Abstract
Pine wood is mainly composed of extractives, lignin, cellulose, and hemicelluloses (which include pentosans and hexosans). In the scope of biorefineries, the utilization of pine wood entails the selective separation of its major components. A sequence of aqueous and delignification treatments enables the selective separation of hemicelluloses (as soluble products from the aqueous fractionation step), lignin (as soluble products from the delignification stage), and cellulose (accumulated in the solid phase leaving the delignification stage). Delignification was done in media containing acetic acid or the ionic liquid 1-butyl-3-methylimidazolium hydrogen sulfate. Both the soluble hemicellulose-derived saccharides and the cellulose-containing solids were found to be suitable substrates for 5-hydroxymethylfurfural production in reaction media containing the ionic liquid 1-butyl-3-methylimidazolium chloride in the presence of Brønsted and/or Lewis acidic catalysts. The processing schemes considered in this work allowed an efficient utilization of the feedstock using environmentally friendly technologies.
Download PDF
Full Article
Production of 5-Hydroxymethylfurfural from Pine Wood via Biorefinery Technologies Based on Fractionation and Reaction in Ionic Liquids
Lucía Penín, Susana Peleteiro, Sandra Rivas, Valentín Santos, and Juan Carlos Parajó *
Pine wood is mainly composed of extractives, lignin, cellulose, and hemicelluloses (which include pentosans and hexosans). In the scope of biorefineries, the utilization of pine wood entails the selective separation of its major components. A sequence of aqueous and delignification treatments enables the selective separation of hemicelluloses (as soluble products from the aqueous fractionation step), lignin (as soluble products from the delignification stage), and cellulose (accumulated in the solid phase leaving the delignification stage). Delignification was done in media containing acetic acid or the ionic liquid 1-butyl-3-methylimidazolium hydrogen sulfate. Both the soluble hemicellulose-derived saccharides and the cellulose-containing solids were found to be suitable substrates for 5-hydroxymethylfurfural production in reaction media containing the ionic liquid 1-butyl-3-methylimidazolium chloride in the presence of Brønsted and/or Lewis acidic catalysts. The processing schemes considered in this work allowed an efficient utilization of the feedstock using environmentally friendly technologies.
Keywords: Biorefinery; Environmentally friendly processes; Furans; Pinus pinaster wood; Renewable resources
Contact information: Department of Chemical Engineering, University of Vigo (Campus Ourense), Faculty of Science, As Lagoas, 32004 Ourense, Spain; *Corresponding author: jcparajo@uvigo.es
INTRODUCTION
The chemical industry is facing important challenges related to sustainability, particularly those factories involved with large-scale utilization of fossil raw material and the resulting CO2emissions. In contrast, the increasing population (that results in increased demand for fuels, chemicals, and materials), the fluctuating prices of fossil resources, and the geostrategic threats regarding their safe supply are important problems affecting the development of the global economy.
From this perspective, the development of new, advanced technologies enabling the replacement of oil-derived products with renewable ones is essential (Stocker 2008; Gullón et al. 2010). These new processes must be able to reach an integral benefit of the raw materials, operating according to the green chemistry principles and the “sustainable development” concept (Octave and Thomas 2009; Cheng and Zhu 2009), limiting the generation of wastes, and extracting the highest possible added-value from the feedstocks.
Vegetal biomass is the most important renewable source of organic carbon in earth, and it represents a key renewable resource for sustainable development, standing out for its high generation rate (estimated at 170 ton/year to 200·109 ton/year) (Stark 2011). Other interesting features are its widespread occurrence, low specific price, and wide range of possible applications. The term “lignocellulosic materials” (LCM) stands for the vegetal biomass mainly made up of polysaccharides (cellulose and hemicelluloses) and lignin (an aromatic fraction made up of phenylpropane units). Cellulose, hemicelluloses, and lignin are the structural components of LCM, which jointly account for approximately 85% to 90% of the dry weight of typical feedstocks. The LCM also contain non-structural components (such as extractives, proteins, and inorganic components), which are of minor importance for the purposes of this study.
In terms of abundance, wood is the most important type of LCM. Wood utilization can be conveniently achieved in biorefineries, in which the raw material is processed by physical, chemical, and/or biotechnological methods to yield a scope of commercial products (e.g., chemicals, fuels, or materials). In biorefineries, the structural components of the feedstocks are separated into fractions made up of compounds with related properties that can be used directly or after modification.
The fractionation methods are based on the diverse properties of the various LCM components. For example, hemicelluloses can be separated taking advantage of their comparatively low resistance to hydrolysis, lignin is susceptible to dissolution in organosolvents or in aqueous media containing suitable chemicals, and cellulose is stable in mild alkaline or acidic media.
In Galicia (North West Spain), Pinus pinaster wood is a major resource that is potentially suitable for biorefineries. Integrated processing technologies can be based on consecutive mild acidic and delignification stages (González-Muñoz et al. 2011a,b; Conde et al. 2013).
The mild-acidic stage is performed to selectively solubilize hemicelluloses, in a way that cellulose and lignin remain in the solid phase (Gullón et al. 2011). When performed under suitable conditions, hydrothermal processing (or autohydrolysis, based on the utilization of hot, compressed water) allows an extensive and selective solubilization of hemicelluloses, which are converted into a mixture of monosaccharides and higher saccharides. Wood autohydrolysis can be performed directly, or after an aqueous extraction (performed under mild operational conditions) to recover water-soluble extractives that contain valuable phenolics (Conde et al. 2013). Additionally, the combined extraction-autohydrolysis approach results in the production of saccharide solutions with limited amounts of non-saccharide compounds, facilitating their further utilization.
The soluble saccharides derived from the major hemicellulosic polymers in Pinus pinaster wood (glucomannan and xylan) are potential substrates for manufacturing furans (5-hydroxymethylfurfural, here denoted HMF, and furfural, here denoted F). Both F and HMF have been considered as emerging, renewable platform chemicals and show a broad range of applications, including the manufacture of fine chemicals and plastics (Chheda et al. 2007a,b; Rackemann and Doherty 2011; Lange et al. 2012; Cai et al. 2014; Gullón et al. 2017). However, the generation of furans from sugars or polymers made up of anhydrosugars follows a complicated kinetic pattern, characterized by the participation of non-productive series and parallel reactions. When furans are produced in aqueous media, unwanted rehydration and condensation reactions take place that limit the yields of the target products. Alternatively, the utilization of non-aqueous reaction media for furan production emerges as an attractive approach. In particular, the utilization of ionic liquids (ILs) is an interesting possibility: ILs are salts made up of an organic cation and an organic or inorganic anion, and present favorable properties for chemical utilization, including low melting temperatures, negligible vapor pressure, chemical and thermal stability, and favorable solvation capability. The ILs are considered as “green” solvents, and have applications such as reaction media, separation agents, catalysts, or additives (Peleteiro et al. 2015b, 2016a,b,c).
Imidazolium-based ILs have been employed for biomass processing. Reaction media made up of the IL 1-butyl-3-methylimidazolium chloride ([bmim]Cl) and homogeneous or heterogeneous catalysts have been employed for furan production from biomass or biomass-derived products (Peleteiro et al. 2014, 2015a). Promising results have been reported for experiments dealing with pure sugars or biomass-derived fractions using CrCl3 as a homogeneous catalyst, whereas acidic zeolites have been proposed as suitable heterogeneous catalysts.
The delignification of hemicellulose-free wood resulting from autohydrolysis processing can be achieved using multiple approaches, including the utilization of conventional pulping media (kraft of sulphite), organosolvents (e.g., concentrated acetic acid media), or ionic liquids. The ability of acetic acid-water-HCl media (Acetosolv process) for reaching an extensive lignin removal with good selectivity towards cellulose solubilization wood has been confirmed in previous literature (Parajó et al. 1993, 1995), and the operational conditions have been optimized for Pinus pinaster wood (Parajó et al. 1993). Ionic liquids show potential for multiple biorefinery operations, for example, as agents for physical separations (Peleteiro et al. 2015b), as reaction media for fractionation or delignification (Hou et al. 2017; Liu et al. 2018; Santos et al. 2018), as reaction media for chemical modification of the products resulting from fractionation (Peleteiro et al. 2014, 2016a, and 2016b), or as catalysts (Peleteiro et al. 2016c).
This work deals with the fractionation and the manufacture of furans (HMF and F) from Pinus pinaster wood. Wood fractionation was achieved by consecutive extraction, autohydrolysis, and delignification stages (performed in catalyzed media containing concentrated acetic acid or the ionic liquid [bmim]HSO4) according to the scheme depicted in Fig. 1. The hemicellulosic saccharides solubilized in the autohydrolysis stage were treated in [bmim]Cl in the presence of selected acidic zeolites (acting as heterogeneous catalysts) for furan production; and the cellulose-enriched fractions resulting from delignification treatments were subjected to hydrolysis-dehydration reactions in catalyzed [bmim]Cl for HMF manufacture. The combination of extraction, autohydrolysis, and delignification provides an environmentally friendly alternative for achieving the total fractionation of wood through the separation of aqueous extractives rich in phenolics, cellulose-enriched solids suitable for HMF manufacture, soluble saccharides suitable for furan manufacture, and sulphur-free lignin.
EXPERIMENTAL
Raw Material and Wood Composition
Pinus pinaster wood samples were obtained from a local industry (Orember, Ourense, Spain), air-dried at room temperature, and milled to a particle size below 1 mm. The maximum particle size was selected to ensure that the effects derived from the internal resistance to mass transfer was negligible compared to the reaction kinetics, in a way that the increased yields could compensate the costs derived from milling. Particles were homogenized in a single lot and stored in a dark place until use. The composition of wood was determined using TAPPI standard methods (Peleteiro et al. 2014).
Fig. 1. General scheme of the pine wood processing followed in this study
Methods
Wood fractionation
Wood samples were subjected to extraction, autohydrolysis, and delignification as indicated in Fig. 1. In the extraction stage, water and wood were mixed at an 8:1 mass ratio (based on oven-dry wood), the resulting suspension was heated up to 130 ºC, and then it was immediately cooled. Water-extracted wood was extensively washed with hot water, air-dried, and subjected to a second aqueous treatment with water (autohydrolysis, in absence of other chemicals) operating at an 8:1 mass ratio and 175 ºC for 26 min. These experimental conditions have been reported as optimal for converting hemicelluloses into saccharides, including poly-, oligo-, and mono-saccharides (González-Muñoz et al. 2011a,b). Hemicellulose-free wood was washed (as described before) and subjected to delignification. Two different delignification media were used:
a) Acetosolv pulping medium, an aqueous solution containing concentrated acetic acid (88 wt%) and HCl (0.24 wt%) media. The operation was performed in an autoclave at an 8:1 liquid to solid mass ratio and 121 ºC for 180 min (conditions optimized in preliminary experiments, data not shown).
b) Mixture of the IL 1-butyl-3-methylimidazolium hydrogen sulfate ([bmim]HSO4) and water. The operation was performed in an oil bath at a 5:1 liquid to solid mass ratio and 170 ºC (temperature selected on preliminary experiments, data not shown).
The yields of the various stages and the composition of phases from treatments were measured via the gravimetric and high-performance liquid chromatography (HPLC) using methods reported elsewhere (González-Muñoz et al. 2011a,b; Peleteiro et al. 2014). The HPLC analysis of samples was performed using an 1260 Agilent instrument fitted with refractive index and diode-array detectors (Palo Alto, CA, USA), employing HPX87H (BioRad, Hercules, CA, USA) or CARBOsep CHO 682 (Transgenomic, Omaha, NE, USA) columns operated under the conditions recommended by the manufacturer.
Lignin recovery
Lignin was recovered from the spent Acetosolv pulping solution by precipitation upon water addition (3 g water/g spent pulping solution). For lignin recovery from the [bmim]HSO4 medium, ethanol 96% (5/3 v/v with respect to the medium) was added to the fractionation solution, and the suspension was centrifuged at 5000 rpm for 15 min. The solid phase was washed three times with ethanol and extracted in a Soxhlet apparatus for 15 h. The ethanolic solutions from centrifugation, washing, and Soxhlet extraction were combined and vacuum-evaporated at 40 °C, leaving an ionic liquid/lignin mixture from which lignin was precipitated by water addition (3 g water/g medium).
Furan production from hemicellulose-derived saccharides
The solution of hemicellulosic saccharides obtained in the autohydrolysis stage was freeze-dried, and the resulting solid phase (denoted HS) was employed as a substrate for furan production in media containing [bmim]Cl and CrCl3.6H2O (obtained from Sigma Aldrich, St. Louis, MO, USA), and an acidic zeolite (CP811C-300 or CVB-720, obtained from Zeolyst International, Inc., Conshohocken, PA, USA). The properties of these zeolites were as follows:
- Zeolite CP811C-300: type zeolite-beta; SiO2/Al2O3 mole ratio of 360; nominal cation form, hydrogen; surface area of 620 m2/g; activation procedure: thermal processing at 550 ºC for 5 h;
- CBV-720: type zeolite-Y; SiO2/Al2O3 mole ratio of 30; nominal cation form, hydrogen; surface area of 780 m2/g; activation procedure: thermal processing at 550 ºC for 5 h.
For furan production, oven-dried [bmim]Cl was heated to the target temperature (see below) in a stirred reactor together with CrCl3·6H2O and one of the acidic zeolites, and then the substrate HS was added at the desired concentration. The general operation method and the analysis of samples followed the methods cited above.
Furan production from cellulose-enriched solids
The cellulose-enriched solids from Acetosolv or IL delignification were reacted in media containing [bmim]Cl and CrCl3·6H2O under the conditions indicated below. Reaction and analysis were performed as per Peleteiro et al. (2014). The conversion of potential substrates into HMF was calculated as follows:
Conversion into HMF = 100·(mol of HMF in the reaction medium)/(mol of anhydrohexose units present in the solid substrate at the beginning of the reaction)
RESULTS AND DISCUSSION
Aqueous Processing of Wood (Extraction and Autohydrolysis)
The composition of the pine wood lot employed in experiments, expressed in g/100 g oven-dry wood, was as follows: glucosyl units (including the ones present in cellulose and in hemicelluloses) 38.4; xylosyl units 6.6; galactosyl units 2.7; arabinosyl units 1.9; mannosyl units 10.7; acetyl groups 1.8; Klason lignin 28.5; and other components (including extractives) 9.4.
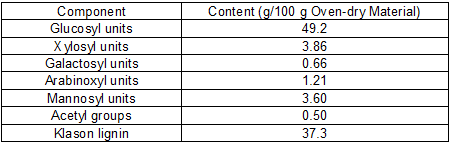
The aqueous extraction stage resulted in 5.06 wt% wood solubilization (oven-dry basis). The water-soluble fraction was not analyzed because it is known to have a complex composition, the major components being simple phenolics, stilbenes, lignans, flavonoids, organic acids, juvabiones, steryl esters, and triglycerides (Conde et al. 2013). The solid phase from the aqueous extraction stage was subjected to autohydrolysis, which provided a solid phase enriched in cellulose and lignin (at 80.1% solid recovery yield, with the compositions indicated in Table 1), and a liquid phase that was freeze-dried to yield the HS concentrate. The liquid phase from autohydrolysis presented a composition following the general patterns reported in previous studies (González-Muñoz et al. 2011a,b): the major products derived from hemicelluloses were monosaccharides (arabinose, xylose, galactose, glucose, and mannose, accounting jointly for 19.2% of the total non-volatile solutes present in the solution), and higher saccharides made up of arabinosyl, xylosyl, galactosyl, glucosyl, and mannosyl moieties that accounted for 67.6% of the total non-volatile solutes present in the solution when expressed as monosaccharide equivalents. Mannose and mannosyl groups in higher saccharides were the most important substrates for HMF manufacture (with an overall monosaccharide equivalent accounting for 38.6% of the non-volatile solutes), whereas xylose and xylosyl groups in higher saccharides were the most important substrate for F manufacture (with a monosaccharide equivalent accounting for 18.3% of the non-volatile solutes). The higher saccharides also contained acetyl groups, which accounted for 5.94 g of equivalent acetic acid/100 g non-volatile solutes. The major volatile component derived from hemicelluloses was acetic acid (yield, 4.23 g/100 g oven-dry wood), whereas low amounts of F and HMF (2.22 g/100 g oven-dry wood and 0.96 g/100 g oven-dry wood, respectively) were also generated.
Table 1. Composition of the Solid Phase from the Autohydrolysis Stage
Acetosolv Delignification of Autohydrolyzed Wood
The organosolv stage enabled an extensive delignification, with a solid recovery yield accounting for 59.9 g/100 g of autohydrolyzed wood. The delignified solid was mainly made up of glucosyl units (73.4 g/100 g of oven-dry material), which were ascribed to cellulose because glucomannan was extensively removed from the solid phase, and Klason lignin (accounting for 17.1 g/100 g oven-dry material). No noticeable amounts of galactosyl, arabinosyl, or mannosyl units were present in the delignified solid, which presented a low proportion of xylosyl groups (1.7 g/100 g oven-dry material). In addition, this material contained a substantial proportion of acetyl groups (with an acetic acid equivalent of 6.16 g/100 g oven-dry material), confirming that partial acetylation took place in the Acetosolv media along the delignification process.
The cellulose-enriched fraction resulting from Acetosolv delignification was employed as a substrate for HMF production.
Delignification of Autohydrolyzed Wood in Media Containing [bmim]HSO4
In the set of experiments performed in [bmim]HSO4 media, the effects of the reaction time (up to 110 min) on the fractionation of autohydrolyzed wood were explored at 170 °C. The percentage of solid recovery steadily decreased with the reaction time from 61.9 g/100 g substrate at 30 min to 42.8 g/100 g substrate at 110 min. The compositional data of the treated solids are listed in Table 2.
The data in Fig. 2 confirm that the fractionation method based on [bmim]HSO4 was suitable for reaching extensive lignin removal together with high glucan recovery. Based on this information, 90 min was considered as the optimal reaction time, and the solid obtained under these conditions was utilized as a substrate for HMF manufacture.
Table 2. Composition of Solids Leaving the [bmim]HSO4 Delignification Stage
Fig. 2. Percentages of glucan recovery and delignification achieved in the experiments performed in [bmim]HSO4 media
Concerning the utilization of ionic liquid for lignocellulose fractionation and enhancement of cellulose digestibility, the suitability of 1,3-dialkylimidazolium ionic liquids for this purpose was confirmed by Brandt et al. (2011). The degrees of delignification reached in this work were in the range reported by Gschwend et al. (2018) and Brandt-Talbot et al. (2017) for the pretreatment of Miscanthus (a more susceptible substrate than the one employed in this study) in media containing water and triethylammonium hydrogen sulfate. Xu et al. (2018) reported approximately 50% delignification of autohydrolyzed Tamarix austromongolica; whereas Sun et al. (2014) studied the effects of switchgrass processing with ionic liquids made up of 1-ethyl-3-methylimidazolium or cholinium cations and acetate or lysinate anions, reaching delignification degrees in the range of 16% to 80%. Working with the same raw material, Li et al. (2010) reported 69% lignin removal in a 1-ethyl-3-methylimidazolium acetate medium. In comparison, the lignin content of Eucalyptus wood decreased from 26.3% to 19.5 to 9.7% through treatment with the ionic liquid tetrabutylammonium hydroxide in reactors with a water bath or microwave heating (Hou et al. 2019).
Furan Production from Hemicellulose-derived Saccharides
The HS concentrate obtained by freeze-drying of the autohydrolysis liquors was reacted for the desired reaction time at 130 °C, 145 °C, or 160 °C in media containing [bmim]Cl and catalysts (chromium trichloride and an acidic zeolite, either CP811C-300 or CBV-720). The combination of two catalysts is expected to improve the results of the hydrolysis-dehydration reaction by facilitating both the isomerization of the various monosaccharides into suitable intermediates and the further dehydration of these latter into the target furan. Because the reaction media contained pentoses, hexoses, and higher oligosaccharides made up of anhydropentoses or anhydrohexoses, both F and HMF were potential target products. The media were prepared using 1 g HS/15 g [bmim]Cl, with catalyst charges of 1 g zeolite/g substrate and 0.05 g CrCl3·6H2O/g substrate.
In experiments with zeolite CBV-720, the highest F concentration (32.5 mmol/L) was achieved at 160 ºC. Slightly lower concentrations (31.4 mmol/L and 32.3 mmol/L) were achieved in the experiments performed at 130 °C and 145 °C, respectively. The reaction rate markedly increased with temperature, in a way that the reaction time needed to achieve the maximum F concentration decreased from 45 min at 130 ºC to 5 min at 160 °C. The experiments conducted with zeolite CP811C-300 presented different kinetics, as the maximum F concentration (34.5 mmol/L) was achieved at 130 °C after 120 min. In comparison, the highest concentrations achieved in experiments at 145 °C and 160 °C were 32.8 mmol/L and 32.1 mmol/L, respectively. At a given temperature, zeolite CP811C-300 required a higher reaction time than zeolite CBV-720 for achieving the optimal F concentration. For example, operating at 160 °C, the optimal reactions times were 10 min and 5 min, respectively.
Concerning the generation of HMF, the productive substrates were limited to glucose and mannose, because galactose reacts through a different intermediate (tagatose) that does not produce HMF in further reactions. Figures 3a and 3b show the concentration profiles determined for HMF in selected experiments.
The major experimental trends were similar in experiments with the two zeolites employed in this study, in a way that increasing temperature from 130 °C up to 160 °C resulted in faster kinetics and improved yields. In comparative terms, the zeolite CVB-720 gave the best results: the maximum concentration at 130 ºC was reached at a shorter time (150 min), and the optimal concentrations at the three temperatures considered (38.9 mmol/L, 48.9 mmol/L, and 56.7 mmol/L at 130 °C, 145 °C, and 160 °C, respectively) were higher than the ones reached with the CP811C-300 catalyst.
In related studies, the production of furans in imidazolium ionic liquids in the presence of catalysts (chromium or aluminum salts, alone or in combination with Brønsted acids or LiCl) has been assayed starting from native raw materials, including pine wood, pine sawdust, corn stover, wheat straw, grass, and corncob (Binder and Raines 2009; Zhang and Zhao 2010; Wang et al. 2011; Zhang et al. 2013), or from pure saccharides (Ståhlberg et al. 2011). The results were strongly dependent on the type of substrate employed and on the operational conditions, leading to yields of HMF and F that varied within broad ranges (6.4% to 57.9 % and 7.0% to 41.1%, respectively).
HMF Production from Cellulose-enriched Solids from Delignification
The solid phases from the delignification in the two types of media assayed (Acetosolv and [bmim]HSO4) were employed as substrates for HMF manufacture. Both substrates were reacted in [bmim]Cl at 150 °C, 160 °C, or 170 °C with a 10 wt% loading with respect to the IL, in the presence of chromium trichloride (catalyst charge of 7.4 g CrCl3·6H2O/100 g substrate).
The experimental data determined for the Acetosolv-delignified solid (containing 73.4 wt% cellulose) are shown in Fig. 4a. In agreement with the behavior observed in the experiments with hemicellulose-derived saccharides, the HMF concentration profiles presented maximal values for the defined reaction times (40 min in the assay performed at 170 °C, in comparison with 85 min and 150 min in the assays at 160 °C and 150 °C, respectively). The general variation pattern was similar to the one shown in Fig. 3, with HMF behaving as a reaction intermediate due to its participation in a complex reaction scheme involving both productive and parasitic reactions. In comparative terms, HMF generation from the cellulose-enriched phase presented slower kinetics. This was ascribed to the specific properties of the cellulosic substrate, including the limited accessibility to the glycosidic bonds caused by the presence of residual lignin, the heterogeneous nature of the reaction media (involving additional steps of mass transfer and diffusion of reagents and products in the solid matrix), and to the polymeric and recalcitrant nature of cellulose, which possess an ordered structure and needs to be hydrolyzed into glucose before HMF production. Similar HMF concentrations (134 mmol/L to 138 mmol/L) and conversions (35% to 38%, based on the potential substrates) were observed at the optimal reaction times in the experiments performed at 150 °C and 160 °C, respectively; whereas no improvement was observed when the temperature was increased up to 170 °C.
In comparison, the [bmim]HSO4-delignified solid, containing 77.6 wt% cellulose, behaved as a better substrate for HMF manufacture (Fig. 4b). Although the reaction pattern was closely related to the one described for the Acetosolv-delignified solid, the kinetics, which are affected by the physicochemical properties of the substrate and so by the previous processing of the sample, showed slight differences: for example, in the experiments performed at 150 °C, the maximum HMF concentrations were achieved after 150 min in the case of the Acetosolv solid, in comparison with 125 min for the [bmim]HSO4-delignified solid. In the same way, operating at 160 °C, this latter substrate yielded the maximum HMF concentration after 65 min, in comparison with 85 min needed for the other substrate (Fig. 4a). Similar HMF conversions (within the range of 51% to 52 %) and HMF concentrations (within the range of 202 mmol/L to 206 mmol/L) were achieved at the optimal reaction times in the experiments performed at 150 °C, 160 °C, or 170 °C.
Fig. 3. HMF concentration profiles determined for the conversion of HS in [bmim]Cl media containing chromium trichloride and an acidic zeolite: a) Experiments performed with the zeolite CP811C-300 and b) experiments performed with the zeolite CBV-720
In the experiments summarized in Figs. 4a and 4b, F was obtained as a co-product at minimal concentrations, resulting from the conversion of the limited residual xylan contained in the delignified solids.
Literature has been reported on the HMF manufacture from pure cellulose or cellulose-containing materials in chromium-catalyzed [bmim]Cl or [emim]Cl. Starting from cotton linters, Binder and Raines (2009) reached HMF conversions in the range of 33% to 53% operating at 140 °C in [emim]Cl catalyzed with both CrCl2 and HCl. Qi et al. (2010) reported HMF conversions in the range of 9% to 54% for the reaction of cellulose in chromium-catalyzed [bmim]Cl at 120 °C to 150 °C; whereas Zhou et al. (2015) employed the same reaction media to complete the hydrolysis-dehydration of cellulosic substrates (microcrystalline cellulose, filter paper, or cotton) at 120 ºC, reaching 53%, 40%, and 12% HMF conversions of the substrates, respectively.
Fig. 4. Concentration profiles and conversion yields determined for the production of HMF from cellulose-enriched solids: a) Obtained by Acetosolv processing and b) obtained by reaction in [bmim]HSO4
CONCLUSIONS
- Coupling aqueous processing (extraction and autohydrolysis) with delignification (in Acetosolv or [bmim] HSO4 media) allowed an extensive and selective fractionation of Pinus pinaster wood.
- The proposed fractionation scheme yielded extractives, hemicellulose-derived saccharides, cellulose-enriched solids, and soluble lignin fragments as valuable fractions.
- The reaction system [bmim] Cl-CrCl3 (in the presence or absence of an acidic zeolite) was suitable for achieving the conversion of both the hemicellulose-derived saccharides and the cellulose-enriched solids into furans.
- The best HMF yields determined in this study for both types of substrates confirmed the interest of the fractionation scheme proposed in this work for the selective separation of the major components of Pinus pinaster wood, a highly lignified substrate poorly susceptible to fractionation.
ACKNOWLEDGMENTS
The authors are grateful to the Spanish “Ministry of Economy and Competitivity” for supporting this study in the framework of the research project, “Modified aqueous media for wood biorefineries” (reference CTQ2017-82962-R), partially funded by the “Fondo Europeo para el Desarrollo Regional” (FEDER) program of the European Union.
REFERENCES CITED
Binder, J. B., and Raines, R. T. (2009). “Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals,” J. Am. Chem. Soc. 131(5), 1979-1985. DOI: 10.1021/ja808537j
Brandt, A., Ray, M. J., To, T. Q., Leak, D. J., Murphy, R. J., and Welton, T. (2011). “Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid-water mixtures,” Green Chem.13(9), 2489-2499. DOI: 10.1039/C1GC15374A
Brandt-Talbot, A., Gschwend, F. J. V., Fennell, P. S., Lammens, T. M., Tan, B., Weale, J., and Hallett, J. P. (2017). “An economically viable ionic liquid for the fractionation of lignocellulosic biomass,” Green Chem. 19(13), 3078-3102. DOI: 10.1039/C7GC00705A
Cai, C., Zhang, T., Kumar, R., and Wyman, C. E. (2014). “Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass,” J. Chem. Technol. Biot.89(1), 2-10. DOI: 10.1002/jctb.4168
Cheng, S., and Zhu, S. (2009). “Lignocellulosic feedstock biorefinery – The future of the chemical and energy industry,” BioResources 4(2), 456-457. DOI: 10.15376/biores.4.2.456-457
Chheda, J. N., Huber, W., and Dumesic, J. A. (2007a). “Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals,” Angew. Chem. Int. Edit.46(38), 7164-7183. DOI: 10.1002/anie.200604274
Chheda, J. N., Román-Leshkov, Y., and Dumesic, J. A. (2007b). “Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides,” Green Chem. 9(4), 342-350. DOI: 10.1039/B611568C
Conde, E., Fang, W., Hemming, J., Willför, S., Moure, A., Domínguez, H., and Parajó, J. C. (2013). “Water-soluble components of Pinus pinaster wood,” BioResources 8(2), 2047-2063. DOI: 10.15376/biores.8.2.2047-2063
González-Muñoz, M. J., Alvarez, R., Santos, V., and Parajó, J. C. (2011a). “Production of hemicellulosic sugars from Pinus pinaster wood by sequential steps of aqueous extracton and acid hydrolysis,” Wood Sci. Technol. 46(1-3), 271-285. DOI: 10.1007/s00226-011-0408-0
González-Muñoz, M. J., Santos, V., and Parajó, J. C. (2011b). “Purification of oligosaccharides obtained from Pinus pinaster hemicelluloses by diafiltration,” Desalin. Water Treat. 27(1-3), 48-53. DOI: 10.5004/dwt.2011.2047
Gschwend, F. J. V., Malaret, F., Shinde, S., Brandt-Talbot, A., and Hallett, J. P. (2018). “Rapid pretreatment of Miscanthus using the low-cost ionic liquid triethylammonium hydrogen sulfate at elevated temperatures,” Green Chem. 20(15), 3486-3498. DOI: 10.1039/C8GC00837J
Gullón, B., Dávila, I., García-Torreiro, M., Yáñez, R., Labidi, J., and Gullón, P. (2017). “Production and emerging applications of bioactive oligosaccharides from biomass hemicelluloses by hydrothermal processing,” in: Hydrothermal Processing in Biorefineries, H. A. Ruiz, M. Thomsen, H. L. Trajano (eds.), Springer International Publishing, New York, NY, USA, pp. 253-283. DOI: 10.1007/978-3-319-56457-9
Gullón, P., Conde, E., Moure, A., Domínguez, H., and Parajó, J. C. (2010). “Selected process alternatives for biomass refining: A review,” The Open Agriculture Journal 4, 135-144. DOI: 10.2174/1874331501004010135
Gullón, P., Romaní, A., Vila, C., Garrote, G., and Parajó, J. C. (2011). “Potential of hydrothermal treatments in lignocellulose biorefineries,” Biofuel. Bioprod. Bior. 6(2), 219-232. DOI: 10.1002/bbb.339
Hou, Q., Ju, M., Li, W., Liu, L., Chen, Y., and Yang, Q. (2017). “Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems,” Molecules 22(3), 490-513. DOI: 10.3390/molecules22030490
Hou, X., Wang, Z., Sun, J., Li, M., Wang, S., Chen, K., and Gao, Z. (2019). “A microwave-assisted aqueous ionic liquid pretreatment to enhance enzymatic hydrolysis of Eucalyptus and its mechanism,” Bioresource Technol. 272, 99-104. DOI: 10.1016/j.biortech.2018.10.003
Lange, J. P., Heide, E., Buijtenen, J., and Price, R. (2012). “Furfural – A promising platform for lignocellulosic biofuels,” ChemSusChem 5(1), 150-166. DOI: 10.1002/cssc.201100648
Li, C., Knierim, B., Manisseri, C., Arora, R., Scheller, H. V., Auer, M., Vogel, K. P., Simmons, B. A., and Singh, S. (2010). “Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification,” Bioresource Technol. 101(13), 4900-4906. DOI: 10.1016/j.biortech.2009.10.066
Liu, E., Li, M., Das, L., Pu, Y., Frazier, T., Zhao, B., Crocker, M., Ragauskas, A. J., and Shi, J. (2018). “Understanding lignin fractionation and characterization from engineered switchgrass treated by an aqueous ionic liquid,” ACS Sustain. Chem. Eng. 6(5), 6612-6623. DOI: 10.1021/acssuschemeng.8b00384
Octave, S., and Thomas, D. (2009). “Biorefinery: Toward an industrial metabolism,” Biochimie 91(6), 659-664. DOI: 10.1016/j.biochi.2009.03.015
Parajó, J. C., Alonso, J. L., and Santos, V. (1995). “Kinetics of catalyzed organosolv processing of pine wood,” Ind. Eng. Chem. Res. 34(12), 4333-4342. DOI: 10.1021/ie00039a025
Parajó, J. C., Alonso, J. L., Vázquez, D., and Santos, V. (1993). “Optimization of catalysed Acetosolv fractionation of pine wood,” Holzforschung 47(3), 188-193. DOI: 10.1515/hfsg.1993.47.3.188
Peleteiro, S., Garrote, G., Santos, V., and Parajó, J. C. (2014). “Furan manufacture from softwood hemicelluloses by aqueous fractionation and further reaction in a catalyzed ionic liquid: A biorefinery approach,” J. Clean. Prod. 76, 200-203. DOI: 10.1016/j.jclepro.2014.04.034
Peleteiro, S., Lopes, A. M., Garrote, G., Parajó, J. C., and Lukasik, R. (2015a). “Manufacture of furfural in biphasic media made up of an ionic liquid and a co-solvent,” Ind. Crop. Prod.77, 163-166. DOI: 10.1016/j.indcrop.2015.08.048
Peleteiro, S., Rivas, R., Alonso, J. L., Santos, V., and Parajó, J. C. (2015b). “Utilization of ionic liquids in lignocellulose biorefineries as agents for separation, derivatization, fractionation or pretreatment,” J. Agr. Food Chem. 63(37), 8093-8102. DOI: 10.1021/acs.jafc.5b03461
Peleteiro, S., Rivas, S., Alonso, J. L., Santos, V., and Parajó, J. C. (2016a). “Furfural production using ionic liquids: A review,” Bioresource Technol. 202, 181-191. DOI: 10.1016/j.biortech.2015.12.017
Peleteiro, S., Santos, V., Garrote, G., and Parajó, J. C. (2016b). “Furfural production from Eucalyptus wood using an acidic ionic liquid,” Carbohyd. Polym. 146, 20-25. DOI: 10.1016/j.carbpol.2016.03.049
Peleteiro, S., Santos, V., and Parajó, J. C. (2016c). “Furfural production in biphasic media using an acidic ionic liquid as a catalyst,” Carbohyd. Polym. 153, 421-428. DOI: 10.1016/j.carbpol.2016.07.093
Qi, X., Watanabe, M., Aida, T. M., and Smith, R. L. (2010). “Fast transformation of glucose and di-polysaccharides into 5-hydroxymethylfurfural by microwave heating in an ionic liquid/catalyst system,” ChemSusChem 3(9), 1071-1077. DOI: 10.1002/cssc.201000124
Rackemann, D. W., and Doherty, W. O. (2011). “The conversion of lignocellulosics to levulinic acid,” Biofuel. Bioprod. Bior. 5(2), 198-214. DOI: 10.1002/bbb.267
Santos, J. H. P. M., Trigo, J. P., Maricato, E., Nunes, C., Coimbra, M. A., and Ventura, S. P. M. (2018). “Fractionation of Isochrysis galbana proteins, arabinans, and glucans using ionic-liquid-based aqueous biphasic systems,” ACS Sustain. Chem. Eng. 6(11), 14042-14053. DOI: 10.1021/acssuschemeng.8b02597
Ståhlberg, T., Rodríguez-Rodríguez, S., Fristrup, P., and Riisager, A. (2011). “Metal‐free dehydration of glucose to 5‐(hydroxymethyl) furfural in ionic liquids with boric acid as a promoter,” Chem. Eur. J. 17(5), 1456-1464. DOI: 10.1002/chem.201002171
Stark, A. (2011). “Ionic liquids in the biorefinery: A critical assessment of their potential,” Energ. Environ. Sci. 4(1), 19-32. DOI: 10.1039/C0EE00246A
Stocker, M. (2008). “Biofuels and biomass-to-liquid fuels in the biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials,” Angew. Chem. Int. Edit. 47(48), 9200-9211. DOI: 10.1002/anie.200801476
Sun, N., Parthasarathi, R., Socha, A. M., Shi, J., Zhang, S., Stavila, V., Sale, K. L., Simmons, B. A., and Singh, S. (2014). “Understanding pretreatment efficacy of four cholinium and imidazolium ionic liquids by chemistry and computation,” Green Chem. 16(5), 2546-2557. DOI: 10.1039/C3GC42401D
Wang, P., Yu, H., Zhan, S., and Wang, S. (2011). “Catalytic hydrolysis of lignocellulosic biomass into 5-hydroxymethylfurfural in ionic liquid,” Bioresource Technol. 102(5), 4179-4183. DOI: 10.1016/j.biortech.2010.12.073
Xu, J., Hou, H., Hu, J., and Liu, B. (2018). “Coupling of hydrothermal and ionic liquid pretreatments for sequential biorefinery of Tamarix austromongolica,” Appl. Energ. 229, 745-755. DOI: 10.1016/j.apenergy.2018.08.038
Zhang, L., Yu, H., Wang, P., Dong, H., and Peng, X. (2013). “Conversion of xylan, D-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid,” Bioresource Technol. 130, 110-116. DOI: 10.1016/j.biortech.2012.12.018
Zhang, Z., and Zhao, Z. K. (2010). “Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid,” Bioresource Technol. 101(3), 1111-1114. DOI: 10.1016/j.biortech.2009.09.010
Zhou, L., He, Y., Ma, Z., Liang, R., Wu, T., and Wu, Y. (2015). “One-step degradation of cellulose to 5-hydroxymethylfurfural in ionic liquid under mild conditions,” Carbohyd. Polym.117, 694-700. DOI: 10.1016/j.carbpol.2014.10.062
Article submitted: March 3, 2019; Peer review completed: April 20, 2019; Revisions accepted: April 24, 2019; Published: April 25, 2019.
DOI: 10.15376/biores.14.2.4733-4747
