Abstract
This review will focus on recent progress regarding the mechanisms of light-induced discoloration of mechanical and chemimechanical pulps and on the proposed preventive treatments. It is evident that the mechanisms behind photoyellowing of lignin-rich pulps are complex and that several types of reaction pathways may coexist. Photoyellowing proceeds via one initial fast phase and a slower following phase. The fast phase has been ascribed to oxidation of free phenolic groups and/or hydroquinones and catechols to photoproducts of mainly quinonoid character. A multitude of reactions involving several lignin subunits are possible. Important intermediates are phenoxyl radicals, and to some extent ketyl radicals. The importance of the phenacyl aryl ether pathway might be more important than previously thought, even though the original content of such groups is low in lignin. Even though many preventive methods against photoyellowing have been suggested, no cost-efficient treatment is available to hinder photoreversion of lignin-containing paper permanently. Suggested methods for stabilization include chemical modification (etherification and esterification), coating the paper product, addition of radical scavengers, excited state quenchers, or ultraviolet absorbing compounds.
Download PDF
Full Article
Review: Light-induced yellowing of lignocellulosic pulps – mechanisms and preventive methods
Magnus Paulsson a,b,* and Jim Parkås c
This review will focus on recent progress regarding the mechanisms of light-induced discoloration of mechanical and chemimechanical pulps and on the proposed preventive treatments. It is evident that the mechanisms behind photoyellowing of lignin-rich pulps are complex and that several types of reaction pathways may coexist. Photoyellowing proceeds via one initial fast phase and a slower following phase. The fast phase has been ascribed to oxidation of free phenolic groups and/or hydroquinones and catechols to photoproducts of mainly quinonoid character. A multitude of reactions involving several lignin subunits are possible. Important intermediates are phenoxyl radicals, and to some extent ketyl radicals. The importance of the phenacyl aryl ether pathway might be more important than previously thought, even though the original content of such groups is low in lignin. Even though many preventive methods against photoyellowing have been suggested, no cost-efficient treatment is available to hinder photoreversion of lignin-containing paper permanently. Suggested methods for stabilization include chemical modification (etherification and esterification), coating the paper product, addition of radical scavengers, excited state quenchers, or ultraviolet absorbing compounds.
Keywords: Chemimechanical pulp; Discoloration; Light-induced; Lignin; Mechanical pulp; Stabilization; Yellowing
Contact information: a: AkzoNobel Pulp and Performance Chemicals, SE-44580, Bohus, Sweden; b: Mid Sweden University, FSCN, SE-85170 Sundsvall, Sweden; c: Södra Innovation, Södra Cell Värö, SE-43024 Väröbacka, Sweden; *Corresponding author: magnus.paulsson@akzonobel.com
This paper is dedicated to the memory of Professor Knut Lundquist.
INTRODUCTION AND SCOPE OF THE REVIEW
Enhanced production and efficient utilization of lignocellulosic products are issues of critical importance to both industry and society. Lignin-containing, high-yield mechanical and chemimechanical pulps such as stone ground wood (SGW) pulp, thermomechanical pulp (TMP), and chemithermomechanical pulp (CTMP) have a number of advantages when compared with chemical pulps. Examples of such advantages are the efficient utilization of the available virgin fiber resource and the possibility to produce pulps with a unique combination of light scattering, bulk, and strength properties that render it possible to produce high-quality and low basis weight printing papers at a low cost (Höglund and Wilhelmsson 1993; Heikkurinen et al. 2009). The main unresolved problems associated with the aforementioned pulp types are the high electrical refining energy needed for fiber separation and development of properties and the inferior brightness and brightness (color) stability that occurs upon exposure to daylight or indoor illumination. The latter hinders high-yield pulps being used as the major fiber component in long-life, high-quality paper (and board) products. Therefore, mechanical and chemimechanical pulps are currently used in short-life graphical paper qualities such as newsprint, and uncoated and coated magazine papers. Newsprint production has decreased by about 20% since the year 2000 (Fig. 1) and it is likely that this decrease will continue due to rapidly changing use of media, altered reading habits, and competition from electronic publications. Alternative value-added products from high-yield pulps are therefore needed, and it is likely that these products, e.g., fine paper, require that the lignin-containing pulps have improved brightness (color) stability, especially if these pulps are to be used as the main fiber component.
Fig. 1. The production and production forecast of wood-containing graphic papers worldwide (source: RISI)
Light-induced discoloration has been extensively studied since the mid-20th century and is one of the most studied wood chemistry problems. Extensive research efforts in North America and Europe during the 15 years starting from the beginning of the 1990s were devoted to this recalcitrant problem. The vast amount of new information is reflected in the number of scientific journal and conference articles published in this time period (Fig. 2). The research activities have, however, declined since then, and it is reasonable to expect that more efforts are needed in the future if the utilization of lignin-containing pulps is going to be extended.
Brightness (color) reversion, also known as yellowing, discoloration, or ageing, of pulp and paper products can be caused by several factors e.g., light, heat (Gellerstedt et al. 1983; McLellan et al. 1990; Paulsson and Ragauskas 1998a), or air pollution (Adelstein et al. 2003). A number of excellent reviews dealing with various aspects of these phenomena have previously been published (Gratzl 1985; Heitner 1993; Leary 1994; Davidson 1996; Forsskåhl 2000; Lanzalunga and Bietti 2000; Heitner 2010).
Fig. 2. The number of journal and conference articles in the databases Paperbase (1988-2010) and SciFinder (1996-2010) dealing with light-induced discoloration of lignocellulosic materials
The scope of this review is to focus on the progress regarding the light-induced discoloration of mechanical and chemimechanical pulps and to discuss the underlying mechanisms for the color formation of these pulps. Further, an overview of the preventive photostabilizing treatments proposed will also be given.
EARLY REPORTS OF LIGHT-INDUCED YELLOWING
The rapid discoloration of groundwood-based papers was reported as early as the end of the 19th century by Cross (1897) and Evans (1898), and it was concluded that oxygen participates in the discoloration process. A few years later, Klemm (1903) introduced light as a contributing factor, and it was also early proposed that the discolora-tion was due to an oxidation of the non-cellulosic constituents in the pulp such as fats, waxes, resins, and lignin (Sindall 1920) and that papers made from chemical pulps were more stable compared to papers produced from mechanical pulps (Schoeller 1912). Further, in many of the early observations, rosin and iron were said to be important factors that influenced the extent of discoloration of both chemical and mechanical pulps. The chemical changes associated with the discoloration and degradation of lignin and lignocellulosic materials were first studied in the 1940s. Irradiation of Brauns’ lignin and spruce woodmeal with ultraviolet (UV) radiation generated light-absorbing compounds, and lignin underwent chemical changes that were reflected in lower methoxyl and Klason lignin contents (Forman 1940). Vanillin (one of the products of lignin degradation) underwent a rapid discoloration when irradiated in solution and caused extensive yellowing of impregnated filter paper. Demethoxylation of lignin was later reported for irradiated milled wood lignin (MWL) (Sjöholm et al. 1992), groundwood-based papers (Lewis and Fronmuller 1945; Leary 1967; Hemmingson and Morgan 1990), jute (Callow and Speakman 1949), and radiata pine wood (Sinclair and Vincent 1964). Light-induced ageing of groundwood pulp in different atmospheres showed that atmospheric oxygen increased the discoloration rate and consequently that oxygen participates (directly or indirectly) in chromophore formation (Nolan et al. 1945; van den Akker et al. 1949). It was also found that discoloration was accelerated by an increased temperature. Further, the discoloration was not directly proportional to the irradiation time, and most of the chromophore formation occurred rapidly during irradiation (Lewis et al. 1945; Francis et al. 1991; Ek et al. 1992). The initial phase was followed by a slower, less detrimental phase. Nolan et al. (1945) stated that the photochemical reactions were of at least two types: 1) discoloration that occurs at wavelengths shorter than 385 nm and 2) bleaching by wavelengths longer than 385 nm. Efforts to find a photostabilizing treatment also began in the 1940s. Reineck and Lewis (1945) studied a large number (>100) of organic compounds as inhibitors for light-induced discoloration, but none of the studied com-pounds improved the stability to any noticeable extent. The early research did reveal that lignin is primarily responsible for the light-induced discoloration of groundwood pulps, that oxygen participates in the reactions, and that pH, moisture, and temperature influence the extent of reversion. A more detailed presentation of early research efforts is summarized in a number of review articles (Walton 1929; Spinner 1962; Kringstad 1969; Rapson and Spinner 1979).
FACTORS AFFECTING LIGHT-INDUCED YELLOWING
The wavelength distribution of the radiation that strikes a paper surface is of utmost importance for the extent of discoloration. Most of the ultraviolet component of sunlight that reaches the earth’s surface has a wavelength between 293 and 400 nm. The total radiation energy at the surface of the earth in the 300 to 350 nm range is about one-third of that between 350 and 400 nm (Hirt et al. 1960). For photoyellowing reactions indoors, it is worth noting that ordinary window glass filters out most of the radiation below about 315 nm (Hirt and Searle 1967) and that office papers when subjected to only fluorescent light are exposed to no radiation below about 350 nm (McGarry et al. 2004). Different spectral regions of radiation are responsible for either yellowing (ultraviolet radiation) or bleaching (visible light), as already stated (Nolan et al. 1945; Leary 1967; Andtbacka et al. 1989; Forsskåhl and Janson 1991; Andrady et al. 1991; Andrady and Searle 1995). The transition between discoloration and bleaching is not fixed to a certain wavelength but is a function of the wood raw material, pulping process, and stabilizing treatment. For paper indoors, it is expected that the most extensive yellowing is obtained when sheets are subjected to radiation between 330 and 385 nm. The photochemical effect is a function of the absorption by the substrate (the Grotthus-Draper law), the efficiency of the photochemical process (the quantum yield), and the intensity and wavelength of radiation. The primary process of photochemistry includes the absorption of a photon by a molecule that activates (excites) the molecule, which in turn will lose its energy in different ways, i.e., by heat, by fluorescence or phosphorescence, by chemical changes within the molecule, by breaking of chemical bonds (photolytic cleavage), or by transferring its energy to another atom or molecule. The secondary process (post-irradiation effects) is when the transfer of energy starts a chain of events, for example, the generation of singlet oxygen from an excited molecule and that singlet oxygen in turn oxidizing a chemical constituent. Factors such as temperature, amount of oxygen present, and humidity are normally not important for the primary process but can be essential for the secondary process and thus the outcome of the irradiation (Hirt et al. 1960; Feller 1994). Light-induced yellowing is largely a surface phenomenon, with about 50% of the discoloration occurring at a depth of about 20 m (Forsskåhl et al. 1995). The depth of discoloration is, however, influenced by the wavelength of irradiation, and since many materials absorb more radiation as the wavelength decreases, the depth of penetration is lower at short wavelengths (Feller 1994). Continuous irradiation may also lead to different results compared to intermittent exposure (altering light and dark exposure), since the moisture content and temperature may be different (Launer 1948). Thus, the procedure (spectral distribution of the light source, humidity, air recirculation, temperature, etc.) chosen for studying the light-induced yellowing of lignocellulosic materials will have an influence on the outcome. For example, the irradiation source used has an influence on the severity of the initial phase of discoloration (Paulsson and Ragauskas 1998b). Previous investigations have shown the importance of choosing a light source that resembles the actual reversion situation as closely as possible, if realistic light-induced reversion results are to be obtained (Paulsson and Ragauskas 2000; Paulsson et al. 2002; McGarry et al. 2004, Fjellström et al. 2007a). This is especially true for chemically modified or additive-treated, high-yield pulps. The usage of non-standard-ized ageing conditions complicates the interpretation of the results reported in the literature both for mechanistic and photostabilizing studies. If an inadequate irradiation source and ageing setup is used, the type of deterioration obtained and the conclusions drawn from it will most likely have little relevance for the photochemical processes that occur when a paper is used under normal conditions. The influence of ageing method is not within the scope of the present review, and a deeper discussion of the problems associated with this will, with a few exceptions, not be discussed further.
Metal ions present in the pulp could influence the stability, both as directly involved in the reactions or as an enhancer of color by forming colored complexes with lignin and extractives (Gupta 1970; Polcin and Rapson 1972; Ni et al. 1999; Peart and Ni 2001). Transition metals such as ferrous, ferric, and copper ions especially aggravate light-induced discoloration (Janson and Forsskåhl 1989; Ni et al. 1998). Effects of pH on the extent of light-induced yellowing have also been claimed (Lewis et al. 1945) with maximum brightness stability at pH 3.0 to 4.5 for unbleached groundwood pulp. However, another study has shown that a somewhat higher pH (6.0) could be more beneficial (Gellerstedt and Pettersson 1977). On the other hand, more recent experiments could not verify this fact for highly bleached groundwood pulp (Andtbacka et al. 1989); no significant difference in the yellowing rate in the pH interval 2 and 10 was obtained (Holmbom 1988). However, it has been reported that acidic papers containing mechanical pulp are more prone to discoloration than alkaline papers (Bond et al. 1999, 2001; Forsskåhl and Tylli 2001). Light-induced discoloration is accompanied by acidification (Andrady and Searle 1995; Forsskåhl and Tylli 2001), which contributes to a reduced mechanical performance. Further, the extent of discoloration also depends on the prevailing humidity and temperature conditions during ageing. It is generally agreed that the presence of moisture will speed up discoloration (Nolan et al. 1945; Spinner 1962; Forsskåhl 1995), even though others have reported this effect to be minor (Andtbacka et al. 1989). The temperature will influence the relative humidity and the oxygen content of the atmosphere, and the temperature should be kept low during irradiation to minimize heat-induced discoloration.
Oxygen participates in the photoyellowing reactions even though its exact role has not been fully elucidated. Several studies have shown that an oxygen-free atmosphere only marginally changes the extent of reversion of lignin model compounds or lignin-containing papers (Nolan et al. 1945; van den Akker et al. 1949; Weir et al. 1995a; Li and Ragauskas 2000; Paulsson et al. 2001), whereas others have shown that oxygen is important for the photodegradation of lignin and lignin model compounds, as well as for the formation of chromophores (Leary 1968a, b; Lin and Kringstad 1971; Fukagawa and Ishizu 1991). This indicates that atmospheric oxygen is not of sole importance for light-induced discoloration or that only a trace of oxygen is necessary to cause the discoloration (Andtbacka et al. 1989).
The wood species, pulping method, and bleaching characteristics have all been claimed to have an influence on the discoloration rate. Hardwood pulps that have lower lignin contents have been reported to be more photostable than softwood pulps (Janson and Forsskåhl 1989; Johnson 1989; Paulsson and Ragauskas 1998b; Fjellström et al. 2007a). The pulping method used will also affect the stability, and it has been proposed that mechanochemical changes of the lignin macromolecule can generate new photo-sensible structures (Wu et al. 1994; Johansson et al. 2002). Groundwood pulps that are subjected to lower temperatures during fiber release seem to be somewhat more photostable (Fjellström et al. 2007a). The results regarding the effect of sulfonation are more unclear; some studies have reported that softwood CTMPs are more photostable than softwood TMPs (Heitner and Min 1987; Johnson 1989), whereas others have reported the opposite (Gellerstedt et al. 1985; Agnemo et al. 1991; Tylli et al. 1992). Bleached high-yield pulps lose more brightness units during ageing than unbleached; (Forsskåhl 2000; Fjellström et al. 2007a), an effect that is partly due to the consequence of the Kubelka-Munk relationship and partly due to the generation of light-sensitive structures during lignin-retaining bleaching. The difference in photostability between oxidatively (hydrogen peroxide) bleached and reductively (dithionite, bisulfite) bleached pulps is not fully understood, and different opinions regarding the effect of reductive and oxidative bleaching exist (Jansen and Lorås 1968; Abou-State 1976; Janson and Forskåhl 1991; Janson 1993; Kuys and Abbot 1996; Forsskåhl 2000). The discrepancy in reported observations could be due to differences in exposure conditions, light sources, exposure times, grammage of the exposed samples, and procedures chosen for quantifying the color reversion (Schmidt and Heitner 1993a; Kimura et al. 1994; Paulsson and Ragauskas 1998b).
New proposed bleaching agents, such as sodium percarbonate and amineborane (Leduc et al. 2002), hydrogen peroxide additives such as tetra-acetyl-ethylenediamine (Agrawal et al. 2005; Hu et al. 2011), tetraalkylammonium salts (Hu 2003a), fluorescent whitening agents (Zhang et al. 2009a), a combination of reductive and oxidative bleaching (Petit-Conil and de Choudens 1994; Kuys and Abbot 1996; Pan 1999), or alternative alkali sources for hydrogen peroxide bleaching (Savoye et al. 2011) have been tested with very limited photostabilizing effects. A recent study in which different types of mechanical and chemimechanical pulps were subjected to accelerated or ambient ageing showed that neither the pre-treatment procedure (sulfite or alkaline hydrogen peroxide) nor the bleaching method (dithionite or hydrogen peroxide) altered the photostability in any decisive way (Fjellström et al. 2007a; Holmbom 1988). It is, however, evident that no matter how mechanical or chemimechanical pulps are produced, they undergo extensive photoyellowing. The difference is how rapid the discoloration occurs and since hardwood (especially aspen) pulps are more photostable, these pulps are the first choice for usage in high-quality, long-life products.
THE CHEMISTRY OF LIGHT-INDUCED YELLOWING
It is important to remember that much of the knowledge gathered related to the mechanism of light-induced yellowing originates from experiments where fairly simple lignin model compounds were irradiated in organic solvents. Despite the fact that important knowledge can be and has been gained by this approach, there are some drawbacks which must be acknowledged when evaluating and comparing results in the literature:
- A low molecular weight lignin model compound in solution does not fully simulate the same lignin functionality in a true ageing case, i.e. in solid state in the presence of carbohydrates and other lignin moieties.
- The supply of oxygen might differ between solution and solid-state experiments (solubility, diffusivity).
- The choice of solvent might influence the outcome of the reactions.
- The mobility of lignin units is different in a solution than in a restricted solid matrix, which is important when contact between different functionalities is needed for the reactions to occur or when a radical pair formed by homolytic bond cleavage separates.
- The lifetime of intermediate radicals could be different in solid state compared to in solution state.
In addition to these drawbacks in using simple lignin model compounds in solution, the intensity and spectral distribution of the irradiation source used often varies between different investigations in the literature. This might lead to different conclusions as discussed above. Due to the complexity of lignin and the challenges to analyze lignin in the solid state as in mechanical pulp or as an isolated lignin sample, work with simple lignin models has been and still is inevitable. Analysis of lignin in the solid state, e.g., in wood or pulp, is difficult (due to its complex structure and interference from other wood components), and efforts to isolate lignin without affecting the chemical structure often results in low yield. Experiments performed by subjecting lignin model compounds to irradiation in solid state (adsorbed to filter paper or pulp sheets) might lead to low recovery yields due to oligomerization and reactions with the substrate. Even though this might better simulate a real ageing case, this approach is challenging from an analytical perspective.
The main mechanism of photoyellowing of lignin-containing materials as we know it today can broadly be divided into three stages:
- Energy absorption [ultraviolet and visible (UV/VIS) radiation] by photo-active groups (chromophores) in the lignin.
- Formation of intermediate structures, e.g., phenoxyl radicals (several suggested mechanisms).
- Formation of chromophores absorbing visible light from the initially formed intermediates.
In addition, other reactions, not necessarily involving phenoxyl radicals, could influence the formation of color and the photodegradation of lignin. The basic knowledge surrounding the mechanisms will be reviewed in subsequent sections.
Energy Absorption
The near-ultraviolet wavelength region of 300 to 400 nm of the sunlight spectrum is most important to light-induced yellowing, since:
- Radiation in this region is quite energy-rich (E
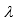 -1) and capable of inducing significant photochemical changes.
-1) and capable of inducing significant photochemical changes. - The amount of radiation below 300 nm reaching earth is low.
- Most lignin chromophores absorb radiation effectively in this region.
The main chromophores (here UV/VIS absorbing groups) in wood and mechan-ical pulps are lignin moieties of various kinds and, to some extent, certain extractives (Norrström 1969; Lorås and Wilhelmsen 1973). The main chromophoric groups in native wood lignin are various kinds of ring-conjugated ethylenic groups, such as cinnamyl alcohol and cinnamaldehyde groups together with carbonyl groups, such as ![]() -carbonyl groups and quinones (Pew and Connors 1971; Imsgard et al. 1971; Hon and Glasser 1979; Gratzl 1985). In addition to the chromophores present in native wood lignin, the mechanical treatment of wood during the pulping process may cause mechanochemical changes in the lignin macromolecule. For instance, it might lead to an increase in
-carbonyl groups and quinones (Pew and Connors 1971; Imsgard et al. 1971; Hon and Glasser 1979; Gratzl 1985). In addition to the chromophores present in native wood lignin, the mechanical treatment of wood during the pulping process may cause mechanochemical changes in the lignin macromolecule. For instance, it might lead to an increase in ![]() -carbonyl groups (and quinones) (Lee and Sumimoto 1990; Itho et al. 1993; Johansson et al. 2002) and the formation of UV/VIS-absorbing stilbenes from phenylcoumaran (
-carbonyl groups (and quinones) (Lee and Sumimoto 1990; Itho et al. 1993; Johansson et al. 2002) and the formation of UV/VIS-absorbing stilbenes from phenylcoumaran (![]() -5) and 1,2-diarylpropane units (
-5) and 1,2-diarylpropane units (![]() -1) (Lee et al. 1990; Wu et al. 1991). Also, the use of chemicals in an optional pre-treatment stage or in bleaching stages influences the distribution of chromophores and leucochromophores (precursors to colored groups) in the lignin. Examples of this phenomenon are reactions of enals and enones with sulphite during impregnation and pre-heating prior to refining (Gellerstedt et al. 1977; Suckling 1991; Lundquist et al. 2007), formation of stilbenes (Gellerstedt and Agnemo 1980a; Gellerstedt and Zhang 1992), and hydroquinones via Dakin-type reactions (from phenolic
-1) (Lee et al. 1990; Wu et al. 1991). Also, the use of chemicals in an optional pre-treatment stage or in bleaching stages influences the distribution of chromophores and leucochromophores (precursors to colored groups) in the lignin. Examples of this phenomenon are reactions of enals and enones with sulphite during impregnation and pre-heating prior to refining (Gellerstedt et al. 1977; Suckling 1991; Lundquist et al. 2007), formation of stilbenes (Gellerstedt and Agnemo 1980a; Gellerstedt and Zhang 1992), and hydroquinones via Dakin-type reactions (from phenolic ![]() -carbonylic structures) during hydrogen peroxide bleaching (Gellerstedt and Agnemo 1980b; Lee and Sumimoto 1991; Gellerstedt and Zhang 1992), and degradation of cinnamaldehyde end groups by hydrogen peroxide (Gellerstedt and Agnemo 1980b; Gellerstedt and Zhang 1992).
-carbonylic structures) during hydrogen peroxide bleaching (Gellerstedt and Agnemo 1980b; Lee and Sumimoto 1991; Gellerstedt and Zhang 1992), and degradation of cinnamaldehyde end groups by hydrogen peroxide (Gellerstedt and Agnemo 1980b; Gellerstedt and Zhang 1992).
To some extent, alkaline hydrogen peroxide degrades quinones but could also induce hydroxylation of quinones, resulting in hydroxyquinones that are more stable against degradation (Gellerstedt et al. 1980; Abbot 1995; Svensson-Rundlöf et al. 2006). Further, metal ions, especially transition metal ions such as iron, can form strongly colored complexes with lignin moieties (e.g. phenols, catechols) and extractives (e.g. flavonoids) (Gupta 1970; Polcin and Rapson 1972; Ni et al. 1999; Peart and Ni 2001).
The main chromophoric groups in lignin-rich pulps are shown in Fig. 3 with their approximate absorption wavelength maxima. It is, however, important to know that the absorption maxima could be altered if the chromophoric group is in a solid lignicellulosic matrix. For example, it has been reported that the absorption bands of differently substituted o– and p-quinone model compounds were intensively red shifted (by 30 to 150 nm) when impregnated onto lignin-rich pulp (Zhang and Gellerstedt 1994a). Further, it has also been suggested that phenoxyl radicals, which are known to be very stable in a solid matrix (days), could act as chromophores, absorbing energy in the range of 370 to 410 nm (Wan and Depew 1996; Kónyaand Scaiano 1996).
Fig. 3. Lignin-related chromophores in lignin-rich pulps and their approximate absorption maxima wavelengths (based on Gratzl 1985)
In this context, it is worth mentioning that the wavelength distribution of the energy source is of great importance relative to which groups can absorb energy and the outcome of the reactions, since it is the wavelength that determines the energy of the photons emitted. Several simultaneous reactions occur, and there is a balance between photoyellowing caused by short wavelength radiation (![]() < 385 nm) and photobleaching caused by longer wavelength radiation (
< 385 nm) and photobleaching caused by longer wavelength radiation (![]() > 385 nm). This implies that irradiation with a light source that has a wavelength distribution simulating the actual aging case (window-filtered sunlight or indoor fluorescent light) is preferred.
> 385 nm). This implies that irradiation with a light source that has a wavelength distribution simulating the actual aging case (window-filtered sunlight or indoor fluorescent light) is preferred.
Formation of Radicals as Intermediate Products
One of the early mechanistic insights regarding photoyellowing was that free phenolic groups are involved (Leary 1968a). Possible reactions of the free phenolic groups are shown in Fig. 4. Note that the aromatic nuclei are drawn as guaiacyl units, i.e., the predominating type in softwood lignin, in all figures below. This is to simplify the presentation and does not exclude other nuclei present in wood or non-wood lignin. The hydrogen atom in a free phenolic group can be abstracted by singlet (excited) oxygen (Matsuura et al. 1972; Brunow and Sivonen 1975), oxygen-based radicals such as the hydroxyl and hydroperoxyl radicals (Gratzl 1985), or an excited carbonyl group (Kringstad and Lin 1970; Brunow and Eriksson 1971; Gellerstedt and Pettersson 1977). Singlet oxygen can be formed by oxygen quenching of photo-excited carbonyl groups as suggested by Brunow and Sivonen (1975) or photo-excited triplet ring-conjugated ethylenic structures (Gellerstedt and Pettersson 1975).
Singlet oxygen could, in theory, be important as an energy mediator with higher mobility than excited groups in lignin that are restricted in their movement by the solid matrix. If mediated by singlet oxygen, the excited carbonyl and phenolic groups do not need to get in direct contact for the reaction to take place (Fischer et al. 1995). The importance of singlet oxygen for photoyellowing and photodegradation of lignin and lignin model compounds has been put forward in several publications (Barclay et al. 1998, 2003; Bonini et al. 1998; Crestini and D’Auria 1996, 1997).
Fig. 4. Possible reactions involving the free phenolic group in lignin during light-induced yellowing (the free phenolic path). L is a connection to lignin. Main references: Kringstad and Lin 1970; Brunow and Eriksson 1971; Matsuura et al. 1972; Brunow and Sivonen 1975; Gellerstedt and Pettersson 1977; Gratzl 1985
It has been reported that hydroxyl radicals form during irradiation of mechanical pulp sheets (Agnemo et al. 1991), although others have claimed that the formation of hydroxyl radicals during irradiation is minor (Beyer et al. 1995). Direct photolysis of phenols in structures absorbing UV/VIS radiation in the region above 300 nm has also been researched (Kringstad and Lin 1970; Fornier de Violet et al. 1989; Castellan et al. 1990a; Jaeger et al. 1993). The reactions summarized in Fig. 4 can be called the free phenolic path. This pathway leads to phenoxyl radicals either by phenolic hydrogen atom abstraction by radicals or excited structures or alternatively by direct photolysis when permitted by the UV/VIS absorption characteristics of the substrate.
Figure 5 is a scheme showing the photoreactions of carbonyl groups exemplified by an ![]() -carbonyl group. A carbonyl group can, when excited, be quenched by oxygen (giving singlet oxygen and the ground-state carbonyl), undergo C
-carbonyl group. A carbonyl group can, when excited, be quenched by oxygen (giving singlet oxygen and the ground-state carbonyl), undergo C![]() -C
-C![]() cleavage (Norrish Type I), or abstract a hydrogen atom, resulting in a ketyl radical. The carbonyl group can act as a photocatalyst since the ketyl radical, formed by hydrogen atom abstraction, can react with oxygen (or hydroxyl radicals, Francis et al. 1991) to regenerate the carbonyl group. However, the ketyl radical might also abstract another hydrogen atom (photo-reduction of the carbonyl group). Excited aryl alkyl ketones might undergo Norrish Type I cleavage, initially leading to an acyl radical and an alkyl radical. The importance of this reaction depends on the conditions (presence or absence of hydrogen donors) and the substitution pattern of the ketone. In the case of
cleavage (Norrish Type I), or abstract a hydrogen atom, resulting in a ketyl radical. The carbonyl group can act as a photocatalyst since the ketyl radical, formed by hydrogen atom abstraction, can react with oxygen (or hydroxyl radicals, Francis et al. 1991) to regenerate the carbonyl group. However, the ketyl radical might also abstract another hydrogen atom (photo-reduction of the carbonyl group). Excited aryl alkyl ketones might undergo Norrish Type I cleavage, initially leading to an acyl radical and an alkyl radical. The importance of this reaction depends on the conditions (presence or absence of hydrogen donors) and the substitution pattern of the ketone. In the case of ![]() -carbonyls, this would eventually lead to formation of end-groups of p-hydroxybenzaldehyde and p-hydroxybenzoic acid types. Structures of both types are common photoproducts from lignin model compounds and substrates like mechanical pulps (Hemmingson and Wong 1989; Sjöholm et al. 1992; Pan et al. 1992, 1993, 1994; Argyropoulos and Sun 1996). Note that these latter types of photoproducts can be formed from other structures as well (see below).
-carbonyls, this would eventually lead to formation of end-groups of p-hydroxybenzaldehyde and p-hydroxybenzoic acid types. Structures of both types are common photoproducts from lignin model compounds and substrates like mechanical pulps (Hemmingson and Wong 1989; Sjöholm et al. 1992; Pan et al. 1992, 1993, 1994; Argyropoulos and Sun 1996). Note that these latter types of photoproducts can be formed from other structures as well (see below).
Fig. 5. Basic photoreactions of carbonyls exemplified by an ![]() -carbonyl group. L is a connection to lignin. Main references: Kringstad and Lin 1970; Brunow and Eriksson 1971; Brunow and Sivonen 1975;Gellerstedt and Pettersson 1977; Francis et al. 1991
-carbonyl group. L is a connection to lignin. Main references: Kringstad and Lin 1970; Brunow and Eriksson 1971; Brunow and Sivonen 1975;Gellerstedt and Pettersson 1977; Francis et al. 1991
A special type of carbonyl group in lignin is the group in phenacyl aryl ethers (2-aryloxy-1-arylpropanones), made of lignin units with side-chains carrying both an ![]() -carbonyl group and a
-carbonyl group and a ![]() -O-4 linkage. Phenacyl aryl ethers have been widely studied as lignin model compounds irradiated both in solution and in solid state (Gierer and Lin 1972; Vanucci et al. 1988; Castellan et al. 1989; Netto-Ferreira et al. 1990; Schmidt et al. 1991; Fukagawa and Ishizu 1991; Hon 1992; Argyropoulos and Sun 1996; Parkås et al. 2004a; Lanzalunga and Bietti 2000). This type of structure can, when excited, undergo homolytic
-O-4 linkage. Phenacyl aryl ethers have been widely studied as lignin model compounds irradiated both in solution and in solid state (Gierer and Lin 1972; Vanucci et al. 1988; Castellan et al. 1989; Netto-Ferreira et al. 1990; Schmidt et al. 1991; Fukagawa and Ishizu 1991; Hon 1992; Argyropoulos and Sun 1996; Parkås et al. 2004a; Lanzalunga and Bietti 2000). This type of structure can, when excited, undergo homolytic ![]() -ether bond cleavage forming a phenacyl-phenoxyl radical pair (Fig. 6), in addition to the reactions pictured in Fig. 5. This is known as the phenacyl aryl ether pathway.
-ether bond cleavage forming a phenacyl-phenoxyl radical pair (Fig. 6), in addition to the reactions pictured in Fig. 5. This is known as the phenacyl aryl ether pathway.
Hydrogen abstraction by the initially formed phenacyl radical leads to formation of a ketone that might further act as a sensitizer or undergo C![]() -C
-C![]() cleavage. The initially formed radical pair can recombine forming the original
cleavage. The initially formed radical pair can recombine forming the original ![]() -O-4 linkage or another type of inter-unit bond (
-O-4 linkage or another type of inter-unit bond (![]() -5). These types of rearrangement reactions have been shown to occur in lignin model compounds irradiated both in solution and in solid state (Fukagawa and Ishizu 1991; Argyropoulos and Sun 1996; Parkås et al. 2004a).
-5). These types of rearrangement reactions have been shown to occur in lignin model compounds irradiated both in solution and in solid state (Fukagawa and Ishizu 1991; Argyropoulos and Sun 1996; Parkås et al. 2004a).
Fig. 6. Reactions of the phenacyl aryl ether units (2-aryloxy-1-aryl-propanones). L is a connection to lignin. Main references: Gierer and Lin 1972; Vanucci et al. 1988; Castellan et al. 1989; Netto-Ferreira et al. 1990; Schmidt et al. 1991; Fukagawa and Ishizu 1991; Hon 1992; Argyropoulos and Sun 1996; Parkås et al. 2004a
Phenacyl aryl ether cleavage can potentially result in a depolymerization of the lignin and the formation of new phenolic groups. Depolymerization (decrease in average molecular weight) of milled wood lignin isolated from softwood mechanical pulp upon irradiation in the presence of oxygen has been reported (Koch et al. 1994; Destiné et al. 1996; Wang et al. 1998). The depolymerisation reaction is, however, to some extent counteracted by condensation reactions due to recombination of the initial radicals, forming new ![]() -5 linkages (Fig. 6). This explains, at least partly, the increase in C-5 condensed phenolic groups observed during irradiation of lignin in the solid state (Argyropoulos and Sun 1996; Li and Ragauskas 1999). Note that in hardwood lignin, a considerable amount of aromatic rings are of the syringyl type, that is, with a methoxyl group in position 5. This, of course, affects the possibility to form
-5 linkages (Fig. 6). This explains, at least partly, the increase in C-5 condensed phenolic groups observed during irradiation of lignin in the solid state (Argyropoulos and Sun 1996; Li and Ragauskas 1999). Note that in hardwood lignin, a considerable amount of aromatic rings are of the syringyl type, that is, with a methoxyl group in position 5. This, of course, affects the possibility to form ![]() -5 linkages. Due to the low amount of these phenacyl aryl ether structures in lignin, less than 2% of the phenylpropane units in spruce and typical hardwood milled wood lignins (Lundquist 1992; Zhang and Gellerstedt 1999; Brunow and Lundquist 2010), a major depolymer-ization of lignin during irradiation can hardly be explained by the cleavage of the original phenacyl aryl ether bonds in lignin, even though the content of these groups might increase during refining. A suggested alternative to the direct homolysis from the excited state in Fig. 6 is that the cleavage of the
-5 linkages. Due to the low amount of these phenacyl aryl ether structures in lignin, less than 2% of the phenylpropane units in spruce and typical hardwood milled wood lignins (Lundquist 1992; Zhang and Gellerstedt 1999; Brunow and Lundquist 2010), a major depolymer-ization of lignin during irradiation can hardly be explained by the cleavage of the original phenacyl aryl ether bonds in lignin, even though the content of these groups might increase during refining. A suggested alternative to the direct homolysis from the excited state in Fig. 6 is that the cleavage of the ![]() -O-4 bond is from the ketyl radical formed after hydrogen atom abstraction of the triplet excited state carbonyl group (Scaiano et al. 1991, 1994). Two phenacyl radicals can, if in contact, form dimers. However in the restricted environment of mechanical pulp with a low content of phenacyl aryl ethers, this is not likely to happen. The recombination of initially-formed phenacyl and phenoxyl radicals is, however, more likely due to their close proximity. An excited triplet state ring-conjugated carbonyl of this type could also react according to the so-called Norrish Type I reaction, leading to C
-O-4 bond is from the ketyl radical formed after hydrogen atom abstraction of the triplet excited state carbonyl group (Scaiano et al. 1991, 1994). Two phenacyl radicals can, if in contact, form dimers. However in the restricted environment of mechanical pulp with a low content of phenacyl aryl ethers, this is not likely to happen. The recombination of initially-formed phenacyl and phenoxyl radicals is, however, more likely due to their close proximity. An excited triplet state ring-conjugated carbonyl of this type could also react according to the so-called Norrish Type I reaction, leading to C![]() -C
-C![]() bond cleavage and resulting in the formation of benzaldehyde structures (for example, vanillin and syringaldehyde end-groups). The substituent pattern of the ketone is of importance for reactivity, and the Norrish Type I cleavage of the phenacyl aryl ethers might be slowed down by
bond cleavage and resulting in the formation of benzaldehyde structures (for example, vanillin and syringaldehyde end-groups). The substituent pattern of the ketone is of importance for reactivity, and the Norrish Type I cleavage of the phenacyl aryl ethers might be slowed down by ![]() -phenyl quenching. The formation of these types of photoproducts has, however, been determined upon irradiation of lignin model compounds of the
-phenyl quenching. The formation of these types of photoproducts has, however, been determined upon irradiation of lignin model compounds of the ![]() -carbonyl
-carbonyl ![]() -ether type in solid state (Argyropoulos and Sun 1996; Parkås et al. 2004a). Lignin models of the
-ether type in solid state (Argyropoulos and Sun 1996; Parkås et al. 2004a). Lignin models of the ![]() -carbonylic
-carbonylic ![]() -1 lignin type (deoxy-veratroin) have been shown to partly react according to Norrish Type I cleavage (Castellan et al. 1990b).
-1 lignin type (deoxy-veratroin) have been shown to partly react according to Norrish Type I cleavage (Castellan et al. 1990b).
Another proposed reaction scheme for formation of phenoxyl radicals and depolymerisation of lignin through ![]() -O-4 bond cleavage is the ketyl radical pathway (Schmidt and Heitner 1993b), shown in Fig. 7.
-O-4 bond cleavage is the ketyl radical pathway (Schmidt and Heitner 1993b), shown in Fig. 7.
Fig. 7. Formation of ketyl radicals in lignin during photoyellowing and the further reactions either by direct ether cleavage of the ketyl radical (Path A, Scaiano et al. 1991; Schmidt and Heitner 1993b) or by oxygen quenching of the ketyl radical (Path B, Huang et al. 1995; Andersen and Wayner 1999; Fabbri et al. 2005). Ar is an aromatic unit.
This pathway involves the most common type of inter-unit bond in lignins, i.e., the arylglycerol -aryl ether bond. This inter-unit bond represents around 35% of the side-chains in softwood lignin and 45% of the side-chains in a typical hardwood lignin (Brunow and Lundquist 2010). Cleavage of these ether bonds might thus explain a large degradation in molecular weight as opposed to bond cleavage of the original phenacyl ether bonds. Oxygen- and carbon-centered radicals formed during irradiation are thought to abstract the hydrogen atom from the benzylic group, leading to a ![]() -ether ketyl radical (Fig. 7). It was originally thought that the ketyl radical quickly undergoes
-ether ketyl radical (Fig. 7). It was originally thought that the ketyl radical quickly undergoes ![]() -ether cleavage, resulting in the formation of a phenoxyl radical and an enol (Path A) that, in turn, quickly tautomerizes to the corresponding ketone (Scaiano et al. 1991; Schmidt and Heitner 1993b). However, this mechanism has been challenged (Huang et al. 1995; Andersen and Wayner 1999; Fabbri et al. 2005) based on later experiments that instead indicate that the primary photoproduct from the ketyl radical is the
-ether cleavage, resulting in the formation of a phenoxyl radical and an enol (Path A) that, in turn, quickly tautomerizes to the corresponding ketone (Scaiano et al. 1991; Schmidt and Heitner 1993b). However, this mechanism has been challenged (Huang et al. 1995; Andersen and Wayner 1999; Fabbri et al. 2005) based on later experiments that instead indicate that the primary photoproduct from the ketyl radical is the ![]() -carbonylic compound formed by oxygen quenching (Path B) and that the ketone and the phenoxyl radical are secondary photoproducts formed according to the phenacyl aryl ether pathway (Fig. 6 and 7).
-carbonylic compound formed by oxygen quenching (Path B) and that the ketone and the phenoxyl radical are secondary photoproducts formed according to the phenacyl aryl ether pathway (Fig. 6 and 7).
The newly formed ketone can, of course, act as a photosensitizer, leading to further reactions. An excited carbonyl group might also abstract a hydrogen atom from a benzylic carbon, leading to a pair of ketyl radicals, both of which could form carbonyl groups under the action of oxygen or hydroxyl radicals (Francis et al. 1991) or photoreduce by abstracting additional hydrogen atoms. Direct photooxidation of secondary alcohols to ketones, although slow, could be quite important due to the ability of carbonyl groups to catalyze photoyellowing (Balsells and Frasca 1982; Omori et al. 1991) and the low amount of carbonyl groups needed to initiate the reactions. All in all, recent data presented in the literature suggests that the phenacyl aryl ether pathway might be of more importance than previously thought.
Formation of Chromophores
So far, the reactions detailed above have resulted in formation of phenoxyl radicals and, to some extent, perhaps depolymerisation of the lignin macromolecule. To display the yellow color associated with photoreversion, the initially formed structures need to form chromophores absorbing visible light in the blue-green region. It was suggested early on that these colored photoproducts might be quinones, quinone methides, and cyclohexadienones formed from intermediate phenoxyl radicals (Leary 1968a, b). The formation of o-quinones was suggested based on the observation that the methoxyl group content decreased during irradiation (Leary 1968a, b). Then, the formation of both o– and p-quinones from phenoxyl radicals was suggested by Lin and Kringstad (1971), based on the product pattern determined after irradiating lignin model compounds in the presence of oxygen. Their suggested mechanisms are shown in Fig. 8. Quinones are formed under the action of oxygen (and/or oxygen-centred radicals) and the different resonance forms of the stabilized phenoxyl radical. For formation of o-quinones, either oxygen or a hydroxyl radical reacts in position three of the aromatic ring, followed by a loss of the methoxyl group. The p-quinone product could be formed after a reaction in position one in the aromatic ring, resulting in side-chain displacement (Fig. 8). Quinones are present in wood lignins in amounts that are typically less than 2% of the lignin units (Brunow and Lundquist 2010). Mislankar et al. (1997) showed, using confocal laser scanning microscopy, that both black spruce and jack pine wood contained o-quinones. The highest concentrations were in the torus, middle lamella, cell corners, and ray cells. Even after treating thin wood shavings with alkaline hydrogen peroxide, o-quinones were detected, despite the fact that quinones are susceptible to oxidation by alkaline hydrogen peroxide (Gellerstedt et al. 1980; Zhang and Gellerstedt 1999). By using solid state 31P NMR spectroscopy, Lebo et al. (1990) could quantify the o-quinone content in white spruce refiner mechanical pulp before (5-6 quinone units/100 C9-units) and after irradiation (10/100 C9-units). This surprisingly high absolute value for quinones in refiner mechanical pulps was later proposed by Argyropoulos and Heitner (1994) to be caused by the presence of non-ionized carboxylic groups in the samples that Lebo et al. (1990) had not accounted for. Later studies showed that the content of o-quinones was only 0.7/100 C9-units in black spruce refiner mechanical pulp (Argyropoulos and Heitner 1994) and 0.4/100 C9-units in black spruce MWL (Argyropoulos and Zhang 1998). An increase in the o-quinone content during irradiation of unbleached and hydrogen peroxide-bleached SGW pulp and isolated spruce MWL has been shown in several investigations using 31P NMR spectroscopy (Argyropoulos et al. 1995; Zhang and Gellerstedt 1999). It was also observed with fluorescence spectroscopy on hydrogen peroxide-bleached and irradiated mechanical pulp (Zhu et al. 1995).
Fig. 8. Formation of o– and p-quinones from initially formed phenoxyl radicals (Lin, Kringstad 1971)
The rapid formation of o-quinones in the early phase of irradiation is followed by reactions leading to colored but non-quinonoid structures, based on the fact that the o-quinone content seems to go through a maximum (Argyropoulos et al. 1995; Argyropoulos and Sun 1996). The generation of p-quinones during photoyellowing has also been suggested to contribute to the formed color (Gellerstedt and Pettersson 1977; Forsskåhl et al. 1991; Hirashima and Sumimoto 1994; Jääskeläinen et al. 2006). Based on FT-Raman and FT-IR spectroscopic analyses of yellowed spruce thermomechnical pulp, Agarwal (1998) found support for a mechanism based on the p-hydroquinone/p-quinone redox couple as one of the major leucochromophoric/chromophoric systems (Hirashima and Sumimoto 1994). Forsskåhl and Janson (1991) reported that two bands in the UV/VIS difference reflectance spectra of bleached mechanical pulp before and after irradiation were interrelated. Irradiation in the band at 370 nm gave a response at 430 nm and vice versa, a behaviour that was either ascribed to a hydroquinone/quinone system, charge transfer complexes, quinone methides, or E/Z isomerisation of ring-conjugated double bonds (Forsskåhl and Janson 1991; Ek et al. 1992; Castellan et al. 1993a; Agarwal 1998). Agarwal and Atalla (1990) suggested that cis-ferulic acid is a chromo-phore formed during photoyellowing of white spruce mechanical pulp. To summarize, quinoid groups have been shown to form during light-induced yellowing, but they are also in themselves photoactive and could, apart from giving color, catalyze other photo-reactions or react to form chromophores of both quinoid and non-quinoid types (Gierer and Lin 1972; Neumann and Machado 1989; Ek 1992; Ragauskas 1993a; Forsskåhl et al. 1993; Castellan et al. 1993b; Lennholm et al. 1994).
Other Reactions
In this section, reactions of cinnamaldehyde and cinnamyl alcohol end-groups will be discussed with reactions involving miscellaneous structures. The content of cinnamaldehyde units in spruce-milled wood lignin is around 4 per 100 C9-units (one phenolic and three etherified). Approximately the same amount is found in unbleached softwood groundwood pulp (Adler and Marton 1959; Marton et al. 1961; Hirashima and Sumimoto 1987; Brunow and Lundquist 2010). Cinnamaldehydes are efficiently sulfonated by sodium sulphite (CTMP-production) and are degraded during alkaline hydrogen peroxide-bleaching although, in industrial practice, probably not completely (Gellerstedt et al. 1977; Hirashima and Sumimoto 1987; Suckling 1991; Gellerstedt and Zhang 1992; Lundquist et al. 2007). Cinnamyl alcohol groups are present in lignins (hardwood and softwood) in amounts of about 2% of the side-chains (Brunow and Lundquist 2010). These groups seem to be stable during refining and bleaching of mechanical pulp. The fate of the unsaturated end-groups during irradiation was studied by Gellerstedt and Pettersson (1975) by irradiating solutions of the methyl ethers of coniferyl alcohol, isoeugenol, and coniferaldehyde. The product pattern led to the suggested mechanism shown in Fig. 9. Singlet oxygen reacts with the double bond, initially giving a peroxiran, which, after rearrangement to a dioxetane, gives an aldehydic structure (p-hydroxybenzaldehyde type end-group) that in turn can oxidize to the corresponding carboxylic group. Gellerstedt and Pettersson (1975) found the methyl ether of coniferaldehyde to be stable during irradiation both in solution and solid state. Hirashima and Sumimoto (1987) also found that filter paper impregnated with coniferaldehyde (and its methyl ether) in amounts corresponding to the levels found in untreated groundwood pulp did not show a brightness loss when irradiated. This indicates that the photoproducts from this type of end-group do not contribute significantly to the total color change upon irradiation of mechanical pulps, but as a photo-initiator, it could be more important due to the clear absorption of UV/VIS by cinnamaldehydes. On the other hand, the methyl ethers of coniferaldehyde and coniferyl alcohol deposited on filter paper both gave decreased brightness when irradiated, and in both cases veratraldehyde and veratric acid were formed as photoproducts together with oligomeric/polymeric material (Castellan et al. 1991; Omori et al. 1992; Parkås et al. 2004a). These oligomerizations are not likely in a true ageing case with few end-groups in direct contact. However, indications that model compounds of this type react with the cellulosic substrate might be important (Jaeger et al. 1993).
Fig. 9. Reactions of cinnamaldehyde and cinnamyl alcohol end-groups exemplified by their guaiacyl analogue. Main references: Gellerstedt and Pettersson 1975; Castellan et al. 1991; Omori et al. 1992; Pan et al. 1992; Parkås et al. 2004a,b,c)
Other reactions that can occur during irradiation of cinnamyl alcohol structures, as shown by etherified model compound studies in solid state are: oxidation of cinnamyl alcohol to cinnamaldehyde and E/Z-isomerization of both cinnamaldehydes and cinnamyl alcohols (Parkås et al. 2004a). Oxidation of cinnamyl alcohol groups to cinnamaldehydes could explain the increased amounts of cinnamaldehydes found after irradiation of bleached spruce TMP (Pan et al. 1992) or 13C-labelled dehydrogenation polymers in solid state (Parkås et al. 2004b, c). The different results regarding the behaviour of cinnamal-dehydes during photoageing might also be due to the different irradiation sources used in the various investigations.
Some important leucochromophores that might be formed during refining and bleaching of mechanical pulps have been briefly mentioned above. Examples of these are stilbenes formed from ![]() -5 and
-5 and ![]() -1 structures in lignin during refining and hydrogen peroxide bleaching, phenylcoumarones formed from
-1 structures in lignin during refining and hydrogen peroxide bleaching, phenylcoumarones formed from ![]() -5 units, and stilbene para-hydroquinones from stilbenes via the Dakin reaction during alkaline hydrogen peroxide bleaching. The formation and photoreactions of these structures are outlined in Fig. 10. Side chains attached to phenolic units, either by a conjugated double-bond or an
-5 units, and stilbene para-hydroquinones from stilbenes via the Dakin reaction during alkaline hydrogen peroxide bleaching. The formation and photoreactions of these structures are outlined in Fig. 10. Side chains attached to phenolic units, either by a conjugated double-bond or an ![]() -carbonylic group, can, during bleaching with alkaline hydrogen peroxide, form hydro-quinones by side chain displacement (Gellerstedt and Agnemo 1980b). This process is shown in Fig. 10a. Hydroquinones are, in general, easily oxidized to corresponding p-quinones (Castellan et al. 1993a). This rapid oxidation of leucochromophoric hydro-quinones (or catechols) to p-quinones and o-quinones might help explain the initial fast brightness loss seen during irradiation and could also explain the higher sensitivity of hydrogen peroxide-bleached mechanical pulps to photoyellowing when compared to unbleached pulps. Gellerstedt and Zhang (1992) concluded that diguaiacyl stilbenes, mainly from
-carbonylic group, can, during bleaching with alkaline hydrogen peroxide, form hydro-quinones by side chain displacement (Gellerstedt and Agnemo 1980b). This process is shown in Fig. 10a. Hydroquinones are, in general, easily oxidized to corresponding p-quinones (Castellan et al. 1993a). This rapid oxidation of leucochromophoric hydro-quinones (or catechols) to p-quinones and o-quinones might help explain the initial fast brightness loss seen during irradiation and could also explain the higher sensitivity of hydrogen peroxide-bleached mechanical pulps to photoyellowing when compared to unbleached pulps. Gellerstedt and Zhang (1992) concluded that diguaiacyl stilbenes, mainly from ![]() -1 units, were the leucochromophores responsible for the fast initial photoyellowing of hydrogen peroxide-bleached pulps (Fig. 10b) based on mild hydrolysis of spruce wood and several unbleached, hydrogen peroxide-bleached, and irradiated spruce mechanical pulps and the subsequent identification of released lignin-derived monomers, dimers, and some trimers. Based on this, a model compound trial was performed with two monophenolic diguaiacyl stilbenes irradiated in solid state, deposited on filter paper (Zhang and Gellerstedt 1994b). The only colored structure identified was the corresponding stilbene ortho-quinone. Apart from the quinone, products from cyclo-dimerization [2
-1 units, were the leucochromophores responsible for the fast initial photoyellowing of hydrogen peroxide-bleached pulps (Fig. 10b) based on mild hydrolysis of spruce wood and several unbleached, hydrogen peroxide-bleached, and irradiated spruce mechanical pulps and the subsequent identification of released lignin-derived monomers, dimers, and some trimers. Based on this, a model compound trial was performed with two monophenolic diguaiacyl stilbenes irradiated in solid state, deposited on filter paper (Zhang and Gellerstedt 1994b). The only colored structure identified was the corresponding stilbene ortho-quinone. Apart from the quinone, products from cyclo-dimerization [2![]() +2
+2![]() ] of excited and ground-state stilbenes in the form of tetraphenyl-cyclobutanes were found. Although theoretically interesting, the relative scarcity of the stilbenes in pulp lignin makes this reaction less important from a practical ageing perspective. Since excited stilbenes also can undergo cycloaddition with other ethylenic structures (e.g. cinnamyl alcohols or cinnamaldehydes), similar reactions might have some importance. However, they do not primarily lead to the formation of color. Fast color formation upon irradiation of stilbene model compounds was also reported by Weir et al. (1995b). On the other hand, Ruffin et al. (1998) concluded that the para-stilbene phenols formed from
] of excited and ground-state stilbenes in the form of tetraphenyl-cyclobutanes were found. Although theoretically interesting, the relative scarcity of the stilbenes in pulp lignin makes this reaction less important from a practical ageing perspective. Since excited stilbenes also can undergo cycloaddition with other ethylenic structures (e.g. cinnamyl alcohols or cinnamaldehydes), similar reactions might have some importance. However, they do not primarily lead to the formation of color. Fast color formation upon irradiation of stilbene model compounds was also reported by Weir et al. (1995b). On the other hand, Ruffin et al. (1998) concluded that the para-stilbene phenols formed from ![]() -1 structures are scarce in hydrogen peroxide-bleached pulp and that they could not explain the rapid yellowing of bleached mechanical pulps. After refining and hydrogen peroxide bleaching,
-1 structures are scarce in hydrogen peroxide-bleached pulp and that they could not explain the rapid yellowing of bleached mechanical pulps. After refining and hydrogen peroxide bleaching, ![]() -5 structures might form leucochromophoric groups of stilbene hydroquinone type (Lee and Sumimoto 1991, Fig. 10c). Model compound studies in solid state have shown that the highly colored corresponding quinone is formed rapidly upon irradiation (Ruffin and Castellan 2000). In solution state, additional reactions leading to phenanthrene quinone by photocyclization of the Z-stilbene hydroquinone were found. The photochemistry of phenolic and methylated lignin models of the phenylcoumarone type have been studied by Noutary et al. (1995a, b). Both models were photoactive, the phenolic more than the etherified, forming colored coupling products as well as phenylcoumarone o-quinone. This demethylation reaction is possible, whereas a coupling between two phenylcoumarones is very unlikely to occur in pulp. The frequency of
-5 structures might form leucochromophoric groups of stilbene hydroquinone type (Lee and Sumimoto 1991, Fig. 10c). Model compound studies in solid state have shown that the highly colored corresponding quinone is formed rapidly upon irradiation (Ruffin and Castellan 2000). In solution state, additional reactions leading to phenanthrene quinone by photocyclization of the Z-stilbene hydroquinone were found. The photochemistry of phenolic and methylated lignin models of the phenylcoumarone type have been studied by Noutary et al. (1995a, b). Both models were photoactive, the phenolic more than the etherified, forming colored coupling products as well as phenylcoumarone o-quinone. This demethylation reaction is possible, whereas a coupling between two phenylcoumarones is very unlikely to occur in pulp. The frequency of ![]() -1 structures is higher in hardwood lignin than in softwood lignin, while the occurrence of
-1 structures is higher in hardwood lignin than in softwood lignin, while the occurrence of ![]() -5 (phenylcoumaranes) is higher in softwood than hardwood lignin (Brunow and Lundquist 2010).
-5 (phenylcoumaranes) is higher in softwood than hardwood lignin (Brunow and Lundquist 2010).
Fig. 10. Formation of hydroquinones, stilbenes, and quinones during refining, hydrogen peroxide bleaching, and irradiation of mechanical pulp. Main references: Gellerstedt and Agnemo 1980b; Lee and Sumimoto 1991; Gellerstedt and Zhang 1992; Castellan et al. 1993a; Zhang and Gellerstedt 1994b; Ruffin and Castellan 2000)
The discovery of the dibenzodioxocin structure in lignin (Karhunen et al. 1995) has not passed unnoticed in the field of photoyellowing. Even though the mechano-chemical reactions and the reactions during alkaline peroxide bleaching of this structure are poorly known, model compounds of this type have been irradiated in solution and studied (Gardrat et al. 2004, 2005; da Hora Machado et al. 2006). An etherified dibenzodioxocin model in benzene reacted during irradiation through homolytic cleavage of both ![]() -O-4 and
-O-4 and ![]() -O-4 bonds, implying a potential mechanism for lignin depolymer-ization at a branching point in lignin (Fig. 11).
-O-4 bonds, implying a potential mechanism for lignin depolymer-ization at a branching point in lignin (Fig. 11).
Fig. 11. Photochemistry of an etherified lignin model compound of the dibenzodioxocin type irradiated in solution (benzene). Adapted from Gardrat et al. (2004)
The formation of colored materials such as 2,2’-diphenoquinone and oligomeric colored material was suggested (Gardrat et al. 2004). Irradiation of the corresponding phenolic lignin model compound in ethanol resulted in lower degradation efficiency (lower quantum yield), possibly due to the reversible formation of the quinone methide (Gardrat et al. 2005). In recent years, analytical techniques designed to capture radicals and analyze stable products by 31P NMR spectroscopy and gas chromatography-mass spectrometry have been further developed (Argyropoulos et al. 2006; Zoia and Argyropoulos 2009, 2010). More experiments using this technique in the study of light-induced yellowing might reveal further important knowledge regarding the mechanism of discoloration.
PHOTOSTABILIZING METHODS FOR LIGHT-INDUCED YELLOWING
A large number of photostabilizing treatments to protect lignin-rich pulps against ultraviolet and visible radiation have been proposed and evaluated during the last seventy years. The treatments can be divided into three main categories (Fig. 12). The first is characterized by hindering ultraviolet radiation from reaching the photosensible struc-tures in lignin, which can be done by adding UV-absorbing compounds or by coating the paper. The second category includes treatments that function by suppressing the formation of chromophores by chemical modification of functional groups in the lignin moiety that can absorb UV radiation or that can generate reactive intermediates (e.g. phenoxyl and ketyl radicals). The last category is characterized by disrupting the color forming reactions by addition of inhibitors, such as quenchers, which inactivate excited molecules and/or antioxidants, which trap intermediate radicals.
Fig. 12. Schematic description of the different proposed inhibition methods for light-induced yellowing of lignocellulosic materials
The extent of photostabilization needed is dependent on the lifetime expectancies of the lignocellulosic product, on the conditions under which the product is used, and on the amount of high-yield pulp (i.e. amount of lignin) used in the product. The UV/VIS radiation, temperature, and humidity conditions may vary considerably between different end-use applications and geographical locations, and the extent of stabilization needed will thus also vary. It is, however, a prerequisite that a photostabilizing treatment is environmentally, technically, and economically feasible to be considered an option. Even though there are possibilities to more or less completely retard the light-induced discoloration, no treatment that can inhibit photoyellowing of a product composed of a substantial amount of high-yield pulp and can fulfill all of the demands above exists today. An overview of the proposed photostabilizing treatments, divided according to the categories in Fig. 12, is given below. Special attention will be focused on the photo-stabilization characteristics of products, where a part of the fiber content is of high-yield origin, since this is the highest potential for growth of mechanical and chemimechanical pulps in long-life paper and board products.
Absorption or Scattering of Ultraviolet Radiation
One obvious way of improving the photostability is to protect the lignin against exposure to ultraviolet radiation, i.e., to prevent the radiation from reaching the chromophores. This can be done by introducing an ultraviolet absorbing compound on the paper surface or in the paper itself, in order to introduce a compound that converts the absorbed radiation to non-harmful thermal energy. It is also possible that UV absorbers may function as antioxidants, as triplet quenchers, or via photoionization (Castellan et al. 1994; Weir and Miller 2000). Efficient UV screens are hydroxybenzophenone and benzotriazole derivatives (Kringstad 1969, 1973; Gellerstedt et al. 1983; Fornier de Violet et al. 1990; Li and Ragauskas 2000; Li et al. 2004). The performance of 2,4-dihydroxybenzophenone has been improved by the introduction of cationic groups, which facilitate the location of the benzophenone derivative to lignin-rich areas of the fiber material (Castellan et al. 1994). Novel water-soluble hydroxybenzophenones containing ionic or neutral amino groups have been synthesized and proven to be efficient photostabilizers (Argyropoulos et al. 2000). A favorable synergistic effect between hydroxybenzophenone or hydroxyphenylbenzotriazole type ultraviolet absorbers and thiols or sodium hypophosphite has been reported (Davidson et al. 1995; Trichet et al. 1996) and ascribed to the regeneration of the UV absorber by reduction (Noutary et al. 1994, Gugumus 1993), whereas others have reported that the improved stability is not due to a regeneration mechanism (Cook and Ragauskas 1997). A combination of a UV screen (benzotriazole derivative), polyethylene oxide dithiol, and high charge of sucrose has also been used to photostabilize different types of high-yield pulps with some success (Petit-Conil et al. 1998). Another effective additive combination that has been explored for stabilizing hardwood and softwood pulps is the system 2,4-dihydroxybenzophenone/ thiol/ascorbic acid (Pan et al. 1996). The addition of a UV screen in combination with in situ peroxyborate bleaching has been suggested as a possible photostabilizating system for high-yield pulps (Liu and Argyropoulos 1998; Moore and Argyropoulos 2000). A dual component system that has been thoroughly examined and that efficiently inhibits yellowing of mechanical pulps is the system consisting of a benzotriazole derivative (UV absorber) and a hindered nitroxide radical scavenger (4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl) (McGarry et al. 2000; Beaton and Argyropoulos 2001; Yuan et al. 2002; 2003). The effectiveness of the system is claimed to be partly due to an increased photostability of the ultraviolet absorber in the presence of the nitroxide and partly due to inhibition of the cleavage of -O-4-aryl ether linkages (Beaton and Argyropoulos 2001). These additives have, however, limited ability to absorb on pulp fibers and therefore need to be applied in a size press or as an additive in the coating layer. Hindered nitroxide and hydroxylamine radical scavengers with a higher affinity for mechanical pulps have been synthesized (Hu 2003b, 2004; Hu et al. 2005). These inhibitors may be suitable for treating the high-yield pulp separately; something that could improve the economic performance of the dual system for papers containing only part mechanical pulp since the chemical pulp fraction in the papers can be left untreated. Ultraviolet absorbers used alone or in combination with other inhibitors have the poten-tial to improve the photostability of lignin-rich pulps, but to be a realistic alternative, the efficiency/cost ratio needs to be improved.
The use of fluorescent whitening agents (FWAs) to retard photoyellowing has been reported by several researchers (e.g. Bourgoing et al. 2001; Ragauskas et al. 2001; Li and Ragauskas 2001; Zhang et al. 2009a, b). Fluorescent whitening agents are used in the pulp and paper industry to improve the optical properties and are often derivatives of diaminostilbene sulfonic acids or distyrylbiphenyl compounds that absorb radiation in the ultraviolet range (max ~350-360 nm) and re-emit the energy within the visible blue region (max ~440 nm), i.e., these compounds act as UV screens. It has been proposed that FWAs are most effective in retarding the slow phase of reversion (Ragauskas et al. 2001). FWAs can be added in the wet-end of the paper machine, in a size press or coating layer, or in the bleach plant. Although fluorescent whitening agents retard discoloration, relatively large amounts are needed, and the effect is only temporary. The fluorescent whitening agent is photochemically degraded, creating a loss of fluorescence over time as a result (Bourgoing et al. 2001). Nevertheless, FWA is an important additive for high-brightness papers containing part high-yield pulps, and it has been reported that fluorescent whitening agents, especially in combination with fillers, significantly retard the discoloration for these products (He et al. 2010; Perng and Wang 2010). Different types of fillers have also been examined for their inhibiting capacity. Zinc oxide and titanium dioxide that both absorb and scatter ultraviolet radiation can significantly retard photoyellowing of hydrogen peroxide-bleached softwood TMP (Kang and Ni 2006). However, the required addition amounts are high, and these pigments are much more expensive than other fillers.
Paper and board are often coated to improve their printability and optical properties. Coatings also block the damaging ultraviolet radiation by absorbing or scattering radiation or by concealing a part of the discoloration of the base paper (Fjellström et al. 2007b). It is most likely necessary to coat a paper if pulps containing lignin are to be used as the main fiber component in the furnish. The coat-weight will affect the ability of the radiation to pass through the coating (Johnson 1989, 1991; Ghosh et al. 2002; Yuan et al. 2006; Fjellström et al. 2007b). The amount and type of pigment in the coating color will affect the protective capacity; titanium dioxide and zinc oxide pigments being the most effective, followed by kaolin, precipitated, and ground calcium carbonate pigments (Gellerstedt et al. 1983; Reinhart and Arneberg 1988; Luo and Göttsching 1991; Beyer et al. 2006a; Fjellström et al. 2009; Li et al. 2011). A narrow pigment size distribution is beneficial for reducing the amount of ultraviolet radiation that reaches the base paper (Fjellström et al. 2009; Lindblad et al. 1989). The binders, thickeners, and additives, including FWA and carriers for FWA, such as polyvinyl alcohol, polyethylene glycol, carboxymethylcellulose, and polyvinylpyrrolidone, may also affect the stability of the coated paper (Reinhardt and Arneberg 1988; Luo and Göttsching 1991; Fjellström et al. 2009). Low- and medium-weight coating layers provide only partial protection against deterioration by light, and it is necessary to add other inhibiting compounds for successful stabilization. Good results have been reported with a combination of a radical scavenger (hydroxylamine-type) and a UV absorber (benzotriazole derivative or titanium dioxide) at moderate coat weights (El-Sadi et al. 2002; Yuan et al. 2004 a, b). Even though coating a lignin-containing paper is one of the most promising stabilizing treatments, the costs associated with the ultraviolet absorbing/scattering pigments and inhibitors necessary are still too high if a substantial amount of the chemical pulp is to be replaced with a mechanical or chemimechanical pulp. A high substitution degree will negatively impact the photostability of the product and a higher coat weight or charge of additives will therefore be needed (Johnson 1989, 1991; Paulsson et al. 2002; Fjellström et al. 2007a; He et al. 2009).
Chemical Modification
The chemical modification approach of potential chromophoric or leucochromo-phoric groups in lignin involves the reduction of carbonyl groups, hydrogenation of ring-conjugated ethylenic groups, and acylation or alkylation of hydroxyl groups. Ring-conjugated carbonyl groups participate in several of the proposed reaction sequences leading to discoloration. Therefore, many attempts have been made to remove these groups by reductive treatments. Sodium borohydride reduction of spruce SGW pulp (Manchester et al. 1960; Leary 1968a; Ek et al. 1990), spruce TMP (Tschirner and Dence 1988; Francis et al. 1991; Schmidt and Heitner 1991; Paulsson et al. 1995), or softwood CTMP (Fornier de Violet et al. 1989; Castellan et al. 1992a) did not have any dramatic effect on the overall discoloration rate, despite the fact that milled wood lignin or carbonyl-containing lignin model compounds were substantially stabilized by the chemical treatment (Lin and Kringstad 1970). Other reducing agents (dithionite, thiourea dioxide, dithionite, diboran) were also ineffective in suppressing the light-induced discoloration (Manchester et al. 1960; Andrews and DesRosiers 1966; Tschirner and Dence 1988). The low efficiency of reductive treatments may be due to incomplete reduction of carbonyl groups, and since only a few carbonyl groups are needed to catalyze photoyellowing reactions, care must be taken when evaluating the results from the studies. A further explanation of the ineffectiveness is the presence of other potential chromophoric structures not removable by reduction or the formation of new carbonyl groups during irradiation (see Fig. 7). A method for selective removal of ![]() -carbonyl and
-carbonyl and ![]() -hydroxyl groups has been proposed (Hu et al. 1999a), and could, according to the authors, possibly hinder ketyl radical formation during photoyellowing.
-hydroxyl groups has been proposed (Hu et al. 1999a), and could, according to the authors, possibly hinder ketyl radical formation during photoyellowing.
Reduction in combination with hydrogenation has been reported to be effective in stabilizing lignin model compounds and milled wood lignin towards photoinduced degradation (Lin and Kringstad 1970; Castellan et al. 1992b), but was ineffective in reducing the discoloration of spruce TMP (Tschirner and Dence 1988). It is possible that the discrepancy in obtained results is related to the difficulty in reducing vinylic double bonds in the fiber structure. A method for mild catalytic hydrogenation of lignin aromatics in lignin model compounds and MWL has been described (Hu et al. 1997a, b, 1999b) and if a catalyst that can diffuse into the fibers and catalyze the hydrogenation of pulp lignin substrate could be developed, photoyellowing might be inhibited permanently.
One of the most effective treatments tested so far has been to chemically modify hydroxyl groups in softwood or hardwood lignins through etherification or esterification reactions. A variety of alkylation and acylation modifications including acetylation, methylation, benzoylation, and hydroxyethylation have been tested. Many researchers have shown that these treatments not only increase the photostability of high-yield pulp, but often brighten and, in some cases, also photobleach the pulp. Methylation has been reported to inhibit photoyellowing (Peill 1946; Callow 1947; Andrews and DesRosiers 1966; Leary 1968a) but not to affect the rate of chromophore formation during photoyellowing (Schmidt and Heitner 1991). Acetylation with acetic anhydride was reported early on to increase the photostability of jute fibers (Peill 1946; Callow 1947, 1952), and acetylation was later found to photostabilize, and in some cases, also brighten high-yield pulps (Manchester et al. 1960; Leary 1968a; Lorås 1968; Ek et al. 1992; Paulsson et al. 1995; Hirashima and Sumimoto 1996; Itoh et al. 1997, Agarwal 1998; Paulsson and Ragauskas 1998b; Agarwal 1999). Acetylation has also been reported to increase the photostability of MWL (Agarwal et al. 1999; Heitner et al. 2001), isolated aspen and softwood bleached CTMP lignins (Pu et al. 2003; Pu and Ragauskas 2005), and wood (Dunningham et al. 1992; Plackett et al. 1992; Hon 1995; Ohkoshi 2002). Acetylation is thought to be a single-site reaction that is acid- or base-catalyzed. Acetic acid is formed as a byproduct during the reaction. Examples of catalysts that have been used are pyridine, zinc chloride, dimethylformamide, urea-ammonium sulfate, magnes-ium perchlorate, trifluoroacetic acid, boron trifluoride, sodium acetate, potassium hydrogen phosphate, potassium acetate, and ![]() -rays (Rowell 1982). Acetylation can, however, also be performed without any catalyst. The photostabilizing effects of acetylation are most pronounced in the initial phase of derivatization. Therefore, structures that are important for discoloration can be expected to be found among those that react during this initial phase. Lignin model compound studies have shown that phenols, hydroquinones, and catechols were easily acetylated, although not completely (Paulsson et al. 1996a, b). This is in agreement with the observations that most of the free phenolic hydroxyl groups in unbleached and hydrogen peroxide-bleached, high-yield pulps are removed at a moderate extent of derivatization (Paulsson et al. 1995). The
-rays (Rowell 1982). Acetylation can, however, also be performed without any catalyst. The photostabilizing effects of acetylation are most pronounced in the initial phase of derivatization. Therefore, structures that are important for discoloration can be expected to be found among those that react during this initial phase. Lignin model compound studies have shown that phenols, hydroquinones, and catechols were easily acetylated, although not completely (Paulsson et al. 1996a, b). This is in agreement with the observations that most of the free phenolic hydroxyl groups in unbleached and hydrogen peroxide-bleached, high-yield pulps are removed at a moderate extent of derivatization (Paulsson et al. 1995). The ![]() -hydroxyl groups in models of the
-hydroxyl groups in models of the ![]() -O-4 type and coniferyl alcohol type are compar-atively rapidly acetylated, whereas
-O-4 type and coniferyl alcohol type are compar-atively rapidly acetylated, whereas ![]() -hydroxyl groups in
-hydroxyl groups in ![]() -O-4 models seem to be more resistant towards acetylation (Paulsson et al. 1996a, b). Later, similar results were reported for acetylated hydrogen peroxide-bleached aspen lignin; the order of reactivity was found to be phenolic OH >>
-O-4 models seem to be more resistant towards acetylation (Paulsson et al. 1996a, b). Later, similar results were reported for acetylated hydrogen peroxide-bleached aspen lignin; the order of reactivity was found to be phenolic OH >> ![]() -side chain OH >
-side chain OH > ![]() -side chain OH (Pu and Ragauskas 2005; Haque and Hill 2000). Others have not, however, found any evidence for selective acetylation of phenolic hydroxyl groups in MWL at a low degree of derivatization (Heitner et al. 2001). Further, o-quinoidic model compounds were rapidly decomposed and to a large extent decolored on acetylation, whereas p-quinoidic model compounds were essentially unaffected by the chemical treatment (Paulsson et al. 1996b). Other alkylation and acylation agents such as ethylene oxide, propylene oxide, butylene oxide, benzoyl chloride, methyl chlorocarbonate, and propionic anhydride have also been found to be effective in photostabilizing high-yield pulps (Singh 1966; Wallis and Wearne 1982; Paulsson and Parkås 2000). Removal of phenolic hydroxyl groups via catalytic hydrogenolysis has been reported to result in a significant yellowing inhibition of lignin model compounds (Hu et al. 2000).
-side chain OH (Pu and Ragauskas 2005; Haque and Hill 2000). Others have not, however, found any evidence for selective acetylation of phenolic hydroxyl groups in MWL at a low degree of derivatization (Heitner et al. 2001). Further, o-quinoidic model compounds were rapidly decomposed and to a large extent decolored on acetylation, whereas p-quinoidic model compounds were essentially unaffected by the chemical treatment (Paulsson et al. 1996b). Other alkylation and acylation agents such as ethylene oxide, propylene oxide, butylene oxide, benzoyl chloride, methyl chlorocarbonate, and propionic anhydride have also been found to be effective in photostabilizing high-yield pulps (Singh 1966; Wallis and Wearne 1982; Paulsson and Parkås 2000). Removal of phenolic hydroxyl groups via catalytic hydrogenolysis has been reported to result in a significant yellowing inhibition of lignin model compounds (Hu et al. 2000).
Although successful photostabilization has been achieved by derivatizing lignin hydroxyl groups in lignocellulosic materials, no treatment has yet become technically or economically feasible due to the large amounts of chemicals required for successful protection and due to the deterioration in strength and optical properties accompanying some of the treatments. Furthermore, many of the chemicals used (e.g. for methylation and epoxidation modifications) are highly toxic, and the use of these chemicals is therefore highly questionable.
Inhibiting Compounds
The photoyellowing process involves a series of radical reactions and free radical scavengers, which often function as proton donators and have therefore been extensively explored. Sodium citrate and sodium ascorbate were reported early on to increase the photostability of high-yield pulps (Kringstad 1969). Later, ascorbic acid was found to be an efficient stabilizer for different types of high-yield pulps (Fornier de Violet et al. 1990; Agnemo et al. 1991; Schmidt and Heitner 1991; Wan et al. 1993). However, the inhibition was only temporary, and the inherent thermal instability of ascorbic acid is a serious limitation for the use of this antioxidant (Ragauskas 1994; Schmidt and Heitner 1997).
Sulfur and phosphorous compounds, often used as antioxidants in polymers, have been tested as inhibitors, and examples of compounds with a photostabilizing effect are: 1-thioglycerol, dimercaptoacetate, thioglycolic acid, 2,2’-oxydiethanethiol, ethylene glycol bisthioglycolate, thiosulfinates, and 1-dodecanethiol (Cole and Sarkanen 1987; Janson and Forsskåhl 1989; Fischer 1990; Daneault et al. 1991; Pan and Ragauskas 1995a, b; Cook et al. 1996; Li et al. 1999; Barnett et al. 2002). The stabilizing effect of sulfur-containing compounds decreases over time, and the unpleasant odor is a serious problem that makes these compounds unsuitable for most applications. However, a group of novel non-odorous thiols, isolated from biomaterials or synthesized, have been found to be effective in retarding the discoloration of aspen CTMP (Beyer et al. 2006b). The sulfur-containing inhibitors were found to protect the lignin-containing paper for a long time when combined with an ultraviolet absorber and as part of a coating layer.
Different types of water-soluble, colorless, and odorless phosphinates have also been proposed and tested with moderate inhibition of photoyellowing of bleached softwood CTMP (Guo and Gray 1996; Guo et al. 1997). Addition of radical scavengers, such as phenyl-N-t-butylnitrone, phthalic hydrazide, 1-hexanol, or colloidal sulfur during milling of TMP significantly reduced photoyellowing (Wu et al. 1994), and it was proposed that the mechanochemical conversion of lignin to photoactive structures was hindered or stopped. This has, however, not been verified in later studies where different types of antioxidant and radical scavengers were added during refining (Zhu et al. 1997).
Naturally occurring antioxidants, e.g., quercetin, catechin, -tocopherol, and avenanthramides, have been evaluated for their capacity to inhibit light-induced discoloration with very limited success (Fagerlund et al. 2003). Polymers such as polyethylene glycol, polyvinyl-pyrrolidone, and polytetrahydrofuran with different molecular weights and end groups can retard photoyellowing if relatively large amounts are used (Minemura 1978; Forsskåhl and Janson 1992; Cole et al. 1993; Hortling et al. 1993; Rättö et al. 1993; Davidsson et al. 1995; Janson and Forsskåhl 1996). The exact mechanism behind the stabilization obtained by polymers is not known.
Another class of inhibitors is quenchers, i.e., molecules that can relax excited chemical structures. Naphthalene derivatives that function as -carbonyl triplet quenchers (Fornier de Violet et al. 1990) and 1,4-diazabicyclo[2.2.2]octane (DABCO) that quenches singlet oxygen (Janson and Forsskåhl 1989) have shown no or very limited inhibiting effect when applied to pulp. DABCO could, however, decrease the photooxidative degradation of MWL (Wang et al. 1996) and suppress the formation of quinones during photolysis of lignin in solution (Neumann and Machado 1989). Hexadienol trans,trans-2,4-hexadiene-1-ol has been reported to retard yellowing as efficiently as ascorbic acid (Ragauskas 1993b; Harvey and Ragauskas 1996). The photostabilizing activities of diene-type structures are believed to be due in part to radical scavenging properties and in part by quenching the excited state of lignin (Schmidt et al. 1991; Pan et al. 1996).
Since transition metal ions present in pulp or paper aggravate discoloration, chelating agents have also been tested as inhibitors. The addition of diethylene-triaminepentaacetic acid, either by spraying (paper) or in situ (pulp), was found to reduce both the light-induced and heat-induced discoloration of maple CTMP (Ni et al. 1998). Others have, however, found that chelating agents photostabilize mechanical pulps only if the chelants are thoroughly washed from the fiber material prior to ageing (Janson and Forsskåhl 1989).
Although some of the proposed inhibiting compounds have the possibility to retard discoloration, relatively large amounts of the additive are needed, and they will only temporarily delay the discoloration. Synergistic effects have been reported for certain inhibitor mixtures (Davidson et al. 1995; Cook and Ragauskas 1997; McGarry et al. 2000; Ragauskas et al. 2001), and it is has been proposed that a successful photo-stabilization technology needs an ultraviolet absorber, an excited state quencher, and a radical scavenger (Pan et al. 1996). Such technologies are, with the inhibitors known today, not cost-effective.
CURRENT STATUS: PHOTOYELLOWING MECHANISMS AND PREVENTIVE METHODS
Acetylation of lignin-rich mechanical and chemimechanical pulps removes most of the free phenolic hydroxyl groups after a short reaction time, and the photostabilizing effect is most pronounced at the initial phase of acetylation (Paulsson and Simonson 2002). Model compound studies have shown that phenols, hydroquinones, catechols, and o-quinones are rapidly acetylated, whereas benzylic hydroxyl groups (and p-quinones) are more resistant to acetylation (Paulsson et al. 1996 a, b; and Ragauskas 2005). There is nothing to suggest that ![]() -hydroxyl groups should be more reactive in the pulp than in the model compound studied. The number of underivatized benzylic hydroxyl groups present in the lignin moiety at an acetylation level, which to a large extent inhibits the photo-yellowing of high-yield pulps, can thus be expected to be fairly high. It is therefore difficult to explain the stabilizing effect obtained by acetylation as a protection of the
-hydroxyl groups should be more reactive in the pulp than in the model compound studied. The number of underivatized benzylic hydroxyl groups present in the lignin moiety at an acetylation level, which to a large extent inhibits the photo-yellowing of high-yield pulps, can thus be expected to be fairly high. It is therefore difficult to explain the stabilizing effect obtained by acetylation as a protection of the ![]() -O-4 aryl ether bond, since it has been suggested that most of the degradation of such bonds proceeds via cleavage of arylglycerol
-O-4 aryl ether bond, since it has been suggested that most of the degradation of such bonds proceeds via cleavage of arylglycerol ![]() -aryl ether structures (Fig. 7, Path A). The acetylation of
-aryl ether structures (Fig. 7, Path A). The acetylation of ![]() -hydroxyl and phenolic hydroxyl groups could possibly influence the stability of the
-hydroxyl and phenolic hydroxyl groups could possibly influence the stability of the ![]() -O-4 aryl ether bond and thereby retard the subsequent breakdown of these lignin units. Further work is needed to evaluate this possibility. Acetylation of the phenolic hydroxyl group in a lignin model compound of the 2-aryloxy-l-arylpropanone type could, however, not prevent the cleavage of the
-O-4 aryl ether bond and thereby retard the subsequent breakdown of these lignin units. Further work is needed to evaluate this possibility. Acetylation of the phenolic hydroxyl group in a lignin model compound of the 2-aryloxy-l-arylpropanone type could, however, not prevent the cleavage of the ![]() -O-4 aryl ether structure upon irradiation (Hon 1995). This suggests that the phenacyl aryl ether pathway (Fig. 6) is still a possible source of phenoxyl radicals (and thereby chromophore formation) upon irradiation of acetylated high-yield pulps. A photo-Fries rearrangement, i.e., homolytic cleavage of the ArO-COCH3 bond, which releases the phenolic hydroxyl group and forms ortho and para rearrangement products, has been reported to occur upon photoyellowing of acetylated phenolic lignin model compounds (Braga and Hampp 2005) and could be one possible reason for the incomplete photostability obtained by acetylation. The cleavage of original 2-aryloxy-l-arylpropanone structures is probably responsible for only a small part of the total discoloration of high-yield pulps, since the number of such units in lignin is low compared with the number of phenolic hydroxyl groups. However, as can be seen in Fig. 7 (Path B), these structures could be generated from initially formed ketyl radicals and later give rise to new phenolic groups (phenoxyl radicals) by homolytic cleavage of the
-O-4 aryl ether structure upon irradiation (Hon 1995). This suggests that the phenacyl aryl ether pathway (Fig. 6) is still a possible source of phenoxyl radicals (and thereby chromophore formation) upon irradiation of acetylated high-yield pulps. A photo-Fries rearrangement, i.e., homolytic cleavage of the ArO-COCH3 bond, which releases the phenolic hydroxyl group and forms ortho and para rearrangement products, has been reported to occur upon photoyellowing of acetylated phenolic lignin model compounds (Braga and Hampp 2005) and could be one possible reason for the incomplete photostability obtained by acetylation. The cleavage of original 2-aryloxy-l-arylpropanone structures is probably responsible for only a small part of the total discoloration of high-yield pulps, since the number of such units in lignin is low compared with the number of phenolic hydroxyl groups. However, as can be seen in Fig. 7 (Path B), these structures could be generated from initially formed ketyl radicals and later give rise to new phenolic groups (phenoxyl radicals) by homolytic cleavage of the ![]() -O-4 bond according to the phenacyl aryl ether pathway (Fig. 6). Further, it has been found that the content of noncondensed
-O-4 bond according to the phenacyl aryl ether pathway (Fig. 6). Further, it has been found that the content of noncondensed ![]() -aryl ether units in bleached spruce thermomechanical pulp was essentially unchanged even after irradiation that caused an extensive brightness loss (Pan et al. 1992). It is worth mentioning that the monomeric thioacidolysis product coming from
-aryl ether units in bleached spruce thermomechanical pulp was essentially unchanged even after irradiation that caused an extensive brightness loss (Pan et al. 1992). It is worth mentioning that the monomeric thioacidolysis product coming from ![]() -ethers only stems from noncondensed
-ethers only stems from noncondensed ![]() -ether units. This means that a decrease in yield upon prolonged irradiation, reported by some, could be due to either
-ether units. This means that a decrease in yield upon prolonged irradiation, reported by some, could be due to either ![]() -ether cleavage and/or condensation reactions (Pan et al. 1992, 1993). On the contrary, the decrease in absolute amount of isolated dimeric thioacidolysis products after irradiation also points to a significant increase in the condensation degree (Pan et al. 1992).
-ether cleavage and/or condensation reactions (Pan et al. 1992, 1993). On the contrary, the decrease in absolute amount of isolated dimeric thioacidolysis products after irradiation also points to a significant increase in the condensation degree (Pan et al. 1992).
The importance of cleavage of ![]() -ethers for the formed color during photo-yellowing is probably minor; reactions involving arylglycerol
-ethers for the formed color during photo-yellowing is probably minor; reactions involving arylglycerol ![]() -aryl ether structures are less probable reaction pathways in chromophore formation during photoyellowing than reaction pathways involving photooxidation of free phenolic hydroxyl groups (Fig. 4 and 8), hydroquinones, catechols, or cleavage of 2-aryloxy-l-arylpropanone structures (Fig. 6), at least during the initial and most detrimental phase of photoyellowing. However, from a photostabilizing perspective,
-aryl ether structures are less probable reaction pathways in chromophore formation during photoyellowing than reaction pathways involving photooxidation of free phenolic hydroxyl groups (Fig. 4 and 8), hydroquinones, catechols, or cleavage of 2-aryloxy-l-arylpropanone structures (Fig. 6), at least during the initial and most detrimental phase of photoyellowing. However, from a photostabilizing perspective, ![]() -ether cleavage is more severe since it enables the formation of free phenols (source of phenoxyl groups), even if all free phenols are derivatized prior to ageing. The importance of structures that can initiate discoloration of lignin-containing pulps and participate in a cyclic process must also be taken into consideration. The significance of o-quinonoid compounds or some other still unknown structure needs to be investigated further.
-ether cleavage is more severe since it enables the formation of free phenols (source of phenoxyl groups), even if all free phenols are derivatized prior to ageing. The importance of structures that can initiate discoloration of lignin-containing pulps and participate in a cyclic process must also be taken into consideration. The significance of o-quinonoid compounds or some other still unknown structure needs to be investigated further.
Many photostabilizing treatments have been proposed, and some of the treatments are efficient in retarding discoloration. Although ultraviolet absorbers in combination with antioxidants have the potential to inhibit photoyellowing, the efficiency/cost ratio needs to be improved if these additives are going to be used. Derivatization of hydroxyl groups by chemical modification has been proven to be effective, but the deterioration in strength and optical properties and the technical difficulties in executing the chemical treatments in the pulp or paper mill makes chemical modification not a viable option. The best approach to protecting lignin-containing paper or board products against photo-yellowing is by coating them alone or with inhibiting additives. The use of fluorescent whitening agents could, at least for some time, slow down the discoloration. However, there exists today no photostabilizing treatment that is economically and/or technically feasible for a product in which the main part of the fiber material is of mechanical or chemimechanical origin.
ACKNOWLEDGMENTS
The authors want to thank Gunilla Fridholm, Eka Chemicals for valuable help with information retrieval.
REFERENCES CITED
Abbot, J. (1995). “Reactions of ortho-quinones and the model compound 4-t-butyl-1,2-benzoquinone in alkaline peroxide,” Res. Chem. Intermed. 21(3-5), 535-562.
Abou-State, M. A. (1976). “Effect of light on high-yield pulps,” Indian Pulp Pap. 31(2), 21, 23-28.
Adelstein, P. Z., Zinn, E. D., and Reilly, J. M. (2003). “Effect of atmospheric pollution on paper stability,” J. Pulp Pap. Sci. 29(1), 21-28.
Adler, E., and Marton, J. (1959). “Zur Kenntnis der Carbonylgruppen im Lignin. I.,” Acta Chem. Scand. 13(1), 75-96.
Agarwal, U. P. (1998). “Assignment of the photoyellowing-related 1675 cm-1 Raman/IR band to p-quinones and its implications to the mechanism of color reversion in mechanical pulps,” J. Wood Chem. Technol. 18(4), 381-402.
Agarwal, U. P. (1999). “On the importance of hydroquinone/p-quinone redox system in the photoyellowing of mechanical pulps,” 10th International Symposium on Wood and Pulping Chemistry, Yokohama, Japan, Yokohama, Japan, June 7-10, vol. 1, pp. 694-697.
Agarwal, U. P., and Atalla, R. H. (1990). “Formation and identification of cis/trans ferulic acid in photoyellowed white spruce mechanical pulp,” J. Wood Chem. Technol. 10(2), 169-190.
Agarwal, U. P., McSweeny, J. D., and Ralph, S. A. (1999). “An FT-Raman study of softwood, hardwood, and chemically modified black spruce MWLs,” 10th International Symposium on Wood and Pulping Chemistry, Yokohama, Japan, June 7-10, vol. 2, pp. 136-140.
Agnemo, R., Francis, R. C., Alexander, T. C., and Dence, C. W. (1991). “Studies on the mechanism of the photoyellowing of bleached mechanical and chemimechanical pulps. III. The role of hydroxyl radicals,” Holzforschung 45(Suppl.), 101-108.
Agrawal, C., Hsieh, J. S., Xu, H. X., Matthews, J., and Kokoszka, J. (2005). “The effectiveness of TAED on the peroxide bleaching of mechanical, chemical, and recycled pulp,” Eng. Pulp. Environ. Conf., Philadelphia, PA, USA, August 28-31, 26.
Andersen, M. L., and Wayner, D. D. M. (1999). “Electrochemistry of electron transfer probes. -Aryloxyacetoveratrones and implications for the mechanism of photo-yellowing of pulp,” Acta Chem. Scand. 53, 830-836.
Andrady, A. L., Song, Y., Parthasarathy, V. R., Fueki, K., and Torikai, A. (1991). “Photoyellowing of mechanical pulp. Part 1: Examining the wavelength sensitivity of light-induced yellowing using monochromatic radiation,” Tappi J. 74(8), 162-168.
Andrady, A. L., and Searle, N. D. (1995). “Photoyellowing of mechanical pulps. Part 2: Activation spectra for light-induced yellowing of newsprint paper by polychromatic radiation,” Tappi J. 78(5), 131-138.
Andrews, D. H., and DesRosiers, P. (1966). “The influence of wood components on the bleach response of jack pine groundwood to peroxide,” Pulp Pap. Mag. Can. 67, 119-128.
Andtbacka, A., Holmbom, B., and Gratzl, J. S. (1989). “Factors influencing light-induced yellowing and bleaching of spruce groundwood,” 5th Int. Symp. Wood Pulp. Chem., Raleigh, N.C., USA, May 22-25, 347-351.
Argyropoulos, D. S., and Heitner, C. (1994). “31P NMR spectroscopy in wood chemistry. Part IV. Solid state 31P NMR of trimethyl phosphate derivatives of chromophores and carboxylic acids present in mechanical pulps; A method for the quantitative determination of ortho-quinones,” Holzforschung 48(Suppl.), 112-116.
Argyropoulos, D. S., Heitner, C., and Schmidt, J. A. (1995). “Observation of quinonoid groups during the light-induced yellowing of softwood mechanical pulp,” Res. Chem. Intermed. 9(3-5), 263-274.
Argyropoulos, D., and Sun, Y. (1996). “Photochemically induced solid-state degradation, condensation, and rearrangement reactions in lignin model compounds and milled wood lignin,” Photochem. Photobiol. 64(3), 510-517.
Argyropoulos, D., and Zhang, L. (1998). “Semiquantitative determination of quinonoid structures in isolated lignins by 31P nuclear magnetic resonance,” J. Agric. Food Chem. 46(11), 4628-4634.
Argyropoulos, D. S., Halevy, P., and Peng, P. (2000). “Photoyellowing inhibition of bleached high yield pulps using novel water-soluble UV screens,” Photochem. Photobiol. 71(2), 141-148.
Argyropoulos, D. S., Li, H., Gaspar, A. R., Smith, K., Lucia, L. A., and Rojas, O. R. (2006). “Quantitative 31P NMR detection of oxygen-centered radical species,” Bioorg. Med. Chem.14(12), 4017-4028.
Balsells, R. E., and Frasca, A. R. (1982). “Photochemical reaction of alcohols-II. Irradiation of aromatic alcohols,” Tetrahedron 38(16), 2525-2538.
Barclay, L. R. C., Grandy, J. K., MacKinnon, H. D., Nichol, H. C., and Vinqvist, M. R. (1998). “Peroxidations initiated by lignin model compounds: Investigating the role of singlet oxygen in photo-yellowing,” Can. J. Chem. 76(12), 1805-1816.
Barclay, L. R. C., Basque, M.-C., and Vinqvist, M. R. (2003). “Singlet-oxygen reactions sensitized on solid surfaces of lignin or titanium dioxide: Product studies from hindered secondary amines and from lipid peroxidation,” Can. J. Chem. 81(6), 457-467.
Barnett, V., Banham, P., and Yates, B. (2002). “Inhibition of photo-induced colour reversion of TMP and peroxide-bleached TMP by mercaptans,” Appita J. 55(1), 27-34, 48.
Beaton, C. R., and Argyropoulos, D. S. (2001). “Photostabilizing milled wood lignin with benzotriazoles and hindered nitroxide,” Photochem. Photobiol. A. 73(6), 605-610.
Beyer, M., Koch, H., and Fischer, K. (1995). “Photoyellowing of high yield pulps – Looking for oxygen radicals,” 8th Int. Symp. Wood Pulp. Chem., Helsinki, Finland, June 6-9, vol. 3, 5-8.
Beyer, M., Gerds, A., Bäurich, C., and Fisher, K. (2006a). “Stabilisierung von holzhaltigem Papier gegen lichtinduzierte Vergilbung durch Zusätze in Streichfarbe und Oberflächenleimung,” Wochenbl. Papierfabr. 134(7), 371-375.
Beyer, M., Fischer, K., and Süss, H.-U. (2006b). “Brightness stabilization of bleached high-yield pulps by novel sulfur-containing inhibitors,” Holzforschung 60(3), 223-230.
Bond, J. S., Atalla, R. H., Agarwal, U. P., and Hunt, C. G. (1999). “The ageing of lignin rich papers upon exposure to light: Its quantification and prediction,” 10th Int Symp. Wood Pulp. Chem., Yokohama, Japan, June 7-10, vol. 3, 500-504.
Bond, J. S., Yu, X., Agarwal, U. P., Atalla, R. H., and Hunt, C. G. (2001). “The ageing of printing and writing papers upon exposure to light: Part 1 – Optical and chemical changes due to long-term light exposure,” 11th Int. Symp. Wood Pulp. Chem., Nice, France, June 11-14, vol. 2, 209-213.
Bonini, C., D’Auria, M., D’Alessio, L., Mauriello, G., Tofani, D., Viggiano, D., and Zimbardi, F. (1998). “Singlet oxygen degradation of lignin,” J. Photochem. Photobiol. A 113(2), 119-124.
Bourgoing, S., Leclerc, É., Martin, P., and Robert, S. (2001). “Use of fluorescent whitening agents to inhibit light-induced colour reversion of unbleached mechanical pulps,” J. Pulp Pap. Sci. 27(7), 240-244.
Braga, D., and Hampp, N. (2005). “Protection of thermomechanical pulps (TMP) against photo-yellowing,” 59th Appita Ann. Conf. Exhib., Auckland, New Zealand, May 16-19, vol. 3, 41-44.
Brunow, G., and Eriksson, B. (1971). “-Carbonyl groups as sensitizers in the photo-dehydrogenation of phenolic structures in lignin,” Acta Chem. Scand. 25(7), 2779-2781.
Brunow, G., and Sivonen, M. (1975). “The yellowing of lignin. Part II. The participation of oxygen in the photodehydrogenation of lignin model compounds,” Paperi ja Puu 57(4a), 215-220.
Brunow, G., and Lundquist, K. (2010). “Functional groups and bonding patterns in lignin (Including the lignin-carbohydrate complexes),” Lignin and lignans. Advances in Chemistry, C. Heitner, D. Dimmel, and J. Schmidt (eds.), CRC Press, Taylor Francis Group, New York, NY, USA, 267-299.
Callow, H. J. (1947). “Action of light upon jute,” Nature 159(4035), 309.
Callow, H. J., and Speakman, J. B. (1949). “The action of light on jute,” J. Soc. Dyers Colorists 65(12), 758-763.
Callow, H.J. (1952). “Acetylation of jute,” J. Text. Inst. 43(8), 423-432.
Castellan, A., Colombo, N., Cucuphat, C., and Fornier de Violet, P. (1989). “Photodegradation of lignin: A photochemical study of a phenolic -carbonyl -O-4 lignin model dimer 4-hydroxy-3-methoxy--(2’-methoxyphenoxy)-acetophenone,” Holzforschung 43(3), 179-185.
Castellan, A., Colombo, N., Nourmamode, A., Zhu, J. H., Lachenal, D., Davidson, D. S., and Dunn, L. (1990a). “Discoloration of -carbonyl-free lignin model compounds under UV light exposure,” J. Wood Chem. Technol. 10(4), 461-493.
Castellan, A., Colombo, N., Vanucci, C., Fornier de Violet, P., and Bouas-Laurent, H. (1990b). “A photochemical study of an O-methylated -carbonyl -1 lignin model dimer: 1,2-di(3’,4’-dimethoxyphenyl)ethanone (deoxyveratroin),” J. Photochem. Photobiol. A. 51(3), 451-467.
Castellan, A., Nourmamode, A., Colombo, N., Jaeger, C., Noutary, C., and Zhu, J. H. (1991). “Discoloration of phenolic carbonyl-free lignin model molecules, milled wood lignin (MWL) and peroxide MWL in the solid state; Approach to the mechanism of protection of high-yield pulps against photoyellowing by reducing agents using 3,4-dimethoxy--(2’-methoxyphenoxy)acetophenone as a lignin model,” 6th Int. Symp. Wood Pulp. Chem., Melbourne, Australia, April 29-May 3, vol. 1, 151-158.
Castellan, A., Nourmamode, A., Noutary, C., and Lachenal, D. (1992a). “Improvement of the photostability of peroxide bleached chemithermomechanical pulps using cationic phase transfer catalyst and hydroxyl blocking agents,” Cell. Chem. Technol. 26(4), 451-459.
Castellan, A., Nourmamode, A., Fornier de Violet, P., Colombo, N., and Jaeger, C. (1992b). “Photoyellowing of milled wood lignin and peroxide-bleached milled wood lignin in solid 2-hydroxypropylcellulose films after sodium borohydride reduction and catalytic hydrogenation in solution: An UV/VIS absorption spectroscopic study,” J. Wood Chem. Technol. 12(1), 1-18.
Castellan, A., Nourmamode, A., Jaeger, C., and Forsskåhl, I. (1993a). “Photochemistry of methoxy-p-benzoquinone and methoxyhydroquinone in solid 2-hydroxypropylcellulose films and on filter paper. UV/Vis absorption and diffuse reflectance spectroscopy studies,” Nord. Pulp Pap. Res. J. 8(2), 239-244.
Castellan, A., Nourmamode, A., Jaeger, C., and Forsskåhl, I. (1993b). “Photochemistry of quinones and hydroquinones in solid 2-hydroxypropylcellulose films and on filter paper,” Photochemistry of Lignocellulosic Materials, C. Heitner, and J. C. Scaiano (eds.), ACS Symposium Series 531, Washington, DC, USA, 60-76.
Castellan, A., Noutary, C., and Davidson, R. S. (1994). “Attempts to photostabilize paper made from high-yield pulp by application of UV screens containing groups to aid their compatibility with cellulose and lignin,” J. Photochem. Photobiol. A. 84(3), 311-316.
Cole, B. J. W., and Sarkanen, K. V. (1987). “Bleaching and brightness stabilization of high-yield pulps by sulfur-containing compounds,” Tappi J. 70(11), 117-122.
Cole, B. J. W., Huth, S. P., and Runnels, P. S. (1993). “Modification of high yield pulps with polyethylene glycols. I. Model compound and isolated lignin studies,” J. Wood Chem. Technol.13(1), 59-72.
Cook, C. M., Pan, X., and Ragauskas, A. J. (1996). “Brightness reversion of mechanical pulps VII: Photostabilization studies of thiol additives for lignocellulosic materials,” J. Wood Chem. Technol. 16(3), 327-345.
Cook, C. M., and Ragauskas, A. J. (1997). “Brightness reversion of mechanical pulps VIII Investigation of synergistic photostabilization methods for high-yield pulp,” J. Photochem. Photobiol. A. 104(1-3), 217-224.
Crestini, C., and D’Auria, M. (1996). “Photodegradation of lignin: The role of singlet oxygen,” J. Photochem. Photobiol. A. 101(1), 69-73.
Crestini, C., and D’Auria, M. (1997). “Singlet oxygen in the photodegradation of lignin models,” Tetrahedron 53(23), 7877-7888.
Cross, C. F. (1897). “The industrial use of cellulose,” J. Royal Soc. Arts 45, 684-696.
Daneault, C., Robert, S., and Lévesque, M. (1991). “The prevention of light-induced yellowing of paper: The inhibition of reversion by mercaptans of TMP and CTMP pulp from balsam fir (Abies balsamea) and black spruce (Picea mariana),” J. Pulp Pap. Sci. 17(6), 187-193.
Davidson, R. S. (1996). “The photodegradation of some naturally occurring polymers,” J. Photochem. Photobiol. B. 33(1), 3-25.
Davidson, R. S., Dunn, L., Castellan, A., and Nourmamode, A. (1995). “Attempts to photostabilize paper made from high-yield pulp by application of UV screens and control of pH,” J. Photochem. Photobiol. A. 86(1-3), 275-282.
Destiné, J.-N., Wang, J., Heitner, C., and St. John Manley, R. (1996). “The photodegradation of milled wood lignin. Part I: The role of oxygen,” J. Pulp Pap. Sci. 22(1), 24-30.
Dunningham, E. A., Plackett, D. V., and Singh, A. P. (1992). “Weathering of chemically modified wood. Natural weathering of acetylated radiata pine: Preliminary results,” Holz Roh- Werkst. 50(11), 429-432.
Ek, M. (1992). Some Aspects on the Mechanisms of Photoyellowing of High-Yield Pulps, Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden.
Ek, M., Lennholm, H., and Iversen, T. (1990). “A comment on the effect of carbonyl groups on the light-induced reversion of groundwood pulp,” Nord. Pulp Pap. Res. J. 5(4), 159-160.
Ek, M., Lennholm, H., Lindblad, G., and Iversen, T. (1992). “A study on the mechanism of the photo-yellowing of partially acetylated groundwood pulps,” Nord. Pulp Pap. Res. J. 7(3), 108-112.
El-Saidi, H., Yuan, Z., Esmail, N., and Schmidt, J. (2002). “Factors affecting the inhibition of light-induced yellowing of a coated BTMP paper,” J. Pulp Pap. Sci. 28(12), 400-405.
Evans, J. (1898). “Report of the committee on the deterioration of paper,” J. Royal Soc. Arts 46, 597-601.
Fabbri, C., Bietti, M., and Lanzalunga, O. (2005). “Generation and reactivity of ketyl radicals with lignin related structures. On the importance of the ketyl radical pathway in the photoyellowing of lignin containing pulps and papers,” J. Org. Chem. 70(7), 2720-2728.
Fagerlund, A., Shanks, D., Sunnerheim, K., Engman, L., and Frisell, H. (2003). “Protective effects of synthetic and naturally occurring antioxidants in pulp products,” Nord. Pulp Pap. Res. J. 18(2), 176-181.
Feller, R. L. (1994). Accelerated Ageing. Photochemical and Thermal Aspects. D. Berland (ed.), Edwards Bros., Ann Arbor, MI, USA.
Fisher, K. (1990). “Vergilbung von Hochausbeutezellstoff,” Papier 44(10A), 11-18.
Fischer, K., Beyer, M., and Koch, H. (1995). “Photo-induced yellowing of high yield pulps,” Holzforschung 49(3), 203-210.
Fjellström, H., Höglund, H., and Paulsson, M. (2007a). “Light-induced yellowing of mechanical and chemimechanical pulp sheets – Influence of wood raw material, process and ageing method,” Nord. Pulp Pap. Res. J. 22(1), 117-123, 275.
Fjellström, H., Höglund, H., Forsberg, S., and Paulsson, M. (2007b). “Inhibition of light-induced brightness reversion of high-yield pulps: The UV-screening properties of coating layers containing kaolin or calcium carbonate pigments,” Nord. Pulp Pap. Res. J. 22(3), 350-355.
Fjellström, H., Höglund, H., Forsberg, S., and Paulsson, M. (2009). “The UV-screening properties of coating layers: The influence of pigments, binders and additives,” Nord. Pulp Pap. Res. J. 24(2), 206-212.
Fornier de Violet, P., Nourmamode, A., Colombo, N., and Castellan, A. (1989). “Study of brightness reversion of bleached chemithermomechanical pulp by solid state electronic spectroscopy,” Cell. Chem. Technol. 23(5), 535-544.
Fornier de Violet, P., Nourmamode, A., Colombo, C., Zhu, J., and Castellan, A. (1990). “Photochemical brightness reversion of peroxide bleached mechanical pulps in the presence of various additives,” Cell. Chem. Technol. 24(2), 225-235.
Forsskåhl, I. (1995). “Temperature and humidity effects on the light-induced changes in lignocellulosic pulps,” 8th Int. Symp. Wood Pulp. Chem., Helsinki, Finland, June 6-9, vol. 2, 9-14.
Forsskåhl, I. (2000). “Brightness reversion,” Forest Products Chemistry, P. Stenius (ed.), Fapet Oy, Helsinki, Finland, 278-332.
Forsskåhl, I., and Janson, J. (1991). “Irradiation of mechanical pulps with monochromatic light at selected wavelength,” 6th Int. Symp. Wood Pulp. Chem., Melbourne, Australia, April 29-May 3, vol. 1, 255-262.
Forsskåhl, I., Tylli, H., Olkkonen, C., and Janson, J. (1991). “Photochemistry of quinones and hydroquinones on solid matrices,” 6th Int. Symp. Wood Pulp. Chem., Melbourne, Australia, April 29-May 3, vol. 2, 325-331.
Forsskåhl, I., and Janson, J. (1992). “Photostabilization of mechanical pulps from spruce and aspen by treatment with polyethylene glycol,” Pap. Puu 74(7), 553-559.
Forsskåhl, I., Tylli, H., and Olkkonen, C. (1993). “Influence of quinoid and aromatic chromophores on the light-induced yellowing of high-yield pulps,” 7th Int. Symp. Wood Pulp. Chem., Beijing, China, May 25-28, vol. 2, 750-758.
Forsskåhl, I., Olkkonen, C., and Tylli, H. (1995). “Depth profiling of a photochemically yellowed paper. Part I. UV-Visible reflectance and fluorescence spectroscopy,” Appl. Spectrosc. 49(1), 92-97.
Forsskåhl, I., and Tylli, H. (2001). “Accelerated ageing studies of printing and writing papers: Part I. Irradiation with filtered xenon light,” 11th Int. Symp. Wood Pulp. Chem., Nice, France, June 11-14, 181-184.
Francis, R. C., Dence, C. W., Alexander, T. C., Agnemo, R., and Omori, S. (1991). “Photostabilization of thermomechanical pulps by alkylation and borohydride reduction,” Tappi J.74(12), 127-133.
Fukagawa, N., and Ishizu, A. (1991). “Photoreaction of phenacyl aryl ether type lignols,” J. Wood Chem. Technol. 11(3), 263-289.
Gardrat, C., Ruggiero, R., Hoareau, W., Nourmamode, A., Grelier, S., Siegmund, B., and Castellan, A. (2004). “Photochemical study of an o-ethyl dibenzodioxocin molecule as a model for the photodegradation of non-phenolic lignin units of lignocellulosics,” J. Photochem. Photobiol. A. 167(2-3), 111-120.
Gardrat, C., Ruggiero, R., Hoareau, W., Damigo, L., Nourmamode, A., Grelier, S., and Castellan, A. (2005). “Photochemical study of 4-(4,9-dimethoxy-2,11-n-dipropyl-6,7-dihydro-5,8-dioxa-dibenzo[a,c]cycloocten-6-yl)-2-methoxyphenol, a lignin model of phenolic dibenzodioxocin unit,” J. Photochem. Photobiol. A. 169(3), 261-269.
Gellerstedt, G., and Pettersson, E.-L. (1975). “Light-induced oxidation of lignin. The behaviour of structural units containing a ring-conjugated double bond,” Acta Chem. Scand. 29B(10), 1005-1010.
Gellerstedt, G., and Pettersson, E.-L. (1977). “Light-induced oxidation of lignin. Part 2. The oxidative degradation of aromatic rings,” Svensk Papperstidn. 80(1), 15-21.
Gellerstedt, G., Gierer, J., and Pettersson, E.-L. (1977). “The reactions of lignin during neutral sulphite pulping. Part VII. The behaviour of structural elements containing carbonyl groups,” Acta Chem. Scand. 31(9), 735-741.
Gellerstedt, G., and Agnemo, R. (1980a). “The reactions of lignin with alkaline hydrogen peroxide. Part V. The formation of stilbenes,” Acta Chem. Scand. 34B(6), 461-462.
Gellerstedt, G., and Agnemo, R. (1980b). “The reactions of lignin with alkaline hydrogen peroxide. Part III. The oxidation of conjugated carbonyl structures,” Acta Chem. Scand. 34B(4), 275-280.
Gellerstedt, G., Hardell, H.-L., and Lindfors, E.-L. (1980). “The reactions of lignin with alkaline hydrogen peroxide. Part IV. Products from oxidation of quinone model compounds,” Acta Chem. Scand. 34B(9), 669-673.
Gellerstedt, G., Pettersson, I., and Sundin, S. (1983). “Light-induced and heat-induced yellowing of mechanical pulps,” Svensk Papperstidn. 86(15), 157-163.
Gellerstedt, G., Hammar, L.-Å., Olsson, A., Pettersson, I., and Sundin, S. (1985). “Brightness and bleachability of chemimechanical pulps from spruce,” Svensk Papperstidn. 88(6), 68-77.
Gellerstedt, G., and Zhang, L. (1992). “Formation and reactions of leucochromophoric structures in high yield pulping,” J. Wood Chem. Technol. 12(4), 387-412.
Ghosh, T., Cogswell, D., Cunningham, A., and Raue, D. (2002). “Inhibition of brightness reversion in coated groundwood sheets: Effect of coatweight, application method and coating ingredients,” Coat. Graph. Arts Conf. Trade Fair, Orlando, FL, USA, May 5-8, 11.
Gierer, J., and Lin, S. Y. (1972). “Photodegradation of lignin. Contribution to the mechanism of chromophore formation,” Svensk Papperstidn. 75(7), 233-239.
Gratzl, J. S. (1985). “Lichtinduzierte Vergilbung von Zellstoffen – Ursachen und Verhütung,” Papier 39(10A), 14-23.
Gugumus, F. (1993). “Re-evaluation of the stabilization mechanisms of various light stabilizer classes,” Polym. Degrad. Stabil. 39(1), 117-135.
Guo, J. G., and Gray, D. G. (1996). “Inhibition of light-induced yellowing of high-yield pulps by sodium hydroxymethylphosphinate,” J. Pulp Pap. Sci. 22(2), 64-70.
Guo, J. X., Lin, Y. C., and Gray, D. G. (1997). “Sodium -hydroxyalkyl phosphinates as inhibitors of photoyellowing of high-yield pulps,” J. Pulp Pap. Sci. 23(7), 311-317.
Gupta, V. N. (1970). “Effect of metal ions on brightness, bleachability and colour reversion of groundwood,” Pulp Pap. Mag. Can. 71(18), 69-77.
Haque, M. N., and Hill, C. A. S. (2000). “Chemical modification of model compounds, wood flour and fibre with acetic anhydride,” J. I. Wood Sci. 15(3), 109-115.
Harvey, L. C., and Ragauskas, A. J. (1996). “Mechanistic investigations into the brightness stabilization effects of hexadienol,” J. Wood Chem. Technol. 16(1), 79-93.
He, Z., Hui, L., Liu, Z., Ni, Y., and Zhou, Y. (2010). “Impact of high-yield pulp substitution on the brightness stability of uncoated wood-free paper,” Tappi J. 9(3), 15-20.
Heikkurinen, A., Leskelä, L., Heinemann, S., and Vehniäinen, A. (2009). “The character and properties of mechanical pulps,” Mechanical Pulping, B. Lönnberg (ed.), Paper Engineers’ Association/Paperi ja Puu Oy, Helsinki, Finland, 456-514.
Heitner, C. (1993). “Light-induced yellowing of wood-containing papers: An evolution of the mechanism,” Photochemistry of Lignocellulosic Materials, C. Heitner and J. C. Scaiano (eds.), ACS Symposium Series 531, Washington, DC, USA, 2-25.
Heitner, C. (2010). “The photochemistry of lignin,” Lignin and Lignans. Advances in Chemistry, C. Heitner, D. R. Dimmel, and J. A. Schmidt (eds.), CRC Press, Taylor and Francis Group, Boca Raton, FL, USA, 555-584.
Heitner, C., and Min, T. (1987). “The effect of sulphite treatment on the brightness and bleachability of chemithermomechanical pulp,” Cell. Chem. Technol. 21(3), 289-296.
Heitner, C., St. John Manley, R., Ahvazi, B., and Wang, J. (2001). “The effect of acetylation on the photodegradation of milled wood lignin,” J. Pulp Pap. Sci. 27(10), 325-329.
Hemmingson, J. A., and Wong, H. (1989). “Characterization of photochemically degraded newsprint solubles by 13C NMR and IR spectroscopy,” Holzforschung 43(2), 141-147.
Hemmingson, J. A., and Morgan, K. R. (1990). “A CP/MAS carbon-13 NMR study of photodegraded newsprint,” Holzforschung 44(2), 127-131.
Hirashima, H., and Sumimoto, M. (1987). “Behaviors of coniferyl aldehyde type structures in pulp lignin,” Mokuzai Gakkaishi 33(1), 31-41.
Hirashima, H., and Sumimoto, M. (1994). “A basic chromophore and leucochromophore in mechanical pulps: Possible repetition of photochemical reduction-oxidation-reduction,” Tappi J. 77(1), 146-154.
Hirashima, H., and Sumimoto, M. (1996). “Fundamental properties of mechanical pulp lignins IV. Photostabilization of H2O2-bleached mechanical pulp sheets,” Mokuzai Gakkaishi 42(5), 506-514.
Hirt, R. C., Schmitt, R. G., Searle, N. D., and Sullivan A. P. (1960). “Ultraviolet spectral energy distributions of natural sunlight and accelerated test light sources,” J. Opt. Soc. Am. 50(7), 706-713.
Hirt, R. C., and Searle, N. Z. (1967). “Weatherability of plastics,” Weatherability of Plastic Materials, Symposium held at National Bureau of Standards, Gaithersburg, MD, USA, February 8-9. M. R. Kamal (ed.), Interscience Publishers, New York, NY, USA, 61-83.
Holmbom, B. (1988). “Factors influencing the brightness reversion of high-yield pulps,” Symposium on Brightness Reversion, Espoo, Finland, March 22-23, 10.
Hon, D. N.-S. (1992). “Thermomechanical pulp and light – Photoreactivity of -carbonyl group in lignin,” J. Wood Chem. Technol. 12(2), 179-196.
Hon, D. N.-S. (1995). “Stabilization of wood color: Is acetylation blocking effective?,” Wood Fiber Sci. 27(4), 360-367.
Hon, D. N.-S., and Glasser, W. (1979). “On possible chromophoric structures in wood and pulps – A survey of the present state of knowledge,” Polymer-Plast. Technol. Eng. 12(2), 159-179.
da Hora Machado, A. E., De Paula, R., Ruggiero, R., Gardrat, C., and Castellan, A. (2006). “Photophysics of dibenzodioxocins,” J. Photochem. Photobiol. A. 180(1-2), 165-174.
Hortling, B., Rättö, M., Forsskåhl, I., and Viikari, L. (1993). “The interaction of polyvinylpyrrolidone (PVP) and groundwood pulp,” Holzforschung 47(2), 155-162.
Hu, T. Q. (2003a). “Improvement of brightness stability of peroxide-bleached mechanical pulps by the use of tetraalkylammonium salts,” 12th Int. Symp. Wood Pulp. Chem., Madison, WI, USA, June 9-12, vol. 3, 227-230.
Hu, T. Q. (2003b). “Discovery and use of simple fibre-reactive yellowing inhibitors,” J. Pulp Pap. Sci. 29(8), 267-274.
Hu, T. Q. (2004). “A new yellowing inhibitor for peroxide-bleached mechanical pulps,” J. Pulp Pap. Sci. 30(6), 153-158.
Hu, T. Q., James, B. R., and Lee, C.-L. (1997a). “Towards inhibition of yellowing of mechanical pulps. Part I: Catalytic hydrogenation of lignin model compounds under mild conditions,” J. Pulp Pap. Sci. 23(4), 153-156.
Hu, T. Q., James, B. R., and Lee, C.-L. (1997b). “Towards inhibition of yellowing of mechanical pulps. Part II: Water-soluble catalysts for the hydrogenation of lignin model compounds,” J. Pulp Pap. Sci. 23(5), 200-205.
Hu, T. Q., Leary, G., and Wong, D. (1999a). “A new approach towards yellowing inhibition of mechanical pulps. Part I: Selectively removal of -hydroxyl and -carbonyl groups in lignin model compounds,” Holzforschung 53(1), 43-48.
Hu, T. Q., James, B. R., and Wang, Y. (1999b). “Towards inhibition of yellowing of mechanical pulps. Part III: Hydrogenation of milled wood lignin,” J. Pulp Pap. Sci. 25(9), 312-317.
Hu, T. Q., Cairns, G. R., and James, B. R. (2000). “Removal of phenolic hydroxyl groups and its effect on photostability,” Holzforschung 54(2), 127-132.
Hu, T. Q., Williams, T., Pikulik, I. I., and Schmidt, J. A. (2005). “Design, synthesis and studies of yellowing inhibitors with high affinity to mechanical pulps,” J. Pulp Pap. Sci. 31(3), 109-115.
Hu, T. Q., Daneault, C., and Robert, S. (2011). “Use of nitrogen centered peroxide activators to increase the brightening potential of peroxide. II. Optimized conditions,” Cell. Chem. Technol. 45(5-6), 381-388.
Huang, Y., Page, D., Wayner, D. D. M., and Mulder, P. (1995). “Radical-induced degradation of a lignin model compound. Decomposition of 1-phenyl-2-phenoxyethanol,” Can. J. Chem. 73(11), 2079-2085.
Höglund, H., and Wilhelmsson, K. (1993). “The product must determine the choice of wood type in mechanical pulping,” Int. Mech. Pulp Conf., Oslo, Norway, June 15-17, 1-22.
Imsgard, F., Falkehag, S. I., and Kringstad, K. P. (1971). “On possible chromophoric structures in spruce wood,” Tappi 54(10), 1680-1684.
Itho, K., Sumimoto, M., and Tanaka, H. (1993). “Mechanochemistry of syringylglycerol--guaiacyl ether and its similarity to the conventional bleaching of mechanical pulps,” J. Wood Chem. Technol. 13(4), 463-479.
Itoh, K., Adachi, Y., Tsubota, H., Oki, T., and Tachibana, S. (1997). “Studies on biological treatment of mechanical pulps (IX). Effect of phenolic hydroxyl groups in mechanical pulps on depression of color reversion of mechanical pulps,” Jpn. Tappi J. 51(7), 1090-1097.
Jaeger, C., Nourmamode, A., and Castellan, A. (1993). “Photodegradation of lignin: A photochemical study of phenolic coniferyl alcohol lignin model molecules,” Holzforschung 47(5), 375-390.
Jansen, O., and Lorås, V. (1968). “Bleached mechanical pulp – Its paper printability and colour reversion,” Norsk Skogind. 22(10), 342-353.
Janson, J. (1993). “Kann die Vergilbung von Papier vermieden werden?,” Papier 47(10A), 47-52.
Janson, J., and Forsskåhl, I. (1989). “Color changes in lignin-rich pulps on irradiation by light,” Nord. Pulp Pap. Res. J. 4(3), 197-205.
Janson, J., and Forsskåhl, I. (1991). “Influence of sulphonation and bleaching on colour and colour reversion of mechanical pulps,” 6th Int. Symp. Wood Pulp. Chem., Melbourne, Australia, April 29-May 3, vol. 3, 627-630.
Janson, J., and Forsskåhl, I. (1996). “Polytetrahydrofuran – A polymer that counteracts colour formation,” Nord. Pulp Pap. Res. J. 11(1), 10-14.
Johansson, M., Zhang, L., and Gellerstedt, G. (2002). “On chromophores and leucochromophores formed during the refining of wood,” Nord. Pulp Pap. Res. J. 17(1), 5-8.
Johnson, R. W. (1989). “Brightness stability of mechanical pulps. Relating laboratory data to performance,” Tappi J. 72(12), 181-187.
Johnson, R. W. (1991). “CTMP in fine papers: On-machine surface treatments for improved brightness stability,” Tappi J. 74(5), 209-217.
Jääskeläinen, A.-S., Saariaho, A.-M., Vyörykkä, J., Vuorinen, T., Matousek, P., and Parker, A. W. (2006). “Application of UV-VIS and resonance Raman spectroscopy to study bleaching and photoyellowing of thermomechanical pulps,” Holzforschung 60(3), 231-238.
Kang, G., and Ni, Y. (2006). “Photostabilization of bleached mechanical pulps with zinc oxide,” 3rd Int. Symp. Emerg. Technol. Pulp. Papermak., Guangzhou, China, October 8-10, 321-325.
Karhunen, P., Rummako, P., Sipilä, J., and Brunow, G. (1995). “Dibenzodioxocins: A novel type of linkage in softwood lignins,” Tetrahedron Lett. 36(1), 169-170.
Kimura, F., Kimura, T., and Gray, D. G. (1994). “FT-IR study of the effect of irradiation wavelength on the colour reversion of thermomechanical pulp,” Holzforschung 48(4), 343-348.
Klemm, P. (1903). Paper Makers´ Monthly J. 41, 4, quoted by: Walton, R. P. (1929). “Causes and prevention of deterioration in book materials,” Paper Trade J. 89(6), 53-58.
Koch, H., Hübner, K., and Fischer, K. (1994). “The influence of light on the molecular mass of lignin,” J. Wood Chem. Technol. 14(3), 339-349.
Kónya, K., and Scaiano, J. C. (1996). “High-intensity laser generation of ortho-quinones from 2-methoxyphenols; Relevance to the question of color reversion in pulp and paper,” J. Photochem. Photobiol. A. 100(1-3), 119-124.
Kringstad, K. (1969). “Degradation of wood and high-yield pulps by light. A survey of the present state of knowledge,” Tappi 52(6), 1070-1074.
Kringstad, K. P. (1973). “Einige mögliche Reaktionen in der lichtinduzierten Degradierung von ligninreichen Hochausbeutezellstoffen,” Papier 27(10A), 462-469.
Kringstad, K. P., and Lin, S. Y. (1970). “Mechanism in the yellowing of high-yield pulps by light. Structure and reactivity of free radical intermediates in the photodegradation of lignin,” Tappi 53(12), 2296-2301.
Kuys, K., and Abbot, J. (1996). “Bleaching of mechanical pulps with sodium bisulfite,” Appita 49(4), 269-273.
Lanzalunga, O., and Bietti, M. (2000). “Photo- and radiation chemical induced degrada-tion of lignin model compounds,” J. Photochem. Photobiol. B. 56(2-3), 85-108.
Launer, H. F. (1948). “Light-sensitive papers as controls for textile colorfastness and sta-bility of materials under arc lamp exposure,” J. Res. Nat. Bur. Stand. 41(3), 169-177.
Leary, G. J. (1967). “The yellowing of wood by light,” Tappi 50(1), 17-19.
Leary, G. J. (1968a). “The yellowing of wood by light: Part II,” Tappi 51(6), 257-260.
Leary, G. J. (1968b). “Photochemical production of quinoid structures in wood,” Nature 217(5129), 672-673.
Leary, G. J. (1994). “Recent progress in understanding and inhibiting the light-induced yellowing of mechanical pulps,” J. Pulp Pap. Sci. 20(6), 154-160.
Lebo, S. E., Lonsky, W. F. W., McDonough, T. J., Medvecz, P. J., and Dimmel, D. R. (1990). “The occurrence and light-induced formation of ortho-quinonoid lignin structures in white spruce refiner mechanical pulp,” J. Pulp. Pap. Sci. 16(5), 139-143.
Leduc, C., Garceau, M., Daneault, C., and Robert, S. (2002). “Bleaching of a mechanical pulp with sodium percarborate and amineborane – Bleaching response and brightness stability,” J. Pulp Pap. Sci. 28(5), 171-175.
Lee, D. Y., Matsuoka, M., and Sumimoto, M. (1990). “Mechanochemistry of lignin. IV. Mechanochemical reactions of phenylcoumaran models,” Holzforschung 44(6), 415-418.
Lee, D. Y., and Sumimoto, M. (1990). “Mechanochemistry of lignin. III. Mechanochem-ical reactions of -O-4 lignin model compounds,” Holzforschung 44(5), 347-350.
Lee, D. Y., and Sumimoto, M. (1991). “Mechanochemistry of lignin. V. An intensive chromophore in mechanical pulps bleached with alkaline hydrogen peroxide,” Holzforschung45(Suppl.), 15-20.
Lennholm, H., Rosenqvist, M., Ek, M., and Iversen, T. (1994). “Photoyellowing of groundwood pulps,” Nord. Pulp Pap. Res. J. 9(1), 10-15.
Lewis, H. F., and Fronmuller, D. (1945). “The “fading” of groundwood by light. III. The changes taking place in the nature of the compounds present in groundwood as the result of fading,” Paper Trade J. 121(14), 25-28.
Lewis, H. F., Reineck, E. A., and Fronmuller, D. (1945). “The “fading” of groundwood by light. I. A study on the relation between the variables in sheet formation and fading,” Paper Trade J. 121(8), 44-48.
Li, C., and Ragauskas, A. J. (1999). “Brightness reversion of mechanical pulps. Part XIII: Photoinduced degradation of lignin on cellulose matrix,” J. Wood Chem. Technol. 19(1-2), 43-60.
Li, C., Cook, C. M., and Ragauskas, A. J. (1999). “Brightness reversion of mechanical pulps XI: Photostabilization of high-yield pulps by thiosulfinates,” J. Wood Chem. Technol. 19(1-2), 27-41.
Li, C., and Ragauskas, A. J. (2000). “Brightness reversion of mechanical pulps. Part XVII: Diffuse reflectance study on brightness stabilization by additives under various atmospheres,” Cellulose 7(4), 369-385.
Li, C., and Ragauskas, A. J. (2001). “Brightness reversion of mechanical pulps. Part XVI: The effect of oxygen on photostabilization of high-yield mechanical pulps treated with UV absorbers and a fluorescent whitening agent,” J. Pulp Pap. Sci. 27(6), 202-206.
Li, C., Kim, D. H., and Ragauskas, A. J. (2004). “Brightness reversion of mechanical pulps. XIX. Photostabilization of mechanical pulps by UV absorbers: Surface photochemical studies using diffuse reflectance technique,” J. Wood Chem. Technol. 24(1), 39-53.
Li, X., Zang, Y., Wu, Z., and Xu, Y. (2011). “Application of TiO2 to improve the brightness stability of HYP containing coated paper,” Adv. Mat. Res. 236-238, 1367-1371.
Lin, S. Y., and Kringstad, K. P. (1970). “Stabilization of lignin and lignin model compounds to photodegradation,” Tappi 53(9), 1675-1677.
Lin, Y., and Kringstad, K. (1971). “Some reactions in the photoinduced discoloration of lignin,” Norsk Skogind. 25(9), 252-256.
Lindblad, G., Iversen, T., Bergenblad, H., and Rigdahl, M. (1989) “Light scattering ability of coatings as a tool to protect paper and board against photo-oxidation,” Nord. Pulp Pap. Res. J. 4(4), 253-257.
Liu, Z., and Argyropoulos, D. S. (1998). “Maintaining the brightness of mechanical pulps with solid-state perborate bleaching,” Holzforschung 52(3), 319-324.
Lorås, V. (1968). “Bleaching and stabilizing of flash-dried mechanical pulp,” Pulp Pap. Mag. Can. 69(1), 57-63.
Lorås, V., and Wilhelmsen, G. (1973). “Lyshet i granved,” Norsk Skogind. 27(12), 346-349.
Lundquist, K. (1992). “1H NMR spectral studies of lignins. Results regarding the occurrence of -5 structures, - structures, non-cyclic benzyl aryl ethers, carbonyl groups and phenolic groups,” Nord. Pulp Pap. Res J. 7(1), 4-8.
Lundquist, K., Parkås, J., Paulsson, M., and Heitner, C. (2007). “Reactions of lignin chromophores of the enal and enone types with sulfite,” BioResources 2(3), 334-350.
Luo, C., and Göttsching, L. (1991). “Technologische massnahmen zur Verringerung der lichtinduzierten Vergilbung holzhaltiger Papiere. Teil 2: Schutzwirkung von Pigmentstrichen,” Wochenbl. Papierfabr. 119(17), 635-644.
Manchester, D. F., McKinney, J. W., and Pataky, A. A. (1960). “The brightening of groundwood,” Svensk Papperstidn. 63(20), 699-706.
Marton, J., Adler, E., and Persson, K.-I. (1961). “Carbonyl groups in lignin. IV. Infrared absorption studies and examination of the volumetric borohydride method,” Acta Chem. Scand. 15(2), 384-392.
Matsuura, T., Yoshimura, N., Nishinaga, A., and Saito, I. (1972). “Photoinduced reactions-LVI: Participation of singlet oxygen in the hydrogen abstraction from a phenol in the photosensitized oxygenation,” Tetrahedron 28(19), 4933-4938.
McGarry, P., Heitner, C., Schmidt, J., Seltzer, R., Cunkle, G., and Wolf, J.-P. (2000). “Hindered nitroxide: A new yellowing inhibitor for mechanical pulps,” J. Pulp Pap. Sci. 26(2), 59-66.
McGarry, P. F., Schmidt, J. A., and Heitner, C. (2004). “Accelerated light exposure of wood-containing pulps. Part I: Effects of wavelength and intensity,” Tappi J. 3(10), 18-24.
McLellan, F., Colodette, J. L., Fairbank, M. G., and Whiting, P. (1990). “Factors affect-ting ambient thermal reversion of high-yield pulps,” J. Pulp Pap. Sci. 16(6), 173-179.
Minemura, N. (1978). “Control of the photo-induced discoloration of mechanical pulp with polyethylene glycol,” Mokuzai Gakkaishi 24(8), 587-588.
Mislankar, A., Darabie, A., and Reeve, D. W. (1997). “Detection of ortho-quinones in wood using confocal laser scanning microscopy,” J. Pulp Pap. Sci. 23(2), 73-76.
Moore, D. B., and Argyropoulos, D. S. (2000). “A pilot plant trial using in situ peroxyborate for the brightness retention of TMP,” Tappi J. 83(11), 14.
Netto-Ferreira, J. C., Avellar, I. G. J., and Scaiano, J. C. (1990). “Effect of ring substitution on the photochemistry of -(aryloxy)acetophenones,” J. Org. Chem. 55(1), 89-92.
Neumann, M. G., and Machado, E. H. (1989). “The role of oxygen in the photodegradation of lignin in solution,” J. Photochem. Photobiol. B. 3(4), 473-481.
Ni, Y., Ghosh, A., Li, Z., Heitner, C., and McGarry, P. (1998) “Photostabilization of bleached mechanical pulps with DTPA treatment,” J. Pulp Pap. Sci. 24(8), 259-263.
Ni, Y., Ng, A., and Mosher, M. (1999). “A model compound study: The formation of colored metallic extractive complexes and their effect on brightness of TMP pulp,” J. Wood Chem. Technol. 19(3), 213-223.
Nolan, P., van den Akker, J. A., and Wink, W. A. (1945). “The fading of groundwood by light. II. The physical mechanism of fading,” Paper Trade J. 121(11), 33-37.
Norrström, H. (1969). “Light absorbing properties of pulp and pulp components. Part 2. Sulfite pulp,” Svensk Papperstidn. 72(2), 32-38.
Noutary, C., Castellan, A., and Davidson, R. S. (1994). “The use of alkyl cinnamates as UV screens for preventing the photo-yellowing of lignin-rich pulps,” J. Photochem. Photobiol. A. 84(3), 317-326.
Noutary, C., Fornier de Violet, P., Vercauteren, J., and Castellan, A. (1995a). “Photochemical studies on a phenolic phenylcoumarone lignin model molecule in relation to the photodegradation of lignocellulosic materials. Part 1. Structure of the photoproducts,” Res. Chem. Intermed. 21(3-5), 223-245.
Noutary, C., Fornier de Violet, P., Vercauteren, J., and Castellan, A. (1995b). “Photochemical studies on a phenolic phenylcoumarone lignin model molecule in relation to the photodegradation of lingocellulosic materials. Part 2. Photophysical and photochemical studies,” Res. Chem. Intermed. 21(3-5), 247-262.
Ohkoshi, M. (2002). “FTIR-PAS study of light-induced changes in the surface of acetylated or polyethylene glycol-impregnated wood,” J. Wood Sci. 48(5), 394-401.
Omori, S., Francis, R. C., and Dence, C. W. (1991). “Studies on the mechanism of the photoyellowing of bleached mechanical and chemimechanical pulps. II. The role of photosensitizers,” Holzforschung 45(Suppl.), 93-100.
Omori, S., Takagi, H., Francis, R. C., and Dence, C. W. (1992). “Studies on the mechanism of the photoyellowing of bleached mechanical and chemimechanical pulps. IV. The role of ring-conjugated ethylenic groups,” Holzforschung 46(1), 47-52.
Pan, G. X. (1996). “A comparison of bleaching sequence configuration on aspen CTMP with respect to brightness gain and stability,” Tappi Pulp. Conf., Orlando, FL, USA, October 21-November 4, vol. 1, 213-219.
Pan, X., Lachenal, D., Lapierre, C., and Monties, B. (1992). “Structure and reactivity of spruce mechanical pulp lignins. Part I. Bleaching and photoyellowing of in situ lignins,” J. Wood Chem. Technol. 12(2), 135-147.
Pan, X., Lachenal, D., Lapierre, C., and Monties, B. (1993). “Structure and reactivity of spruce mechanical pulp lignins. Part III. Bleaching and photoyellowing of isolated lignin fractions,” J. Wood Chem. Technol. 13(2), 145-165.
Pan, X., Lachenal, D., Neirinck, V., and Robert, D. (1994). “Structure and reactivity of spruce mechanical pulp lignins. IV. 13C-NMR spectral studies of isolated lignins,” J. Wood Chem. Technol. 14(4), 483-506.
Pan, X., and Ragauskas, A. J. (1995a). “Brightness reversion of mechanical pulps. Part 3: Mechanistic studies of mercapto-stabilizers for high-brightness mechanical pulps,” J. Wood Chem. Technol. 15(1), 135-152.
Pan, X., and Ragauskas, A. J. (1995b). “Brightness reversion of mechanical pulps. Part IV: A study on the action of thiols and disulphides on hardwood BCTMP,” J. Pulp Pap. Sci. 21(1), 25-29.
Pan, X., Harvey, L. C., and Ragauskas, A. J. (1996). “Brightness reversion of mechanical pulps. Part VI: Cooperative photostabilization approaches for high-yield pulps,” J. Pulp Pap. Sci.22(4), 135-140.
Parkås, J., Paulsson, M., Li, S., Lundquist, K., and Westermark, U. (2004a). “Photoyellowing behavior of non-phenolic lignin models representative of end groups and β-ether structures,” Nord. Pulp Pap. Res. J. 19(2), 146-154.
Parkås, J., Paulsson, M., Terashima, N., Westermark, U., and Ralph, S. (2004b). “Light-induced yellowing of selectively 13C-enriched dehydrogenation polymers (DHPs). Part 1. Side-chain 13C-enriched DHP (-, -, and -13C),” Nord. Pulp Pap. Res. J. 19(1), 29-36.
Parkås, J., Paulsson, M., Terashima, N., Westermark, U., and Ralph, S. (2004c). “Light-induced yellowing of selectively 13C-enriched dehydrogenation polymers (DHPs). Part 2. NMR assignments and photoyellowing of aromatic ring 1-, 3-, 4- and 5-13C DHPs,” Nord. Pulp Pap. Res. J. 19(1), 44-52.
Paulsson, M., Simonson, R., and Westermark, U. (1995). “Chemical modification of lignin-rich paper. Part 2. Photostabilization by acetylation of paper made from spruce TMP and aspen CTMP,” Nord. Pulp Pap. Res. J. 10(1), 62-67.
Paulsson, M., Li, S., Lundquist, K., Simonson, R., and Westermark, U. (1996a). “Chemical modification of lignin-rich paper. Part 3. Acetylation of lignin model compounds representative of -O-4 structures of the -guiacyl ether type,” Nord. Pulp Pap. Res. J. 11(2), 109-114.
Paulsson, M., Li, S., Lundquist, K., Simonson, R., and Westermark, U. (1996b). “Chemical modification of lignin-rich paper. Part 4. Elimination of chromophoric and leucochromophoric structures by acetylation,” Nord. Pulp Pap. Res. J. 11(4), 220-226.
Paulsson, M., and Ragauskas, A. J. (1998a). “Chemical modification of lignin-rich paper. Part 9. Effect of dry heat and moist heat on accelerated yellowing of untreated and acetylated pulps,” Nord. Pulp Pap. Res. J. 13(2), 191-197.
Paulsson, M., and Ragauskas, A. J. (1998b). “Chemical modification of lignin-rich paper. Part 8. Effect of light source on the accelerated light-induced yellowing of untreated and acetylated high-yield pulps,” Nord. Pulp Pap. Res. J. 13(2), 132-142.
Paulsson, M., and Parkås, J. (2000). “Chemical modification of chemithermomechanical pulps Part 1: Mechanical, optical, and ageing properties of propionylated spruce CTMP,” J. Wood Chem. Technol. 20(2), 205-224.
Paulsson, M., and Ragauskas, A. J. (2000). “Chemical modification of lignin-rich paper. Light-induced changes of softwood and hardwood chemithermomechanical pulps. The effect of irradiation source,” In: Lignin: Historical, Biological, and Material Perspectives, W. G. Glasser, R. A. Northey, and T. P. Schultz (eds.), Oxford University Press, Washington, DC, USA, 490-504.
Paulsson, M., Lucia, L. A., Ragauskas, A. J., and Li, C. (2001). “Photoyellowing of untreated and acetylated aspen chemithermomechanical pulp under argon, ambient, and oxygen atmospheres,” J. Wood Chem. Technol. 21(4), 343-360.
Paulsson, M., and Simonson, R. (2002). “Acetylation of lignin and photostabilization of lignin-rich mechanical wood pulp and paper,” Chemical Modification, Properties, and Usage of Lignin, T.Q. Hu (ed.), Kluwer Academic/Plenum Publishers, New York, NY, USA, 221-245.
Paulsson, M., Parkås, P., and Ragauskas, A. J. (2002). “Long-time natural ageing of untreated and additive treated aspen CTMP,” 7th European Workshop on Lignocellulosic and Pulp, Åbo, Finland, August 26-29, 325-328.
Peart, C., and Ni, Y. (2001). “UV-VIS spectra of lignin model compounds in the presence of metal ions and chelants,” J. Wood Chem. Technol. 21(2), 113-125.
Peill, P. L. D. (1946). “Permanent bleaching of ligno-cellulosic materials,” Nature 128(4016), 554.
Perng, Y.-S., and Wang, E. I.-C. (2010). “Effect of BCTMP content in furnish on the performance of fluorescent optical brightening agents,” 64th Appita Ann. Conf. Exhib., Melbourne, Australia, April 18-21, 17-24.
Petit-Conil, M., and de Choudens, C. (1994). “Influence of pulping and bleaching on the photoyellowing of refiner mechanical and chemimechanical pulps,” Cell. Chem. Technol. 28(2), 195-204.
Petit-Conil, M., de Choudens, C., Castellan, A., Grelier, S., and Davidson, R. S. (1998). “Prevention of photoyellowing of high-yield pulps using ternary mixtures containing a UV screen, a polyethylene oxide dithiol and sucrose,” J. Pulp Pap. Sci. 24(6), 167-172.
Pew, J. C., and Connors, W. J. (1971). “Color of coniferous lignin,” Tappi 54(2), 245-251.
Plackett, D. V., Dunningham, E. A., and Singh, A. P. (1992). “Weathering of chemically modified wood. Accelerated weathering of acetylated radiata pine,” Holz Roh- Werkst. 50(4), 135-140.
Polcin, J., and Rapson, H. (1972). “Sapwood and heartwood groundwood of western hemlock and jack pine. Part III. Influence of solvent extraction on the bleaching of pulps,” Pulp Pap. Mag. Can. 73(1), 86-92.
Pu, Y., Anderson, S., Lucia, L., and Ragauskas, A. J. (2003). “Fundamentals of photobleaching lignin. Part I: Photobehaviours of acetylated softwood BCTMP lignin,” J. Pulp Pap. Sci. 29(12), 401-406.
Pu, Y., and Ragauskas, A. J. (2005). “Structural analysis of acetylated hardwood lignins and their photoyellowing properties,” Can. J. Chem. 83(12), 2132-2139.
Ragauskas, A. J. (1993a). “Photoreactivity of ligninlike quinoid structures,” Photochemistry of Lignocellulosic Materials, C. Heitner and J.C. Scaiano (eds.), ACS Symposium Series 531, Washington, DC, USA, 77-85.
Ragauskas, A. J. (1993b). “Photoyellowing of mechanical pulp. Part 1: Inhibition of brightness reversion by unsaturated compounds,” Tappi J. 76(12), 153-157.
Ragauskas, A. J. (1994). “Brightness reversion of mechanical pulps. II Thermal ageing of ascorbic acid impregnated lignin-retaining pulps,” Cell. Chem. Technol. 28(3), 265-272.
Ragauskas, A. J., Allison, L., Lucia, L. A., and Li, C. (2001). “Brightness reversion of mechanical pulps XIV: Application of FWAs for high-brightness, high-yield pulps,” Solutions 84(11), 11.
Rapson, W. H., and Spinner, I. H. (1979). “Brightness reversion in bleached pulps,” The Bleaching of Pulp, R. P. Sing (ed.), TAPPI Press, Atlanta, GA, USA, 357-391.
Reineck, E. A., and Lewis, H. F. (1945). “The fading of groundwood by light. IV. A search for an inhibitor to prevent the fading of groundwood,” Paper Trade J. 121(20), 27-30.
Reinhardt, B., and Arneberg, U. (1988). “Die Vergilbung gestrichener Papiere unter Licht und Temperatur,” Wochenbl. Papierfabr. 116(5), 179-184, 186-177.
Rowell, R. M. (1982). “Distribution of acetyl groups in southern pine reacted with acetic anhydride,” Wood Sci. 15(2), 172-182.
Ruffin, B., Castellan, A., Grelier, S., Nourmamode, A., Riela, S., and Trichet, V. (1998). “Evaluation of the contribution of lignin stilbene phenol units in the photoyellowing of peroxide-bleached lignin-rich pulps,” J. Appl. Pol. Sci. 69(13), 2517-2531.
Ruffin, B., and Castellan, A. (2000). “Photoyellowing of peroxide-bleached lignin-rich pulps: A photochemical study on stilbene-hydroquinone chromophores issued from -5 units of lignin during refining and (or) bleaching,” Can. J. Chem. 78(1), 73-83.
Rättö, M., Forsskåhl, I., Janson, J., and Viikari, L. (1993). “Photostabilization of mechanical pulps by polyvinylpyrrolidone,” Tappi J. 76(6), 67-70.
Savoye, L., Petit-Conil, M., and Meyer, V. (2011). “Mechanisms of TMP peroxide bleaching using Mg-based alkalis,” Holzforschung 65(6), 797-803.
Scaiano, J. C., Netto-Ferreira, J. C., and Wintgens, V. (1991). “Fragmentation of ketyl radicals derived from -phenoxyacetophenone: An important mode of decay for lignin-related radicals?,” J. Photochem. Photobiol. A. 59(2), 265-268.
Scaiano, J. C., Wittlesey, M. K., Berinstain, A. B., Malenfant, P. R. L., and Schuler, R. H. (1994). “Pulse radiolysis and laser flash photolysis studies of the lignin model compound -(p-methoxyphenoxy)-p-methoxyacetophenone and related compounds,” Chem. Mater. 6(6), 836-843.
Schmidt, J. A., and Heitner, C. (1991). “Light-induced yellowing of mechanical and ultra-high yield pulps. Part 1. Effect of methylation, NaBH4 reduction and ascorbic acid on chromophore formation,” J. Wood Chem. Technol. 11(4), 397-418.
Schmidt, J. A., Berinstain, A. B., de Rege, F., Heitner, C., Johnston, L. J., and Scaiano,
J. C. (1991). “Photodegradation of the model -guaiacoxyacetoveratrone, unusual effects of solvent, oxygen and singlet state participation,” Can. J. Chem. 69(1), 104-107.
Schmidt, J. A., and Heitner, C. (1993a). “Use of UV-visible diffuse reflectance spectroscopy for chromophore research on wood fibers: A review,” Tappi J. 76(2), 117-123.
Schmidt, J. A., and Heitner, C. (1993b). “Light-induced yellowing of mechanical and ultra-high yield pulps. Part 2. Radical-induced cleavage of etherified guaiacylglycerol--arylether groups is the main degradative pathway,” J. Wood Chem. Technol. 13(3), 309-325.
Schmidt, J. A., and Heitner, C. (1997). “Thermal yellowing of lignin-containing pulps: Acceleration by ascorbic acid,” J. Pulp Pap. Sci. 23(11), 532-538.
Schoeller, V. (1912). “Ueber Vergilben von Papier,” Wochenblatt für Papierfabrikation 43, 3222-3225, 3408-3409, 3489-3491, 3673-3675, 3963-3965, 4148-4150, 4336-4338, quoted by: Walton, R. P. (1929). “Causes and prevention of deterioration in book materials,” Paper Trade J. 89(6), 53-58.
Sinclair, R. M., and Vincent, T. A. (1964). “Yellowing of radiate pine timber,” New Zealand J. Sci. 7(2), 196-206.
Sindall, R. W. (1920). Paper Technology: An Elementary Manual on the Manufacture, Physical Qualities and Chemical Constituents of Paper and of Paper-Making Fibres, Charles Griffin and Co, London, England.
Singh, R. P. (1966). “Bleaching of groundwood pulp: Investigation of methods based on chemical modification of the woodpulp,” Tappi 49(7), 281-286.
Sjöholm, R., Holmbom, B., and Åkerback, N. (1992). “Studies of the photodegradation of spruce lignin by NMR spectroscopy,” J. Wood Chem. Technol. 12(1), 35-52.
Spinner, I. H. (1962). “Brightness reversion. A critical review with suggestions for further research,” Tappi 45(6), 495-514.
Suckling, I. D. (1991). “The effect of coniferaldehyde sulfonation on the brightness and absorption spectra of chemithermomechanical pulps,” 6th Int. Symp. Wood Pulp. Chem., Melbourne, Australia, April 29-May 3, vol. 1, 587-593.
Svensson-Rundlöf, E., Zhang, E., Zhang, L., and Gellerstedt, G. (2006). “The behavior of chromophoric structures in softwood mechanical pulp on bleaching with alkaline hydrogen peroxide,” Nord. Pulp Pap. Res. J. 21(3), 359-364.
Trichet, V., Grelier, S., Castellan, A., Choudhury, H., and Davidson, R. S. (1996). “Attempt to photostabilize paper made from high-yield pulp by application of UV screens in conjunction with thiols,” J. Photochem. Photobiol. A. 95(2), 181-188.
Tschirner, U., and Dence, C. W. (1988). “Attempts to photostabilize Norway spruce TMP by chemical modification,” Pap. Puu 70(4), 338-346.
Tylli, H., Forsskåhl, I., and Olkkonen, C. (1992). “A fluorescence spectroscopic study of chemically and photochemically treated high-yield pulps,” J. Photochem. Photobiol. A. 67(1), 117-131.
Van den Akker, J. A., Lewis, H. F., Jones, G. W., and Buchanan, M. A. (1949). “The nature of the color changes in groundwood,” Tappi 32(4), 187-192.
Vanucci, C., Fornier de Violet, P., Bouas-Laurent, H., and Castellan, A. (1988). “Photodegradation of lignin: A photophysical and photochemical study of a non-phenolic -carbonyl -O-4 lignin model dimer, 3,4-dimethoxy--(2’-methoxy-phenoxy)acetophenone,” J. Photochem. Photobiol. A. 41(2), 251-265.
Wallis, A. F. A., and Wearne, R. H. (1982). “Hydroxyethylation of lignin in mechanical pulps,” Appita 36(3), 192-196.
Walton, R. P. (1929). “Causes and prevention of deterioration in book materials,” Paper Trade J. 89(6), 53-58.
Wan, J. K. S., Tse, M. Y., and Heitner, C. (1993). “An ESR and time-resolved CIDEP study of the light-induced yellowing process of TMP paper,” J. Wood Chem. Technol. 13(3), 327-348.
Wan, J. K. S., and Depew, M. C. (1996). “Some mechanistic insights in the behaviour of thiol containing antioxidant polymers in lignin oxidation processes,” Res. Chem. Intermed. 22(3), 241-253.
Wang, J., Heitner, C., and St. John Manley, R. (1996). “The photodegradation of milled-wood lignin. Part II. The effect of inhibitors,” J. Pulp Pap. Sci. 22(2), 58-63.
Wang, J., Heitner, C., and St. John Manley, R. (1998). “The photodegradation of milled wood lignin. Part III: The effect of time and media,” J. Pulp Pap. Sci. 24(11), 337-340.
Weir, N. A., Arct, J., and Ceccarelli, A. (1995a). “Chromophore production in lignin model compounds – Anaerobic reactions,” Res. Chem. Intermed. 21(3-5), 353-371.
Weir, N. A., Arct, J., and Ceccarelli, A. (1995b). “Photodegradation of lignin model compounds: Part 2 – Substituted stilbenes,” Polym. Degrad. Stabil. 47(2), 289-297.
Weir, N. A., and Miller, B. (2000). “Retardation of photo-yellowing of lignin-rich pulp,” Polym. Degrad. Stabil. 69(1), 121-126.
Wu, Z.-H., Matsuoka, M., Lee, D.-Y., and Sumimoto, M. (1991). “Mechanochemistry of lignin. VI. Mechanochemical reactions of -1 lignin model compounds,” Mokuzai Gakkaishi37(2), 164-171.
Wu, Z.-H., Sumimoto, M., and Tanaka, H. (1994). “Mechanochemistry of lignin. XVIII. A novel approach to improve brightness stability of H2O2-bleached high-yield pulps by addition of radical scavengers to the pulping process,” Holzforschung 48(5), 400-404.
Yuan, Z., Schmidt, J., and Heitner, C. (2002). “Compatibility of yellowing inhibitors with several surface-sizing agents,” J. Pulp Pap. Sci. 28(10), 354-358.
Yuan, Z., Schmidt, J., Heitner, C., and Fairbank, M. (2003). “Application of yellowing inhibitors to improve the brightness stability of coated mechanical papers,” Tappi J. 2(5), 9-15.
Yuan, Z., Gilbert, D., Schmidt, J., and Heitner, C. (2004a). “Improving the brightness stability of a machine-finished coated mechanical paper. Part I. Laboratory evaluation,” J. Pulp Pap. Sci. 30(7), 196-202.
Yuan, Z., Schmidt, J., Gilbert, D., Aspler, J., Heitner, C., Cunkle, G., and Ghosh, T. (2004b). “Improving the brightness stability of a machine-finished coated mechanical paper. Part II. A mill trial,” Pulp Pap. Can. 105(10), 235-241.
Yuan, Z., Schmidt, J., Heitner, C., and Zou, X. (2006). “Coating improves the brightness stability of wood-free coated papers containing high-yield pulp,” Tappi J. 5(1), 9-13.
Zhang, L., and Gellerstedt, G. (1994a). “Quinoid lignin chromophores and their contribution to photoyellowing,” 3rd European Workshop on Lignocellulosics and Pulp, Stockholm, Sweden, August 28-31, 293-295.
Zhang, L., and Gellerstedt, G. (1994b). “Reactive structures in wood and high-yield pulps. IV. Daylight-induced oxidation of stilbene structures in the solid state,” Acta Chem. Scand. 48(6), 490-497.
Zhang, L., and Gellerstedt, G. (1999). “Detection and determination of carbonyls and quinones by modern NMR techniques,” 10th Int. Symp. Wood Pulp. Chem., June 7-10, Yokohama, Japan, vol. 2, 164-170.
Zhang, R., Ni, Y., Wong, D., Schmidt, J., Heitner, C., and Jordan, B. (2009a). “Interactions of optical brightening agents with high-yield pulps,” J. Wood Chem. Technol. 29(4), 358-370.
Zhang, H., Hu, H., and Xu, Z. (2009b). “Use of fluorescent whitening agents against light-induced colour reversion of aspen BCTMP,” Appita J. 62(5), 355-359.
Zhu, J. H., Olmstead, J. A., and Gray, D. G. (1995). “Fluorescent detection of o-quinones formed in lignin-containing pulps during irradiation,” J. Wood Chem. Technol. 15(1), 43-64.
Zhu, J. H., Archer, C., MacNab, F., Andrews, M. P., Kubes, G., and Gray, D. G. (1997). “The influence of mechanochemical action on the photosensitivity of refiner pulps,” J. Pulp Pap. Sci. 23(7), 305-310.
Zoia, L., and Argyropoulos, D. S. (2009). “Phenoxy radical detection using 31P NMR spin trapping,” J. Phys. Org. Chem. 22(11), 1070-1077.
Zoia, L., and Argyropoulos, D. S. (2010). “Characterization of free radical spin adducts of 5-diisopropyloxy-phosphoryl-5-methyl-1-pyrrolidine-N-oxide using mass spectrometry and 31P nuclear magnetic resonance,” Eur. J. Mass Spectrom. 16(2), 175-185.
Article submitted: May 9, 2012; Peer review completed: August 16, 2012; Revised version received: August 21, 2012; Accepted: August 22, 2012: Published: August 27, 2012.
