Abstract
This study characterized the lignin peroxidase (LiP) activity of soil via an enzyme assay to determine the reaction rates and activation energies for 5 wt%, 10 wt%, 15 wt%, and 20 wt% lignin loads in urea crosslinked starch biocomposites. The results revealed that a mixed mode of LiP inhibition occurred after the soil was mixed with these biocomposites with different loads of lignin. Loading of lignin at 5 wt% and 10 wt% lignin resulted in higher values of catalytic activity of LiP: -39.58 and 49.14 µM h-1 g-1 soil, respectively. In comparison, with higher loading of lignin at 15 wt% and 20 wt%, decreases in the catalytic activity of LiP were found and were 28.72 to 37.25 µM h-1 g-1 soil, respectively. The activation energy of LiP increased approximately 1.11- to 1.22-fold when 15 and 20 wt% of lignin was loaded in biocomposites. Research findings established the possibility of unfavorable binding of the LiP to lignin with an increase in the load of lignin, possibly due to the complex structure of intact lignin and presence of inhibitory biodegradation products of lignin accumulates during lignin biodegradation in biocomposites. It was concluded that higher lignin contents (15 wt% and 20 wt%) were effective in reducing the activity of the soil LiP. Hence, higher lignin content possibly protects against losses of lignin, while acting as a filler in the formulation of biocomposites.
Download PDF
Full Article
Reduction in Lignin Peroxidase Activity Revealed by Effects of Lignin Content in Urea Crosslinked Starch under Aerobic Biodegradation in Soil
Zahid Majeed,a,b* Zainab Ajab,c Qingjie Guan,c Abdul Zahir Abbasi,a Qaisar Mahmood,d Mater H. Mahnashi,e Bandar A. A. Alyami,e Ali O. Alqarni,e Yahya S. Alqahtani,e and Nurlidia Mansor b
This study characterized the lignin peroxidase (LiP) activity of soil via an enzyme assay to determine the reaction rates and activation energies for 5 wt%, 10 wt%, 15 wt%, and 20 wt% lignin loads in urea crosslinked starch biocomposites. The results revealed that a mixed mode of LiP inhibition occurred after the soil was mixed with these biocomposites with different loads of lignin. Loading of lignin at 5 wt% and 10 wt% lignin resulted in higher values of catalytic activity of LiP: -39.58 and 49.14 µM h-1 g-1 soil, respectively. In comparison, with higher loading of lignin at 15 wt% and 20 wt%, decreases in the catalytic activity of LiP were found and were 28.72 to 37.25 µM h-1 g-1 soil, respectively. The activation energy of LiP increased approximately 1.11- to 1.22-fold when 15 and 20 wt% of lignin was loaded in biocomposites. Research findings established the possibility of unfavorable binding of the LiP to lignin with an increase in the load of lignin, possibly due to the complex structure of intact lignin and presence of inhibitory biodegradation products of lignin accumulates during lignin biodegradation in biocomposites. It was concluded that higher lignin contents (15 wt% and 20 wt%) were effective in reducing the activity of the soil LiP. Hence, higher lignin content possibly protects against losses of lignin, while acting as a filler in the formulation of biocomposites.
Keywords: Activation energy; Biocomposites; Lignin peroxidase; Lignin; Michaelis-Menten; Urea crosslinked starch
Contact information: a: Department of Biotechnology, University of Azad Jammu and Kashmir, Chehla Campus, Muzaffarabad, Azad Kashmir Pakistan; b: Chemical Engineering Department, Universiti Teknologi PETRONAS, Seri Iskandar, Tronoh, Malaysia; c: Key Laboratory of Saline-alkali Vegetation Ecology Restoration, College of Life Science, Northeast Forestry University, No.26 Hexing Road Xiangfang District, Harbin 150040 P.R. China; d: Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus, Pakistan; e: Department of Pharmaceutical Chemistry, College of Pharmacy, Najran University, Najran 61441 Saudi Arabia;
* Corresponding author: zahidfdb@gmail.com; zahid.majeed@ajku.edu.pk
INTRODUCTION
Lignin is made up of three phenylpropane units, which are syringyl, guaiacyl, and p-hydroxyphenyl. These units build up the complex aromatic structure of the lignin, which has an important role in maintaining the integrity of plant’s cell wall. Lignin also is known to provide protection to plants against pathogens. Lignin peroxidase (LiP) is the first enzyme that has ligninolytic activity, and it was purified from a fungal species, Phanerochaete chrysosporium.
LiP contains a heme cofactor that is able to break bonds of aromatic rings present in lignin (Romero et al. 2019). LiP is capable of oxidizing lignin directly at the protein surface by a long-range electron transfer process (Johjima et al. 1999). This electron transfer involves the His–Asp⋅⋅⋅proximal-His motif, which routes the electrons to oxidize polymeric lignin. In the motif, His-239 acts as a possible lignin-binding site on the surface, which is linked to Asp-238. This Asp residue is hydrogen-bonded to the proximal His-176. In another study, energy changes demonstrated that the interface between Trp171 of LiP is more favorable with veratryl alcohol cation radical (VA•+) than with veratryl alcohol (VA). VA•+ stabilization at the LiP surface. This difference has been linked to the increase in enzyme turnover in the form of final product veratraldehyde, after the rapid reaction of VA•+ with O2; these events also make possible its participation in lignin degradation as a redox mediator (Houtman et al. 2018; Romero et al. 2019). The catalytic activity (Kcat) of LiP is also strongly associated with specific ratios of monomers, e.g., cinnamyl/vanillin, and syringyl:cinnamyl:vanillin/vanillin, in lignin. The maximum reaction velocity (Vmax) and enzyme-substrate affinity (Km) are tightly modulated by the nature of the source of the lignin. LiP has shown low Vmax and Km values, but high catalytic efficiency (kcat = Vmax/Km) estimates in lignin-rich soils (Wang et al. 2012). In enzyme reactions, activation energy (Ea) has also been viewed as a degree of conformational stability of enzymes in soil (Wallenstein et al. 2010). A low-quality carbon substrate, e.g., lignocellulose, requires a higher net Ea for biotransformation (Kleber 2010).
Lignin is an important bioresource that is recommended as a hydrophobic filler for hydrophilic and bioabsorbent types of biocomposite matrices (Ariyanti et al. 2012). Lignin also protects the biocomposites degradation from microorganisms present in soil (Wool et al. 2000). In addition, the hydrophilic fraction of phenolic compounds has a low molecular weight, which may also affect the biodegradation of lignin fillers in biocomposites. The biodegradation of the lignin filler in starch biocomposites has not been reported in previous literature, which establishes the novel nature of this proposed research. If lignin biodegrades, then use as filler will impact negatively on the starch biocomposite in terms of its optimal mechanical properties. Therefore, the biodegradation of hydrophobic lignin in a hydrophilic starch matrix would not be effective in reducing the biodegradation of starch biocomposites. Furthermore, little research has been reported on the biodegradation of fillers in starch biocomposites. Therefore, lack of systematic studies pertaining to how the rates of enzyme reactions and their activation energies change during the filler biodegradation process warrants further research on this topic. In addition, the purpose of this study is to answer the question that how lignin constrain the activity of LiP in starch biocomposites that are carrying the increasing loads of lignin as filler. Thus, the objective of this work was to determine the reaction rates and activation energies of LiP, in response to low (5 wt% and 10 wt%) and high (15 wt% and 20 wt%) lignin loaded urea crosslinked starch (UcS) biocomposites.
EXPERIMENTAL
Materials
The following materials were used: di-sodium tetraborate heptahydrate (99.9%) (Merck, Darmstadt, Germany), sodium potassium tartrate (99%) (HmbG Chemicals, Hamburg, Germany), L(+)-tartaric acid (99.5%) (Merck), trisodium citrate dihydrate (>99%) (Merck), citric acid monohydrate (>99%) (Merck), veratryl alcohol (96%) (Sigma-Aldrich, St. Louis, MO), hydrogen peroxide (30% w/v) (Thermo Fisher Scientific, Waltham, MA), urea (46% N) (PETRONAS Chemicals Fertilizer Kedah Sdn Bhd, Kedah, Malaysia), alkaline kraft lignin (Sigma-Aldrich, St. Louis, MO, USA), and tapioca starch (Cap Kapal ABC brand) (Thye Huat Chan Sdn Bhd, Penang, Malaysia). Loamy sand soil used in this work was collected from Titi Gantung (4.36° North, 100.84° East), Perak, Malaysia (Majeed et al. 2017).
Lignin loading process for urea crosslinked starch biocomposites
The lignin-loaded UcS biocomposites were prepared according to the authors’ previous published report (Majeed et al. 2017). The UcS with lignin were termed biocomposites and designated names based on the basis of lignin loadings which were UcS5%L, UcS10%L, UcS15%L, and UcS20%L (the L stands for lignin). The UcS0%L was used as control for the comparison of reaction rates and Ea of LiP.
LiP reaction rates and activation energies
The aerobic soil microcosm was constructed at laboratory scale at pH 4.8 to 5.0 and 50% water holding capacity (Majeed et al. 2017) for the LiP kinetic studies. Approximately 2.5 g of aerobic soil was separately extracted in a 50 mL sodium tartrate–citric acid buffer (100 mM, pH 2.5) for 30 minutes in an ice cooled water bath. The LiP activity was assayed by measuring the conversion of 0 to 300 µM of veratryl alcohol (substrate) into veratryl aldehyde (product) quantified at 310 nm via UV-Visible spectrophotometer (Shimadzu, Kyoto, Japan) (Ramírez et al. 2010). The unit activity of LiP is defined as the oxidative cleavage of 1 µM of veratryl alcohol to veratryl aldehyde in one minute. The molar extinction coefficient of veratryl aldehyde (9300 M-1 cm-1) was used to determine the unit activity of LiP (German et al. 2011). The Michaelis-Menten kinetic was used to calculate the Vmax (maximum reaction velocity), Km (substrate affinity), and Kcat (catalytic efficiency) (Johnson and Goody 2011). OriginPro software (OriginLab Corporation, Northampton, MA) was used to fit the data into a Michaelis-Menten kinetic, a non-linear regression model, for predicting the Vmax and Km values for LiP. The Ea was calculated to measure the temperature sensitivities of soil LiP at three different temperatures (20 °C, 30 °C, and 40 °C). The temperature and LiP reaction rates were analysed by fitting the data into the Arrhenius Equation (Arrhenius 1889).
Statistical analysis
One-way ANOVA was applied and followed by a post hoc Dunnett’s test to determine whether the addition of lignin into biocomposites significantly reduced the reaction rates and Ea of LiP found in soil.
RESULTS AND DISCUSSION
Effect of Lignin’s Loadings on Reaction Rates of LiP
Figure 1 shows the change in LiP reaction rates in response to different loads of lignin in starch biocomposites. The reaction rates of LiP decreased with the increase of lignin loads from 5 to 20 wt% in starch biocomposites. At 5 and 10 wt% of lignin loads, the reaction rates of LiP were higher than the control. By contrast, for 15 and 20 wt% of lignin loads, reaction rates were lower than the control in aerobic soil. However, the lignin loads at all concentrations showed a non-significant change in the reaction rates of LiP (One-way ANOVA, F5,42 = 0.91, p-value greater than 0.05) in comparison to control. These findings showed that higher loads of lignin have observable impact on reducing the activity of LiP and hence protects the biodegradation of the lignin in biocomposites. The difference in reaction rates for LiP can be explained by the composition of lignin monomers, i.e., syringyl, cinnamyl, and vanillin. Vanillyl-phenols are reported to be more resistant as compared to syringyl type of phenols in lignin (Thevenot et al. 2010). In the authors’ earlier work, biodegradation analysis revealed that higher starch to lignin ratios in low wt% lignin loaded biocomposites increased the hydrophilicity of the degraded lignin, which caused fast turnover of the lignin’s acid (Majeed et al. 2016).
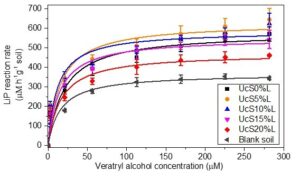
Fig. 1. The reaction rates of LiP in aerobic soil mixed with lignin reinforced biocomposites
In addition, low wt% of lignin loads could easily be oxidized due to better electron transference from the lignin to the LiP. This transfer of electrons is assumed to be favored by the higher starch to lignin ratio in biocomposites with low wt% of the lignin. The oxidation state of hydrophilic starch is lower than the oxidation state of hydrophobic lignin (Voitkevich et al. 2012; Kabo et al. 2013). This possibly increases the electron transfer efficiently to lignin. Insoluble high molecular weight lignin has been reported to be involved in long-range electron transfer and to serve as a reservoir of electrons known for facilitating the activity of oxidative enzymes (Westereng et al. 2015). It is therefore possible that the proportionally higher starch in 5 and 10 wt% compared to 15 and 20 wt% lignin biocomposites increased the electron transfer efficiently from the lignin to LiP. Therefore, a low concentration of lignin is possibly easily oxidized in biocomposites. This concept is supported by the observed higher reaction rates of LiP. In fact, LiP needs the electron transfer from the lignin (substrate) in order to form the oxidized intermediate forms of the LiP, i.e., LiP I and LiP II. In fact, dimeric lignins or monolignolic analogs containing free-hydroxyl phenolic groups are reported as unfavourable substrates and a potent inhibitor of the LiP. Therefore, higher loads of lignin (15 and 20 wt%) carry higher concentration of free hydroxyl phenolic moieties, which are known to reduce the enzymatic degradation rate of lignin by misbalancing the electron transfer between LP I and LiP II (Johjima et al. 1999; Eom and Kim 2014).
In Table 1, the values of the reaction rates of LiP are shown, which were calculated in aerobic soil. The fold change in activity of LiP for lignin loaded biocomposites assessed through the comparison with known activity for control. It was found that increasing the lignin concentration in the biocomposites had a negative correlation with the Vmax of LiP. The Vmax of LiP was approximately 1.01 to 1.08 times higher for 5 and 10 wt% lignin loaded biocomposites compared to the activity observed against control. The LiP activity observed for 15 and 20 wt% loads of lignin was approximately 1.05 to 1.22 times less than the activity found for control. The Km of LiP did not show much difference between the various lignin loadings in the biocomposites under aerobic soil conditions.
Addition of lignin into the biocomposites reduced Km of LiP to approximately 1.26 to 1.73 times less than the control. This can be explained if it is assumed that the LiP has higher affinity for lignin as its substrate. However, the Km of LiP did not change much despite the different loads of lignin. The difference in Vmax was not well correlated with the change in Km, which indicated the formation of LiP-lignin complexes involved mix mode of inhibition mechanism (Berg et al. 2012). It was concluded that at low concentration of lignin, LiP has higher affinity for lignin, but at higher concentration of lignin the activity of LiP is constrained.
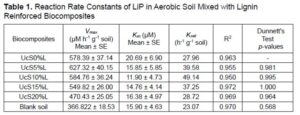
The Kcat of LiP was approximately 1.02 to 1.75 times greater in lignin-loaded biocomposites as compared to the control. When comparing the lignin loading amounts in the biocomposites in terms of the Kcat values, it was found that the Kcat of LiP decreased as the lignin wt.% in biocomposites increased. This decrease in Kcat could be due to either the direct participation of the lignin in regulating the expression of LiP in soil residing microorganisms or the indirect involvement of the starch to lignin ratio in lignin-loaded biocomposites. First, lignin may be directly used as a substrate, which stimulates the soil microorganisms to produce more LiP. The expressed LiP is possibly inhibited by excess of lignin due to mixed-type of inhibition effect, which reduces the Kcat of LiP. This is further validated by the fact that the presence of additional recalcitrant vanillyl units in lignin can possibly reduce Kcat of peroxidases (Triebwasser-Freese et al. 2015). Second, starch could contribute to the lignin directed indirect inhibition of LiP and reduce the Kcat estimates. As starch provides glucose, which is an essential energy molecule, it is metabolized intracellularly to produce energy and promote cellular activities in microorganisms. However, the wt% of starch proportionally decreased in the lignin reinforced biocomposites in response to the stepwise increase in the amount of lignin added. Thus, ultimately less starch was available for microorganisms to metabolize and produce sufficient LiP to biodegrade the higher loads of lignin compared to lower loads of lignin in biocomposites.
Activation Energy of LiP
Figure 2 shows the reactions rates (ln k vs. 1/T (K) and the slope determined by the Ea. Table 2 summarizes the Ea values of the soil LiP obtained from Fig. 2, which was calculated after conducting the biodegradation experiments in aerobic soil for each biocomposite with different lignin wt%. Compared to the control biocomposites, the Ea of LiP generally increased in proportion to the increase in lignin wt% in biocomposites. This trend is in agreement with reports, which showed that starch biocomposites with 30 wt% toluene diisocyanate modified cellulose reduced the velocity rate constant of amylase enzymes by approximately 40% and doubled the Ea (as high as 23.93 KJ/mol to 25.35 KJ/mol) in comparison to the unmodified starch biocomposite (Spiridon et al. 2015). Thus, under the current research work, the addition of lignin into starch reduced the rates of reaction but increased the Ea for LiP, which was in agreement with the literature. Thus, the increase in Ea of LiP was approximately 1.11 to 1.22 times greater in biocomposites than the control.

The effect of different wt% of lignin on the Ea of LiP in biocomposites showed a non-significant difference (one-way ANOVA F5,12 = 2.55, p-value less than 0.05). The increase in Ea indicated that LiP activity greatly depends on the temperature, in terms of the depolymerization of lignin, in comparison to increasing lignin wt.% in the biocomposites. A lower lignin wt% in the biocomposite was possibly related to a lower temperature sensitivity of the reactions catalyzed by LiP, which was needed in order to pass the energy barrier for the conversion of the lignin into their biodegradation products.
CONCLUSIONS
- Soil lignin peroxidase (LiP) responded differently to low and high wt% of lignin added as a filler to starch termed as biocomposites. Higher amounts, e., 15 and 20 wt% of lignin, can more effectively reduce the reaction velocity of LiP due to constraint of the lignin on LiP affinity, which ultimately reduces its catalytic activity.
- The reaction rates of LiP corresponded to the higher energy of activation (Ea) in 15 and 20 wt% lignin-reinforced biocomposites, which explained why the LiP needed a greater temperature to have equal reaction rates observed in the 5 and 10 wt% lignin loaded biocomposites.
- Based on this work, it is recommended to employ a higher lignin load (>15%) for lignin reinforced biocomposites in order to effectively constrain the LiP activity to reduce the depolymerization of lignin, e., reduce the loss of filler properties in the biocomposites.
ACKNOWLEDGEMENTS
All authors acknowledge the Universiti Teknologi PETRONAS, Malaysia for extending cooperation for the use of research facilities and providing graduate assistantship. We also admit the Deanship of Scientific Research at Najran University, Najran, Saudi Arabia for financial support to publish this research work in Journal of BioResources successfully.
REFERENCES CITED
Ariyanti, S., Man, Z., and Azmi, B. M. (2012). “Improvement of hydrophobicity of urea modified tapioca starch film with lignin for slow release fertilizer,” Advanced Material Research 626, 350-354. DOI: 10.4028/www.scientific.net/AMR.626.350
Arrhenius, S. (1889). “Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren,” Z. Physikalische Chem. 4, 226-248.
Berg, J. M., Tymoczko, J. L., and Stryer, L. (2012). Biochemistry, J. M. Berg, J. L. Tymoczko, L. Stryer, and G. J. Gatto, Jr. (eds.), W. H. Freeman, New York.
Eom, M.-H., and Kim, Y. H. (2014). “Inactivating effect of phenolic unit structures on the biodegradation of lignin by lignin peroxidase from Phanerochaete chrysosporium,” Enzyme and Microbial Technology 61, 48-54. DOI: 10.1016/j.enzmictec.2014.04.013
German, D. P., Weintraub, M. N., Grandy, A. S., Lauber, C. L., Rinkes, Z. L., and Allison, S. D. (2011). “Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies,” Soil Biology and Biochemistry 43(7), 1387. DOI: 10.1016/j.soilbio.2011.03.017
Houtman, C. J., Maligaspe, E., Hunt, C. G., Fernández-Fueyo, E., Martínez, A. T., and Hammel, K. E. J. (2018). “Fungal lignin peroxidase does not produce the veratryl alcohol cation radical as a diffusible ligninolytic oxidant,” Journal of Biological Chemistry 293(13), 4702-4712. DOI: 10.1074/jbc.RA117.001153
Johjima, T., Itoh, N., Kabuto, M., Tokimura, F., Nakagawa, T., Wariishi, H., and Tanaka, H. (1999). “Direct interaction of lignin and lignin peroxidase from Phanerochaete chrysosporium,” Proceedings of the National Academy of Sciences 96(5), 1989-1994. DOI: 10.1073/pnas.96.5.1989
Johnson, K. A., and Goody, R. S. (2011). “The original Michaelis constant: Translation of the 1913 Michaelis–Menten paper,” Biochemistry 50(39), 8264. DOI: 10.1021/bi201284u
Kabo, G. J., Voitkevich, O. V., Blokhin, A. V., Kohut, S. V., Stepurko, E. N., and Paulechka, Y. U. (2013). “Thermodynamic properties of starch and glucose,” The Journal of Chemical Thermodynamics 59, 87-93. DOI: 10.1016/j.jct.2012.11.031
Kleber, M. (2010). “What is recalcitrant soil organic matter?” Environmental Chemistry 7(4), 320-332. DOI: 10.1071/EN10006
Majeed, Z., Mansor, N., Ajab, Z., and Man, Z. (2017). “Lignin macromolecule’s implication in slowing the biodegradability of urea crosslinked starch films applied as slow release fertilizer,” Starch‐Stärke 68, 1600362. DOI: 10.1002/star.201600362
Majeed, Z., Mansor, N., Man, Z., and Wahid, a. S. A. (2016). “Lignin reinforcement of urea-crosslinked starch films for reduction of starch biodegradability to improve slow nitrogen release properties under natural aerobic soil condition,” e-Polymers 16(2), 159-170. DOI: 10.1515/epoly-2015-0231
Ramírez, D. A., Muñoz, S. V., Atehortua, L., and Michel Jr, F. C. (2010). “Effects of different wavelengths of light on lignin peroxidase production by the white-rot fungi Phanerochaete chrysosporium grown in submerged cultures,” Bioresource Technology 101(23), 9213-9220. DOI: 10.1016/j.biortech.2010.06.114
Romero, J. O., Fernández-Fueyo, E., Avila-Salas, F., Recabarren, R., Alzate-Morales, J., and Martínez, A. T. J. C. (2019). “Binding and catalytic mechanisms of veratryl alcohol oxidation by lignin peroxidase: A theoretical and experimental study,” 17, 1066-1074. DOI: 10.1016/j.csbj.2019.07.002
Spiridon, I., Anghel, N., and Bele, A. (2015). “Behavior of biodegradable composites based on starch reinforced with modified cellulosic fibers,” Polymers for Advanced Technologies 26(9), 1189-1197. DOI: 10.1002/pat.3553
Thevenot, M., Dignac, M.-F., and Rumpel, C. J. S. B. (2010). “Fate of lignins in soils: A review,” Biochemistry 42(8), 1200-1211. DOI: 10.1016/j.soilbio.2010.03.017
Triebwasser-Freese, D. J., Tharayil, N., Preston, C. M., and Gerard, P. G. (2015). “Catalytic kinetics and activation energy of soil peroxidases across ecosystems of differing lignin chemistries,” Biogeochemistry: 1-17. DOI: 10.1007/s10533-015-0086-3
Voitkevich, O. V., Kabo, G. J., Blokhin, A. V., Paulechka, Y. U., and Shishonok, M. V. (2012). “Thermodynamic properties of plant biomass components. Heat capacity, combustion energy, and gasification equilibria of lignin,” Journal of Chemical & Engineering Data 57(7), 1903-1909. DOI: 10.1021/je200270t
Wallenstein, M., Allison, S. D., Ernakovich, J., Steinweg, J. M., and Sinsabaugh, R. (eds.), (2010). Controls on the temperature sensitivity of soil enzymes: A key driver of in situ enzyme activity rates,” in: Soil Enzymology, G. Shukla and A. Varma (eds.), Springer, Berlin, Germany, Springer. DOI: 10.1007/978-3-642-14225-3_13
Wang, G., Post, W. M., Mayes, M. A., Frerichs, J. T., and Sindhu, J. (2012). “Parameter estimation for models of ligninolytic and cellulolytic enzyme kinetics,” Soil Biology and Biochemistry 48, 28-38. DOI: 10.1016/j.soilbio.2012.01.011
Westereng, B., Cannella, D., Agger, J. W., Jørgensen, H., Andersen, M. L., Eijsink, V. G., and Felby, C. J. S. r. (2015). “Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer,” Sci. Rep. 5(1), 1-9. DOI: 10.1038/srep18561
Wool, R., Raghavan, D., Wagner, G., and Billieux, S. (2000). “Biodegradation dynamics of polymer–starch composites,” Journal of Applied Polymer Science 77(8), 1643-1657. DOI: 10.1002/1097-4628(20000822)77:8<1643::AID-APP1>3.0.CO;2-8
Article submitted: Sept. 11, 2020; Peer review completed: December 5, 2020; Revised version received and accepted: January 23, 2021; Published: January 26, 2021.
DOI: 10.15376/biores.16.1.1940-1948
