adhesion between never-dried cellulose surfaces be exploited when manufacturing lignocellulosic materials? Restricting the discussion to aqueous manufacturing processes for products that are mainly lignocellulose, two cases can be imagined: the cellulosic fibers or nano materials are first treated with one or more adhesives and then they are formed into the final shape, or the shape is formed first and then the adhesive polymer is added. Both approaches present challenges. The shape forming processes could include filtration, as in papermaking, spin coating, casting, templated casting, printing, etc.
With the first method, pretreating with an adhesive may cause the aggregation of fibers or nanocellulose before the final shape is formed. In this situation, some repulsion between adhesive-coated surfaces is desirable to prevent inadvertent aggregation before the shape formation step. However, repulsion must be sufficiently weak that it can be overcome during joint lamination. A subtle balance is required.
In the second method, the structure is formed first and then the adhesive is introduced. This approach avoids the problems of unintended aggregation. However, introducing the polymer can be a challenge. In papermaking, which is susceptible to problems from polymer-induced fiber aggregation, adhesive polymers can be added after the wet paper is formed by spraying polymers on the wet web (Allan et al. 1978; Vishtal and Retulainen 2014) or passing the web through a bath (Wu and Farnood 2014).
In this review, the importance of the organization of adhesive polymers in joints is emphasized. Figures 2 and 7 illustrate the idealized cases. While all of these joint types can be assembled in the laboratory, only a few are easily implemented on a large scale. Limited to joint assembly from aqueous processing, the primary operation is polymer adsorption onto a solid/water interface. Adsorption is usually driven by the release of counterions when charged polymers adhere to oppositely charged surfaces. Adsorption is irreversible in most cases, and usually ceases after the formation of an adsorbed monolayer. Most of the idealized joints in Figs. 2 and 7 involved surfaces with saturated layers of adsorbed polymers. Sub-monolayer adsorption requires extremely sensitive and responsive process control.
The αα, αββα, and LbL joints can be manufactured by consecutive adsorption and washing steps, followed by joint formation or lamination. The PEC joints can be prepared by forming PECs, removing excess polymer not present in the complexes, adsorbing the complexes, and forming the joints.
The d-Γ laminates can be prepared by dispersing fibers or nanocellulose particles in polymer solutions to produce the objects and evaporate the water. Alternatively, first forming the object, followed by impregnation with polymer solutions will also result in d-Γ joints.
Finally, α and αβα joints are the most difficult to prepare because they require bringing together two surfaces with different coatings. The α joint is made by laminating a polymer-coated surface with an untreated surface. Therefore, these joints can only be manufactured with macroscopic substrates, such as films or webs that are treated separately and then laminated.
CONCLUDING THOUGHTS
This article has attempted to distill the findings from the literature and to recast them into design rules for polymers that increase cellulose never-dried adhesion.
Joints employing one water-soluble adhesive polymer
Based on the literature, the following requirements are proposed for an effective one-component never-dried cellulose wet adhesive:
- The polymer in contact with the cellulose surface must form covalent grafts or some other linkage with an equivalent strength. For never-dried joints, grafting must occur in the presence of water.
- The only way to achieve never-dried strength with a highly charged and hydrophilic polyelectrolyte is by forming α joints that have a single layer of grafting polymer simultaneously attached to both substrate surfaces. With αα joints, where both surfaces are polymer-coated, electrosteric repulsion inhibits polymer-polymer contact and thus inhibits cohesion within the polymer layer. Weakly charged adhesives that have the potential to crosslink with themselves and graft to cellulose can result in never-dried adhesion with αα joints. Cationic aldehyde starch is the best example of this.
- Once-dried joints with a strong wet strength are easier to achieve because drying removes electrosteric repulsion, which allows polymer-coated surfaces to come into molecular contact. However, the contacting polymer layers must have adequate cohesion after rewetting in water. Not all water-soluble polymers are cohesive. For example, once-dried PVAm results in an intermediate wet cohesion, whereas gCMC provides none (Myllytie et al. 2009b).
- With thick adhesive layers, obtainable by casting cellulose with adhesive solutions, viscous dissipation within the water-swollen polymer layer contributes to the work required for once-dried joint failure. However, thick layers of non-crosslinked polymers swell slowly, which causes the joints to weaken over time.
Joints employing two polymers added in multiple steps
The αββα and LbL joint structures were considered. The main design rules are:
- When there are more polymer layers, the work required to sever the joint increases.
- When forming a joint between two LbL-coated surfaces, a cationic exterior layer results in the strongest joints.
- Primary amines are more effective than quaternary amine functional groups on a cationic polymer.
- A grafted α layer of CMC causes strong wet αββα joints if combined with an appropriate β polymer, such as PVAm, GCPAM, or chitosan.
Joints employing dispersed adhesive particles
There is not much never-dried data for this category. The short list includes works with crosslinked microgels and some AFM-CP studies involving PECs, and possibly some of the chitosan results at a high pH. This is a category of cellulose adhesives with unexploited potential. The rules so far are:
- Crosslinked microgels bearing surface moieties capable of grafting to cellulose result in some never-dried adhesion. However, the crosslinks prevent the viscous dissipation of energy when the joint is challenged.
- The PECs with excess cationic groups cause never-dried adhesion, whereas net negatively charged PECs are unlikely to be effective.
- The presence of excess cationic polymer competes with PEC microgel particles for adsorption on cellulose surfaces, which complicates experiments and lowers performance.
Looking Ahead
This review focused on water-soluble polymers that increase wet adhesion between cellulose surfaces. Furthermore, most of the polymers discussed above were commodity commercial polymers that were developed for the paper industry and have been available for decades. Future breakthroughs are likely to come from developing biomedical adhesive materials and biomimetic adhesive research. There are many examples of wet adhesion or underwater adhesion in nature, which stimulate attempts both to understand the mechanisms and create synthetic polymers to mimic nature. Mussels and sandcastle worms have been intensively studied (Stewart et al. 2011a), which has led to synthetic analogues (Shao et al. 2009; Zhong et al. 2014; Zhao et al. 2016). Natural adhesives can display instantaneous tack in water by forming coacervates, followed by more permanent covalent crosslinking (Stewart et al. 2011b). Cellulose-specific binding agents have been studied, including cellulose binding domain proteins from cellulase (Shoseyov et al. 2006) and cellulose binding DNA aptamers (Su et al.2007; Boese et al. 2008). The challenge is to learn from these biological examples to design green and cost-effective adhesives for new lignocellulosic materials, thus contributing to the bioeconomy (Staffas et al. 2013).
ACKNOWLEDGEMENTS
This review includes results from over a decade of studies at McMaster University performed by many talented students and post-doctoral fellows. The authors thank the Canadian Natural Sciences and Engineer Research Council and the Canada Foundation for Innovation for funding, as well as BASF and Proctor & Gamble, who acted as industrial partners.
REFERENCES CITED
Aarne, N., Vesterinen, A.-H., Kontturi, E., Seppälä, J., and Laine, J. (2013). “A systematic study of non-crosslinking wet strength agents,” Ind. Eng. Chem. Res. 52(34), 12010-12017. DOI: 10.1021/ie401417e
Alince, B., Vanerek, A., de Oliveira, M. H., and van de Ven, T. G. M. (2006). “The effect of polyelectrolytes on the wet-web strenght of paper,” Nord. Pulp Pap. Res. J. 21(5), 653-658. DOI: 10.3183/NPPRJ-2006-21-05-p653-658
Allan, G. G., Fox, J. R., Crosby, G. D., and Sarkanen, K. V. (1978). “Chitosan, a mediator for fibre-water interactions in paper,” in: Fibre-water Interactions in Papermaking Symposium Transactions, Oxford, UK, pp. 765-794.
Ankerfors, C., Lingström, R., Wågberg, L., and Odberg, L. (2009). “A comparison of polyelectrolyte complexes and multilayers: Their adsorption behaviour and use for enhancing tensile strength of paper,” Nord. Pulp Pap. Res. J. 24(1), 77-86. DOI: 10.3183/NPPRJ-2009-24-01-p077-086
Belle, J., and Odermatt, J. (2016). “Initial wet web strength of paper,” Cellulose 23(4), 2249-2272. DOI: 10.1007/s10570-016-0961-7
Boese, B. J., Corbino, K., and Breaker, R. R. (2008). “In vitro selection and characterization of cellulose-binding RNA aptamers using isothermal amplification,” Nucleos. Nucleot. Nucl. 27(8), 949-966. DOI: 10.1080/15257770802257903
Celzard, A., Fierro, V., and Kerekes, R. (2009). “Flocculation of Cellulose Fibres: New Comparison of Crowding Factor with Percolation and Effective-Medium Theories.” Cellullose, 16(6), 983.DOI: doi.org/10.1007/s10570-009-9314-0
Cervin, N. T., Aulin, C., Larsson, P. T., and Wågberg, L. (2012). “Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids,” Cellulose 19(2), 401-410. DOI: 10.1007/s10570-011-9629-5
Chen, N., Hu, S., and Pelton, R. (2002). “Mechanisms of aldehyde-containing paper wet-strength resins,” Ind. Eng. Chem. Res. 41(22), 5366-5371. DOI: 10.1021/ie020355m
Chen, W., Leung, V., Kroener, H., and Pelton, R. (2009a). “Polyvinylamine-phenylboronic acid adhesion to cellulose hydrogel,” Langmuir 25(12), 6863-6868. DOI: 10.1021/la900131g
Chen, W., Pelton, R., and Leung, V. (2009b). “Solution properties of polyvinylamine derivatized with phenylboronic acid,” Macromolecules 42(4), 1300-1305. DOI: 10.1021/ma802402z
Chen, W., Lu, C., and Pelton, R. (2006c). “Polyvinylamine boronate adhesion to cellulose hydrogel,” Biomacromolecules 7(3), 701-702. DOI: 10.1021/ma802402z
Claesson, P. M., Dedinaite, A., and Rojas, O. J. (2003). “Polyelectrolytes as adhesion modifiers,” Adv. Colloid Interfac. 104(1-3), 53-74. DOI: 10.1016/S0001-8686(03)00036-8
Claesson, P. M., Poptoshev, E., Blomberg, E., and Dedinaite, A. (2005). “Polyelectrolyte-mediated surface interactions,” Adv. Colloid Interfac. 114-115, 173-187. DOI: 10.1016/j.cis.2004.09.008
de Gennes, P. G. (1996). “Soft adhesives,” Langmuir 12(19), 4497-4500. DOI: 10.1021/la950886y
Decher, G., and Schlenoff, J. B. (2002). Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, Wiley-VCH, Weinheim, Germany.
Delgado, E., Lopez-Dellamary, F. A., Allan, G. G., Andrade, A., Contreras, H., Regla, H., and Cresson, T. (2004). “Zwitterion modification of fibres: Effect of fibre flexibility on wet strength of paper,” J. Pulp Pap. Sci. 30(5), 141-144.
DiFlavio, J.-L., Bertoia, R., Pelton, R., and Leduc, M. (2005). “The mechanism of polyvinylamine wet-strengthening,” in: Advances in Paper Science and Technology: Transactions of the 13th Fundamental Research Symposium, Cambridge, UK, pp. 1293-1316.
Ducker, W. A., Senden, T. J., and Pashley, R. M. (1992). “Measurement of forces in liquids using a force microscope,” Langmuir 8(7), 1831-1836. DOI: 10.1021/la00043a024
Erlandsson, J., Pettersson, T., Ingverud, T., Granberg, H., Larsson, P. A., Malkoch, M., and Wågberg, L. (2018). “On the mechanism behind freezing-induced chemical crosslinking in ice-templated cellulose nanofibril aerogels,” J. Mater. Chem. A 6(40), 19371-19380. DOI: 10.1039/C8TA06319B
Espy, H. H. (1995). “The mechanism of wet-strength development in paper: A review,” Tappi J. 78(4), 90-99.
Feng, X., Pouw, K., Leung, V., and Pelton, R. (2007). “Adhesion of colloidal polyelectrolyte complexes to wet cellulose,” Biomacromolecules 8(7), 2161-2166. DOI: 10.1021/bm070307r
Fleer, G. J., Cohen Stuart, M. A., Scheutjens, J. M. H. M., Cosgrove, T., and Vincent, B. (1993). Polymers at Interfaces, Chapman & Hall, London, England.
Gärdlund, L., Forsström, J., Andreasson, B., and Wågberg, L. (2005). “Influence of polyelectrolyte complexes on the strength properties of papers from unbleached kraft pulps with different yields,” Nord. Pulp Pap. Res. J. 20(1), 36-42. DOI: 10.3183/NPPRJ-2005-20-01-p036-042
Gernandt, R., Wågberg, L., Gärdlund, L., and Dautzenberg, H. (2003). “Polyelectrolyte complexes for surface modification of wood fibres: I. Preparation and characterisation of complexes for dry and wet strength improvement of paper,” Colloid. Surface. A 213(1), 15-25. DOI: 10.1016/S0927-7757(02)00335-7
Gustafsson, E., Pelton, R., and Wågberg, L. (2016). “Rapid development of wet adhesion between carboxymethylcellulose modified cellulose surfaces laminated with polyvinylamine adhesive,” ACS Appl. Mater. Inter. 8(36), 24161-24167. DOI: 10.1021/acsami.6b05673
Hubbe, M. A. (2005). “Dry-strength development by polyelectrolyte complex deposition onto non-bonding glass fibres,” J. Pulp Pap. Sci. 31(4), 159-166.
Israelachvili, J. (1991). Intermolecular and Surface Forces, Academic Press, Cambridge, MA.
Jin, H., Nishiyama, Y., Wada, M., and Kuga, S. (2004). “Nanofibrillar cellulose aerogels,” Colloid. Surface. A 240(1-3), 63-67. DOI: 10.1016/j.colsurfa.2004.03.007
Johansson, E., Blomberg, E., Lingström, R., and Wågberg, L. (2009a). “Adhesive interaction between polyelectrolyte multilayers of polyallylamine hydrochloride and polyacrylic acid studied using atomic force microscopy and surface force apparatus,” Langmuir 25(5), 2887-2894. DOI: 10.1021/la803628w
Johansson, E., Lundström, L., Norgren, M., and Wågberg, L. (2009b). “Adsorption behavior and adhesive properties of biopolyelectrolyte multilayers formed from cationic and anionic starch,” Biomacromolecules 10(7), 1768-1776. DOI: 10.1021/bm900191s
Junka, K., Sundman, O., Salmi, J., Österberg, M., and Laine, J. (2014). “Multilayers of cellulose derivatives and chitosan on nanofibrillated cellulose,” Carbohyd. Polym. 108, 34-40. DOI: 10.1016/j.carbpol.2014.02.061
Kargl, R., Mohan, T., Bračič, M., Kulterer, M., Doliška, A., Stana-Kleinschek, K., and Ribitsch, V. (2012). “Adsorption of carboxymethyl cellulose on polymer surfaces: Evidence of a specific interaction with cellulose,” Langmuir 28(31), 11440-11447. DOI: 10.1021/la302110a
Kendall, K. (2001). Molecular Adhesion and Its Applications – The Sticky Universe, Plenum Press, London, England.
Kitaoka, T., Isogai, A., and Onabe, F. (1999). “Chemical modification of pulp fibers by TEMPO-mediated oxidation,” Nord. Pulp Pap. Res. J. 14(4), 279-284. DOI: 10.3183/NPPRJ-1999-14-04-p279-284
Kontturi, E., Tammelin, T., and Österberg, M. (2006). “Cellulose-model films and the fundamental approach,” Chem. Soc. Rev. 35(12), 1287-1304. DOI: 10.1039/B601872F
Kurosu, K., and Pelton, R. (2004). “Simple lysine-containing polypeptide and polyvinylamine adhesives for wet cellulose,” J. Pulp Pap. Sci. 30(8), 228-232.
Laine, J., Lindström, T., Nordmark, G. G., and Risinger, G. (2000). “Studies on topochemical modification of cellulosic fibres. Part 1. Chemical conditions for the attachment of carboxymethyl cellulose onto fibres,” Nord. Pulp Pap. Res. J. 15(5), 520-526. DOI: 10.3183/NPPRJ-2000-15-05-p520-526
Laine, J., Lindström, T., Nordmark, G. G., and Risinger, G. (2002). “Studies on topochemical modification of cellulosic fibres. Part 3. The effect of carboxymethyl cellulose attachment on wet-strength development by alkaline-curing polyamide-amine epichlorohydrin resins,” Nord. Pulp Pap. Res. J. 17(1), 57-60. DOI: 10.3183/NPPRJ-2002-17-01-p057-060
Laleg, M., Pikulik, I. I., Ono, H., Barbe, M., and Seth, R. S. (1991). “The effect of starch on the properties of groundwood papers,” Pap. Technol. Ind. 32(5), 24-29.
Laleg, M., and Pikulik, I. I. (1991a). “Improving paper machine runnability and paper properties by a polymeric additive,” J. Pulp Pap. Sci. 17(6), J206-J216.
Laleg, M., and Pikulik, I. I. (1991b). “Wet-web strength increase by chitosan,” Nord. Pulp Pap. Res. J. 6(3), 99-103. DOI: 10.3183/NPPRJ-1991-06-03-p099-103
Laleg, M., and Pikulik, I. I. (1993a). “Modified starches for increasing paper strength,” J. Pulp Pap. Sci. 19(6), J248-J255.
Laleg, M., and Pikulik, I. I. (1993b). “Unconventioanal strength additives,” Nord. Pulp Pap. Res. J. 8(1), 41-47. DOI: 10.3183/NPPRJ-1993-08-01-p041-047
Lindström, T., and O’Brien, H. (1986). “On the mechanism of sizing with alkylketene dimers. Part 2. The kinetics of reaction between alkylketene dimers and cellulose,” Nord. Pulp Pap. Res. J. 1(1), 26-33. DOI: 10.3183/NPPRJ-1986-01-01-p034-042
Lindström, T., Wågberg, L., and Larsson, T. (2005). “On the nature of joint strength in paper – A review of dry and wet strength of paper during paper manufacturing,” in: Symposium on Advances in Paper Science and Technology: Transactions of the 13th Fundamental Research Symposium, Cambridge, UK, pp. 457-462.
Lingström, R., Notley, S. M., and Wågberg, L. (2007). “Wettability changes in the formation of polymeric multilayers on cellulose fibres and their influence on wet adhesion,” J. Colloid Interf. Sci. 314(1), 1-9. DOI: 10.1016/j.jcis.2007.04.046
Liu, Z., Choi, H., Gatenholm, P., and Esker, A. R. (2011). “Quartz crystal microbalance with dissipation monitoring and surface plasmon resonance studies of carboxymethyl cellulose adsorption onto regenerated cellulose surfaces,” Langmuir 27(14), 8718-8728. DOI: 10.1021/la200628a
Lofton, M. C., Moore, S. M., Hubbe, M. A., and Lee, S. (2005). “Deposition of polyelectrolyte complexes as a mechanism for developing paper dry strength,” Tappi J. 4(9), 3-8.
Luner, P., Eriksson, E., Vemuri, K. P., and Leopold, B. (1967). “The effect of chemical modification on the mechanical properties of paper. 1. Oxidation and reduction of rayon fibers,” Tappi 50(1), 37-39.
Marais, A., Pendergraph, S., and Wågberg, L. (2015). “Nanometer-thick hyaluronic acid self-assemblies with strong adhesive properties,” ACS Appl. Mater. Inter. 7(28), 15143-15147. DOI: 10.1021/acsami.5b03760
McLaren, A. D. (1948). “Adhesion of high polymers to cellulose. Influence of structure, polarity, and tack temperature,” J. Polym. Sci. 3(5), 652-663. DOI: 10.1002/pol.1948.120030507
Moore, E. E. (1976). “Charge relationships of dual polymer retention aids,” Tappi 59(6), 120-122.
Myllytie, P., Salmi, J., and Laine, J. (2009a). “The influence of pH on the adsorption and interaction of chitosan with cellulose,” BioResources 4(4), 1647-1662. DOI: 10.15376/biores.4.4.1647-1661
Myllytie, P., Yin, J., Holappa, S., and Laine, J. (2009b). “The effect of different polysacharides on the development of paper strength during drying,” Nord. Pulp Pap. Res. J. 24(4), 469-477. DOI: 10.3183/NPPRJ-2009-24-04-p469-477
Nikolaeva, M. (2010). Measurement and Improvement of Wet Paper Web Strength, Master’s Thesis, Lappeenranta University of Technology, Lappeenranta, Finland.
Notley, S. M., Eriksson, M., and Wågberg, L. (2005). “Visco-elastic and adhesive properties of adsorbed polyelectrolyte multilayers determined in situ with QCM-D and AFM measurements,” J. Colloid Interf. Sci. 292(1), 29-37. DOI: 10.1016/j.jcis.2005.05.057
Notley, S. M., Pettersson, B., and Wågberg, L. (2004). “Direct measurement of attractive van der Waals’ forces between regenerated cellulose surfaces in an aqueous environment,” J. Am. Chem. Soc. 126(43), 13930-13931. DOI: 10.1021/ja045992d
Notley, S., Chen, W., and Pelton, R. (2009). “The extraordinary adhesion of phenylboronic acid derivatives of polyvinylamine to wet cellulose: A colloidal probe microscopy investigation,” Langmuir 25(12), 6898–6904. DOI:10.1021/la900256s
Österberg, M. (2000). “The effect of a cationic polyelectrolyte on the forces between two cellulose surfaces and between one cellulose and one mineral surface,” J. Colloid Interf. Sci.229(2), 620-627. DOI: 10.1006/jcis.2000.7054
Page, D. H. (1993). “A Quantitative Theory of the Strength of Wet Webs.” J. Pulp Paper Sci., 19(4), 175-6
Pettersson, T., Pendergraph, S. A., Utsel, S., Marais, A., Gustafsson, E., and Wågberg, L. (2014). “Robust and tailored wet adhesion in biopolymer thin films,” Biomacromolecules 15(12), 4420-4428. DOI: 10.1021/bm501202s
Pikulik, I. I. (1997). “Wet-web properties and their effect on picking and machine runnability – Paper machines with open draws in the press section require adequate wet-web strength,” Pulp Pap.-Canada 98(12), 161-165.
Poptoshev, E., Rutland, M. W., and Claesson, P. M. (2000). “Surface forces in aqueous polyvinylamine solutions. 2. Interactions between glass and cellulose,” Langmuir 16(4), 1987-1992. DOI: 10.1021/la990961v
Retulainen, E., and Salminen, K. (2009). “Effects of furnish related factors on tension and relaxation of wet webs,” in: Advances in Pulp and Paper Research, 14th Fundamental Research Symposium, Oxford, UK, pp. 1019-1038.
Saito, T., and Isogai, A. (2006). “Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation,” Colloid. Surface. A 289(1-3), 219-225. DOI: 10.1016/j.colsurfa.2006.04.038
Saito, T., and Isogai, A. (2007). “Wet strength improvement of TEMPO-oxidized cellulose sheets prepared with cationic polymers,” Ind. Eng. Chem. Res. 46(3), 773-780. DOI: 10.1021/ie0611608
Salmi, J., Österberg, M., and Laine, J. (2007). “The effect of cationic polyelectrolyte complexes on interactions between cellulose surfaces,” Colloid. Surface. A 297(1-3), 122-130. DOI: 10.1016/j.colsurfa.2006.10.036
Seth, R. S. (1995). “The effect of fiber length and coarseness on the tensile strength of wet webs: A statistical geometry explanation,” Tappi J. 78(3), 99-102.
Shallhorn, P. M. (2002). “Effect of moisture content on wet-web tensile properties,” J. Pulp Pap. Sci. 28(11), 384-387.
Shao, H., Bachus, K. N., and Stewart, R. J. (2009). “A water-borne adhesive modeled after the sandcastle glue of P. californica,” Macromol. Biosci. 9(5), 464-471. DOI: 10.1002/mabi.200800252
Shoseyov, O., Shani, Z., and Levy, I. (2006). “Carbohydrate binding modules: Biochemical properties and novel applications,” Microbiol. Mol. Biol. R. 70(2), 283-295. DOI: 10.1128/MMBR.00028-05
Siqueira, G., Kokkinis, D., Libanori, R., Hausmann, M. K., Gladman, A. S., Neels, A., Tingaut, P., Zimmermann, T., Lewis, J. A., and Studart, A. R. (2017). “Cellulose nanocrystal inks for 3D printing of textured cellular architectures,” Adv. Funct. Mater. 27(12). DOI: 10.1002/adfm.201604619
Solarek, D. B., Jobe, P. E., and Tessler, M. M. (1987). Polysaccharides Containing Acetal Groups, Their Preparation from the Corresponding Acetals and Use as Paper Additives, National Starch and Chemical Corporation, Bridgewater, NJ.
Staffas, L., Gustavsson, M., and McCormick, K. (2013). “Strategies and policies for the bioeconomy and bio-based economy: An analysis of official national approaches,” Sustainability-Basel 5(6), 2751-2769. DOI: 10.3390/su5062751
Stewart, R. J., Ransom, T. C., and Hlady, V. (2011a). “Natural underwater adhesives,” J. Polym. Sci. Pol. Phys. 49(11), 757-771. DOI: 10.1002/polb.22256
Stewart, R. J., Wang, C. S., and Shao, H. (2011b). “Complex coacervates as a foundation for synthetic underwater adhesives,” Adv. Colloid Interfac. 167(1-2), 85-93. DOI: 10.1016/j.cis.2010.10.009
Su, S., Nutiu, R., Filipe, C. D. M., Li, Y., and Pelton, R. (2007). “Adsorption and covalent coupling of ATP-binding DNA aptamers onto cellulose,” Langmuir 23(3), 1300-1302. DOI: 10.1021/la060961c
Syverud, K., Pettersen, S. R., Draget, K., and Chinga-Carrasco, G. (2015). “Controlling the elastic modulus of cellulose nanofibril hydrogels—Scaffolds with potential in tissue engineering,” Cellulose, 22, 473-481. DOI: 10.1007/s10570-014-0470-5
Tejado, A., and van de Ven, T. G. M. (2010). “Why does paper get stronger as it dries?,” Mater. Today 13(9), 42-49. DOI: 10.1016/S1369-7021(10)70164-4
Träger, A., Pendergraph, S. A., Pettersson, T., Halthur, T., Nylander, T., Carlmark, A., and Wågberg, L. (2016). “Strong and tuneable wet adhesion with rationally designed layer-by-layer assembled triblock copolymer films,” Nanoscale 8(42), 18204-18211. DOI: 10.1039/C6NR05659H
Trout, P. E. (1951). “The mechanism of the improvement of the wet strength of paper by polyethylenimine,” Tappi 34, 539-544.
Vishtal, A., and Retulainen, E. (2014). “Improving the extensibility, wet web and dry strength of paper by addition of agar,” Nord. Pulp Pap. Res. J. 29(3), 434-443. DOI: 10.3183/NPPRJ-2014-29-03-p434-443
Wågberg, L., and Björklund, M. (1993). “On the mechanism behind wet strength development in papers containing wet strength resins,” Nord. Pulp Pap. Res. J. 8(1), 53-58. DOI: 10.3183/NPPRJ-1993-08-01-p053-058
Wågberg, L., Forsberg, S., Johansson, A., and Juntti, P. (2002). “Engineering of fibre surface properties by application of the polyelectrolyte multilayer concept. Part I: Modification of paper strength,” J. Pulp Pap. Sci. 28(7), 222-228.
Wen, Q., and Pelton, R. (2012). “Microgel adhesives for wet cellulose: Measurements and modeling,” Langmuir 28(12), 5450-5457. DOI: 10.1021/la2050493
Wicklein, B., Kocjan, A., Salazar-Alvarez, G., Carosio, F., Camino, G., Antonietti, M., and Bergström, L. (2015). “Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide,” Nat. Nanotechnol. 10(3), 277-283. DOI: 10.1038/nnano.2014.248
Wu, T., and Farnood, R. (2014). “Cellulose fibre networks reinforced with carboxymethyl cellulose/chitosan complex layer-by-layer,” Carbohydr. Polym. 114, 500-505. DOI: 10.1016/j.carbpol.2014.08.053
Yang, D. (2018). Adhesives with Controllable Degradability for Wet Cellulosic Materials, Ph.D. Thesis, McMaster University, Hamilton, Canada.
Yang, D., DiFlavio, J.-L., Gustafsson, E., and Pelton, R. (2018a). “Wet-peel: A tool for comparing wet-strength resins,” Nord. Pulp Pap. Res. J. DOI: 10.1515/npprj-2018-0013
Yang, D., Gustafsson, E., Stimpson, T. C., Esser, A., and Pelton, R. H. (2017). “Hydrazide-derivatized microgels bond to wet, oxidized cellulose giving adhesion without drying or curing,” ACS Appl. Mater. Inter. 9(24), 21000-21009. DOI: 10.1021/acsami.7b04700
Yang, D., Stimpson, T. C., Soucy, J., Esser, A., and Pelton, R. H. (2018b). “Increasing wet adhesion between cellulose surfaces with polyvinylamine,” Cellulose, on line.
Young, R. A. (1978). “Bonding of oxidized cellulose fibers and interaction with wet strength agents,” Wood Fiber Sci. 10(2), 112-119.
Zauscher, S., and Klingenberg, D. J. (2001). “Friction between cellulose surfaces measured with colloidal probe microscopy,” Colloid. Surface. A 178(1-3), 213-229. DOI: 10.1016/S0927-7757(00)00704-4
Zauscher, S., and Klingenberg, D. J. (2000). “Normal forces between cellulose surfaces measured with colloidal probe microscopy,” J. Colloid Interf. Sci. 229(2), 497-510. DOI: 10.1006/jcis.2000.7008
Zhang, D., Tanaka, H., and Pelton, R. (2007). “Polymer assembly exploiting three independent interactions,” Langmuir 23(17), 8806-8809. DOI: 10.1021/la700711a
Zhao, Q., Lee, D. W., Ahn, B. K., Seo, S., Kaufman, Y., Israelachvili, J. N., and Waite, J. H. (2016). “Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange,” Nat. Mater. 15(4), 407-412. DOI: 10.1038/nmat4539
Zhong, C., Gurry, T., Cheng, A. A., Downey, J., Deng, Z., Stultz, C. M., and Lu, T. K. (2014). “Strong underwater adhesives made by self-assembling multi-protein nanofibres,” Nat. Nanotechnol. 9(10), 858-866. DOI: 10.1038/nnano.2014.199
Article submitted: October 26, 2018; Peer review completed: January 1, 2019; Revised version received and accepted: January 2, 2019; Published: January 15, 2019.
DOI: 10.15376/biores.14.1.Pelton
APPENDIX
Table A1. Acronyms and Abbreviations
