Abstract
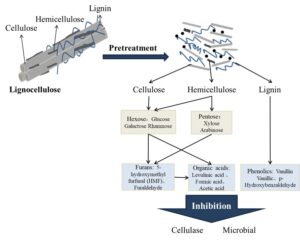
Lignocellulosic biomass is a renewable resource that is widely abundant and can be used to produce biofuels such as methanol and ethanol. Because biofuels have the potential to alleviate shortages of energy in today’s world, they have attracted much research attention. The pretreatment of lignocellulose is an important step in the conversion of biomass products. The pretreatment can destroy the crosslinking effect of lignin and hemicellulose on cellulose, remove lignin, degrade hemicellulose, and change the crystal structure of cellulose. The reaction area between the enzyme and the substrate is enlarged, and the yield of subsequent enzymatic hydrolysis and microbial fermentation products is significantly increased. Conventional pretreatment methods help convert lignocellulosic material to sugars, but the treatments also produce some inhibitors, which are mainly organic acids, aldehydes, phenols, and other substances. They may affect the subsequent saccharification and growth of fermentation microorganisms, thereby reducing the bioconversion of the lignocellulose. It is therefore necessary to take effective means of detoxification. This paper reviews lignocellulose pretreatment methods, with an emphasis on inhibitors and their management. A summary is provided of detoxification methods, and the future use of lignocellulosic biomass for fuels prospects.
Download PDF
Full Article
Production and Detoxification of Inhibitors during the Destruction of Lignocellulose Spatial Structure
Xingxing Luo,a Baiquan Zeng,a,* Yanan Zhong,a and Jienan Chen c
Lignocellulosic biomass is a renewable resource that is widely abundant and can be used to produce biofuels such as methanol and ethanol. Because biofuels have the potential to alleviate shortages of energy in today’s world, they have attracted much research attention. The pretreatment of lignocellulose is an important step in the conversion of biomass products. The pretreatment can destroy the crosslinking effect of lignin and hemicellulose on cellulose, remove lignin, degrade hemicellulose, and change the crystal structure of cellulose. The reaction area between the enzyme and the substrate is enlarged, and the yield of subsequent enzymatic hydrolysis and microbial fermentation products is significantly increased. Conventional pretreatment methods help convert lignocellulosic material to sugars, but the treatments also produce some inhibitors, which are mainly organic acids, aldehydes, phenols, and other substances. They may affect the subsequent saccharification and growth of fermentation microorganisms, thereby reducing the bioconversion of the lignocellulose. It is therefore necessary to take effective means of detoxification. This paper reviews lignocellulose pretreatment methods, with an emphasis on inhibitors and their management. A summary is provided of detoxification methods, and the future use of lignocellulosic biomass for fuels prospects.
DOI: 10.15376/biores.17.1.Luo
Keywords: Pretreatment; Inhibitors; Detoxification
Contact information: a: College of Life Science and Technology, Central South University of Forestry & Technology, Changsha, 410018, China; b: Ministry of Forestry Bioethanol Research Center, Changsha, 410018, China; c: Hunan Engineering Research Center for Woody Biomass Conversion, Changsha 410018, China; *Corresponding author: baiquanzhn@163.com
GRAPHICAL ABSTRACT

INTRODUCTION
In the era of rapid development of industrialization, mankind’s demand for energy is increasing. Shortages are anticipated in oil, coal, natural gas, and other resources, and pollution-related energy usage can lead to serious ecological environment problems. Vigorously developing renewable energy is of great significance for avoiding the usage of fossil fuels and improving the ecological environment. Lignocellulosic biomass is the only predictable sustainable resource in the world that can provide humans with materials and fuels. It has abundant reserves and wide sources, which can effectively overcome the oncoming shortage of traditional fuels (Raud et al. 2016; Valdivia et al. 2016). In recent years, research on the pretreatment of wood fiber and bioenergy conversion technology of its products has attracted continuous attention at home and abroad (Xavier et al. 2010). Biomass resources are widely distributed on the earth and are dozens of times as much as fossil energy, but their development is slow due to a low utilization rate. Common lignocellulosic substances include waste rice husk, straw, poplar sawdust, etc. (Yu et al. 2018). Due to the complex chemical structure of lignocellulose, the components of cellulose, hemicellulose, and lignin are cross-linked with each other, and it is difficult to separate the components under normal conditions, which affects the conversion of lignocellulose to biomass fuels. The limitation is that only in the presence of a catalyst will it be hydrolyzed into sugars (Rastogi and Shrivastava 2017), and then microbial fermentation and other methods can be used to produce the required products (Bajwa et al. 2011).
LIGNOCELLULOSIC PRETREATMENT METHODS
Basic Composition of Lignocellulose
Lignocellulose is composed of three main components: cellulose, hemicellulose, and lignin (Koddenberg 2016), and the biotransformation process is shown in Fig. 1. Among them, cellulose is composed of glucose through glycosidic bonds, and its properties are relatively stable (Abdelaziz and Hulteberg 2017).
Hemicellulose is a branched heteropolysaccharide, of which different versions are composed of xylose, arabinose, galactose, glucose, mannose, etc., which can carry acetyl groups (Cullis and Mansfield 2010). It is interconnected with cellulose to form a hard network. The hemicellulose structure contains branched chains, so the crystallinity is lower and more unstable, and it is more prone to degradation (Emmel et al. 2003).
Lignin is a high-molecular amorphous polymer formed by three phenylpropane units connected to each other in a non-linear manner (Diwan et al. 2017). Usually, lignin is mainly located between hemicellulose and cellulose (Emmel et al. 2003), which constitutes a lignin-carbohydrate complex, provides structural support, and plays a role in resisting pressure. The higher the proportion of lignin, the less likely it is to be degraded by chemical reagents and enzymes.
Fig. 1. A schematic view of a lignocellulosic conversion process
Types of Pretreatment
Through the pretreatment of lignocellulose, the structure is damaged to varying degrees, which is beneficial to hydrolysis. According to a single preprocessing method or a combination of multiple methods, the preprocessing can be divided into traditional preprocessing and new preprocessing classification. Table 1 lists common pretreatment methods.
Table 1. Pretreatment Methods and Influence on Lignocellulose
Traditional Pretreatment Methods
Mechanical pulverization is a commonly used physical method. It mainly reduces the size of lignocellulose particles through traditional mechanical pulverization and makes them loosely arranged to increase the reaction area between lignocellulose and subsequent reagents, thereby improving utilization efficiency. However, mechanical pulverization only has the effect of reducing the size of lignocellulose particles, without destroying the chemical structure of lignin, and has almost no effect on the degradation of hemicellulose and lignin (Zeng et al. 2007; Xu et al. 2016).
Acid pretreatment is a relatively mature pretreatment method of biomass energy. It usually takes concentrated or dilute acid (Emmel et al. 2003; Karapatsia et al. 2017). Under certain temperatures and pressure, acid molecules will break the glycosidic bond between cellulose and hemicellulose, thereby partially or completely hydrolyzing hemicellulose into monosaccharides, leading to the destruction of the structure of lignocellulose (Tan 2015). In the pretreatment process, when the acid concentration is more than 10%, it is regarded as concentrated acid, the temperature is usually less than 100 ℃, the dilute acid concentration is usually less than 5%, and the reaction temperature is relatively high, i.e. 100 to 240 ℃ (Jönsson and Martín 2016). Acid pretreatment has been widely used due to its low cost, rapid reaction, and good treatment effect, but it has the disadvantage of strong corrosiveness (Hang et al. 2021). Song et al. (2014) used 1% H2SO4, 2% HCl, 3% H2O2 and 4% CH3COOH to pretreat corn stalks at 100 °C for 7 days and then produce methanol. The yields were increased by 62.4%, 74.6%, and 44.2%, respectively, compared with the untreated. Geddes et al. (2010) treated sugarcane with dilute phosphoric acid at 160 ℃, and the degradation rate of hemicellulose was greatly improved. However, in the process of acid hydrolysis, lignocellulosic will produce a large number of inhibitory substances that affect the subsequent fermentation, which restricts the development of the fuel industry and related industries (Zaldivar et al. 2000).
Alkaline pretreatment is also widely used in the pretreatment process. Compared with acid treatment, it is relatively mild (Silverstein et al. 2007; Kamran et al. 2020). Commonly used alkaline reagents include NaOH, Ca(OH)2, KOH, and ammonia. Alkaline pretreatment successfully removes lignin from biomass by destroying the biomass. The OH– ion can destroy lignin and xylan, ester bonds between hemicellulose, and other components, thus increasing the porosity of lignocellulose and reducing its crystallinity (McIntosh and Vancov 2010). These changes favor an amorphous wood fiber structure, thereby reducing the crystallinity and polymerization degree of cellulose. Among the commonly used alkaline reagents, NaOH is the most studied and the most common (Chen et al. 2012). An et al. (2021) used 2% NaOH to pretreat at 121 °C for 10 minutes, as well as enzymatic hydrolysis. The glucose content (GC) and enzymatic hydrolysis degree (ED) were 46.7% and 55.3%, respectively. After being pretreated with NaOH, it was 2.4 times and 2.5 times higher than the control (untreated). Continuing to use Saccharomyces cerevisiae K35 for ethanol fermentation, the results showed that the highest ethanol yield can reach 41.3 g, which is 2.4 times higher than that of the control group. Jiang et al. (2020) studied the pretreatment of reeds with NaOH and Ca(OH)2. The highest sugar yields of NaOH and Ca(OH)2 pretreatment were 44.9% and 25.8%, respectively. These were 85% and 70% higher than untreated giant reeds, respectively.
The hydrothermal method is a kind of green pretreatment method. Robinson et al. (2015) used hot water to pretreat bagasse and bamboo at different temperatures and different times, and after optimization, the reducing sugar content reached 26.5 and 17.98 g/L, respectively. Harahap and Kresnowati (2018) used oil palm empty fruit bunch (OPEFB) as raw material to prepare xylitol and autohydrolysis at 1.5 barg/127.9 °C for 60 minutes to obtain the best yield. The yield of xylose was 0.085 g xylose/g OPEFB. At the same time, because of its natural pH, the hydrolysate can be used directly as a fermentation substrate. Meilany et al. (2020) used oil palm empty fruit bunches (OPEFBs) as raw materials; the effects of different pretreatment processes on the sugar recovery of lignocellulosic biomass were studied. The hydrothermal process was used to enzymatically hydrolyze OPEFB. The effects of temperature, solid-phase loading, and pretreatment time on the hydrothermal of OPEFB were investigated. The concentration of xylose in the hydrolysate of OPEFB was analyzed by high-performance liquid chromatography. The results showed that the pretreatment time for maximum xylose recovery was 165◦C, and the xylose recovery rate of pretreated OPEFB was 0.061 g/g (35%)
The organosolv method is also commonly used as a pretreatment method. Lignin can be effectively dissolved by organic solvents (Mesa et al. 2011). After the material is pretreated, it is recycled and reused by distillation, and the pretreated product can be used as a chemical raw material (Hamidah et al. 2017). Zhang et al. (2018) under the conditions of 120 ℃, 10 min, and microwave irradiation, used methanol/dioxane binary solvent pretreated poplar while achieving over 99% of conversion of enzymatic saccharification. Amiri et al. (2014) used rice straw as raw material, pretreated with 1% sulfuric acid in 75% ethanol aqueous solution at 150 °C for 60 min, and the total enzymatic sugar concentration was as high as 31 g/L. Compared with alkaline pretreatment, the lignin dissolved by ethanol had less structural degeneration and a more uniform molecular weight. However, organic solvents such as ethanol have the disadvantages of high volatility, high temperature, high requirement for equipment, and a long time requirement (Tang et al. 2017).
New Pretreatment Methods
The disadvantages of traditional pretreatment methods are long times, high equipment requirements (Chin et al. 2020), high economic cost, low enzymatic hydrolysis efficiency, and the fact that they may cause a certain degree of corrosion of the instrument. Therefore, some new pretreatment methods have been developed and utilized. The conditions employed are relatively mild in these new pre-treatment methods, which implies low equipment requirements. In addition, there have been improvements in efficiency and the hydrolysis reaction period had been shortened.
Steam explosion takes place at high temperature and pressure. the rapid heating method using saturated steam dissolving lignocellulose, broad applicability, is an environmentally friendly pretreatment (Yoo et al. 2011; Ewanick and Bura 2011; de Albuquerque Wanderlay et al. 2013). It can damage the structure of the arrangement and degrade the hemicellulose component of lignocellulose, thus effectively improving the efficiency of enzymatic hydrolysis and enhancing the conversion efficiency (Martín-Sampedro et al. 2012; Yu et al. 2020; Cubas-Cano et al. 2020). For example, Borand et al. (2020) used pine to conduct steam explosion treatment at 190 ℃ for 10 min, and the final glucose yield was 97.7%, whereas xylose, mannose, galactose, and arabinose were 85.6%, 87.8%, 86.4%, and 90.3%, respectively. He et al. (2019) used steam explosion pretreatment at a pressure of 1.5 MPa to pre-treat seabuckthorn; it was found that a reaction of 20 minutes could remove 77.2% of hemicellulose, and the sugar yield after enzymatic hydrolysis was 4.54 times that of the untreated control.
Alkali hydrogen peroxide pretreatment (AHP) is one of the most promising pretreatment methods (Liu et al. 2017). AHP mainly uses H2O2 to remove lignin in an alkaline medium, while increasing the dissolution of hemicellulose. The degree of dissolution depends on the pH of the reaction. Chen et al. (2020b) established an improved method of analytic hierarchy process (AHP) for delignification; at 100 ℃ the removal percentage of lignin reached 80.0%, which was higher than the 74.9% level that was achieved in the pretreatment without ethanol. The effect of alkaline hydrogen peroxide (AHP) pretreatment on bamboo stalk and bamboo stalk structure changes and enzymatic hydrolysis was studied. The results showed that after AHP pretreatment, compared with the chemical composition of raw materials, cellulose increased by 36.9%, hemicellulose decreased by 50.7%, lignin decreased by 37.9%, and 370 mg/g reducing sugars were obtained after enzymolysis (Nasir et al. 2020).
Wang et al. (2018) used ozone-NaOH combined pretreatment of corn stover to achieve a removal percentage of lignin up to 84.4%, which could not only reduce the amount of NaOH but also achieve a maximum enzymatic hydrolysis efficiency of up to 91.7%. Dai et al. (2015) adopted the pretreatment method combining bacteria with NaOH-urea to improve the enzymatic hydrolysis performance of the rice straw. The results showed that combined pretreatment increased the yield of reducing sugar and glucose by 1.40 and 1.37 times, respectively, compared with single pretreatment.
The method of combining organic solvent and cellulose solvent has been applied for pretreatment. Zhang et al. (2007) treated rice straw with ethanol and concentrated phosphoric acid under the condition of atmospheric pressure and 50 ℃, which could greatly break the crystallinity of lignin, make the substrate become irregular, and remove hemicellulose, thus improving the efficiency of enzymatic conversion. Compared with a single pretreatment method, it greatly reduces the number of enzymes used, and the enzymatic hydrolysis time is significantly reduced, which has good application value.
Electrochemistry is a method that uses the phenomena of charged interfaces between conductors and the changes that occur (Huang et al. 2012). Electrochemical pretreatment can destroy the refractory bonding structure of lignin, which is an economical and effective method for lignin degradation. This process has mild reaction conditions, no need to add chemicals, and the ability to be used over a wide range. Accordingly, it can be regarded as a green pollution-free pretreatment process. Cui et al. (2016) proposed a technology for synergistic degradation of lignin that combines electrochemical oxidation (ECO) and biodegradation (BD). The results showed that after ECO treatment, the biodegradability of lignin increased from the range 0.20-0.25 to 0.31-0.37. As a result, the subsequent biodegradation time was reduced. However, the technology is not mature enough and is currently in the laboratory stage. Future research of this technology is needed to establish and improve the electrochemical pretreatment technology so that it can be used in industrial production.
Inhibitor Production during Pretreatment and their Inhibitory Effects
Lignocellulosic substances will produce a series of low-molecular-weight fermentation inhibitors during the hydrolysis process (Hidayatullah et al. 2020). Table 2 shows several common types of inhibitors, which seriously affect the fermentation and utilization of substrates by microorganisms.
Inhibition Mechanism of Acids
Formic acid, acetic acid, and a small amount of levulinic acid is a common combination of inhibitory substances (Parawira and Tekere 2011). The main formation mechanism of these inhibitors is that acid can degrade hemicellulose, which generates pentylene under the action of acid. Sugar, acetic acid (Ravindran and Jaiswal 2016), pentoses, and hexoses are dehydrated to form furfural and 5-hydroxymethylfurfural (5-HMF) (Wang et al. 2019; Cola et al. 2020). 5-HMF and furfural will decompose with the generation of formic acid (van der Pol et al. 2014; Chen et al. 2019). Studies have shown that under the same pH value, formic acid has a lower pKa value than acetic acid and levulinic acid, and the degree of dissociation of formic acid is also smaller than that of acetic acid and levulinic acid. It is easy to enter the cell membrane in molecular form and inhibit microbial activity. Therefore, the toxicity of formic acid is higher than that of acetic acid and levulinic acid (Hyland et al. 2013). Generally, there will be no separate acid inhibitors, and if furan aldehydes are or phenols are present, they would inhibit the enzyme activity; in addition, the presence of microorganisms (Li et al. 2016) would influence enzymatic hydrolysis and fermentation.
Table 2. Types, Sources, and Inhibitory Effects of Inhibitors
Inhibition Mechanism of Phenolic Substances
Phenolic substances in lignocellulosic pretreatment solution are mainly lignin degradation products, usually aromatic compounds containing a benzene ring. There are dozens of phenolic substances identified now (Viegas and Sá-Correia 1991; Varga et al. 2004). Although the content of phenolic substances is relatively low, the inhibition effect is more serious and affects the subsequent fermentation of hydrolysate (Palmqvist and Hahn-Hägerdal 2000). Even at low concentrations, phenolics exhibit very strong inhibition of fermentation (Chen and Dou 2016). Examples include vanillin, syringaldehyde, and vanillic acid, which provide the most significant inhibition of fermentation (Moreno et al. 2012). Compared with acid substances, vanillin in the concentration of 4 g/L completely inhibits the sugar utilization in Saccharomyces cerevisiae, and 6 g/L completely inhibits ethanol fermentation (Soudham et al. 2014). Heipieper phenolic compounds have been found to have an important impact on fermentation, and the explanation is that the material can infiltrate into the cell membrane and destroy the integrity of the cell membrane; this inhibits the normal growth of microbial cells, hurts fermentation efficiency, and reduces the efficiency of the fuel ethanol (Yi et al. 2015; Chen et al. 2020c). Different from weak acids and furan aldehydes, phenolic substances not only significantly inhibit the growth of microorganisms, but also significantly reduce the activities of cellulase and hemicellulase (Taherzadeh et al. 2000; Rahikainen et al. 2017).
Phenolic substances are the most significant inhibitors of inhibition in enzymatic hydrolysis or microbial fermentation, and the molecular weight and the position of substituents (meta-position, ortho-position, para-position) are important factors that affect the inhibition effect of phenolic substances (Qin et al. 2016; Ishida et al. 2017). Generally speaking, the lower the molecular weight, the higher the toxicity (Ladeira Ázar et al. 2017).
Inhibition Mechanism of Aldehydes
The aldehyde inhibitor category consists of furfural and 5-HMF (Field et al. 2015), which are the by-products of lignocellulosic pretreatment liquid having the greatest content and the highest toxicity. They are furan derivatives of five-carbon sugars and six-carbon sugars. They enter cells by means of active transport and have little inhibition of cellulase, mainly inhibiting the growth of microorganisms (Palmqvist et al. 1999). Studies have shown that furfural and HMF can affect intracellular respiration and thus affect the glycolytic pathway in vivo (Iwaki et al. 2013). Sárvári et al. (2003) believed that Saccharomyces cerevisiae could reduce furfural to furfuryl alcohol. Such a reduction process is expected to cause a large amount of consumption of coenzyme NADH, leading to an imbalance in xylose metabolism. Antioxidant proteins are also inactivated due to the reduction of available coenzymes, making yeast cells vulnerable to oxidative damage (da Silva et al. 2017). The work of Jung et al. (2019) showed that when only furfural or HMF was present, the ethanol yield of the final fermentation was less affected. Some studies have shown that when furfural and HMF are both present, the normal metabolic activities of microorganisms are inhibited, thus reducing the ethanol yield in the fermentation process (Iwaki et al. 2013). In addition, furfural can also lead to the accumulation of reactive oxygen species in yeast (reactive oxygen species, ROS), resulting in damage to the cell nucleus and may even induce cell death (Song et al. 2017).
Inhibition Mechanism of Metal Ions
In the pretreatment process of lignocellulose, due to corrosion of mechanical equipment or added chemical substances, some metal ions will be solubilized, such as iron and chromium. These ions will inhibit the activities of metabolism-related enzymes of microorganisms, and they are not conducive to the growth and fermentation process of microorganisms (Mussatto and Roberto 2003).
Detoxification of Inhibitors
The inhibitors in the lignocellulose pretreatment liquid seriously affect the subsequent enzymatic hydrolysis and saccharification process of enzymes and microorganisms, which is also one of the main difficulties in the comprehensive utilization of lignocellulose. Therefore, effective measures must be taken to reduce or remove the negative effects of inhibitors and improve the utilization rate of lignocellulose, in order to achieve large-scale production of biomass energy. In recent years, scholars throughout the world have tried various methods around how to get rid of inhibitors and made some new progress. As shown in Table 3, the approaches employed can be divided into chemical, physical, biological, and integrated detoxification methods.
Table 3. Common Detoxification Methods and Their Advantages and Disadvantages
Physical Methods
Physical methods are usually used to remove part of the inhibitor in the pretreatment solution by physical method to reduce or eliminate its inhibitory effect, which is the simplest method of detoxification. Common methods include rotary evaporation (Llano Astuy et al. 2017) adsorption, extraction, and membrane filtration.
Adsorbent Method
The adsorption method usually involves adding an adsorbent to the pretreatment liquid to combine with the inhibitor and precipitate, thereby removing the inhibitor (Llano Astuy et al. 2017). Activated carbon has a strong adsorption effect and low cost, so it has become one of the commonly used adsorbents. In addition, new adsorbents and cross-linked polyethyleneimine (PEI), and other adsorbents are also unique (López-Linares et al. 2016; Huang et al. 2018). The structure and excellent adsorption performance have attracted widespread attention. Ravindran and Jaiswal (2016) used activated carbon to adsorb by-products in the hydrolysate of cotton stalk after sodium hydroxide pretreatment, which reduced the total mass of furfural by 59.1%. However, the disadvantage of this treatment method is the loss of higher reducing sugars. Substances such as adsorption resin and PEI can selectively adsorb furan, fatty acid, and phenolic substances in the lignocellulose pretreatment solution, and then use the desorption mechanism to recover these substances (Carter et al. 2011). Deng et al. (2018) used PEI and poly-diallyldimethylammonium chloride (pDADMAC) to adsorb and recover formic acid, acetic acid, and levulinic acid in the bagasse enzymatic hydrolysate, after dilute ammonia pretreatment, furfural, 5-HMF, and phenolic were reduced. The results showed that the effect was the best when the pH was 4.5, and when the addition amount of PEI and pDADMAC was 15 g/L. It was able to remove 43 per of organic acids, 73 per of total phenolic compounds, and 100 per of furan aldehyde compounds. The loss of fermentable sugar was less than 10%.
Solvent Extraction Method
The solvent extraction method employs solvent to separate the inhibitors from the fermentation broth because of the difference in the solubility of sugars and inhibitors in the extracting agent. The commonly used extractants are n-hexane, chloroform, and ethyl acetate. Studies have shown that extraction with ethyl acetate can remove peracetic acid and all furfural, vanillin, and 4-hydroxybenzoic acid in lignocellulose hydrolysate (Palmqvist and Bärbel 2000). Zhang et al. (2005) studied the effect of several different extractants on inhibitors, and the results showed that among the three organic solvents, n-hexane had a poor removal effect on several inhibitors, while ethyl acetate and chloroform had a higher removal effectiveness for furfural, but the removal effect on phenolic compounds was poor. This is because the polarity of furfural is less than that of phenolic compounds. As a consequence, the solubility of furfural in organic solvents such as ethyl acetate and chloroform is higher, so the removal of furfural is higher than that of phenolic substances when extracted with organic solvents. Because xylose is almost insoluble in organic solvents, extraction with organic solvents has little effect on it.
Membrane Separation Method
The removal of various inhibitors used in nanotechnology has the characteristics of separation and low energy consumption, etc. (Benkun et al. 2012; Abels et al. 2013). At present, most of them are used in the removal of inhibitors in acid pretreatment solution. Brás et al. (2014) used a nano-separation membrane to detoxify the pretreatment solution of olive residue, and the results showed that 99 per of furan aldehydes, acetic acid, and formic acid could be removed, and the loss rate of reducing sugar reached 40%. When Jiang et al. (2018) used nano-separation membrane to detoxify dilute acid pretreatment solution, they found that the removal rate of inhibitors was affected by solution pH, osmotic flux, and concentration of Na2SO4. When treated with low pH and high concentration of Na2SO4 for 35 min, 90 per of the inhibitors could be removed. Chen et al. (2020a) used reverse osmosis (RO) membrane to simultaneously concentrate sugar and remove inhibitors, and the results showed that the removal rates of furfural and acetic acid reached 51.6% and 77.7%, respectively. This method has a great application prospect in the production of bioethanol. Membrane separation technology has unique advantages in the detoxification of lignocellulosic pretreatment solution. However, the loss rate of reducing sugar is high, and the pretreatment inhibition species are complex, so it is difficult to remove all the inhibitors by single membrane separation technology. In addition, the cost of nano-separation membrane is high, so it cannot be industrialized to achieve large-scale production.
Chemical Methods
Chemical methods mainly use chemical reagents to react with inhibitors in hydrolysate to bring about their precipitation. Alternatively, they can work by changing the pH and ionization properties of some inhibitors, so as to reduce the toxicity of inhibitors. Commonly used chemical reagents include alkali and reducing agents. Of these, persulfate has received more study for detoxification in recent years.
Alkaline Method
Adding excess alkaline substances to the hydrolysate to reduce inhibitors is a common alkaline detoxification method. Sodium hydroxide, ammonia, and calcium hydroxide are common alkaline detoxification agents. Alriksson et al. (2005) added NH4OH, Ca(OH)2, Mg(OH)2, Ba(OH)2, and NaOH to the dilute acid hydrolysate of spruce for detoxification, and compared the inhibitor removal effect. Treatment with NH4OH can significantly reduce furfural and 5-hydroxymethyl furfural. Zhang et al. (2012) used calcium hydroxide to detoxify the corn stalk hydrolysate and found that the content of inhibitors in the corn stalk hydrolysate changed significantly. It was found that 38.8% furfural, 45.9 %, 5-hydroxymethyl furfural, and 3 % of total phenolic compounds were removed. Ikram et al. (2018) used 3 kinds of alkali, namely NaOH, NH4OH, and Ca(OH)2, to study the detoxification of pretreated wheat straw. When Ca(OH)2 was immersed in the solution for 2 h, at pH=12 and 80 ℃, 60% of the phenolic compounds were obviously removed, and the sugar conversion was increased 2.4 times. The results of scanning electron microscopy (SEM) on the substrate after alkali pretreatment showed that the lignin cellulose structure had a great change, in which the lignin was degraded, so it was more susceptible to enzymatic hydrolysis. However, the disadvantage of alkali detoxification is that the amount of alkali is large, and neither alkali nor inhibitor can be recovered (Hamidah et al. 2017; Zhang et al. 2018).
Reducing Agent Method
This method works by adding reducing agents to the hydrolysate to achieve the effect of detoxification. Commonly used reducing agents are sulfites and hydrogen peroxide. The reducing agent method is easy to operate, can improve the fermentation effect, and ensure that the reducing sugar in the hydrolysate is not lost, and has a good development prospect. Alriksson et al. (2011) conducted SHF (simultaneous hydrolysis and fermentation) experiments on pretreatment hydrolysates of spruce or bagasse treated with bisulfite and sulfite, and the results showed that the ethanol yield of spruce hydrolysate fermented by bisulfite treatment increased from 0.2 g/(L·h)-1 to 2.5 g/(L·h)-1, and that of bagasse fermented ethanol yield increased from 0.9 g/(L·h)-1 to 3.9 g/(L·h)-1. Soudham et al. (2014) used 2.5 mmol/L FeSO4 and 150 mmol/L H2O2 to treat the hydrolysates of Chinese fir and found that this method could effectively remove 29 per of 5-HMF, 34 per of furfural, and 24 per of phenolic substances, and increase the ethanol yield from 0.4 g/L to 8.3 g/L. The use of reductant is a common method of detoxification, but the disadvantage is that the detoxification range is small, and there is low specificity.
Persulfate Method
Persulfate, which is a strong oxidizer, is relatively stable at room temperature and soluble in water (Cong et al. 2015). Persulfate can produce the sulfate radical, which can selectively oxidize and degrade phenol material. For these reasons, this technique is often used in the environmental engineering field containing phenol wastewater treatment. The treatment of phenol-containing wastewater in the engineering field is similar to treatment of lignocellulose degradation products. In recent years, the persulfate method has been used as a new technology for the detoxification of hydrolysate. UV (Gao et al. 2012), heat (Liang et al. 2003), acids, bases, and transition metals (Yang et al. 2009) are all effective in activating persulfates. Compared with OH–, S2O82− has a wider pH range and longer duration, so it has a broad application prospect in the degradation of hydrolysate inhibitors (Wu et al. 2020). Rong et al. (2016) and others used a new type of detoxification technology-heat activated persulfate advanced oxidation to remove the typical inhibitors in the production process of bioethanol (vanillin, syringaldehyde, 4-hydroxybenzaldehyde, vanillic acid, syringic acid, and 4-hydroxybenzoic acid). Studies have shown that the effect of thermally activated persulfate oxidative degradation products is significant, and the removal percentages of vanillin, 4-hydroxybenzaldehyde, vanillic acid, 4-hydroxybenzoic acid, and syringic acid all reach 100% within 1 h. Similarly, the removal of syringaldehyde can reach 100% within 2 h. Zhou et al. (2021) used activated persulfate to degrade phenols, and the efficiency reached 98.2 per within 120 minutes. The main mechanism is that the sulfate radical reacts with the inhibitor to degrade it into small non-toxic substances.
Biological Methods
Biological methods usually use microorganisms or enzymes to act on the inhibitors in the hydrolysate, and then they react with them to produce low-toxicity or non-toxic substances, so as to achieve the purpose of improving the fermentation efficiency. Biological methods are generally divided into two categories: microbial and enzymatic methods. The advantages of biological methods are specificity, mild conditions, and no introduction of new impurities.
Enzymatic Detoxification
Saravanakumar et al. (2016) used a new class of material – nanofibers – to immobilize laccase and found that furfural, acetosyringone, and coniferous aldehyde in the lignocellulose hydrolysate can be completely removed by reacting at 40 °C for 36 hours. The sugar loss is low. Tramontina et al. (2020) added peroxidase and superoxide dismutase to the bagasse hemicellulose hydrolysate for detoxification. The results showed that butanol was produced by the fermentation of the bacterium Clostridium spp. and ethanol was produced by the action of yeast. Compared with the hydrolyzed solution without detoxification, it increased by 24 times and 2.4 times, respectively. Studies have found that the detoxification effect of laccase is related to the time it is added (Jurado et al. 2009; Oliva-Taravilla et al. 2015).
Microbial Detoxification Method
White rot fungi (WRF) is a commonly used group of microorganisms suitable for detoxification. Specifically, WRF usually degrades furfural, acids, and aromatic aldehyde compounds (Bulter et al. 2003; Hasunuma et al. 2011). Nichols et al. (2008) added the fungus Coniochaeta ligniaria NRRL30616 to the dilute acid hydrolysate of corn stalk to removed the toxic components. Quantitative analysis showed that 5-hydroxymethyl furfural, furfural, and phenolics were effectively removed, and only small amounts of glucose (usually 2.5 g/L or less) were lost.
A breakthrough was made in the study of inhibitor-tolerant strains for maintaining efficient saccharification in the presence of multiple inhibitors. Fonseca et al. (2011) treated bagasse hydrolyzate with the strain of Issatchenkia occidentalis (CCTCC M 206097) and found that the concentration of reducing sugar did not decrease after 24 h of detoxification, while the concentrations of syringaldehyde, ferulic acid, furfural, and 5-HMF decreased by 62%, 67%, 33%, and 85%, respectively. Other studies have found that strain Y-50049, induced by aldehydes and 5-HMF, is activated and expressed in ZWF1 to produce NADPH, which can assist aldehyde reductase to reduce furfural and 5-HMF and can remove furfural aldehydes (Todhanakasem et al. 2018). With the rapid development of modern molecular biology techniques, it is possible to breed and screen out the strains with high efficiency and strong tolerance, and it is possible to use genetic engineering methods to clone the genes of virus-free strains and construct a variety of engineering strains with different types and functions. This is a new trend of using microorganisms.
Compound Detoxification Methods
In recent years, researchers have adopted a combined detoxification method, which combines several single methods for detoxification, achieving better detoxification effects and obtaining more fermentation products. There are a variety of inhibitors in the lignocellulose pretreatment solution. The structural components are complex, and each has a synergistic inhibitory effect. Using a single detoxification method cannot achieve the expected effect, and each method has its own shortcomings. Yücel and Aksu (2015) added activated carbon, activated carbon for beet meal, and fly ash to the beet meal hydrolysate for detoxification. Studies have shown that in the presence of CaO, activated carbon has a better adsorption effect on phenols and furan compounds, and fly ash can remove a large number of furan compounds. In addition, Santos et al. (2014) combined ion exchange resin with activated carbon as a new method for removing inhibitors. At a temperature of 30 °C and a flow rate of 2.5 VB/h, the concentration of most furfural, 5-HMF, and phenols decreased. Similarly, Cheng et al. (2017) used organic acids to detoxify the pretreatment liquid by activated carbon and ion exchange resin, and the results showed that the yield of bacterial cellulose reached 2.86 g/L. Tomek et al. (2015) combined enzymes and liquid-liquid extraction to achieve detoxification of inhibitors in the pretreatment solution, which can effectively remove inhibitors and improve enzymatic hydrolysis and fermentation efficiency.
SUMMARY AND PROSPECTS
The use of lignocellulose to produce biofuels such as methanol and ethanol has become an industry trend, and the implementation of such technology is expected to alleviate the energy shortage in the world today. Seeking effective pretreatment methods and detoxification methods is the core of improving the utilization rate of lignocellulose. In order to improve the utilization rate of wood fiber, breakthroughs can be made from the following aspects:
- Explore more effective pretreatment methods, or develop new reagents to increase the degradation rate of wood fiber while producing as few inhibitors as possible;
- Most of the inhibitors in the existing pretreatment process have been determined, but their inhibitory mechanism on enzymes and cellulose has not been fully elucidated, so more in-depth research will be carried out in this part in the future;
- In addition, the effective removal of inhibitors in the pretreatment solution is also an important aspect of research and development. There are many types of inhibitors. The existing detoxification methods cannot completely remove the inhibitors and cause loss of reducing sugars. Therefore, it is necessary to develop materials for efficiently removing inhibitors or use genetic engineering, cell engineering, etc. Modern biotechnology has genetically modified fermenting microorganisms to screen out microorganisms with higher fermentation tolerance, which can maximize the conversion and utilization of biomass resources.
ACKNOWLEDGMENTS
This work was funded by the National Key Research and Development Program of China (No. 2019YFB1503802) and Scientific Research Project of Department of Education of Hunan Province (No.20K144).
REFERENCES CITED
Abdelaziz, O. Y., and Hulteberg, C. P. (2017). “Physicochemical characterisation of technical lignins for their potential valorisation,” Waste and Biomass Valorization 8, 859-869. DOI: 10.1007/s12649-016-9643-9
Abels, C., Carstensen, F., and Wessling, M. (2013). “Membrane processes in biorefinery applications,” Journal of Membrane Science 444, 285-317. DOI: 10.1016/j.memsci.2013.05.030
Alriksson, B., Cavka, A., and Jönsson, L. J. (2011). “Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in-situ detoxification with reducing agents,” Bioresource Technology 102, 1254-1263. DOI: 10.1016/j.biortech.2010.08.037
Alriksson, B., Horváth, I. S., Sjöde, A., Nilvebrant, N.-O., and Jönsson, L. J. (2005). “Ammonium hydroxide detoxification of spruce acid hydrolysates,”Applied Biochemistry Biotechnology 124, 911-922. DOI: 10.1385/ABAB:124:1-3:0911
Amiri, H., Karimi, K., and Zilouei, H. (2014). “Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production” Bioresource Technology 152, 450-456. DOI: 10.1016/j.biortech.2013.11.038
Bajwa, P. K., Phaenark, C., Grant, N., Zhang, X., Paice, M., Martin, V. J. J., Trevors, J. T., and Lee, H. (2011). “Ethanol production from selected lignocellulosic hydrolysates by genome shuffled strains of Scheffersomyces stipitis,” Bioresource Technology 102(21), 9965-9969. DOI: 10.1016/j.biortech.2011.08.027
Benkun, Q., Luo, J. Q., Chen, G. Q., Chen, X. G., and Wan, Y. H. (2012).“Application of ultrafiltration and nanofiltration for recycling cellulase and concentrating glucose from enzymatic hydrolyzate of steam exploded wheatstraw,” Bioresource Technology 104, 466-472. DOI: 10.1016/j.biortech.2011.10.049
Brás, T., Guerra, V, Torrado, I., Lourenço, P., Cavalheiro, F., Duarte, L. C., and Neves, L. A. (2014). “Detoxification of hemicellulosic hydrolysates from extracted olive pomace by diananofiltration,” Process Biochemistry 49, 173-180. DOI: 10.1016/j.procbio.2013.09.017
Borand, M. N., Isler, K. A., and Karaosmanoglu, F. (2020). “Saccharification yield through enzymatic hydrolysis of the steam-exploded pinewood,” Energies 13, 4552-4552. DOI: 10.3390/en13174552
Bulter, T., Alcalde, M., Sieber, V., Meinhold, P., Schlachtbauer, C., and Arnold, F. H. (2003). “Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution,” Applied and Environmental Microbiology 699, 87-95.
Carter, B., Squillace, P., Gilcrease, P. C., and Menkhaus, T. J. (2011). “Detoxification of a lignocellulosic biomass slurry by soluble polyelectrolyte adsorption for improved fermentation efficiency,” Biotechnology Bioengineering 108, 2053-2060. DOI: 10.1002/bit.23152
Chen, B. Y., Chen, S. W., and Wang, H. T. (2012). “Use of different alkaline pretreatments and enzyme models to improve low-cost cellulosic biomass conversion,” Biomass and Bioenergy 39, 182-191. DOI: 10.1016/j.biombioe.2012.01.012
Chen, R., and Dou, J. (2016). “Biofuels and bio-based chemicals from lignocellulose: metabolic engineering strategies in strain development,” Biotechnology Letters 38, 213-221. DOI: 10.1007/s10529-015-1976-0
Chen, H. Q., Li, J., Wan, C., Fang, Q., Bai, F. W., and Zhao, X. Q. (2019). “Improvement of inhibitor tolerance in Saccharomyces cerevisiae by overexpression of the quinone oxidoreductase family gene,” YCR102C. FEMS Yeast Research 19, 55-67.
Chen, C. H., Kedong, M., Zhu, Q. L., Furong, T., Wang, Y. W., He, M. X., and Hu, G. Q. (2020a). “A method for concentration of monosaccharide and removal of inhibitors during hydrolysate pretreatment for improved bioethanol production,” Journal of Cleaner Production 260, 233-432.
Chen, H., Fang, G. G.,Yu, L. X., Zhou, Y., Meng, X. Z., Deng, Y. J., Shen, K. Z., and Arthur, J. R. (2020b). “Maximizing enzymatic hydrolysis efficiency of bamboo with a mild ethanol-assistant alkaline peroxide pretreatment,” Bioresource Technology 299, 101-156. DOI: 10.1016/j.biortech.2019.122568
Chen, X. X., Zhai, R., Li, Y., Yuan, X. C., Liu, Z. H., and Jin, M. J. (2020c). “Understanding the structural characteristics of water-soluble phenolic compounds from four pretreatments of corn stover and their inhibitory effects on enzymatic hydrolysis and fermentation,” Biotechnology Biofuels 13, 44-57. DOI: 10.1186/s13068-020-01686-z
Cheng, Z., Yang, R. D., Liu, X., Liu, X., and Chen, H. (2017). “Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source,” Bioresource Technology 234, 8-14. DOI: 10.1016/j.biortech.2017.02.131
Chin, D. W. K., Lim, S., Pang, Y. L., Lim, C. H., and Lee, K. M. (2020). “Dataset of alkaline ethylene glycol pretreatment and two-staged acid hydrolysis using oil palm empty fruit bunch,” Data in Brief 30, 105-431. DOI: 10.1016/j.dib.2020.105431
Cola, P., Procopio, D. P., Tabosa de Castro Alves, A., Rebelo Carnevalli, L., Viana Sampaio, I., Labate Vale da Costa, B., and Olitta Basso, T. (2020). “Differential effects of major inhibitory compounds from sugarcane-based lignocellulosic hydrolysates on the physiology of yeast strains and lactic acid bacteria,” Biotechnology Letters 42, 571-582. DOI: 10.1007/s10529-020-02803-6
Cong, J. F., Shi, J. L., and Zhe, L. (2015). “Research progress in removal of organic pollutants from water by persulfate technology,” Environmental Science and Technology 38, 136-141.
Cubas-Cano, E., Gonzalez-Fernandez, C., and Ballesteros, I. (2020). “Efficient utilization of hydrolysates from steam-exploded gardening residues for lactic acid production by optimization of enzyme addition and pH control,” Waste Management 1079, 235-243. DOI: 10.1016/j.wasman.2020.04.003
Cui, X. M., Wang, Y. P., and Liang, J. D. (2016). “Combined degradation of lignin by electrochemical oxidation and biodegradation ( ECO-BD),” Research of Environmental Sciences 29, 434-441.
Cullis, I. F., and Mansfield, S. D. (2010). “Optimized delignification of wood-derived lignocellulosics enzymatic hydrolysis,” Biotechnology and Bioengineering 106, 884-893. DOI: 10.1002/bit.22768
Dai, Y. Z., Si Meng. Y., Chen, Y. H., Zhang, N. L., Zhou, M., Liao, Q., Shi, D. Q., and Liu, Y. (2015). “Combination of biological pretreatment with NaOH/Urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw,” Bioresource Technology 198, 725-731. DOI :10.1016/j.biortech. 2015.09.091
da Silva, T. L., Santo, R., Reis, A., and Passarinho, P. C. (2017). “Effect of furfural on Saccharomyces carlsbergensis growth, physiology and ethanol production,” Applied Biochemistry and Biotechnology 182, 708-720. DOI: 10.1007/s12010-016-2356-5
De Albuguerque Wanderley, M. C., Martín, C., de Moraes Rocha, G. J., and Gouveia, E. R. (2013). “Increase in ethanol production from sugarcane bagasse based on combined pretreatments and fed-batch enzymatic hydrolysis,” Bioresource Technology 128, 448-453. DOI:10.1016/j.biortech.2012.10.131
Deng, F., Cheong, D. Y., and Aita, G. M. (2018). “Detoxification of dilute ammonia pretreated energy cane bagasse enzymatic hydrolysate by soluble polyelectrolyte flocculants,” Forum Modernes Theater 112, 681-690. DOI: 10.1016/j.indcrop.2018.02.030
Diwan, B., Parkhey, P., and Gupta, P. (2017). “Platform study on the development of a nondetoxified rice straw hydrolysate to its application in lipid production from Mortierella alpina,” ACS Sustainable Chemistry and Engineering 6, 1225-1234.
DOI: 10.1016/j.rser.2017.05.225
Emmel, A., Mathias, A. L., Wypych, F., and Ramos, L. P. (2003). “Fractionation of Eucalyptus grandis chips by dilute acid- catalysed steam explosion,” Bioresource Technology 86, 105-115. DOI: 10.1016/S0960-8524(02)00165-7
Ewanick, S., and Bura, R. (2011). “The effect of biomass moisture content on bioethanol yields from steam pretreated switchgrass and sugarcane bagasse,” Bioresource Technology 102, 2651-2658. DOI: 10.1016/j.biortech.2010.10.117
Field, S. J., Ryden, P., Wilson, D., James, S. A., Roberts, I. N., Richardson, D. J., Waldron, K. W., and Clarke, T. A. (2015). “Identification of furfural resistant strains of Saccharomyces cerevisiae and Saccharomyces paradoxus from a collection of environmental and industrial isolates,” Biotechnology for Biofuels 8, 213-235. DOI: 10.1186/s13068-015-0217-z
Fonseca, B. G., Moutta Rde, O., Ferraz Fde, O, Vieira, E. R., Nogueira, A. S., Baratella, B. F., Rodrigues, L. C., Zhang, H.-R., and da Silva, S. S. (2011). “Biological detoxification of different hemicellulosic hydrolysates using Issatchenkia occidentalis CCTCC M 206097 yeast,” Industrial Microbiology Biotechnology 38, 199-207. DOI: 10.1007/s10295-010-0845-z
Gao, Y. Q., Gao, N. Y., Deng, Y., Yang, Y. Q., and Ma, Y. (2012). “Ultraviolet (UV) light-acti9vated persulfate oxidation of sulfamethazine in water,” Chemical Engineering Journal 195/196, 248-253. DOI: 10.1016/j.cej.2012.04.084
Geddes, C. C., Peterson, J. J., Roslander, C., Zacchi, G., Mullinnix, M .T., Shanmugam, K .T., and Ingram, L. O. (2010). “Optimizing the saccharification of sugar cane bagasse using dilute phosphoric acid followed by fungal celluloses,” Bioresource Technology 101, 1851-1857. DOI: 10.1016/j.biortech.2009.09.070
Hamidah, U., Arakawa, T., H’ng, Y. Y., Nakagawa-izumi, A., and Kishino, M. (2017). “Recycled ionic liquid 1-ethyl-3-methylimidazolium acetate pretreatment for enhancing enzymatic saccharification of softwood without cellulose regeneration,” Journal of Wood Science 64, 149-156. DOI: 10.1007/s10086-017-1681-9
Hamidah, U., Arakawa, T., and Yin, Y. (2017). “Recycled ionic liquid 1-ethyl-3-methylimidazolium acetate pretreatment for enhancing enzymatic saccharification of softwood without cellulose regeneration,” Journal of Wood Science 64, 149-156.
Harahap, B. M., and Kresnowati, M. T. A. P. (2018). “Moderate pretreatment of oil palm empty fruit bunches for optimal production of xylitol via enzymatic hydrolysis and fermentation,” Biomass Conversion and Biorefinery 8(2), 255-263. DOI: 10.1007/s13399-017-0299-x
Hang, Z. X., Xia, L., and Yuan, Y. J. (2021). “Developments of pretreatment and high technical value of lignocellulosic,” Biological Bulletin 37, 162-174.
Hasunuma, T., Sung, K, Sanda, T., Yoshimura, K., Matsuda, F., and Kondo, A. (2011). “Efficient fermentation of xylose to ethanol at high formic acid concentrations by metabolically engineered Saccharomyces cerevisiae,” Applied Microbiology and Biotechnology 90, 997-1004. DOI: 10.1007/s00253-011-3085-x
He, L. W., Wang, C., Shi, H. H., Zhou, W., Zhang, Q., and Chen, X. Y. (2019). “Combination of steam explosion pretreatment and anaerobic alkalization treatment to improve enzymatic hydrolysis of Hippophae rhamnoides,” Bioresource Technology 289, 121-693. DOI:10.1016/j.biortech.2019.121693
Hidayatullah, I. M., Setiadi, T., Kresnowati, M. T. A. P., and Boopathy, R. (2020) “Xylanase inhibition by the derivatives of lignocellulosic material.” Bioresource Technology, 300 :122740. DOI: 10.1016/j.biortech.2020.122740.
Huang, Q. L, Zhang, H. R., and Xiong, L. (2018). “Controllable synthesis of styrene-divinylbenzene adsorption resins and the effect of textural properties on removal performance of fermentation inhibitors from rice straw hydrolysate,” Journal of Engineering 57, 5119-5127.
Huang, R. L., Su, R. X., Qi, W., and He, Z. M. (2012). “Understanding the key factors for enzymatic conversion of pretreated lignocellulose by partial least square analysis,” Biotechnology Progress 26, 384-392. DOI: 10.1002/btpr.324
Hyland, P. B., Mun, S. L. S., and Mahadevan, R. (2013). “Prediction of weak acid toxicity in Saccharomyces cerevisiae using genome-scale metabolic models,” Industrial Biotechnology 9, 229-235.
Hyung, E. A., Lee, K. H., Jang, Y. W., Kim, C. B., and Yoo, H. Y. (2021). “Improved glucose recovery from Sicyos angulatus by NaOH pretreatment and application to bioethanol production,” Processes 9, 245-245. DOI: 10.3390/PR9020245
Ikram, H., Yesra, A., Ali, N., Muhammad, A., Asad, R., Hamid, M., Zinnia, M., and Qurratulain, S. (2018). “Removal of phenolic compounds through overliming for enhanced saccharification of wheat straw,” Chemistry Technology Biotechnology 93, 3011-3017. DOI: 10.1002/jctb.5659
Ishida, Y., Ngugen, T. T. M., and Izawa, S. (2017). “The yeast ADH7 promoter enables gene expression under pronounced translation repression caused by the combined stress of vanillin, furfural, and 5-hydroxymethylfurfural,”Journal of Biotechnology 252, 65-72. DOI: 10.1016/j.jbiotec.2017.04.024
Iwaki, A., Kawai, T., Yamamoto, Y., and Izawa, S. (2013). “Biomass conversion inhibitors furfural and 5-hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae,” Applied and Environmental Microbiology 79, 1661-1667. DOI: 10.1128/AEM.02797-12
Jiang, D. P., Xu, M. G., Tian, Z., Zhou, C., Zhi, P. Z., Chao, H., Quan, G. Z., and Ye, B. L. (2020). “Effect of alkaline pretreatment on photo-fermentative hydrogen production from giant reed: Comparison of NaOH and Ca(OH)2,” Bioresource Technology 30, 497-107. DOI: 10.1016/j.biortech.2020.123001
Jiang, K. K., Kuang, H., and Qin, T. T. (2018). “Recovery ofmonosaccharides from dilute acid corncob hydrolysate by nanofiltration: Modeling and optimization,” RSC Advances 81, 2672-12683.
Jönsson, L. J., and Martín, C. (2016). “Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects,” Bioresource Technology 199, 103-112. DOI: 10.1016/j.biortech.2015.10.009
Jung, H. R., Lee, J. H., Moon, Y. M., Choi, T. R., Yang, S. Y., Song, H. S., Park, J. Y., Park, Y. L., Bhatia, S. K., Gurav, R., Ko, B. J., and Yang, Y. H. (2019). “Increased tolerance to furfural by introduction of polyhydroxybutyrate synthetic genes to Escherichia coli,” Microbiology Biotechnology 29, 776–784. DOI: 10.4014/jmb.1901.01070
Jurado, M., Prieto, A., Martínez-Alcalá, Á., and Martínez, A. T. (2009). “Laccase detoxification of steam-exploded wheat straw for second generation bioethanol,” Bioresource Technology 100, 6378-6384. DOI: 10.1016/j.biortech.2009.07.049
Kamran, M., El-Sayed, S., Tae, H. K., and Xiang, K. L. (2020). “Enhanced ethanol production by Saccharomyces cerevisiae fermentation post acidic and alkali chemical pretreatments of cotton stalk lignocellulose,” International Biodeterioration and Biodegradation 147, 443-498. DOI: 10.1016/j.ibiod.2019.104869
Karapatsia, A., Ioannis, P., Giannis, P., Olympia, K., and Costas, K. (2017). “Optimization of dilute acid pretreatment and enzymatic hydrolysis of Phalaris aquatica lignocellulosic biomass in batch and fed-batch processes,” Bioenergy Research 10, 225-236. DOI: 10.1007/s12155-016-9793-4
Keshav, P. K., Shaik, N., Koti, S., and Venkateswar, R. L. (2016). “Bioconversion of alkali delignified cotton stalk using two-stage dilute acid hydrolysis and fermentation of detoxified hydrolysate into ethanol,” Industrial Crops and Products 91, 323-331. DOI: 10.1016/j.indcrop.2016.07.031
Koddenberg, T. (2016). “Handbook of wood chemistry and wood composites,” Journal of Cleaner Production 110, 193-193. DOI: 10.1016/j.jclepro.2015.07.070 .
Ladeira Ázar, T. I. S., Morgan, T., dos Santos, A. C. F., de Aquino Ximenes, E., Ladisch, M. R., and Guimarães, V. M. (2017). “Deactivation and activation of lignocellulose degrading enzymes in the presence of laccase,” Enzyme Microbiology Technology 109, 25-30. DOI: 10.1016/j.enzmictec.2017.09.007
Llano Astuy, T., Quijorna Kyburz, M., and Coz Fernandez, A. (2017). “Detoxification of a lignocellulosic waste from a pulp mill to enhance its fermentation prospects,” Energies 10(3), 348. DOI: 10.3390/en10030348
Li, Y. C., Mitsumasu, K., Gou, Z. X., Gou, M., Tang, Y. Q., Li ,G. Y., Wu, X. Lei., Akamatsu, T., Taguchi, H., and Kida, K. K. (2016). “Xylose fermentation efficiency and inhibitor tolerance of the recombinant industrial Saccharomyces cerevisiae strain NAPX37,” Applied Microbiology Biotechnology 100, 1531-1542.
Liang, C. J., Bruell, C. J., Marley, M. C., and Sperry, K. L. (2003). “Thermally activated persulfate oxidation of trichloroethylene (TCE) and 1,1,1-trichloroethane (TCA) in aqueous systems and soil slurries,” Soil and Sediment Contamination: An International Journal 12, 207-228. DOI: 10.1080/713610970
Liu, T. J., and Li, Z. L. (2017). “An electrogenerated base for the alkaline oxidative pretreatment of lignocellulosic biomass to produce bioethanol,” RSC Advances 7, 47456-47463.
Llano Astuy, T., Quijorna Kyburz, N., and Coz Fernández, A.(2017). “Detoxification of a lignocellulosic waste from a pulp mill to enhance its fermentation prospects,” Energies 10(3), 348. DOI: 10.3390/en10030348
López-Linares, J. C., Romero, I., and Cara, C. (2016). “Bioconversion of rapeseed straw: enzymatic hydrolysis of whole slurry and cofermentation by an ethanologenic Escherichia coli,” Energy Fuels 30(11), 9532–9539. DOI: 10.1021/acs.energyfuels.6b02308
Martín-Sampedro, R., Eugenio, M. E., García, J. C., Lopez, F., Villar, J. C., and Diaz, M. J. (2012). “Steam explosion and enzymatic pre-treatments as an approach to improve the enzymatic hydrolysis of Eucalyptus globulus,” Biomass and Bioenergy 4(2), 97-106. DOI: 10.1016/j.biombioe.2012.03.032
McIntosh, S., and Vancov, T. (2010). “Enhanced enzyme saccharification of Sorghum bicolor straw using dilute alkali pretreatment,” Bioresource Technology 101, 6718-6727. DOI: 10.1016/j.biortech.2010.03.116
Mesa, L., González, C. E., Cara, M., González, E. C., and Mussatto, S. I. (2011). “The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse,” Chemical Engineering Journal 168, 1157-1162. DOI: 10.1016/j.cej.2011.02.003
Meilany, D., Kresnowati, M. T. A. P., Setiadi, T., and Boopathy, R. (2020). “Optimization of xylose recovery in oil palm empty fruit bunches for xylitol production,” Applied Sciences 10(4). DOI: 10.3390/app10041391
Moreno, A. D., Ibarra, D., Fernández, J. L., and Ballesteros, M. (2012). “Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875,” Bioresource Technology 106, 1-9. DOI: 10.1016/j.biortech.2011.11.108
Mussatto, S., and Roberto, I. C. (2003). “Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review,” Bioresource Technology 93, 1-10. DOI:10.1016/j.biortech.2003.10.005
Nasir, A., Giwa, A. S., Abdalla, M., and Liu, X. (2020). “Alkaline hydrogen peroxide pretreatment of bamboo culm for improved enzymatic release of reducing sugars using recombinant cellulases,” Cellulose 27(2), 769-779. DOI: 10.1007/s10570-019-02829-8
Nichols, N. N., Sharma, L. N., Mowery, R. A., Chambliss, C. K., van Walsum, G. P., Dien, B. S., and Iten, L. B. (2008). “Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate,” Enzyme Microbial Technology 42, 624-630. DOI: 10.1016/j.enzmictec.2008.02.008
Oliva-Taravilla, A., Tomás-Pejó, E., Demuez, M., González-Fernández, C., and Balllesteros, M. (2015). “Inhibition of cellulose enzymatic hydrolysis by laccase-derived compounds from phenols,” Biotechnology Progress 31(3),700-706. DOI: 10.1002/btpr.2068
Palmqvist, E., Almeida, J. S., and Hahn-Hägerdal, B. (1999). “Influence of furfural on anaerobic glycolytic kinetics of Saccharomyces cerevisiae in batch culture,” Biotechnology and Bioengineering 62, 447-454.
Palmqvist, E., and Bärbel, H. H. (2000). “Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification,” Bioresource Technology 74, 17-24. DOI: 10.1016/S0960-8524(99)00160-1
Palmqvist, E., and Hahn-Hägerdal, B. (2000). “Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition,” Bioresource Technology 74(1), 25-33. DOI: 10.1016/S0960-8524(99)00161-3
Parawira, W., and Tekere, M. (2011). “Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review,” Critical Reviews in Biotechnology 31, 20-31. DOI: 10.3109/07388551003757816
Qin, L., Li, W. C., Liu, L., Zhu, J. Q., Li, X., Li, B. Z., and Yuan, Y. J. (2016). “Inhibition of lignin-derived phenolic compounds to cellulase,” Biotechnology Biofuels 9, 70-97. DOI: 10.1186/s13068-016-0485-2
Rahikainen, J. L., Martin-Sampedro, G., Heikkinen, H., Rovio, S., Marjamaa, K., Tamminen, T., Rojas, O. J., and Kruus, K. (2017). “Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme,” Bioresource Technology 45, 22-34. DOI: 10.1016/j.biortech.2013.01.075
Ravindran, R., and Jaiswal, A. K. (2016). “A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities,” Bioresource Technology 199, 92-102. DOI:10.1016/j.biortech.2015.07.106
Rastogi, M., and Shrivastava, S. (2017). “Recent advances in second-generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes,” Renewable and Sustainable Energy Reviews 80, 330-340. DOI: 10.1016/j.rser.2017.05.225.
Raud, M., Tutt, M., Olt, J., and Kikas, T. (2016). “Dependence of the hydrolysis efficiency on the lignin content in lignocellulosic material,” International Journal of Hydrogen Energy 41, 16338-16343. DOI: 10.1016/j.ijhydene.2016.03.190
Robinson, T., Mood, M., Balaji, C., Soumya, S., Tamal, B., Vaibhav, V. G. (2015). “Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: A comparative study,” Biomass and Bioenergy 81, 9-18. DOI: 10.1016/j.biombioe.2015.05.006
Rong, Y. Y., Shi, L. L., and Zhang, C. (2016). “Removal of lignin degradation products by thermally activated persulfate oxidation,”Journal of Chemical Industry 67, 2618-2624
Santos, J. C., Marton, J. M., and Felipe, M. G. A. (2014). “Continuous system of combined columns of ion exchange resins and activated charcoal as a new approach for the removal of toxics from sugar cane bagasse hemicellulosic hydrolysate,” Industrial and Engineering Chemistry Research 531, 6494-16501.
Saravanakumar, T., Park, H. S., Mo, A. Y., Choi, M. S., Kim, D. H., and Park, S. M. (2016). “Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers,”Journal of Molecular Catalysis B Enzymatic 134, 196-205. DOI: 10.1016/j.molcatb.2016.11.006
Sárvári, H. I., Franzén, C. J., Taherzadeh, M. J., Niklasson, C., and Lidén, G. (2003). “Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats,” Applied Environmental Microbiology 69, 4076-4086.
Silverstein, R. A., Chen ,Y., Sharma, S. R., Boyette, M. D., and Osborne, J. (2007). “A comparison of chemical pretreatment methods for improving saccharification of cotton stalks,” Bioresource Technology 98, 100-103. DOI: 10.1016/j.biortech.2006.10.022
Song, H. S., Jeon, J. M., Kim, H. J., Bhatia, S. K., Ganesan, S., Kim, J. Y., Hong, J. W., Hong, Y. G., Kim, Y. G., Kim , W., and Yang, Y. H. (2017). “Increase infurfural tolerance by combinatorial overexpressionof NAD salvage pathway enzymes in engineered isobutanol-producing E. coli,” Bioresource Technology 245, 1430–1435.
Song, Z. L., Gaihe,Y., Liu, X. F., Yan, Z. Y., Yuan, Y. X., and Liao, Y. Z. (2014). “Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion,” PLoS One 9, 80-93. DOI: 10.1371/journal.pone.0093801
Soudham, V. P., Brandberg, T., Mikkola, J. P., and Larsson, C. (2014). “Detoxification of acid pretreated spruce hydrolysates with ferrous sulfate and hydrogen peroxide improves enzymatic hydrolysis and fermentation,” Bioresource Technology 166, 112-134. DOI: 10.1016/j.biortech.2014.05.096
Taherzadeh, M. J., Gustafsson, L., Niklasson, C., and Lidén, G. (2000). “Inhibition effects of furfural on aerobic batch cultivation of Saccharomyces cerevisiae growing on ethanol and/or acetic acid,” Journal of Bioscience and Bioengineering 90, 374-380. DOI: 10.1016/S1389-1723(01)80004-9
Tan, L. P. (2015). Pretreatment and Saccharification Fermentation of Oil Palm Empty Fruit Bunch (EFB) for Ethanol Production and Mechanism of Pretreatment, PhD Thesis, Ji’nan: Shandong University, pp 67-87.
Tang, C. L., Shan, J. Q., Chen, Y. J., Zhong, L. X., Shen, T., Zhu, C. J., and Ying, H. J. (2017). “Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin,” Bioresource Technology 232, 222-228. DOI: 10.1016/j.biortech.2017.02.041
Todhanakasem, T., Yodsanga, S., Sowatad, A., Kanokratana, P., Thanonkeo, P., and Champreda, V. (2018). “Inhibition analysis of inhibitors derived from lignocellulose pretreatment on the metabolic activity of Zymomonas mobilis biofilm and planktonic cells and the proteomic responses,” Biotechnology Bioengineering 115, 70-81. DOI: 10.1002/bit.26449
Tomek, K. J., Saldarriaga, C. R. C., Velasquez, F. P. C., Liu, T. J., Hodge, D. B., and Whitehead, T. A. (2015). “Removal and upgrading of lignocellulosic fermentation inhibitors by in situ biocatalysis and liquid-liquid extraction,” Biotechnology Bioengineering 112, 627-632. DOI: 10.1002/bit.25473
Tramontina, R., Brenelli, L. B., Sousa, A., Alves, R., Zetty Arenas, A. M., Marcos Nascimennto, B., Cándida Rabel, S., Freitas, S., Ruller, R., and Marcio Squina, F. (2020). “Designing a cocktail containing redox enzymes to improve hemicellulosic hydrolysate fermentability by microorganisms,” Enzyme Microbiology Technology 135, 109-490. DOI: 10.1016/j.enzmictec.2019.109490
Valdivia, M., Galan, J. L., Laffarga, J., and Ramos, J. L. (2016). “Biofuels 2020: Biorefineries based on lignocellulosic materials,” Microbial Biotechnology 9, 585-594. DOI: 10.1111/1751-7915.12387
van der Pol, E. C., Bakker, R. R., Baets, P., and Eggink, G. (2014). “By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels,” Applied Microbiology and Biotechnology 989, 579-9593. DOI:10.1007/s00253-014-6158-9
Varga, E., Klinke, H. B., Réczey, K., and Thomsen, A. B. (2004). “High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol,” Biotechnology and Bioengineering 88(5), 567-574. DOI: 10.1002/bit.20222
Viegas, C. A., and Sá-Correia, I. (1991). “Activation of plasma membraneATPase of Saccharomyces cerevisiae by octanoic acid,” Journal of General Microbiology 137, 645-651. DOI: 10.1099/00221287-137-3-645
Wang, W. H., Zhang, C. Y., Tong, S. S., Cui, Z. Y., and Liu, P. (2018). “Enhanced enzymatic hydrolysis and structural features of corn stover by NaOH and ozone combined pretreatment,” Molecules 23, 1300-1313. DOI: 10.3390/molecules23061300
Wang, W. T., Wu, B., Qin, H., Liu, P. T., Qin, Y., Duan, G. W., Hu, G. Q., and He, M. X. (2019). “Genome shuffling enhances stress tolerance of Zymomonas mobilis to two inhibitors,” Biotechnology Biofuels 12, 288-294. DOI: 10.1186/s13068-019-1631-4.
Wu, F. F., Hong, Y. D., Guo, S. Y., and Zhang, B. B. (2020). “Study on the removal efficiency of phenol-containing wastewater with different catalysts in persulfate system,” Guangdong Chemical Industry 47, 102-104..
Wu, J., Zhou, Y. J., Zhang, W., Cheng, K. K., Liu, H. J., and Zhang, J. A. (2019). “Screening of a highly inhibitor-tolerant bacterial strain for 2,3-BDO and organic acid production from non-detoxified corncob acid hydrolysate,” AMB Express 9, 153-178. DOI: 10.1186/s13568-019-0879-1
Xavier, A. M. R. B., Correia, M. F., Pereira, S. R., and Evtuguin, D. V. (2010). “Second-generation bioethanol from eucalypt sulphite spentliquor,” Bioresource Technology 101(8), 2755-2761. DOI: 10.1016/j.biortech.2009.11.092
Xu, H., Li, B., and Mu, X. (2016). “Review of alkali-based pretreatment to enhance enzymatic saccharification for lignocellulosic biomass conversion,” Industrial and Engineering Chemistry Research 55, 8691-8705.
Yang, S. Y., Wang, P., Yang, X., Wei, G., Zhang, W. Y., and Shan, L. (2009). “A novel advanced oxidation process to degrade organic pollutants in wastewater: Microwave-activated persulfate oxidation,” Journal of Environmental Sciences 21, 1175-1180. DOI: 10.1016/S1001-0742(08)62399-2
Yi, X., Gu, H. Q., Gao, Q. Q., Lew, Z., and Bao, J. (2015). “Transcriptome analysis of Zymomonas mobilis ZM4 reveals mechanisms of tolerance and detoxification of phenolic aldehyde inhibitors from lignocellulose pretreatment,” Biotechnology for Biofuels 8, 456-678. DOI: 10.1186/s13068-015-0333-9.
Yoo, J.-Y., Alavi, S., Vadlani, P., and Amanor-Boadu, V. (2011). “Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars,” Bioresource Technology 102, 7583-7590. DOI: 10.1016/j.biortech.2011.04.092
Yu, G. W., Guo, T. T., and Huang, Q. D. (2020). “Preparation of rapeseed oil with superhigh canolol content and superior quality characteristics by steam explosion pretreatment technology,” Food Science Nutrual 8, 2271-2278. DOI: 10.1002/fsn3.1502
Yu, X. J., Bao, X. J., Zhou, C. S., Zhang, L., Yagoub, A. E. A., Yang, H. P., and Ma, H. L. (2018). “Ultrasound-ionic liquid enhanced enzymatic and acid hydrolysis of biomass cellulose,” Ultrasonics Sonochemistry 41, 410-418. DOI: 10.1016/j.ultsonch.2017.09.003
Yücel, H. G., and Aksu, Z. (2015). “Ethanol fermentation characteristics of Pichia stipitis yeast from sugar beet pulp hydrolysate: use of new detoxification methods,” Fuel 158, 793–799. DOI: 10.1016/j.fuel.2015.06.016
Zaldivar, J. Martinez, A., and Ingram, L. O. (2000). “Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli,” eEarth 68, 524-530. DOI: 10.1002/(SICI)1097-0290(20000605)68:5<524::AID-BIT6>3.0.CO; 2-T.
Zhang, Y., Wang, Y. H., and Deng, L. H. (2005). “Study on hydrolysis conditions of hemicellulose and detoxification of rice straw by hydrolysate,” Cellulose Science and Technology 13, 38-44
Zhang, Y. H. P., Ding, S. Y., Mielenz, J. R., Cui, J.-B., Elander, R. T., Laser, M., Himmel, M. E., McMillan, J. R., and Lynd, L. R. (2007). “Fractionating recalcitrant lignocellulose at modest reaction conditions,” Biotechnology and Bioengineering 97, 214-223. DOI: 10.1002/bit.21386
Zhang, Q., Li, Z., and Anne, B. T. (2012). “Study on the production of alcohol by detoxification of corn straw hydrolysate,” Transactions of the Chinese Society for Agricultural Machinery 43, 108-111.
Zhang, Y., Xia, C. L., Lu, M. M., and Tu, M. B. (2018). “Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates,” Biotechnology Biofuels 11, 178-156. DOI:10.1186/s13068-018-1182-0
Zhou, J., Cheng, H., Ma, J. F., Peng, M. G., Kong, Y., and Komarneni, S. (2021). “Persulfate activation by MnCuS nanocomposites for degradation of organic pollutants,” Separation and Purification Technology 26, 422-435. DOI: 10.1016/J.SEPPUR.2020.118290
Zhou, Y., Lei, F. H., Li, P. F., and Jiang, J. X. (2018). “Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology,” Biotechnology and Bioengineering 115, 2683-2702. DOI: 10.1002/bit.26788
Article submitted: July 28, 2021; Peer review completed: October 9, 2021; Revised version received: December 8, 2021; Accepted: December 9, 2021; Published: December 15, 2021.
DOI: 10.15376/biores.17.1.Luo
