Abstract
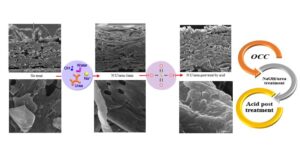
Chemical surface modification is one method for enhancing the mechanical and barrier properties of packaging paper. The NaOH/urea solvent system has been deemed an effective, inexpensive, and cost-effective solvent for paper modification and cellulose dissolution due to its unique self-reinforcing qualities and the fact that it can be utilized on an industrial scale, although it is ineffective for porous paper and requires pre- or post-treatment. This study examined the influence of acid as a post-treatment on the surface modification of paper with NaOH/urea to improve properties relative to packaging uses. The results indicated that NaOH/urea modification on OCC as a semi-crystalline material did not result in materials with superior resistance and barrier qualities. While acid treatment increased tensile and burst strength and air permeability, it was ineffective at increasing tear strength. Properties of control, NaOH/urea treatment, and acidic post treatment papers were respectively 33.31, 29.4, and 37.46 mn/g in the tensile index, 1.7, 1.58, and 1.74 Kpa.m2/g in burst index, 9.94, 9.07 and 8.87 mn.m2/g in tear index, 2.04, 1.34 and 1.32 s-1 in smoothness, 37.2, 38.2 and 45.4 s in air resistance, and 77.5, 90.8 and 80.5 water absorption. Therefore, with or without acidic post-treatment, the sheets became hydrophilic.
Download PDF
Full Article
The Impact of an Acidic Post-Treatment on Surface-Modified Old Corrugated Cardboard (OCC) with NaOH-Urea as a Reinforcing Agent
Negin Ghahrani, Noureddin Nazarnezhad,* Omid Ramezani, and Ghasem Asadpour
Chemical surface modification is one method for enhancing the mechanical and barrier properties of packaging paper. The NaOH/urea solvent system has been deemed an effective, inexpensive, and cost-effective solvent for paper modification and cellulose dissolution due to its unique self-reinforcing qualities and the fact that it can be utilized on an industrial scale, although it is ineffective for porous paper and requires pre- or post-treatment. This study examined the influence of acid as a post-treatment on the surface modification of paper with NaOH/urea to improve properties relative to packaging uses. The results indicated that NaOH/urea modification on OCC as a semi-crystalline material did not result in materials with superior resistance and barrier qualities. While acid treatment increased tensile and burst strength and air permeability, it was ineffective at increasing tear strength. Properties of control, NaOH/urea treatment, and acidic post treatment papers were respectively 33.31, 29.4, and 37.46 mn/g in the tensile index, 1.7, 1.58, and 1.74 Kpa.m2/g in burst index, 9.94, 9.07 and 8.87 mn.m2/g in tear index, 2.04, 1.34 and 1.32 s-1 in smoothness, 37.2, 38.2 and 45.4 s in air resistance, and 77.5, 90.8 and 80.5 water absorption. Therefore, with or without acidic post-treatment, the sheets became hydrophilic.
DOI: 10.15376/biores.18.2.4153-4167
Keywords: Recycled paper; NaOH/urea; Self-reinforcing; Surface-modified paper; Acidic treatment
Contact information: Faculty of Natural Resources, Wood and Paper Science Department, Sari Agricultural Sciences and Natural Resource University, P.O. Box 737, Sari 4818168984 Iran;
* Corresponding author: nazarnezhad91@gmail.com
GRAPHICAL ABSTRACT

INTRODUCTION
Paperboard is used as a raw material for low-cost packaging materials with high mechanical strength and flexibility. Paper applications demand a wide variety of qualities (Zhu et al. 2018). Producers can face different kinds of challenges, limitations, and poor mechanical and barrier performance such as water penetration in paper due to hydrophilicity and porous structures. Paper’s strength and barrier properties can be reduced due to water penetration (Lavoine et al. 2014). Controlling the interactions between water and paper fibers is crucial to paper use. The hydrophobicity of cellulose achieved through internal and surface sizing may not be sufficient to meet the requirements of resistance, particularly water resistance, in packaging materials. Moreover, the coating makes the paper thicker (Samyn et al. 2013). In addition to refining, coating, calendering, laminating, and chemical applications are different methods for enhancing mechanical and barrier qualities.
Surface modification is limited to the superficial depth of the material. In an ideal situation, the modified layer should form many layers of the polymer’s surface, while the deeper (bottom) layers remain unchanged. The surface layers’ properties are different from the other layers, which have the initial qualities. Surface modification is generally a chemical process involving the combination of certain elements, such as oxygen, nitrogen, and fluorine atoms, or functional groups, such as hydroxyl, carbonyl, and carboxyl (Vaswani et al. 2005). Since paper is composed of cellulose and the functional groups in cellulose are hydroxyl groups, these alterations should be restricted to surface OH groups to preserve the fibers’ structural integrity and mechanical strength (Belgacem and Gandini 2005). The cell wall is composed of many layers of cellulose fibrils. According to theory, the poorly aligned nature of the cellulose chains in the outer layers of the cell wall render the cellulose there more soluble in certain solvents. Surface modification can close paper pores and decrease voids to increase barrier qualities (Jiao et al. 2015).
In recent years, aqueous NaOH/urea solution has been regarded as an effective solvent system due to its ability to dissolve a portion of cellulose fast and easily and to form stable cellulose solutions (Wei et al. 2020). Among the existing solvents, NaOH-based aqueous solutions can be regarded as a cheap, non-volatile, non-toxic, and environmentally friendly, with a fast dissolution ability. Using this solvent system, recovered cellulose materials, films, aerogels, hydrogels, etc., have been prepared (Jiang et al. 2014). In the past, it has been common to use safe solvents such as aqueous solutions of sodium hydroxide (7% to 9%) to dissolve cellulose (Sescousse and Budtova 2009). After subsequent precipitation, the resulting regenerated cellulose (RC) can have a honeycomb-like structure with a high number of micro-, and nanopores, which improves the interaction between the filler and a matrix via the physical entanglement effect. Consequently, composites containing regenerated cellulose may display particular mechanical properties (Yu et al. 2017(. By contrast, composites can be produced by the so-called partial or selective dissolution process (Duchemin et al. 2016). By means of a partial dissolution of cellulosic materials, both the reinforcement and the matrix of the resulting composite structure have their origin in the same raw material (also called self-reinforcing composite). In this dissolution, the solvent dissolves the surface or weak areas of the reinforcing fibers and forms the matrix around the structure. It forms primary fibers, and the insoluble fiber content of the composite is controlled by changing the dissolution time (Uusi-Tarkka et al. 2021(. The partial dissolution of NaOH/urea solvent may be good for industrial applications, but if the material is porous, then it may be useless for the intended applications and cannot be compared to other materials such as wood-plastic composites. There are numerous efficient methods for enhancing the dissolution and self-reinforcing capabilities of NaOH/urea, such as the manufacturing process, the degree of polymerization of the materials, and post-treatment techniques (Duchemnin et al. 2016; Hilderbrandt et al. 2017). For example, there may be an advantage to compacting the composite paper, as a post-treatment, with the goal of improving and maximizing its self-reinforcing qualities (Hilderbrandt et al. 2018).
In addition to determining the effect of NaOH/urea surface modification on recycled paper as packaging material with good mechanical and barrier qualities, the purpose of this study is to determine the effect of acidic post-treatment on enhancing these qualities of NaOH/urea surface-modified paper. The fibers become brittle due to acid utilization as a coagulant in the wet-spinning in the regeneration process of the fibers in NaOH/urea (Fu et al. 2018). The question is whether acid may also enhance the physical qualities of paper.
EXPERIMENTAL
Materials
Linerboard sheets produced from OCC in a roll form with a width of 30 cm was supplied by Pouya Ayesh Mazandaran (Babol, Iran). Sodium hydroxide (>99% purity), urea, and sulfuric acid (98% purity) were obtained from Merck. Sodium chlorite (80% purity) was obtained from Sigma.
Methods
Preparation of the handsheets
The OCC linerboard was cut to dimensions of 12 * 15 cm in the paper’s machine direction. The holocellulose component of the material was determined by the method of NREL TP-510 42619. Lignin was determined by the method of ISO 302:2012(E) and ash was calculated by the TAPPI method T 211 om-07.
Surface modification
The papers’ surfaces were modified using the Plafco process (Piltonen et al. 2016) in NaOH/urea solvent (as shown in Table 1). In this process, the prepared paper sample will be immersed in the sodium hydroxide/urea solvent for 1 second and then kept in the created cold place (-3°C) for 1 minute. Then, it was dried in a laboratory dryer and kept for 24 hours at a temperature of 23 °C to prepare for determining the characteristics.
Table 1. Solvent Condition as a Surface Modifier
Post-acid treatment papers
Acid post-treatment was applied as a coagulating agent in the wet spinning of regenerated fibers in NaOH/urea solvent (Fu et al. 2018) immediately after surface modification of paper with NaOH/urea, as per Table 2’s parameters. Note for the acidic post-treatment paper had no 1 min rest time with NaOH/urea and immediately, after NaOH/urea modification, the paper was immersed in acid for 1min.
Table 2. Acid Condition as Post-treatment
Properties of paper
The Properties of papers with and without post-treatment were investigated. The Technical Association of the Pulp and Paper Industry (TAPPI) standards for measuring strength and barrier qualities are shown in Table 3.
Table 3. TAPPI Standards for Measuring Strength and Barrier Qualities
Fourier Transform Infrared Spectroscopy analysis
The infrared spectra of the with and without post-treatment papers were measured using a Cary 630 FTIR spectrometer (Agilent Technologies, Santa Clara, CA, USA). The spectra were measured with a spectral width ranging from 600 to 4000 cm-1 at a resolution of 2 cm-1.
Scanning Electron Microscopy Analysis
The morphology of with and without post-treatment papers was examined via SEM analysis. The SEM micrographs of the samples were taken using an SNE-4500 electron microscope (Mira3, Tescan, Czech Republic).
X-ray diffraction Analysis
The XRD analysis was performed using a Rigaku Uluima IV model (Japan). The samples were scanned with CU Kα = 1.5406 Å radiation at a diffraction angle (2θ) ranging from 5° to 80° with a step size of 1°/mm.
RESULTS AND DISCUSSION
Appearance Properties
Tables 4 and 5 indicate the chemical composition of OCC pulp and the basis weight, density, thickness, and ash content of papers, respectively.
Table 4. Chemical Composition of OCC
As shown in Table 5, the weight of the paper decreased after treatment. This was attributed to the process of partial dissolution, which was accompanies by swelling. It is notable that the alkaline medium is less absorbed and dissolved when paper with a high degree of polymerization and high crystallinity, such as filter paper made of cotton linters (Xiao et al. 2014). In comparison, OCC paper has low crystallinity and DP. According to ash content in Table 5, a portion of the paper’s weight loss can be attributed to the removal of ash. For instance, NaOH/urea surface modification eliminated 3.9% of ash, which might account for a 6% decrease in paper weight. Despite the drop in paper dimensions (length and width) following treatment, the weight loss resulted in a considerable decrease in basis weight. In studies carried out by Piltonen et al. (2016), the identical kind of paper treatment resulted in a reduction in thickness and a rise in density, while unbleached pine pulp rose in thickness. (Hilderbrandt et al. 2017).
Table 5. Appearance Properties of Papers
Mechanical Strength Properties
As shown in Fig. 1, the tensile index in the papers was decreased by NaOH/urea treatment. The tensile index in the control and treated samples were 33.31 and 29.4 mN/g, respectively. However, acidic post-treatment increased the tensile strength from 33.31 to 37.46 mN/g). After surface modification with NaOH/urea, the burst index of the papers fell by 7%, as depicted in Fig. 1. Similarly, acid treatment enhanced the burst strength in comparison to the control sample, although tear strength decreased in both instances.
Fig. 1(A&B). Tensile (A), burst (B), and tear (C) indexes in the papers by NaOH/urea treatment
Fig. 1(C). Tensile (A), burst (B), and tear (C) indexes in the papers by NaOH/urea treatment
The swelling and breakdown of cellulose fibers are anticipated to provide a more compact structure and greater strength in the paper (Xiao et al. 2014). However, the lignin content of OCC paper is 12.95 percent; thus strength properties can be reduced. The presence of lignin, a hydrophobic polymer, prevents the maximization of bonding between the fibers, which is a consequence of the fact that cellulose, the principal component of long-chained fibers, is strongly tied to the physical attributes of paper, particularly tensile strength (Miao et al. 2018). In reality, the increased lignin percentage reduces the self-reinforcing effect because the lignin coating of the fibers prevents cellulose disintegration (Isogai and Atalla 1998; Le Moigne and Navard 2010). In addition, the presence of hemicellulose that binds to cellulose reduces the resistance qualities of the material (Hilderbrandt et al. 2017). In the manufacturing of biocomposites from filter paper (devoid of contaminants) and microfibre films, usage of the NaOH/urea solvent system improved tensile strength (Duchemin et al. 2015). Another study on increasing the strength properties of kraft paper showed that during the dissolution time of one minute, the dissolution was limited to the surface and with the formation of a homogeneous fibrous network on the surface, most of the fibers remained intact and the properties of the paper were preserved. Also, as the dissolution time increases, it affects the bulk of the fiber and changes the crystal structure, which turns into an amorphous state (Uusi-Tarkka et al. 2022). This shows that due to the weak structure of the OCC paper, the duration of one minute was maybe too long and it harmed the paper and it needs a post-treatment.
Additionally, the physical structure of the pulp governed the paper’s strength properties. Non-wood fibers, such as abaca, have significantly smaller lumens than wood fibers (Dinand et al. 2002). Therefore, surface penetration and dissolution are larger in wood fibers than in non-wood fibers, and the process of wood self-reinforcement is enhanced. In contrast, the lowering of resistance qualities is also influenced by the microstructure of the fibers. OCC paper is semi-crystalline, and dissolution happens preferentially in the amorphous portion; the loss of this portion reduces the paper’s resilience (Dormanns et al. 2016). Therefore, the discrepancy between the resistances observed in this investigation and those of other studies can be attributed to variations in the production process, degree of polymerization, and post-treatment techniques (Duchemnin et al. 2015; Hilderbrandt et al. 2017). Due to the weakening of compounds, calendering was ineffective as a post-treatment of the NaOH/urea treatment (Hilderbrandt et al. 2018).
In comparison to the control sample, the tensile strength of the treated sample increased after acidic post-treatment. It appears that the acid-induced swelling disperses the outer cell walls of the fibers and eliminates the short chains of beta-cellulose and gamma-cellulose, forming a layer on the paper’s surface. After neutralization rinsing and during drying, short-chain polysaccharides create a barrier that repositions on and around the fibers, resulting in increased resistance (Reyden et al. 1995). Next comes immersion in sulfuric acid, which changes the wood fibers into an amyloid gel. The gel is then solidified by washing and neutralization, which bonds the fibers into a solvent resistant paper with high initial wet strength (Reyden et al. 1992).
Similar to tensile strength, bursting strength depends on the bondability of the fibers. Consequently, the presence of contaminants and lignin affects the burst resistance in NaOH/urea treatments. The acidic post-treatment enhanced burst and tensile strength. However, due to the increase in bendability and connectivity of fibers, the tear strength was reduced (Koubaa and Koran 2018).
Figure 2 illustrates the reduction in flexural rigidity for both techniques. Paper’s bendability and thickness determine its bending stiffness, which is a measure of its tensile strength. Due to the drop in tensile strength caused by the NaOH/urea treatment, stiffness also decreases. In addition, recycled fibers include an abundance of particles that increase stiffness due to their strong bonding and enhanced strength. The stiffest mixtures of these materials are those containing fillers (Subramanian et al. 2011). Consequently, following modification and washing, a portion of these fillers are extracted from the paper. According to Table 5, due to the fact that the amount of minerals (ash) is effective on the stiffness and also the thickness has a linear relationship with the stiffness. Therefore, despite the reduction of ash, the increase in thickness shows that this modification does not create a significant difference in stiffness.
Fig. 2. Bending strength of untreated and treated papers by NaOH/urea
Barrier Properties
The use of fillers in paper can enhance the surface smoothness of the paper (Hubbe and Gill 2016). As illustrated in Fig. 3A, after surface modification with NaOH/urea, surface smoothness decreased by 34%, indicating the influence of surface modification using aqueous NaOH/urea solvent on the paper’s smoothness decline.
Fig. 3. Smoothing (A), air resistance (B) and Cobb (C) in the papers by NaOH/urea treatment
As can be seen in Fig. 3B, the air resistance increased in both techniques compared to the control sample; however, the acidic post-treatment resulted in an increase by 22%. The 18% improvement in acidic post-treatment to NaOH/urea treatment (acid-free) is attributable to the creation of a membrane on the fiber structure that prevents air from entering the pores and cavities between the fibers (Reyden et al. 1995). Figure 1 demonstrates that the improvement in tensile strength following acid application was also attributable to the previously reported effect of acidic post-treatment on the creation of a layer of short polysaccharide chains.
As depicted in Fig. 3C, both treatments rendered recycled paper hydrophilic, and the contact angles of the materials depicted in Fig. 4 confirm this. Although NaOH/urea can fill holes and eliminate voids in a paper (Jia et al. 2015), the use of this solvent as a strengthening agent on recycled paper increased water absorption and hydrophilicity, hence reducing the smoothness of the paper surface and increasing the absorption. However, acidic post-treatment was able to limit water absorption compared to other methods, which was related to the production of a membrane of short-chain polymers, as reported in the research on tensile strength (Reyden et al. 1995).
Fig. 4. Contact angles in the default OCC papers modified by NaOH/urea treatment and optional acid post-treatment
X-ray Diffraction
The XRD results of the changed sheets are illustrated in Fig. 5. It should be noted that it must be speculated that the amorphous structure of lignin and hemicellulose causes large peaks in the pre-angle range of 20 degrees (13 to 19) that can be ignored (Hildebrandt et al. 2017).
Regarding the peaks at 15.54 and 22.72, the strength of corrective peaks decreased, whereas the intensity of the same peaks in papers with acidic treatment was comparable to that of the control paper. The absence of acid post-treatment of two peaks in grades 11.69 and 20.7 degrees in the control paper shows that cellulose I was converted to cellulose II (Jiao et al. 2015; Xu et al. 2019). The degree of crystallinity of the control sample (without alteration), without acidic post-treatment, and with acidic post-treatment, respectively, was 76.50, 73.88, and 76.07. It appears that the acidic post-treatment allowed for less reduction in crystallinity.
Fig. 5. XRD spectra of acidic post-treatment, without acidic post-treatment and control samples
Increasing the amorphous portion facilitates paper swelling and is followed by enhanced fiber bonding, which results in prolonged water retention in the modified paper (Miao et al. 2018). However, the treatment increases the hydrophilicity of the modified paper. In this investigation, it was determined that the crystalline portions of the paper were not eliminated to make the amorphous sections bondable. In general, crystallinity changes of 2% to 3% suggest that the NaOH/urea solvent did not significantly alter the crystallinity structure and that the reaction zone was limited to the surface of the paper fibers (Zhai and Zhou 2014).
Fourier Transform Infrared Spectroscopy
As demonstrated in Fig. 6, the peaks of acidic post-treatment paper and control paper were comparable. Similar results were seen while modifying TMP paper and filter paper with NaOH/thiourea/urea (Zhai and Zhou 2014; Zhai et al. 2015).
Fig. 6. FTIR spectra of acidic post-treatment, without acidic post-treatment and control samples
Jiao et al. (2015) found broad peaks at 13400 cm-1, showing an increase in hydrogen bonding. These features were more intense and acute in NaOH/urea (acid-free) surface modification, indicating a weak binding. Urea is associated with the peaks at 1635 and 1440 cm-1 (Zhai and Zhou 2014), which were present in both samples. Modification with NaOH/urea also resulted in the displacement of peaks 1111, 1430, and 895 cm-1, which indicates the conversion of a portion of the crystals to amorphous (Jiao et al. 2015). However, the sharpness and narrowness of peaks such as 3400 indicate weak bonding of paper described in the cited article (Zhang et al. 2016). This weakness justifies the low tensile characteristics of NaOH/urea-modified materials.
Scanning Electron Microscopy
Figure 7 represents the SEM results of the cross-sections of the modified papers and the control paper. This figure confirms the increase in thickness noted in Table 5 for the NaOH/urea-modified papers, but the space was more pronounced in non-acidic-treated paper than in acidic-post-treated paper.
In other studies, employing the same technique, after 30 seconds the visible fiber structure on paper made from pine and spruce sulfite paste had completely disappeared, and the matrix and reinforcing fiber phases were indistinguishable, indicating excellent compatibility. This condition was not observed in modified OCC papers (Piltonen et al. 2016). In actuality, the modification of NaOH/urea solvent-reinforced sheets is contingent upon the type of raw material. It was indicated that the presence of lignin in papers made from unbleached pine and eucalyptus pulp changed with less intensity than in unbleached pine and eucalyptus papers (Hildebrandt et al. 2017). Bleaching has a favorable effect on covering the paper’s surface with the dissolved portion of the fibers in NaOH/urea solvent (Tervahartiala et al. 2018). In the comparison of urea and thiourea in this solvent, another study showed that the surface of the cotton paper at a temperature of 8 °C. with thiourea was the same as the surface of the paper at a temperature of -12 with urea. A uniform cross-section with fewer cracks can be observed, which suggests that this was the optimal temperature for using thiourea for partial dissolution and production of the all-cellulosic composite was considered at 8 °C. But the structure of the paper with the presence of urea was denser with less porosity (Hu et al. 2020).
Fig. 7. Cross-section SEM of acidic post-treatment, without acidic post-treatment and control
Figure 8 illustrates the surface SEM. The presence of voids on the surface of the changed paper can confirm the increase in water absorption and decrease in air permeability of paper treated with non-acidic substances. In the surface SEM of acidified post-treated paper, fewer pores are detected, which can be explained by the formation of a membrane on the paper’s surface following the application of acid, which is also noted in the process of improving tensile strength and air permeability. In general, the damage to the paper’s surface caused by the NaOH/urea solvent suggests that this solution has damaged only the surface fibers of the paper and has had a lesser effect on other fibers (Zhai and Zhou 2014). Partially soluble NaOH/urea solvent produced semi-soluble cellulose as an adhesive to bind insoluble fibers during regeneration (Xiao et al. 2014). However, it should be noted that the low dissolution of OCC in NaOH/urea means that it does not adhere well to insoluble fibers and forms a weak, porous structure.
Fig. 8. Surface SEM of acidic post-treatment, without acidic post-treatment, and control samples
Brunauer-Emmett-Teller
The BET method is one of the most important and recognized techniques for determining the porosity and specific surface area of different materials. This method is based on an absorption technique that is fully non-destructive and less expensive than other ways. Furthermore, the capacity to evaluate current data is significantly more precise, straightforward, and effective. The BET-specific surface area and porosity report presented in Table 6 can confirm the alterations observed by SEM in the current study.
Table 6. BET – Specific Surface Area and Porosity of Papers
As demonstrated by the results, the porosity of the paper changed with NaOH/urea solvent rose by approximately 30%, as validated by the material’s surface SEM. Compared to non-acidic post-treatment, acidic post-treatment resulted in a 27% reduction in paper porosity, which was corroborated by the SEM of acidified post-treated paper in Fig. 7.
CONCLUSIONS
- Surface modification of recycled old corrugated container (OCC) paper by a NaOH/urea solvent has not been as effective as it has been for other virgin papers, according to studies. Therefore, OCC is not recommended for this modification method.
- Using 15% sulfuric acid as an acidic post-treatment after surface modification of paper with NaOH/urea enhanced the tensile and burst strengths but decreased the tear strength, which is expected due to the improvement of the other two strengths.
- Despite the increase in paper surface roughness caused by acidic post-treatment, the formation of a layer of short-chain cellulose resulted in increased air resistance and decreased water absorption in the acidic post-treated paper.
REFERENCES CITED
Belgacem, M. N., and Gandini, A. (2005). “The surface modification of cellulose fibers for use as reinforcing elements in composite materials,” Composite Interfaces 12(1-2), 41-75. DOI: 10.1163/1568554053542188
Dinand, E., Vignon, M., Chanzy, H., and Heux, L. (2002). “Mercerization of primary wall cellulose and its implication for the conversion of cellulose I→ cellulose II,” Cellulose 9(1), 7-18. DOI: 10.1023/A: 1015877021688
Dormanns, J. W., Schuermann, J., Müssig, J., Duchemin, B. J., and Staiger, M. P. (2016). “Solvent infusion processing of all-cellulose composite laminates using an aqueous NaOH/urea solvent system. Part A,” Applied Science and Manufacturing 82, 130-140. DOI: 10.1016/j.compositesa.2015.12.002
Duchemin, B., Le Corre, D., Leray, N., Dufresne, A., and Staiger, M. P. (2016). All-cellulose composites based on micro- fibrillated cellulose and filter paper via a NaOH-urea solvent system,” Cellulose 23(1), 593-609. DOI: 10.1007/s10570-015-0835-4
Fu, F., Zhang, W., Zhang, R., Liu, L., Chen, S., Zhang, Y., and Yao, J. (2018). “NaOH/urea solution spinning of cellulose hybrid fibers embedded with Ag nanoparticles: Influence of stretching on structure and properties,” Cellulose 25(12), 7211-7224. DOI: 10.1007/s10570-018-2082-y
Hildebrandt, N. C., Piltonen, P., Valkama, J. P., and Illikainen, M. (2017). “Self-reinforcing composites from commercial chemical pulps via partial dissolution with NaOH/urea,” Industrial Crops and Products 109, 79-84. DOI: 10.1016/j.indcrop.2017.08.014
Hildebrandt, N. C., Piltonen, P., Valkama, J. P., and Illikainen, M. (2018). “The effect of calendering on the mechanical properties of paper-based, self-reinforcing composites,” Cellulose 25(7), 4001-4010. DOI: 10.1007/s10570-018-1831-2
Hu, F., Hu, Y., Zhang, L., Gan, M., Liu, S., Xie, Y., and Feng, Q. (2020). “Preparation and characterization of self-reinforced paper using NaOH/thiourea aqueous solution at room temperature,” BioResources 15(4), 8191-8201. DOI: 10.15376/biores.15.4.8191-8201
Hubbe, M. A., and Gill, R. A. (2016). “Fillers for papermaking: A review of their properties, usage practices, and their mechanistic role,” BioResources 11(1), 2886-2963. DOI: 10.15376/biores.11.1.2886-2963
Isogai, A., and Atalla, R. H. (1998). “Dissolution of cellulose in aqueous NaOH solutions,” Cellulose 5(4), 309-319. DOI: 10.1023/A: 1009272632367
Jiao, L., Ma, J., and Dai, H. (2015). “Preparation and characterization of self-reinforced antibacterial and oil-resistant paper using a NaOH/Urea/ZnO solution,” PLoS One 10(10), article e0140603.DOI: 10.1371/journal.pone.0140603
Koubaa, A., and Koran, Z. (2018). “Effect of press-drying parameters on paper properties,” in: Pulp and Paper Processing, IntechOpen, pp. 87-107. DOI: 10.5772/INTECHOPEN.76508
Lavoine, N., Desloges, I., Khelifi, B., and Bras, J. (2014). “Impact of different coating processes of microfibrillated cellulose on the mechanical and barrier properties of paper,” Journal of Materials Science 49(7), 2879-2893. DOI: 10.1007/s10853-013-7995-0
Le Moigne, N., and Navard, P. (2010). “Dissolution mechanisms of wood cellulose fibers in NaOH–water,” Cellulose 17(1), 31-45. DOI: 10.1007/s10570-009-9370-5
Ma, J., Zhou, X., Xiao, H., and Zhao, Y. (2014). “Effect of NaOH/urea solution on enhancing grease resistance and strength of paper,” Nordic Pulp & Paper Research Journal 29(2), 246-252. DOI: 10.3183/npprj-2014-29-02-p246-252
Miao, Y., Zhi, Y., Zhang, H., Chen, Y., Shan, S., Jia, Q., and Ni, Y. (2018). “Recycled fiber treated with NaOH/urea aqueous solution: Effects on physical properties of paper sheets and on hornification,” Nordic Pulp & Paper Research Journal 33(4), 651-660. DOI: 10.1515/npprj-2018-0014/HTML
Piltonen, P., Hildebrandt, N. C., Westerlind, B., Valkama, J. P., Tervahartiala, T., and Illikainen, M. (2016). “Green and efficient method for preparing all-cellulose composites with NaOH/urea solvent,” Composites Science and Technology 135, 153-158. DOI: 10.1016/j.compscitech.2016.09.022
Samyn, P. (2013). “Wetting and hydrophobic modification of cellulose surfaces for paper applications,” Journal of Materials Science 48(19), 6455-6498. DOI: 10.1007/s10853-013-7519-y
Sescousse, R., & Budtova, T. (2009). “Influence of processing parameters on regeneration kinetics and morphology of porous cellulose from cellulose–NaOH–water solutions,” Cellulose, 16, 417-426.DOI: 10.1007/s10570-009-9287-z
Subramanian, R., Hiltunen, E., and Gane, P. A. (2011). “Potential use of micro-and nano fibrillated cellulose composites exemplified by paper,” in: Cellulose fibers: Bio-and Nano-polymer Composites, Springer, Berlin, Heidelberg, pp. 121-152. DOI: 10.1007/978-3-642-17370-7_5
Tervahartiala, T., Hildebrandt, N. C., Piltonen, P., Schabel, S., and Valkama, J. P. (2018). “The potential of all‐cellulose composites in corrugated board applications: Comparison of chemical pulp raw materials,” Packaging Technology and Science 31(4), 173-183. DOI: 10.1002/pts.2365
Uusi-Tarkka, E. K., Skrifvars, M., and Haapala, A. (2021). “Fabricating sustainable all-cellulose composites,” Applied Sciences 11(21), article 10069
Uusi-Tarkka, E. K., Levanič, J., Heräjärvi, H., Kadi, N., Skrifvars, M., and Haapala, A. (2022). “All-cellulose composite laminates made from wood-based textiles: Effects of process conditions and the addition of TEMPO-oxidized nanocellulose,” Polymers 14(19), article 3959.
Van Der Reyden, D., and Baker, M. (1995). “Genuine vegetable parchment paper: Effects of accelerated aging on some physical and chemical properties,” MRS Online Proceedings Library (OPL) 352. DOI: 10.1557/PROC-352-271
Van Der Reyden, D., Hofmann, C., Baker, M., and Mecklenburg, M. (1992). “Modern transparent papers: Materials, degradation, and the effects of some conservation treatments,” MRS Online Proceedings Library (OPL) 267.DOI: 10.1557/PROC-267-379
Vaswani, S., Koskinen, J., and Hess, D. W. (2005). “Surface modification of paper and cellulose by plasma-assisted deposition of fluorocarbon films,” Surface and Coatings Technology 195(2-3), 121-129. DOI: 10.1016/j.surfcoat.2004.10.013
Wei, Q. Y., Lin, H., Yang, B., Li, L., Zhang, L. Q., Huang, H. D., and Li, Z. M. (2020). “Structure and properties of all-cellulose composites prepared by controlling the dissolution temperature of NaOH/urea solvent,” Industrial & Engineering Chemistry Research 9(15), 4294-4301. DOI: 10.1021/acs.piece.9b07075
Xu, L., Teng, J., Li, L., Huang, H. D., Xu, J. Z., Li, Y., Ren, P.-G., Zhong, G-J., and Li, Z. M. (2019). “Hydrophobic graphene oxide is a promising barrier of water vapor for regenerated cellulose nanocomposite films,” ACS Omega 4(1), 509-517. DOI: 10.1021/acsomega.8b02866
Yu, P., He, H., Luo, Y., Jia, D., and Dufresne, A. (2017). “Reinforcement of natural rubber: the use of in situ regenerated cellulose from alkaline–urea–aqueous system,” Macromolecules 50(18), 7211-7221. DOI: 10.1021/acs.macro mol.7b01663
Zhai, R., Yuan, Y., and Zhou, X. (2015). “Preparation of wet strength paper from filter paper with NaOH-thiourea-urea aqueous solution,” BioResources 10(1), 839-850.
Zhai, R., and Zhou, X. (2014). “Enhanced effect of NaOH/thiourea/urea aqueous solution on paper strength of high-yield pulp,” BioResources 9(2), 2154-2166.
Zhang, W., Jing, Z., Shan, Y., Ge, X., Mu, X., Jiang, Y., and Wu, P. (2016). “Paper reinforced with regenerated cellulose: A sustainable and fascinating material with good mechanical performance, barrier properties and shape retention in water,” Journal of Materials Chemistry A 4(44), 17483-17490. DOI: 10.1039/C6TA07681E
Zhu, R., Liu, X., Song, P., Wang, M., Xu, F., Jiang, Y., and Zhang, X. (2018). “An approach for reinforcement of paper with high strength and barrier properties via coating regenerated cellulose,” Carbohydrate Polymers 200, 100-105. DOI: 10.1016/j.carbpol.2018.07.069
Article submitted: January 4, 2023; Peer review completed: February 4, 2023; Revised version received and accepted: April 9, 2023; Published: April 27, 2023.
DOI: 10.15376/biores.18.2.4153-4167
