Abstract
This work highlights the synthesis of high purity silica from rice husks and the effect of rice husk silica (RHS) loading on the mechanical, physical, and thermal properties of natural rubber (NR) compounds. The RHS was synthesised using the solvent-thermal extraction method, which was adopted from TAPPI T204 (2007) and TAPPI T264 (1997) standards with some modifications. The treatment successfully produced high purity RHS particles, with 99.9% SiO2 content between 100 to 300 nm in size. The high purity RHS was then incorporated in NR compounds at 2, 4, 6, 8, and 10 parts per hundred rubber (phr). Even without any surface modification, the high purity RHS-filled NR compounds showed tremendous improvements in strength-related properties at the optimum loading of 4 phr. In addition, the thermal stability of the NR compounds was remarkably improved with the addition of RHS.
Download PDF
Full Article
The Effect of High Purity Rice Husk Silica Synthesised using Solvent-thermal Extraction Method on the Properties of Natural Rubber Compounds
Zainathul Akhmar Salim Abdul Salim,a,b Aziz Hassan,a and Hanafi Ismail c,*
This work highlights the synthesis of high purity silica from rice husks and the effect of rice husk silica (RHS) loading on the mechanical, physical, and thermal properties of natural rubber (NR) compounds. The RHS was synthesised using the solvent-thermal extraction method, which was adopted from TAPPI T204 (2007) and TAPPI T264 (1997) standards with some modifications. The treatment successfully produced high purity RHS particles, with 99.9% SiO2 content between 100 to 300 nm in size. The high purity RHS was then incorporated in NR compounds at 2, 4, 6, 8, and 10 parts per hundred rubber (phr). Even without any surface modification, the high purity RHS-filled NR compounds showed tremendous improvements in strength-related properties at the optimum loading of 4 phr. In addition, the thermal stability of the NR compounds was remarkably improved with the addition of RHS.
Keywords: Solvent-thermal extraction method; Rice husk silica; Natural rubber; Mechanical properties; Thermal properties
Contact information: a: Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia; b: Faculty of Applied Science, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia; c: School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia Engineering Campus, 14300 Nibong Tebal, Penang, Malaysia; *Corresponding author: ihanafi@usm.my
INTRODUCTION
Natural rubber holds a unique place in rubber technology due to its outstanding tack and strength in its unvulcanised state. Meanwhile, its vulcanisates exhibit high tensile strength, abrasion resistance, and crack growth resistance (Hernandez et al. 2012). Vulcanised rubber that contains the necessary ingredients for the vulcanisation process is known as unfilled or gum vulcanisate (Abdul Salim et al. 2017). Gum-mixed rubber compounds generally show poor processing characteristics, such as producing high shrinkage during calendering and high swelling during the extrusion process. Thus, the addition of fillers is necessary to overcome these shortcomings. Fillers may not only modify the processing performance of rubber and cheapen the rubber compound, but they also tend to increase certain mechanical and physical properties of the vulcanised rubber. The most common fillers used in the rubber industry are carbon black, calcium carbonate, clay, and silica (Abdul Salim et al. 2017). A variety of silica sources have been explored including sodium silicate from quartz sand and silicon tetraethoxide (Jang et al. 2009). However, the production of pure silica from both sources needs high energy consumption (Faizul et al. 2013). Furthermore, silicon tetraethoxide was reported to have high toxicity (Hassan et al. 2014). Therefore, researchers are seeking alternatives of silica from biomass wastes and natural sources. Rice husk is a type of biomass source that has inevitably attracted researchers (Liou and Yang 2011).
The milling process yields 70% rice as the main component, with by-products of 20% rice husk, 8% rice bran, and 2% rice germ (Nagendra et al. 2011). Based on the statistics from the Department of Agriculture Peninsular Malaysia (2014), Malaysia produces approximately 2.6 million metric tonnes of paddy per year. Considering that 20% of paddy production is husks, 0.5 million metric tonnes of this biomass waste can be obtained (Soltani et al. 2015). Due to the abundance of rice husks from the paddy production, the current disposal methods of field dumping and open burning of rice husks have led to environmental issues and serious health problems (Alshatwi et al. 2015). Thus, researchers are searching for productive ways to utilise rice husks in industrial applications. Moreover, the utilisation of rice husks in the rubber industries is quite promising because rice husk ash was reported to have high silica content (Noushad et al. 2012; Dominic et al. 2013). According to Soltani et al. (2015), approximately 20% rice husk ash can be obtained upon combustion of rice husks. The rice husk ash contains 80 to 95% silica with trace amounts of metallic impurities.
To date, several treatment methods for synthesising pure rice husk silica have been reported, which include chemical precipitation, thermal pyrolysis, sol-gel method, and calcination. Among these methods, precipitation with acid, followed by dissolution of sodium silicate from rice husks is preferred to produce silica. Although high purity silica can be produced using this method, the complicated process, huge chemical consumption, and high cost have prevented this method from further development (Gu et al. 2013). Therefore, it is important to develop a simple and practical method of producing high purity silica, with high surface area from rice husks because silica is on demand for many industrial applications, such as fillers to produce various rubber products.
Dominic et al. (2013) demonstrated that rice husk silica (RHS) fillers can lead to better properties compared to commercial silica (CS) fillers when incorporated in the natural rubber matrix. The comparison was made with the addition of 2 phr fillers in a natural rubber compound. The smaller particle size of RHS (252 m2/g) compared to CS (178 m2/g) gave a higher surface area that contributed to improved RHS-NR interaction. The presence of this silica-rubber crosslink delayed the deformation of the RHS-NR compound, thus resulting in higher tensile strength and tear strength values compared to in CS-NR compound. The RHS-NR compound also showed lower abrasion loss compared to the CS-NR compound, thus indicating better reinforcing efficiency of the RHS-filled NR compound. This result is in agreement with the results in a similar study by Lemessa Jembere and Fanta (2017). The authors also suggested that the surface area of the filler is one of the crucial aspects indicating the tensile strength of the composites, which would also increase the modulus of the compound. High modulus contributes to higher stiffness that reduces the elongation at break value.
As shown by Dominic et al. (2013) and Lemessa et al. (2017), a filler’s high surface area could provide better interactions between the fillers and the rubber matrix. Consequently, the overall strength-related properties of the rubber compounds are improved. Therefore, the main interest of this study was to synthesise high purity silica from rice husk as fillers in natural rubber using a simple solvent-thermal treatment with acid leaching. This method was modified from TAPPI T204 (2007) and TAPPI T264 (1997) standards, which are used to treat wood in pulp and papermaking industries. Currently, no other study has reported the effectiveness of this method in extracting silica from rice husks, thus making it a novel method to be explored. Hydrochloric (HCl) acid was used in the leaching process, as many researchers have shown that HCl treatment could effectively remove metallic impurities in rice husks, better than other acid treatments, such as nitric and sulphuric acid (Soltani et al. 2015). The resulting RHS were characterised and incorporated in natural rubber compounds as a filler. The mechanical, physical, and thermal properties of the high purity RHS-filled natural rubber compounds were investigated.
EXPERIMENTAL
Materials
A type of natural rubber (NR), called SMR L, was used in this research, and other compounding ingredients, namely, zinc oxide, stearic acid, sulphur, antioxidants, and N-cyclohexyl-2-benzothiazole sulfenamide (CBS) were purchased from the Malaysian Rubber Board (Lembaga Getah Malaysia, LGM, Sungai Buloh, Malaysia). Solvents and HCl were supplied by BT Science Sdn. Bhd. (Balakong, Selangor, Malaysia), while the rice husks were obtained from a local rice mill (Kuala Selangor, Malaysia).
Methods
Synthesis and characterisation of high purity rice husk silica (RHS)
The solvent-thermal extraction method was adopted from TAPPI T204 (2007) and TAPPI T264 (1997) standards with some modifications. These standards are normally used to extract the extractives from wood in the pulp and papermaking industries. The same mechanism was applied to rice husks to obtain high purity silica, with the finest particle size. A combination of solvents consisting of benzene and ethanol at a ratio of 2:1 v/v is commonly recognised as a standard method to remove most extractives from wood (TAPPI T204 2007). Since benzene is a known carcinogen, numerous researches were conducted to find the alternative solvents to replace it. Sefara and Birkett (2006), and Nasser and Al-Mefarrej (2009) had confirmed that acetone and a combination of toluene and ethanol could remove almost the same amount of wood extractives as the benzene-ethanol mixture. Therefore, a mixture of these three low toxicity solvents; toluene, ethanol, and acetone (3:2:1 v/v) was used in this study to replace benzene to extract extractives in the rice husks.
The solvent mixture with a total volume of 1,800 mL was prepared in a beaker. The mixture was then poured into a 2 L reaction flask containing 180 g of rice husks, which were previously cleaned with distilled water and then dried. The extraction process was conducted for 45 min in an open system at 110 °C. Then, the rice husks were washed with distilled water before being soaked in 1.0 M HCl for a 24 h leaching process. Next, the treated rice husks were thoroughly cleaned with distilled water to remove the remaining acid, followed by oven drying at 110 °C for 24 h. The dried rice husks were incinerated in a furnace at 700 °C for 4 h to obtain white ash silica. Finally, the rice husk ash was ground with an agate mortar until super fine white silica powder was obtained. Figure 1 shows the process flow diagram of the solvent-thermal treatment method with acid leaching to obtain RHS.
The morphology and surface characterisations of RHS samples were conducted using a scanning electron microscope (SEM) (SUPRA 40VP; Carl Zeiss AG, Oberkochen, Germany) with a constant applied voltage of 10 kV. The powder samples were subjected to gold sputtering in a sputter coater (Type SCD 005; Bal-Tec Inc., Balzers, Switzerland) prior to the electron microscopy to prevent any electrostatic charge during the analysis. The Brunauer-Emmett-Teller (BET) method was applied to determine the specific surface area and average pore volume of the RHS particles. Surface area analysis was conducted using a micropore surface area analyser (MIC271002 REV B; Micromeritics, Norcross, USA). Following a degassing procedure for 5 to 6 h, the measurement was conducted under nitrogen adsorption at liquid nitrogen temperature. The average size of the RHS particles was measured using a Zetasizer Nanoseries (Malvern Instruments Ltd, Malvern, United Kingdom) analyser. The RHS powder was dispersed in distilled water, and then sonicated for 30 min prior to analysis of particle size. The elemental composition and purity concentration of the RHS powder was measured using the X-ray fluorescence (XRF) spectrometer (WDXRF; Shimadzu, Kyoto, Japan).
Fig. 1. Process flow diagram showing the solvent-thermal treatment method with acid leaching of RHS
Compounding and curing
The RHS-filled NR compounds were mixed on a two-roll mill according to the formulations shown in Table 1.
Table 1. Formulation used for RHS-filled NR Compounds at Different RHS Loadings
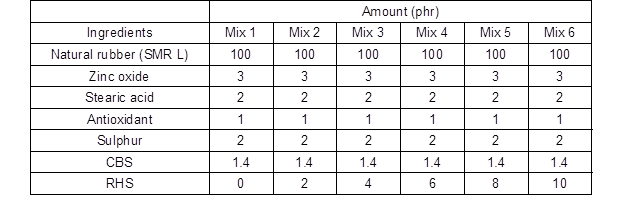
All compounds had the same composition of ingredients, except for the high purity ultrafine RHS. The RHS content was varied at 2, 4, 6, 8, and 10 parts per hundred parts of rubber (phr). The rubber compounds were cured into tensile sheets for tensile properties tests and respective moulds of hardness, resilience, and abrasion at 160 °C using a hot press at 100 bar.
Measuring the properties of RHS-filled NR compounds
The tensile properties of RHS-filled NR compounds were measured using the Instron 5569 tensile tester (Instron, Norwood, MA, USA) with standard dumbbell specimens according to the ASTM D412 (2008) standard. The tests were conducted at room temperature with a load-cell of 2 kN and cross-head speed of 500 mm/min. Approximately five specimens for each compound were tested, and the average values were recorded.
The tensile fracture surface of the RHS-filled NR compounds was examined using a scanning electron microscope (SEM) (SUPRA 40VP; Carl Zeiss AG, Oberkochen, Germany) with a constant applied voltage of 10 kV. The dispersion of the RHS and the rubber-RHS interactions within the NR compounds were observed. A Wallace Akron abrasion tester (Elektron Technology Series, Cambridge, United Kingdom) was used to test the abrasion resistance of the RHS-filled NR compounds in accordance with BS903: Part A9 (1988) methods. The mean volume losses after five test runs were recorded for each specimen. Resilience tests were conducted according to ISO 4662 (2009) requirements using the Wallace Dunlop Tripsometer (Wallace Instruments, Surrey, England, United Kingdom). The average percentage of resilience readings for five repeated impacts for each specimen was recorded. The hardness of the RHS-filled NR compounds was measured using a Wallace IRHD dead load hardness tester (Wallace Instruments, Surrey, England, United Kingdom), set at room temperature according to the ASTM D1415 (2012) method. The average reading from the three different locations on each rubber specimen was recorded.
A thermal gravimetric analyser (TGA) (TG 209 F3 Tarsus; Netzsch, Germany) was used to analyse the thermal decomposition of the RHS-filled NR compounds with a heating rate of 20 °C/min under nitrogen atmosphere. Meanwhile, the dynamic mechanical analysis (DMA) was conducted using rectangular specimens with dimensions 25 × 10 × 2 mm on a DMA machine (DMA 8000; PerkinElmer, Boston, MA, USA). Through DMA, the interaction between the rubber matrix and the RHS filler was studied.
RESULTS AND DISCUSSION
Characterisation of RHS
Acid leaching pre-treatment prior to combustion is the best way to remove most of the metallic impurities contained in rice husks and to produce completely white coloured silica (Soltani et al. 2015). In this study, HCl was used in the leaching treatment prior to calcination. According to most researchers, among other types of acids, such as sulphuric acid (H2SO4) and nitric (HNO3) acid, the application of HCl is extremely effective in removing the metal oxides in the rice husks. It had also led to the production of high purity silica that was white in colour (Chakraverty et al. 1988). Figure 2 shows the appearance of the extracted RHS from untreated rice husks and the extracted RHS from treated and leached rice husks in 1.0 M HCl prior to calcination at 700 °C. The RHS from untreated rice husks was grey in colour, which indicated the presence of metallic impurities, as shown in Fig. 2a. However, the solvent-thermal-treated rice husks leached in HCl showed an incredible result, whereby the ash was completely white in colour after calcination, as shown in Fig. 2b. As reported by Della et al. (2002), the changing colour of the rice husks were due to the complete combustion process, which can be associated with the structural conversion of the silica in the rice ash. Ugheoke and Mamat (2012) had summarised that the transformation from grey raw rice husks to white is critically dependent on the incineration temperature. Temperatures between 300 to 450 °C would produce dark grey carbonised ash, while at higher temperatures of approximately 500 to 650 °C, the rice ash would become light grey and white ash. The disappearance of the grey colour indicated the effectiveness of the treatment method in eliminating any metallic impurities contained in the rice husks, as reported further through the XRF analysis.
Fig. 2. Appearance of RHS samples: (a) RHS extracted from untreated rice husks calcined at 700 °C; and (b) RHS extracted from solvent-thermal treated and leached rice husks in 1.0 M HCl, followed by calcination at 700 °C
The XRF elemental analysis was conducted to determine the percentage compositions of the resulting RHS. As indicated in Table 2, the untreated RHS contained 91.6% of SiO2 and other metallic impurities, namely, K2O, TiO2, CaO, BaO, MnO, Fe2O3, and NiO.
It is known that TiO2 is insoluble in organic solvents. However, Taku et al. (2016) have reported that Cl, TiO2, Cr2O3, MnO, CuO, ZnO, BaO, and Fe2O3 (traced in untreated rice husks) were reduced and eliminated during the calcination process. Even though elements like TiO2 may not be affected by the solvents and HCl during leaching, the thermal treatment after the solvent extraction has, however, managed to improve the pozzolanic properties of RHS. The results showed that the purification through the solvent-thermal treatment method, and the leaching with 1.0 M HCl had successfully eliminated almost all of the metallic impurities, except for Fe2O3 (0.01%). Therefore, the percentage of SiO2 in the treated and leached RHS had increased to 99.99%.
Table 2. XRF Elemental Analysis
The SEM images of the untreated and treated RHS samples are shown in Fig. 3. Figure 3a shows a flat surface with no clear boundaries. The untreated RHS was comprised of non-cellulosic and hemicellulose components that bound the particles together, which resulted in the irregular shape of the silica particles. The solvent-thermal treatment that was used to purify the cellulose components had modified the morphology of the RHS. The solvent mixture, which consisted of non-polar and polar solvents, had influenced the shape of the particles. Noushad et al. (2012) had reported that using non-polar solvents in rice husk treatments could produce irregular silica particles, while polar solvents could produce spherical and well-rounded silica particles. The silica particles were probably solvated by the polar solvent (ethanol), thus increasing the surface area. This condition is believed to be responsible in obtaining the sphere-shaped silica particles, while when a non-polar solvent (toluene) was used, the degree of agglomeration was significantly increased.
As shown in Fig. 3b, the treated RHS was spherical in shape and highly agglomerated. The agglomeration was due to the hydroxyl groups on the surface of RHS, which resulted in strong interactions among the RHS particles. The size distribution of the untreated and treated RHS particles varied from 100 to 300 nm. The BET analysis showed the untreated RHS as having a surface area of 11.4 m2/g, while the surface area of the treated and leached RHS was 234.2 m2/g. Theoretically, a higher surface area would result in a smaller particle size that would perhaps yield a higher reinforcing effect on the rubber matrix.
Fig. 3. SEM images: (a) untreated RHS; and (b) treated RHS and leached with 1.0 M HCl
Fig. 4. Mechanical and physical properties of the NR compounds at various RHS loadings: (a) tensile strength and elongation at break; (b) modulus at 100% elongation (M100) and volume loss; and (c) resilience and hardness values
Measurement of the Properties of RHS-filled NR Compounds
As presented by Fig. 4a, the highest tensile strength value was recorded with the addition of 4 phr of RHS. The tensile strength dropped steadily with further increase in RHS loading. The earlier increment in tensile values was due to the evenly distributed smaller-sized RHS in the rubber matrix, as indicated by Fig. 5a. The smaller RHS particles led to larger surface areas being exposed to the rubber matrix. This condition had encouraged a good interaction between the RHS and the rubber matrix, even with no surface modification of the RHS. At 4 phr loading of RHS, the rough surface and the matrix tearing line of the tensile fractured sample can be observed in Fig. 5b. A good dispersion of RHS particles had altered the crack track of the sample, which then resisted the spread of the crack when the sample was stretched. High resistance to crack propagation indicated a good reinforcement by the RHS, and consequently caused an increase in tensile properties. The SEM images of the tensile fractured surfaces are in good agreement with the results obtained by Idrus et al. (2011), and Surya et al. (2013). These studies claimed that the surface roughness and the matrix tearing line were caused by the high energy used to break the samples, which were the signs of good reinforcement of the compound.
When the RHS filler loading was increased from 6 to 10 phr, the ultrafine fillers became agglomerated. They formed bigger-sized RHS with smaller surface areas, as shown in Figs. 5c and 5d. The surface areas were insufficiently wetted by the rubber matrix, which weakened the interaction between the RHS fillers and the rubber matrix. Subsequently, this condition caused the reduction in tensile strength values at 6 phr loading and onwards, as shown in Fig. 4a.
Fig. 5. SEM images of the tensile fracture surface of the NR compounds, filled with RHS at various loadings: (a) 2 phr of RHS at 500x; (b) 4 phr of RHS at 100x; (c) 10 phr of RHS at 100x; and (d) 10 phr of RHS at 500x magnifications
The elongation at break (Eb) value decreased when the RHS loading was increased. Theoretically, the Eb value is related to the elastic properties of the rubber compounds. A higher elasticity of the rubber compound would result in a higher Eb value. This finding showed that the RHS-filled rubber compounds became less elastic at higher RHS loadings, which led to noticeably reduced the Eb values. The rigidity of the RHS due to the high silica content in the ash increased the stiffness and reduced the elasticity of the rubber compound. Thereby, the incorporation of RHS in the NR restricted the movement of the rubber chains and consequently reduced the Eb value (Tiwari et al. 2015; Jembere et al. 2017).
The incorporation of RHS had also increased the modulus of the NR compounds. The stiffness of the NR compounds was noticeably increased when the filler loading was increased, which resulted in a higher modulus for the NR compounds. The increment in the modulus value affected the reinforcing efficiency of the RHS in the rubber matrix (Ismail and Ramli 2008) due to improved rubber-filler interaction. However, at 6 phr of RHS, the modulus value began to gradually decline, as shown in Fig. 4b. The agglomeration of the RHS particles at higher loadings could have been the cause of this condition, as indicated by the SEM micrograph in Fig. 5d. This result was similar to the results of volume loss, where it showed a gradual increment at 6 phr loading and onwards. Higher volume loss would result in inferior abrasion resistance (Rattanasom et al. 2007). The poor dispersion of RHS at higher loading could have initiated a strong filler-filler interaction that was thought to be the main cause for the increased volume loss.
Harder and stiffer rubber composites are less resilient (Ismail et al. 2013). The addition of fillers would prevent the rubber chains from returning to their original sizes and shapes after stress is removed. This explains the decreasing resilience when the RHS filler loading was increased, which was to the opposite of the hardness values, as indicated in Fig. 4c. The gum rubber with no addition of RHS fillers was more elastic, which led to the highest resilience due to the absence of obstacles between the rubber chains during deformation.
The values of tensile strength, elongation at break, volume loss, and 100% modulus of treated and untreated RHS-filled natural rubber (NR) samples are shown in Table 3. These comparisons were made to prove the effectiveness of the solvent-thermal treatment method in producing purified silica that could reinforce the strength-related properties of NR compounds.
Table 3. Mechanical Properties of Treated RHS and Untreated RHS-filled NR Compounds
As shown in Table 3, the tensile strength of the treated RHS-filled NR was higher than for the untreated RHS-filled NR. As expected, the higher surface area of the treated RHS compared to the lower surface area of the untreated RHS contributed to the improvements of tensile strength, modulus, and abrasion resistance of the rubber compounds. High surface areas would improve the interaction between rubber and RHS fillers. Fillers with high surface areas tend to have more contact areas available to the rubber matrix, and thereby reinforce the rubber chains. Meanwhile, the presence of metallic impurities in the untreated RHS, as previously discussed in the XRF analysis, had decreased the purity and the surface area of the untreated RHS. This would weaken the interaction between the rubber matrix and the untreated RHS fillers, which concurrently reduced the strength-related properties.
The TG and DTG curves of NR compounds filled with RHS are shown in Figs. 6a and 6b, respectively. The thermal decomposition trends for the unfilled NR compound (control) and the NR compounds were comparable. Only one bend was denoted in the TG curves, and one peak was indicated in the DTG curves. The one step thermal decomposition corresponded to the thermal scissions of C-C chain bonds (Chen et al. 2008). Table 4 indicates a clear comparison of the TGA results on the initial (T5%), peak (T50%), and maximum (Tmax) thermal decomposition temperatures, as well as the remaining residue at 900 °C. The temperature at the maximum weight loss (Tmax) was indicated by the lowest peak in the DTG curve. Meanwhile, the RHS content of all RHS-filled NR composites was obtained from the residual weight percentage, as can be seen in Table 4.
Table 4. Characteristics of the Thermal Decomposition Temperatures and Residue of the NR Compounds at Various RHS Loadings
The incorporation of RHS had delayed the decomposition process of the rubber composites. Consequently, the initial degradation temperature of RHS-filled NR compounds had increased to 43 °C compared to the unfilled NR compounds. This increase indicated the strong interaction between RHS and NR molecular chains through several factors, such as the branching effect, particle size, and surface structure. The increasing decomposition temperatures suggested that the thermal aging resistance of the RHS-filled NR compounds was improved. The thermal stability analysis is very important to investigate how much the RHS fillers could withstand the vulcanisation temperature during the curing process of rubber compounds (Jembere et al. 2017).
Figure 6c depicts the temperature dependence of storage modulus (E’) and loss tangent (tan δ) of the unfilled NR compound (control) and the RHS-filled NR compound (4 phr) under a scanning frequency of 10 Hz. The E’ of the unfilled NR compound was 5,297 MPa at -78 °C, which increased considerably to 6,127 MPa with the addition of 4 phr of RHS into the NR compound.
Fig. 6. Thermal properties of NR compounds at various RHS loadings: (a) TG curves; (b) DTG curves; and (c) DMA curves of storage modulus and tan δ of unfilled NR compound (control) and 4 phr of RHS in NR compound
The incorporation of RHS increased the rigidity of the NR compounds, which improved the storage modulus. From the DMA curve, a clear peak from tan δ indicated the glass transition temperature (Tg). The Tg of unfilled NR compound was -46 °C, while the Tg of RHS-filled NR compound (4 phr) was -44 °C. The slight increase in Tg was attributed to the limited movement of the NR molecular chain due to the presence of SiO2 in the NR compound. This observation suggested that SiO2 was physically well dispersed within the NR and had strongly interacted with the NR molecular chains.
CONCLUSIONS
- High purity RHS (99.9% SiO2 content) with a size distribution of 100 to 300 nm and surface area of 234.25 m2/g was successfully prepared using the solvent-thermal extraction method.
- NR compounds filled with RHS fillers displayed remarkable enhancements in overall properties at the optimum loading of 4 phr.
- The SEM images of the tensile fractured surface showed uniform dispersions at 2 phr and 4 phr of RHS loadings.
- The strength-related properties of the treated RHS-filled NR had shown better improvement compared to the untreated RHS-filled NR. High purity treated RHS was able to enhance the rubber-filler interaction and strengthen the properties of the rubber compounds.
- The presence of metallic impurities in the untreated RHS filler had reduced its purity and surface area, thus weakening the interaction between the rubber matrix and the untreated RHS filler. This could be the main cause for the poor mechanical properties of the untreated RHS-filled NR compounds.
- The solvent-thermal extraction is an ideal treatment method for producing the purest RHS that could reinforce NR compounds.
- The thermal stability of RHS-filled NR compounds was improved compared to unfilled NR compounds.
- The DMA results proved that RHS had strongly interacted with the molecular chains of NR when there was an increase in the Tg and storage modulus.
ACKNOWLEDGMENTS
The authors wish to acknowledge Universiti Teknologi MARA (UiTM) and the Ministry of Education for the scholarship and to University of Malaya (UM) for the grant (Grant No. PG160-2015A). The authors would also like to express their gratitude to Universiti Teknologi MARA (UiTM), University of Malaya (UM), and Universiti Sains Malaysia (USM) for providing research facilities and technical support.
REFERENCES CITED
Abdul Salim, Z. A. S., Hassan, A., and Ismail, H. (2017). “A review on hybrid fillers in rubber composites,” Polymer-Plastics Technology and Engineering 57(6), 523-539. DOI: 10.1080/03602559.2017.1329432
Alshatwi, A. A., Athinarayanan, J., and Periasamy, V. S. (2015). “Biocompatibility assessment of rice husk-derived biogenic silica nanoparticles for biomedical applications,” Materials Science and Engineering C 42, 8-16. DOI: 10.1016/j.msec.2014.11.005
ASTM D1412 – 06 (2012). “Standard test method for rubber property – International hardness,” ASTM International, West Conshohocken, PA.
ASTM D412 (2008). “Standard test methods for vulcanised rubber and thermoplastic elastomers – tension,” ASTM International, West Conshohocken, PA.
BS 903-A9 (1988). “Methods of testing vulcanized rubber – Part A9: Determination of abrasion resistance,” British Standard Institution, London, United Kingdom.
Chakraverty, A., Mishra, P., and Banerjee, H. D. (1988). “Investigation of combustion of raw and acid-leached rice husk for production of pure amorphous white silica,” Journal of Materials Science, 23(1), 21-24. DOI: 10.1007/BF01174029
Chen, Y., Pen, Z., Kong, L. X., Huang, M. F., and Li, P. W. (2008). “Natural rubber nanocomposite reinforced with nano silica,” Polymer Engineering and Science 48(9), 1674-1677. DOI: 10.1002/pen.20997
Della, V., Kuhn, I., and Hotza, D. (2002). “Rice husk ash as an elemente source for active silicaproduction,” Mater. Lett. 57(4), 818-821. DOI: 10.1016/S0167-577X(02)00879-0
Department of Agriculture Peninsular Malaysia (2014). “Paddy statistics of Malaysia 2014,”_http://www.doa.gov.my/index/resources/aktiviti_sumber/sumber_awam/maklumat_pertanian/perangkaan_tanaman/perangkaan_padi_2014.pdf, Accessed 31 December 2015.
Dominic, M. C. D., Begum, P. M. S., Joseph, R., Joseph, D., Kumar, P., and Ayswarya, E. P. (2013). “Synthesis, characterization and application of rice husk nanosilica in natural rubber,” International Journal of Science, Environment and Technology 2(5), 1027–1035.
Faizul, C. P., Abdullah, C., and Fazlul, B. (2013). “Review of extraction of silica from agricultural wastes using acid leaching treatment,” Advanced Materials Research 626, 997-1000. DOI: 10.4028/www.scientific.net/AMR.626.997
Gu, S., Zhou, J., Luo, Z., Wang, Q., and Ni, M. (2013). “A detailed study of the effects of pyrolysis temperature and feedstock particle size on the preparation of nanosilica from rice husk,” Industrial Crops and Products 50, 540-549. DOI: 10.1016/j.indcrop.2013.08.004
Hassan, A. F., Abdelghny, A. M., Elhadidy, H., and Youssef, A. M. (2014). “Synthesis and characterization of high surface area nanosilica from rice husk ash by surfactant-free sol-gel method,” Journal of Sol-Gel Science and Technology 69(3), 465-472. DOI: 10.1007/s10971-013-3245-9
Hernandez, M., Ezquerra, T. A., Verdejo, R., and Lopez-Manchado, M. A. (2012). “Role of vulcanizing additives on the segmental dynamics of natural rubber,” Macromolecules 45(2), 1070-1075. DOI: 10.1021/ma202325k
Idrus, S. S., Ismail, H., and Palaniandy, S. (2011). “Study of the effect of different shapes of ultrafine silica as fillers in natural rubber compounds,” Polymer Testing 30(2), 251-259. DOI: 10.1016/j.polymertesting.2010.10.002
Ismail, H., and Ramli, R. (2008). “Organoclay filled natural rubber nanocomposites: The effects of filler loading and mixing method,” Journal of Reinforced Plastics and Composites27(16-17), 1909-1924. DOI: 10.1177/0731684407082541
Ismail, H., Salleh, S. Z., and Ahmad, Z. (2013). “The effect of partial replacement of carbon black with halloysite nanotubes on the properties of CB/HNT filled NR nanocomposites,” Journal of Elastomers and Plastics 45(5), 445-455. DOI: 10.1002/app.39543
ISO 4662 (2009). “Rubber, vulcanized or thermoplastic – Determination of rebound resilience,” International Standard, Geneva, Switzerland.
Jang, H. T., Park, Y., Ko, Y. S., Lee, J. Y., and Margandan, B. (2009). “Highly siliceous MCM-48 from rice husk ash for CO2 adsorption,” International Journal of Greenhouse Gas Control 3(5), 545-549. DOI: 10.1016/j.ijggc.2009.02.008
Lemessa Jembere, A., and Fanta, S. W. (2017). “Studies on the synthesis of silica powder from rice husk ash as reinforcement filler in rubber tire tread. Part: Replacement of commercial precipitated silica,” International Journal of Materials Science and Applications 6(1), 37. DOI: 10.11648/j.ijmsa.20170601.16
Liou, T. H., and Yang, C. C. (2011). “Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash,” Materials Science and Engineering B: Solid-State Materials for Advanced Technology 176(7), 521-529. DOI: 10.1016/j.mseb.2011.01.007
Nagendra, P. M. N, Sanjay, K. R., Shravya, K. M., Vismaya, M. N., Nanjunda, S. S (2011). “Health benefits of rice bran – A review,” Journal of Nutrition and Food Sciences 1(3), 1-7. DOI: 10.4172/2155-9600.1000108
Nasser, R. A., and Al-Mefarrej, H. A. (2009). “Non-carcinogenic solvents as alternative to benzene for wood extractives determination,” Alexandria Science Exchange Journal 30(3), 397-405. DOI: 10.21608/asejaiqjsae.2009.3251
Sefara, N. L., and Birkett, M. (2006). “Development of an Alternative Solvent To Replace Benzene in the Determination of Organic Soluble Extractives in Wood,” Technical Association of the Pulp and Paper Industry of South Africa (TAPPSA Journal).
Noushad, M., Rahman, I. A., Husein, A., Mohamad, D., and Ismail, A. R. (2012). “A simple method of obtaining spherical nanosilica from rice husk,” International Journal on Advanced Science Information Technology 2(2), 28-30. DOI: 10.18517/ijaseit.2.2.172
Rattanasom, N., Saowapark, T., and Deeprasertkul, C. (2007). “Reinforcement of natural rubber with silica/carbon black hybrid filler,” Polymer Testing 26(3), 369-377. DOI: 10.1016/j.polymertesting.2006.12.003
Soltani, N., Bahrami, A., Pech-Canul, M. I., and Gonzalez, L. A. (2015). “Review on the physicochemical treatments of rice husk for production of advanced materials,” Chemical Engineering Journal 264, 899-935. DOI: 10.1016/j.cej.2014.11.056
Surya, I., Ismail, H., and Azura, A. R. (2013). “Alkanolamide as an accelerator, filler-dispersant and a plasticizer in silica-filled natural rubber compounds,” Polymer Testing 32(8), 1313-1321. DOI: 10.1016/j.polymertesting.2013.07.015
Taku, J. K., Amartey, Y. D., and Kassar, T. (2016). “Comparative elemental analysis of rice husk ash calcined at different temperatures using X-ray flourescence (XRF) technique,” American Journal of Civil Engineering and Architecture 4(1), 28-31. DOI: 10.12691/ajcea-4-1-4
TAPPI T204 cm-97. (2007). “Solvent extractives of wood and pulp,” TAPPI Press, Atlanta, GA.
TAPPI T264 cm-97. (1997). “Preparation of wood for chemical analysis,” TAPPI Press, Atlanta, GA.
Tiwari, P., Choudhary, S., and Choudhary, M. (2015). “Study on mechanical, thermal and morphological properties of RHA filled PVC composite,” International Journal of Scientific Engineering and Applied Science 1(5), 265-281.
Ugheoke, I. B., and Mamat, O. (2012). “A critical assessment and new research directions of rice husk,” Journal of Science and Technology 6(03), 430-448.
Article submitted: January 24, 2018; Peer review completed: March 11, 2018; Revised version received and accepted: July 26, 2018; Published: July 27, 2018.
DOI: 10.15376/biores.13.3.6936-6951
