Abstract
The production of energy and biochar from the pyrolysis of straw and other agricultural residues is a developing industry that holds the potential to reduce air pollution from in-field burning, recycle nutrients, reduce chemical fertilizer inputs, and improve soil quality. Use of biochar in agriculture is undergoing tests globally. Water-soluble organic compounds from pyrolysis residue containing humic substances, low-molecular weight acids, and neutral compounds and inorganic elements containing macro and micro nutrients have been shown to be beneficial for plant growth. In the present study, crop straw biochars were extracted into hot water and the extracts tested for their effect on growth of Chinese cabbage. The extracts were sprayed 10 times at three different dilutions throughout the growing period. The extracts were characterized for inorganic elements by ICP-MS and for organic compound type by LC-OCD and FTIR. Results showed that extracts of both wheat and maize straw biochar can significantly increase the yield, vitamin C content, and soluble protein content while decreasing the nitrate content of the cabbage at dilutions of 50 or 100 times. Water extract from biochar was found to have great potential as a liquid amendment in agricultural.
Download PDF
Full Article
Water Extract from Straw Biochar Used for Plant Growth Promotion: An Initial Test
Yingmei Lou,a Stephen Joseph,a,b,c,* Lianqing Li,a,* Ellen R. Graber,d Xiaoyu Liu,a and Genxing Pan a
The production of energy and biochar from the pyrolysis of straw and other agricultural residues is a developing industry that holds the potential to reduce air pollution from in-field burning, recycle nutrients, reduce chemical fertilizer inputs, and improve soil quality. Use of biochar in agriculture is undergoing tests globally. Water-soluble organic compounds from pyrolysis residue containing humic substances, low-molecular weight acids, and neutral compounds and inorganic elements containing macro and micro nutrients have been shown to be beneficial for plant growth. In the present study, crop straw biochars were extracted into hot water and the extracts tested for their effect on growth of Chinese cabbage. The extracts were sprayed 10 times at three different dilutions throughout the growing period. The extracts were characterized for inorganic elements by ICP-MS and for organic compound type by LC-OCD and FTIR. Results showed that extracts of both wheat and maize straw biochar can significantly increase the yield, vitamin C content, and soluble protein content while decreasing the nitrate content of the cabbage at dilutions of 50 or 100 times. Water extract from biochar was found to have great potential as a liquid amendment in agricultural.
Keywords: Biochar; Water extractable organic carbon; Humic substance; Chinese cabbage; Yield; Quality
Contact information: a: Institute of Resource, Ecosystem and Environment of Agriculture, Nanjing Agricultural University, Nanjing 210095-China; b: Discipline of Chemistry, University of Newcastle, Callaghan, NSW 2308, Australia; c: School of Materials Science and Engineering, University of New South Wales, Sydney, NSW 2052, Australia; d: Institute of Soil, Water & Environmental Sciences, The Volcani Center, Agricultural Research Organization, POB 6, Bet Dagan 50250, Israel;
* Corresponding author: stephenjoseph@aliyun.com; lqli@njau.edu.cn.
INTRODUCTION
The production of energy and biochar from agricultural wastes in China is an emerging industry (Pan et al. 2011a). This industry is being driven by the need to find alternatives to burning crop residues, primarily straw, in the field (Pan et al. 2011b) and to reduce greenhouse gas emissions from agriculture (NDRC 2014). Biochar was reported to increase crop yields (Liu et al. 2013), raise the resistance to plant disease (Elad et al. 2010), decrease the uptake of heavy metals by plants in contaminated soil (Cui et al. 2011, 2012), and improve soil carbon sequestration (Lehmann et al. 2006). However, the present high cost of biochar is limiting its market and its potential to expand (Clare et al. 2014). The market potential for production of biochar could be increased if high value but small volume biochar products could be developed for use in agriculture. In the manufacture of biochar, a considerable quantity of water is produced from the scrubbing and cooling of biochar. This water may have a high concentration of cations, anions, and organic compounds that can serve as both plant nutrients and growth stimulants.
As a case in point, it has been shown that some organic volatiles given off during the pyrolysis of biomass can function as germination stimulants and bio-pesticides (Light et al. 2009; Kulkarni et al. 2011). The condensed smoke and active ingredients isolated from the smoke can increase seedling resistance to water and heat stress and improve germination in some crops (Daws et al. 2007, 2008), while increasing fruit yield and size in horticulture (Kulkarni et al. 2008). Dilutions of wood vinegar collected during biomass pyrolysis, which contains a large number of organic compounds including ketones, phenols, and olefins, improved vegetable growth and quality when used as a foliar spray (Yan et al. 2011). Moreover, humic-like substances extracted from a greenhouse waste biochar caused a change in plant perception of P nutrition, leading Arabidopsis seedlings growing under P starvation conditions to develop root hairs as if they were growing under P sufficient conditions (Graber et al. 2015).
It would be of significant merit if compounds (both organic and inorganic) rinsed from biochar during its production could be used for plant growth promotion, thus adding value to the pyrolysis/biochar platform and, at the same time, reducing wastes. The present study models that system, by testing hot water extracts of two different biochars as foliar sprays for potted Chinese cabbage (Brassica rapa). Both yield and quality parameters were determined.
EXPERIMENTAL
Materials
Biochars
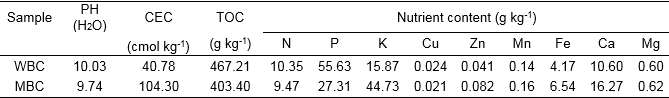
Wheat straw biochar was produced by Sanli New Energy Company, Henan, China in a downdraft gasifier running in pyrolysis mode with a maximum temperature of approximately 480 °C and a yield of approximately 30%. Maize stalk biochar was produced by ShanXi Gongxiao Company, Shanxi, China in a simple batch pyrolyzer. The average maximum pyrolysis temperature was approximately 500 °C, and yields were approximately 33%. The properties of the biochars were analyzed following the methods described in Lu (2000) and are presented in Table S1. The WBC was additionally characterized as described in Joseph et al. (2013).
Methods
Hot water biochar extraction
A foliar spray concentrate was prepared by extracting the biochars as follows: ten grams of each biochar were added to 200 mL of demineralized water and heated in a water bath at 100 °C for 3 h. The mixture was then shaken on a rotatory shaker at 180 rpm at room temperature (25 °C) for 24 h and subsequently vacuum-filtered through a 30-μm ceramic filter. The liquid extracts were stored at 4 °C prior to analysis and pot trials.
Extract analysis
Analysis of the extract for pH, electrical potential (Eh), and electrical conductivity (EC) was carried out using the methods described by Lu (2000). Elements were determined by ICP-MS (Perkin Elmer, USA, NexION 300D with Universal cell technology). Quantification and characterization of organic compounds were carried out by liquid chromatography with organic carbon detection (LC-OCD) using the same equipment and procedure as described in Lin et al. (2011, 2012). In the present study, a Toyopearl TSK HW50S column (Tosoh, USA) was used with a phosphate buffer mobile phase of pH 6.4 at a flow rate of 1.1 mL/min. Injection volumes were 1000 µL. The chromatographic column is a weak cation exchange column containing a polymethacrylate filter. This method subdivides the organic carbon fraction into six sub-fractions, which are assigned to specific classes of compounds: biopolymers, humics, building blocks, low-molecular weight neutrals, and hydrophobic organic carbon (Huber et al. 2011). For FTIR analysis, the concentrated extract was lyophilized at -70 °C using a MODULYOD-230 unit (Thermo Electron Corporation, USA). The freeze-dried material was mixed with KBr powder at a ratio of 1:200 and ground to a fine powder. FTIR spectra were obtained using a Bruker (USA) 66V/S Fourier transform infrared (FTIR) spectrometer with a resolution of 4 cm-1; the range of the spectrum is from 400 to 4000 cm-1.
Greenhouse experiment
Topsoil of a clay-loam soil was collected from a vegetable garden in a suburb of Nanjing (31°58′ N, 118°48′ E) and used for the pot experiment. The topsoil was air-dried and ground to pass through a 5-mm sieve for the pot experiment. To determine the pH, ammonium, and available K and P, the air-dried soil was ground to pass through a 2-mm sieve. To determine the organic matter content and total N, the soil was ground to pass through a 0.149-mm sieve. All the tests followed methods described by Lu (2000). The soil properties were as follows: soil pH in water was 6.03, organic matter content was 16.32 g/kg, total N was 1.19 g/kg, and ammonium (NH4+-N) and available K and P were 8.23 mg/kg, 108.10 mg/kg, and 6.75 mg/kg, respectively.
The greenhouse pot experiments were designed to test cabbage growth as influenced by biochar extracts from wheat straw (SW) and maize stalk (SM) at three dilutions (1:25, 1:50, and 1:100 by volume). Each pot contained 2 kg of dry soil, thoroughly mixed with urea, monoammonium phosphate, and KCl at a rate of N: P2O5: K2O=0.2:0.15:0.2 g/kg of dry soil. Each pot was then watered to 26% of field water holding capacity. Twenty seeds of Chinese cabbage (Brassica rapa) were sown on the surface soil of each pot and then covered with an approximately 2-mm of additional soil. One week after germination, four seedlings with similar size and color were retained per pot (the four together were considered a single biological replicate). There were four replicate pots per treatment.
Pots were weighed and watered to maintain approximately 26% of field water holding capacity during the first week. Two hundred milliliters of diluted liquid extract was sprayed on each pot two times at the beginning of the second and third weeks after germination. Spraying was repeated every three days from the fourth week until harvest. Each pot was sprayed ten times in total over the 45 days of growth. Filtered tap water was sprayed on plants in the control treatment. In addition to the application of the spray, each pot was watered every five days to maintain a 26% field water hold capacity. The pot experiment, in a random block design, was done in a greenhouse with a temperature between 18 and 28 °C on the campus of Nanjing Agriculture University.
Plant analysis
All cabbage plants in a single pot were pooled at harvest, washed with deionized water to remove attached soil particles, the excess water from plant surface was shaken off, and then remaining water was removed using a fan. The moisture free fresh weight was then recorded. Plants were then separated into roots and shoots. A portion of the fresh shoots was refrigerated at 4 °C prior for chemical analysis, and another portion was dried in an air-convention oven at 105 °C for 30 min, followed by 60 °C for another 48 h (Lu 2000), The dry weight was then recorded before other analysis was undertaken.
Fresh roots were cut from the cabbage, washed with deionized water, then blotted with absorbent paper prior to determining root length, surface area, tips, and average diameter using an Epson (Japan) Expression 1640XL with Epson Twain Pro2.10 analysis software. A vegetable crusher was used to mash and blend the fresh shoots for determination of vitamin C, soluble sugar, protein, and nitrite contents via the procedures of Wang (2006). For nutrient measurements, dried and weighed samples were ground in a stainless mill to pass through a 1-mm sieve. A 0.5 g portion of dry sample was solubilized in a 100-mL flask with a 10-mL mixture of HNO3 and HClO4 (8:2, v:v) at room temperature overnight, followed by heating at 100 °C for 30 min and then 250 °C until the solution became clear. The solution was then transferred to a 25-mL volumetric flask and diluted to 25 mL after which is was filtered. The contents of mineral cations were determined using flame atomic absorption spectrometry (FAAS, A3, Persee, China). Reagent blanks and reference materials were used in each batch, and the recovery of the detected metals was between 92% and 110%.
Statistical analysis
All data are shown as mean ± one standard deviation (n=4). Differences between the treatments, comparing the effects of biochar extract type, dilution rate, and their interaction, were examined using a two-way analysis of variance (ANOVA). Then, differences between the whole treatments of one biochar and the control were examined using ANOVA, followed by ad-hoc means comparison using the Tukey Honestly Significant Difference test at p < 0.05. All statistical analyses were carried out using SPSS, version16.0 (SPSS Institute, USA, 2007).
RESULTS
Chemical Properties of Biochar Extracts
The properties and chemical composition of the biochars are shown in Table S1, and the chemical compositions of the extracts are shown in Table 1. Nitrates and ammonium were not detected in the two extracts. The dissolved organic carbon (DOC) and total P for the SW extract was nearly double that of the SM extract. The pH of both extracts was alkaline, and Eh was low and nearly the same for each extract, indicating that both have reducing capability. EC, K, Si, B, Fe, Mg, and Zn were nearly the same in each extract (Table 1) while the concentrations of Ca, Cu, and Mn in the SW extract were significantly greater than those in the SM extract. Concentrations of Na in the SM extract were double compared to those in the SW extract.
As shown in Table 2, the concentrations of DOC, organic N, low-molecular weight neutrals, and humic substances in the SW extract were nearly doubled in comparison to the concentrations in the SM extract. The concentrations of low-molecular weight acids were very low in both extracts. In the two extracts, over 90% of the detected components were hydrophilic carbon, while humics were primarily the compounds in hydrophilic DOC, with 66.5% in SW and 60.4% in SM. The concentration of the dissolved organic nitrogen was very low in the SM but much higher in the SW extract. Most of the N was located in the humic substances, with an N/C ratio of 0.1 for SW and 0.04 for SM.
FTIR spectra (Fig. 1) provided some indications of the differences and similarities between the two extracts. Both extracts had peaks at approximately 2966 cm-1, indicating a C-H stretching of aliphatic compounds and/or PO-H and N-H compounds (Joseph et al. 2013). The presence of two very intense bands at 1630 and 1375 to 1403 cm-1 could have either been C=O vibration of bonded conjugated ketones, quinones, carboxylic esters (Carballo et al. 2008), and phenolics, or a reflection of the increased aromatic characteristics of the humic substances, as they can be assigned to in-plane ring motions of substituted benzene (Francioso et al. 1996). The intensity of these two peaks was greater for the SM extract. The intense peak at 1120 cm-1 was indicative of ν(Si-O) bands, O-P-O bending, and/or aromatic CH out-of-plane deformations of the aromatic humic substances and was greater for the SW extract. This is also reflected in the LC-OCD and ICP-MS analysis of the liquid. The bands at 1030, 990, 930, and 749 cm-1 could be associated with humic acids (Francioso et al. 1996). The significant peaks of both extracts between 885 and 482 cm-1 were indicative of pyridines; the peak at 831 cm-1 indicates primary amine groups (NH2 out-of-plane), and that at 704 cm-1 suggests secondary amine groups (N-H wag).
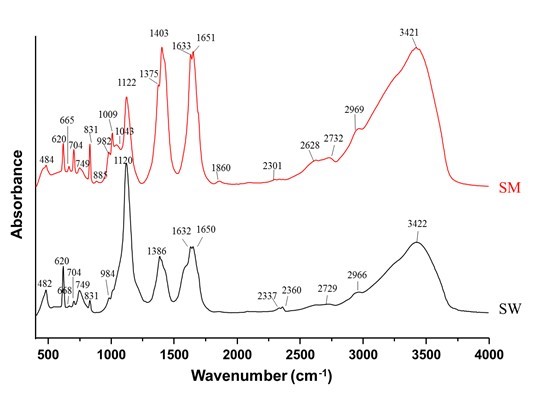
Fig. 1. Stack plot of FTIR absorbance spectra of the two biochar extracts
Effect on Plant Growth
Figures 2 and 3 present the cabbage yield (fresh weight of the shoot) and dry weight of the shoots and roots per pot for all the treatments, respectively. There was no significant difference between the two different extracts (p > 0.05) on these parameters, however, dilution rate did have a significant effect. Yield, shoot dry weight and root dry weight were all significantly higher in the 50-fold dilution of the SW extract than in the water only treatment (control). The 100-fold dilution of the SM extract also gave significantly greater yields and biomass dry weights, with an increase in yield by 68% and shoot dry weight of 62% in comparison to the control.
Table 1. Physico-Chemical Properties of the Biochar Extracts

The contents of I, Mo, Sr, Ba and Al were generally less than 2 mg/L. Others not detectable (< 0.5 mg/L)
Table 2. LC-OCD Quantitative Analysis of Dissolved Organic Carbon and Dissolved Nitrogen Fraction of Biochar Extracts
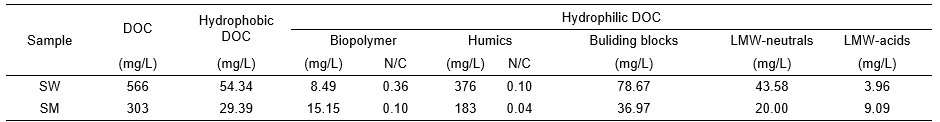
LMW: low molecular weight
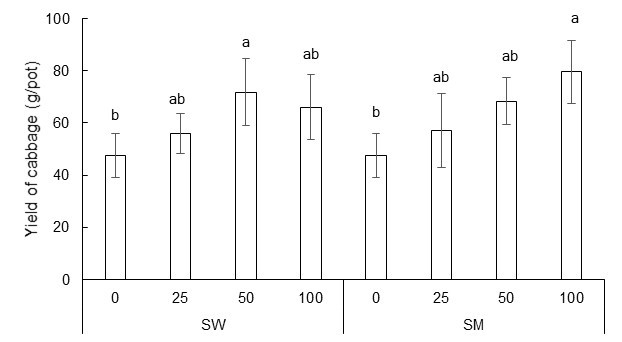
Fig. 2. Effect of biochar extract at various dilution rates on the yield of cabbage per pot. SD is shown as bars (p < 0.05)
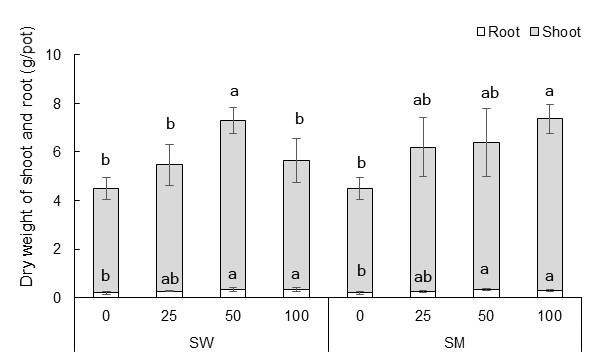
Fig. 3. Effect of biochar extract at various dilution rates on the dry weight of roots and shoots per pot. SD is shown as bars (p < 0.05)
Effect on Plant Quality
Table 3 summarizes the effects of biochar extract and dilution rate on the vitamin C, nitrate, soluble sugar, and soluble protein contents of the cabbage. Two-way ANOVA results showed significant interaction between extract type and dilution rate on all the quality indices with the exception of soluble sugar content. The vitamin C content of the cabbage was significantly higher in SW treated plants (117.7 and 71.0 mg/kg at 50 and 100-fold dilutions, respectively) than in control treated plants (42.2 mg/kg). As for the biomass response, a 50-fold dilution rate of the SW extract resulted in the highest vitamin C content, and the 100-fold dilution of the SM extract gave the highest vitamin C content. Both types of biochar aqueous extracts increased the content of soluble protein in the cabbage, but there was no significant difference between them. There was no difference in soluble protein content between dilution rates for SW. For SM, however, a dilution rate of 25 resulted in the greatest increase in soluble protein content (68%) compared to other dilution rates. A significant reduction in nitrate content was measured at a dilution rate of 50 for SW and of 100 for SM; these values were 21% and 26% lower than the control, respectively.
Table 3. Effect of Biochar Extract at Various Dilution Rates on the Quality Parameters of Cabbage
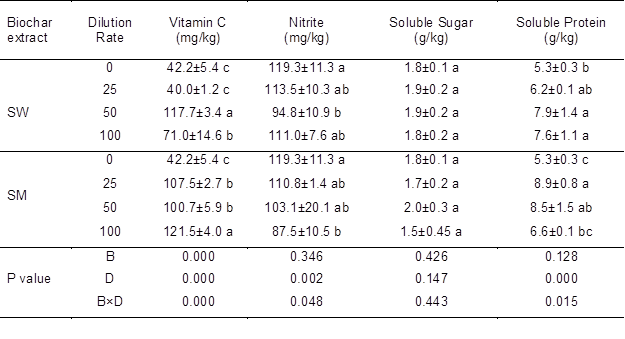
The values presented in the columns are mean ± standard deviation (n=3). Different small letters indicate differences among the four dilution rates for each biochar aqueous extract at p < 0.05. (A dilution rate of 0 means spraying water treated only, called control in the statement; SW: Sanli Wheat straw biochar aqueous extract. SM: Shanxi Maize straw biochar aqueous extract.) Value of P under a two-way ANOVA for the effects of biochar extract (B) and dilution rate (D) on vitamin, nitrate, soluble protein, and soluble sugar contents.
Effect on Root Growth and Leaf Area
The two-way ANOVA showed that dilution rate had a significant effect on root length, surface area, and root volume (Table 4). There were, however, no significant differences between the two different biochar aqueous extracts on these root-related indices. For SW, a dilution rate of 50-fold significantly increased root length, and surface area, 70%, and 80%, respectively, compared to the control. For SM, the highest increase was observed at the same rate as for SW. For leaf area, no difference between extract sources was found, while dilution rate did have a significant effect. The plants treated with the 50-fold dilution of the SW extract exhibited the highest leaf area, while those treated with 100-fold dilution of the SM extract had the highest leaf area; both were nearly 55% higher than the control.
Effect on Plant Nutrients
DISCUSSION
Results from this study showed that cabbage responded positively to foliar application of the two biochar extracts. This was evidenced by the increasing yield and dry weight of roots and shoots. Similar results were found by Taek et al. (2012), who studied the effect of biochar aqueous extract on lettuce germination and seedling growth and determined that high-dilution rate treatments could significantly promote seedling growth. These results were postulated to be a function of the composition and concentration of the cations, anions, and organic compounds in the extract. Chia et al. (2014) and Graber et al. (2010) have shown that biochar labile organic molecules produced at temperatures less than 500 °C consist of a range of low-molecular weight organic acids, phenols, aromatic hydrocarbons, and alkanes. These compounds have also been identified in wood vinegar and smoke water, which have been found to be effective foliar sprays for some plants (Kulkarni et al. 2007; Tuntika et al. 2013). Humic substances can promote the proliferation of lateral roots and increase root length and below-ground biomass (Fagbenro and Agboola 1993; Eyheraguibel et al. 2008), However, in the present study, the effects of both biochar extracts on plant growth and quality were similar, although LC-OCD results showed that SW biochar extract contained a much higher content of DOC and humic substances than SW. It is, therefore, probable that there is an interactional effect with other nutrients.
Both biochar aqueous extracts enhanced micronutrients accumulation in the cabbage in present study. These micronutrients can work in concert to boost photosynthesis and increase carbohydrate production (Bacchus 2010). In general, biochars containing oxygen functional groups, such as carboxyl, hydroxyl, and phenolic, are expected to bind ions, especially heavy metal in soil and aqueous solution (Cui et al. 2011, 2012; Uchimiya et al. 2012). However, biochars can potentially supply soil nutrients and trace metals (Zhao et al. 2013; Gunes et al. 2015). Elements originating from biochar such as Na, Ca, K, Mg, P, and S can be released into soil (Major et al. 2010; Uchimiya et al. 2011). This is also evidenced by the presence of significant amount of mineral elements in the extract.
In addition to increasing yield, the foliar extracts also increased vitamin C and soluble protein content and decreased nitrate content, but had no effect on soluble sugar content. Biochar aqueous extract increased micronutrients content, especially for Fe, Zn and Mn in the cabbage in the present study. These micronutrients might be important for control nitrate content in plant because they could enhance nitrate reductase activity and improving nitrogen metabolism (Ghosh and Srivastava 1994).
Table 4. Effect of Biochar Extract at Various Dilution Rates on Cabbage Leaf Area and Root Development
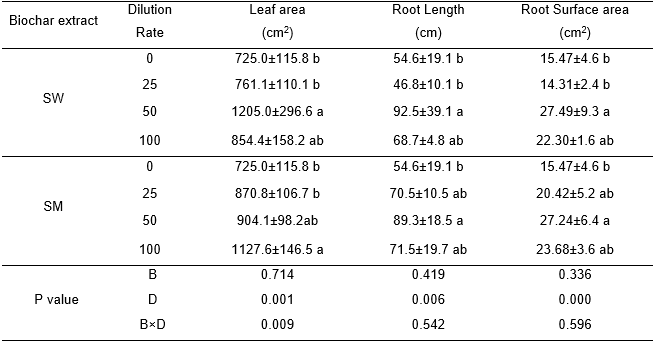
The values presented in the columns are mean ± standard deviation (n=3). Different small letters indicate differences among the four dilution rates for each biochar aqueous extract at p < 0.05. (A dilution rate of 0 is the control where only water was sprayed; SW: Sanli Wheat straw biochar aqueous extract. SM: Shanxi Maize straw biochar aqueous extract.) Value of P derived from a two-way ANOVA shows the effects of biochar extract (B) and dilution rate (D) on leaf area, root length, surface area.
Table 5. Effect of Biochar Extract at Various Dilution Rates on Macro and Micro Nutrient Contents of Cabbage
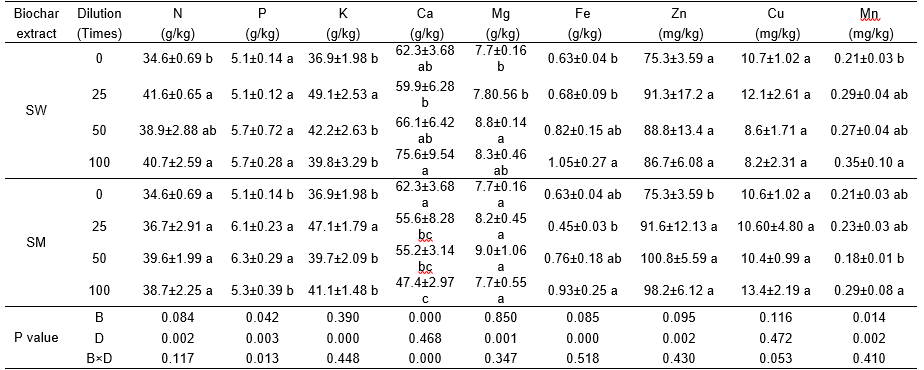
The values presented in the columns are mean ± standard deviation (n=3). Different small letters indicate differences among the four dilution rates for each biochar aqueous extract at p < 0.05. (A dilution rate of 0 is the control where only water was sprayed; SW: Sanli Wheat straw biochar aqueous extract. SM: Shanxi Maize straw biochar aqueous extract.) Value of P derived from a two-way ANOVA for the effects of biochar extract (B) and dilution rate (D) on each element.
Micronutrients also improve the metabolism of carbohydrate, protein, auxin, and pollen formation by being a structural constituent of photosynthetic proteins and enzyme (Marschner 1995; Kumar et al. 2015). Because there are many different organic compounds in biochar aqueous extracts, some of the cationic elements present in the extracts may be complexed with organic species (Graber et al. 2014). The results of FTIR indicated that P and Si were associated with compounds in the liquid. The organic compounds and humic substances are capable of forming chelates with the cations, which would assist in movement through the cuticle and plant growth (Sarhan et al. 2011; Datir et al. 2012).
Very little research has been carried out that determines the effect of concentrations of cations on the yield and quality of Chinese cabbage. However it has been reported that, if the ratio of the components is not balanced and the concentrations of any of the metals are too high, then spraying the liquid extract on leaves could result in lower yield, overall quality, and combined concentrations of vitamin C, proteins, and sugars (Smolen 2012). It is possible that the higher concentrations of one or a number of either Na, Fe, Mn, Cu, and B in the 25-fold dilutions of both extracts were the reason for the lower yields and vitamin C and protein contents. A concentration of Na that is too high could also result in yield reductions and may partly explain why yields were greater in the more diluted SM extract as compared with the SW extract; the concentration of Na was nearly 50% higher in the SM extract as compared with the SW extract. As Smolen (2012) and Kaya et al. (2001) reported, there can also be antagonistic or synergistic effects of cations and anions that are dose- and concentration-related. Further research work needs to be carried out to determine if increasing yield, quality, and nutrient elements are related to increased root development, which in turn may be related to the interactional effect of biochar extracts containing organic and inorganic nutrients.
To cool the hot biochar and gases produced during pyrolysis, a large amount of water is sometimes used. In some instances, biochars made from crop residues that have a high salt content need to be washed so that damage to growing roots can be avoided (Kwapinski et al. 2010; Bastos et al. 2014; Novak et al. 2015). As a result, many large pyrolysis plants need a wastewater treatment system to ensure that external waterways are not polluted by the process water. For electricity-generating plants, water treatment units separate tars, wood vinegar, and a water fraction that contains cations, anions, and a range of soluble organic molecules (John et al. 1992; Dahmen et al. 2012). The tar is burned to provide energy or used for preserving wood, and the wood vinegar is sold as a bio-pesticide (Wang et al. 2012; Tuntika et al. 2013). The remaining water must then be treated to reduce chemical oxygen demand (COD), biological chemical demand (BOD), and salt content before it is either reused in the plant, used in irrigation, or disposed of in a local waterway.
For biochar plants that are not generating electricity, the waste water comes from the cooling and cleaning of the biochar. Such waste water contains little or no tar but has relatively high concentrations of some cations and anions (K, Na, Ca, Mg, P, Cl, SO42+) and small amounts of micronutrients (Fe, Mn, B, Se, Sr, Zn, Cu) and dissolved organic compounds (Lin et al. 2011). Klasson et al. (2014) found that washing biochar with rainwater could uncover surface area and micropores, which would be beneficial for biochar used as sorbents in removing contaminants (Laird et al. 2009). Based on the results of the present study, it may be concluded that biochar could be extracted by hot water to produce plant growth-promoting liquids. Hot waste water is available via biomass pyrolysis and generally not recycled in biochar plants. Therefore, the hot waste water from pyrolysis could be reused for extracting biochar, which can be done when cooling the biochar as it is discharged from the kiln. This could be an innovative way to recycle waste water while adding value through production of a beneficial liquid product.
At present, most biochar that is commercially sold in China has been mixed with either chemical fertilizer or compost to increase crop yields, improve soil properties, and reduce emissions of greenhouse gases (Qian et al. 2015). Extensive research has been undertaken to utilize biochar to remediate contaminated land, although there has been very little commercial use of this application. Vegetables are the most important cash crop in China; the high income potential increasingly prompts the cultivation of vegetables in many regions (Zhang et al. 2013). Vegetable production of China has increased significantly these years; the total cultivated lands for vegetables in China is equivalent to 12.1% of the total national crop-sown area in 2011 (National Bureau of Statistic of China 2012; Ti et al. 2015), and the lands harvested for vegetables in China accounts for 51.5% of the world total (Food and Agriculture Organization 2012). The present study is the first to show that biochar extracts can have a positive effect on cabbage growth and quality. The use of these extracts can also benefit the biochar production process by providing an economic and environmentally friendly method for treating the waste water from washing biochar.
CONCLUSIONS
- Aqueous biochar extracts, obtained via hot water extraction, contain both organic compounds and mineral nutrients that could be beneficial for plant growth. Pot experiments demonstrated an increase in yield and quality of Chinese cabbage using these extracts.
- The extent of the improvement in yield and quality could be as high as 25% or more when used in dilution rates of 50 or 100. The present study suggests that wastewater from washing biochar can become a high-value biochar-associated product.
- More fundamental research is required to determine what specific factors lead to yield increases and improvement in vitamin C and soluble protein contents.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Education, China under grant number 20120097130003; Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization; the Agricultural Science and Technology Achievements Transformation Fund Project (2013GB23600666); China Postdoctoral Science Foundation project (2015M571767), and by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. SJ is a visiting research fellow at IREEA funded by the State Bureau of Foreign Expert Affairs, China under grant number GDW20123200127. LC-OCD was performed by Dr. Khorshed Chinu of the Mark Wainright Analytical Centre, University of NSW, Australia.
REFERENCES CITED
Bacchus, G. L. (2010). “An evaluation of the influence of biodynamic practices including foliar-applied silica spray on nutrient quality of organic and conventional fertilized lettuce (Lactuca sativa L.),”Organic Systems Journal 5(1), 1177-4258.
Bastos, A. C., Prodana, M., Abrantes, N., Keizer, J. J., Soares, A. M. V. M., and Loureiro, S. (2014). “Potential risk of biochar-amended soil to aquatic systems: An evaluation based on aquatic bioassays,” Ecotoxicology 23(9), 1784-1793. DOI: 10.1007/s10646-014-1344-1
Carballo, T., Victoria Gil, M., Gomez, X., Gonzalez-Andres, F., and Moran, A. (2008). “Characterization of different compost extracts using Fourier-transform infrared spectroscopy (FTIR) and thermal analysis,” Biodegradation 19(6), 815-830. DOI: 10.1007/s10532-008-9184-4
Chia, C. H., Singh, B. P., Joseph, S., Graber, E. R., and Munroe, P. (2014). “Characterization of an enriched biochar,” Journal of Analytical and Applied Pyrolysis 108, 26-34. DOI: 10.1016/j.jaap.2014.05.021
Clare, A., Barnes, A., McDonagh, J., and Shackley, S. (2014). “From rhetoric to reality: Farmer perspectives on the economic potential of biochar in China,” International Journal of Agricultural Sustainability 12(4), 440-458. DOI: 10.1080/14735903.2014.927711
Cui, L., Li, L., Zhang, A., Pan, G., Bao, D., and Chang, A. (2011). “Biochar amendment greatly reduces rice Cd uptake in a contaminated paddy soil: A two-year field experiment,” BioResources 6(3), 2605-2618. DOI: 10.15376/biores.6.3.2605-2618
Cui, L., Pan, G., Li, L., Yan, J., Zhang, A., Bian, R., and Chang, A. (2012). “The reduction of wheat Cd uptake in contaminated soil via biochar amendment: A two-year field experiment,” BioResources 7(4), 5666-5676. DOI: 10.15376/biores.7.4.5666-5676
Dahmen, N., Henrich, E., Dinjus, E., and Weirich, F. (2012). “The Bioliq® bioslurry gasification process for the production of biosynfuels, organic chemicals, and energy,” Energy, Sustainability and Society 2(3), 2192-0567. DOI: 10.1186/2192-0567-2-3
Datir, R. B., Apparao, B. J., and Laware, S. L. (2012). “Application of amino acid chelated micronutrients for enhancing growth and productivity in chili (Capsicum annum L.),” Plant Sciences Feed 2(7), 100-105.
Daws, M. I., Davies, J., Pritchard, H. W., Brown, N. A. C., and Van Staden, J. (2007). “Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species,” Plant Growth Regulation 51(1), 73-82. DOI: 10.1007/s10725-006-9149-8
Daws, M. I., Pritchard, H. W., and Van Staden, J. (2008). “Butenolide from plant derived smoke functions as a strigolactone analogue: Evidence from parasitic weed seed germination,” South African Journal of Botany 74(1), 116-120. DOI: 10.1016/j.sajb.2007.09.005
Elad, Y., David, D. R., Harel, Y. M., Borenshtein, M., Kalifa, H. B., Silber, A., and Graber, E. R. (2010). “Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent,” Phytopathology 100(9), 913-921. DOI: 10.1094/PHYTO-100-9-0913
Eyheraguibel, B., Silvestre, J., and Morard, P. (2008). “Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize,” Bioresource Technology 99(10), 4206-4212. DOI: 10.1016/j.biortech.2007.08.082
Fagbenro, J. A., and Agboola, A. A. (1993). “Effect of different levels of humic acid on the growth and nutrient uptake of teak seedlings,” Journal of Plant Nutrition 16(8), 1465-1483. DOI: 10.1080/01904169309364627
Food and Agriculture Organization (2012). FAO statistical databases. Available at http://faostat3.fao.org/home/index.html#DOWNLOAD.
Francioso, O., Sanchez-Cortes, S., Tugnoli, V., Ciavatta, C., Sitti, L., and Gessa, C. (1996). “Infrared, Raman, and nuclear magnetic resonance (1H, 13C, 31P) spectroscopy in the study of fractions of peat humic acids,” Society for Applied Spectroscopy 50(9), 1103-1214. DOI: 10.1366/0003702963905169
Ghosh, M. K., and Srivastava, R. C. (1994). “Effect of Mg, Zn and Mo salts on nitrate reductase activity and soluble protein content in leaves of Quercus serrata,” Biologia Plantarum 36(4), 599-605. DOI: 10.1007/BF02921187
Graber, E. R., Meller-Harel, Y., Kolton, M., Cytryn, E., Silber, A., Rav, D. D., Tsechansky, L., Borenshtein, M., and Elad, Y. (2010). “Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media,” Plant and Soil 337(1-2), 481-496. DOI: 10.1007/s11104-010-0544-6
Graber, E. R.,Tsechansky, L., Lew, B., and Cohen, E. (2014). “Reducing capacity of water extracts of biochar and their solubilization of soil Mn and Fe,” European Journal of Soil Science 65(1), 162-172. DOI: 10.1111/ejss.12071
Graber, E. R.,Tsechansky, L., Mayzlish-Gati, E., Shema, R., and Koltai, H. (2015). “A humic substances product extracted from biochar reduces Arabidopsis root hair density and length under P-sufficient and P-starvation conditions,” Plant and Soil 05/2015. DOI:10.1007/s11104-015-2524-3
Gunes, A., Inal, A., Sahin, O., Taskin, M. B., Atakol, O., and Yilmaz, N. (2015). “Variations in mineral element concentrations of poultry manure biochar obtained at different pyrolysis temperatures, and their effects on crop growth and mineral nutrition,” Soil Use and Management, Article first published online: 1 SEP 2015. DOI: 10.1111/sum.12205
Huber, S. A., Balz, A., Abert, M., and Pronk, W. (2011). “Characterization of aquatic humic and non-humic matter with size-exclusion chromatography-organic carbon detection-organic nitrogen detection (LC-OCD-OND),” Water Research 45(2), 879-885. DOI: 10.1016/j.watres.2010.09.023
John, D. A., Ramesh, K. S., and Narendra, N. B. (1992). “Characterization and stability analysis of wood-derived bio-oil,” Fuel Processing Technology 31(3), 241-256. DOI: 10.1016/0378-3820(92)90023-J
Joseph, S., Graber, E. R., Chia, C., Munroe, P., Donne, S., Thomas, T., Nielsen, S., Marjo, C., Rutlidge, H., Pan, G. X., Li, L., et al. (2013). “Shifting paradigms on biochar: Micro/nano-structures and soluble components are responsible for its plant-growth promoting ability,” Carbon Management 4(3), 323-343. DOI: 10.4155/cmt.13.23
Kaya, C., Higgs, D., and Halil, K. H. (2001). “The effects of high salinity (NaCl) and supplementary phosphorus and potassium on physiology and nutrition development of spinach,” Bulgarian Journal of Plant Physiology 27(3-4), 47-59.
Klasson, K. T., Uchimiya, M., and Lima, I. M. (2014). “Uncovering surface area and micropores in almond shell biochars by rain water wash,” Chemosphere 111, 129-134. DOI: 10.1016/j.chemosphere.2014.03.065
Kulkarni, M. G., Ascough, G. D., and Staden, V. J. (2007). “Effects of foliar applications of smoke-water and a smoke-isolated butenolide on seedling growth of okra and tomato,” HortScience 42(1), 179-182.
Kulkarni, M. G., Ascough, G. D., and Staden, V. J. (2008). “Smoke-water and a smoke-isolated butenolide improve growth and yield of tomatoes under greenhouse conditions,” HortTechnology 18(3), 3449-3454.
Kulkarni, M. G., Light, M. E., and Staden, V. J. (2011). “Plant-derived smoke: Old technology with possibilities for economic applications in agriculture and horticulture,” South African Journal of Botany 77(4), 972-979. DOI: 10.1016/j.sajb.2011.08.006
Kumar, T. D., Shweta, S., Swati, S., Sanjay, M., Chauhan, D. K., and Dubey, N. K. (2015). “Micronutrients and their diverse role in agricultural crops: Advances and future prospective,” Acta Physiologiae Plantarum 37(4), 139. DOI: 10.1007/s11738-015-1870-3
Kwapinski, W., Byrne, C. M. P., Kryachko, E., Wolfram, P., Adiey, C., Leahy, J. J., Novotny, E. H., and Hayes, M. H. B. (2010). “Biochar from biomass and waste,” Waste and Biomass Valorization 1(2), 177-189. DOI: 10.1007/s12649-010-9024-8
Laird, D. A., Brown, R. C., Amonette, J. E., and Lehmann, J. (2009). “Review of the pyrolysis platform for coproducing bio-oil and biochar,” Biofuels, Bioproducts and Biorefining 3(5), 547-562. DOI: 10.1002/bbb.169
Lehmann, J., Gaunt, J., and Rondon, M. (2006). “Biochar sequestration interrestrial ecosystems – A review,” Mitigation and Adaptation Strategies for Global Change 11(2), 395-419. DOI: 10.1007/s11027-005-9006-5
Light, M. E., Daws, M. I., and Staden, J. V. (2009). “Smoke-derived butenolide: Towards understanding its biological effects,” South African Journal of Botany 75(1), 1-7. DOI: 10.1016/j.sajb.2008.10.004
Lin, Y., Munroe, P., Joseph, S., Henderson, R., and Ziolkowski, A. (2011). “Water extractable organic carbon in untreated and chemical treated biochars,” Chemosphere 87(2), 151-157. DOI: 10.1016/j.chemosphere.2011.12.007
Lin, Y., Munroe, P., Joseph, S. D., and Henderson, R. (2012). “Migration of dissolved organic carbon in biochars and biochar-mineral complexes,” Brazilian Agricultural Research 47(5), 677-686. DOI: 10.1590/S0100-204X2012000500007
Liu, X. Y., Zhang, A. F., Ji, C. Y., Joseph, S., Bian, R. J., Li, L. Q., Pan, G. X., and Paz-Ferreiro, J. (2013). “Biochar’s effect on crop productivity and the dependence on experimental conditions – A meta-analysis of literature data,” Plant and Soil 373(1-2), 583-594. DOI: 10.1007/s11104-013-1806-x
Lu, R. K. (2000). Methods of Soil and Agro-Chemical Analysis, China Agricultural Science Technology Press, Beijing, China.
Major, J., Rondon, M., Molina, D., Riha, S. J., and Lehmann, J. (2010). “Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol,” Plant and Soil 333(1), 117-128. DOI: 10.1007/s11104-010-0327-0
Marschner, H. (1995). Mineral Nutrition of Higher Plants, 2nd Edition, Academic Press, San Deigo.
National Bureau of Statistic of China (2012). China statistical yearbook 2012. China Statistic Press, Beijing.
NDRC (2014). “33 low carbon key promotion project,” National Development and Reform Commission, China.
Novak, J. M., Sigua, G. C., Spokas, K. A., Busscher, W. J., Cantrell, K. B., Watts, D. W., Glaz, B., and Hunt, P. G. (2015). “Plant macro- and micronutrient dynamics in a biochar-amended wetland muck,” Water, Air and Soil Pollution 226(1), 1573-2932. DOI: 10.1007/s11270-014-2228-y
Pan, G., Crowley, D., and Lehmann, J. (2011a). “Burn to air or burial in soil: The fate of China’s straw residues,” International Biochar Initiative (http://www.biochar-international.org).
Pan, G., Lin, Z., Li, L., Zhang, A., Zheng, J., and Zhang, X. (2011b). “Perspective on biomass carbon industrialization of organic waste from agriculture and rural areas in China,” Journal of Agricultural Science and Technology 13(1), 75-82.
Qian, K., Kumar, A., Zhang, H., Bellmer, D., and Huhnke, R. (2015). “Recent advances in utilization of biochar,” Renewable and Sustainable Energy Reviews 42, 1055-1064. DOI: 10.1016/j.rser.2014.10.074
Sarhan, T. Z., Mohammad, G. H., and Teli, J. A. (2011). “Effects of humic acid and bread yeast on growth and yield of eggplant (Solanum melongena L),” Journal of Agricultural Science and Technology B 1, 1091-1096.
Smolen, S. (2012). “Foliar nutrition: Current state of knowledge and opportunities,” in: Advances in Citrus Nutrition, Srivastavam, A. K. (ed.), Springer, Netherlands, pp. 41-58. DOI: 10.1007/978-94-007-4171-3_4
Taek, K. O. H., Yoshiyuki, S., Jiro, C., Yong, H. L., and Bongsu, C. (2012). “Effect of aqueous extract of biochar on germination and seedling growth of lettuce (Lactuca sativa L.),” Journal of the Faculty of Agriculture, Kyushu University 57(1), 55-60.
Ti, C. P., Luo, Y. X., Yan, X. Y. (2015). “Characteristics of nitrogen balance in open-air and greenhouse vegetable cropping systems of China,” Environmental Science and Pollution Research International. First online: 04 September 2015. DOI: 10.1007/s11356-015-5277-x
Tuntika, M., Thawan, K., Sumran, P., Banyong, T., and Darunee, J. (2013). “Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.),” Scientia Horticulturae 154, 66-72. DOI: 10.1016/j.scienta.2013.02.020
Uchimiya, M., Cantrell, K. B., Hunt, P. G., Novak, J. M., and Chang, S. (2012). “Retention of heavy metals in a Typic Kandiudult amended with different manure-based biochars,” Journal of Environmental Quality 41(4), 1138-1149. DOI: 10.2134/jeq2011.0115
Uchimiya, M., Chang, S., and Thomas K. K. (2011). “Screening biochars for heavy metal retention in soil: Role of oxygen functional groups,” Journal of Hazardous Materials 190, 432-441. DOI: 10.1016/j.jhazmat.2011.03.063
Wang, X. K. (2006). Principles and Technology of Plant Physiological and Biochemical, Higher Education Press, Beijing, China.
Wang, H. F., Wang, J. L., Wang, C., Zhang, W. M., Liu, J. X., and Dai, B. (2012). “Effect of bamboo vinegar as an antibiotic alternative on growth performance and fecal bacterial communities of weaned piglets,” Livestock Science 144(1-2), 173-180. DOI: 10.1016/j.livsci.2011.11.015
Yan, Y., Lu, X., Li, L., Zheng, J., and Pan, G. (2011). “Components of pyroligneous solution from straw pyrolysis and its effect on growth and quality of pepper spice,” Journal of Nanjing Agriculture University 34(5), 58-62.
Zhang, G. S., Hu, X. B., Zhang, X. X., and Li, J. C. (2013). “Effect of plastic mulch and winter catch crop on water availability and vegetable yield in a rain-fed vegetable cropping system at mid-Yunnan plateau, China,” Scientia Horticulturae 164, 333-339. DOI: 10.1016/j.scienta.2013.09.053
Zhao, L., Cao, X., Wang, Q., Yang, F., and Xu, S. (2013). “Mineral constituents profile of biochar derived from diversified waste biomasses: implications for agricultural applications,” Journal of Environmental Quality 42(2), 545-552. DOI: 10.2134/jeq2012.0232
Article submitted: March 25, 2015; Peer review completed: August 24, 2015; Revised version received and accepted: October 9, 2015; Published: November 16, 2015.
DOI: 10.15376/biores.11.1.249-266
APPENDIX
Table S1. Basic Properties and Composition of the Studied Biochar