Abstract
The pulp and paper mill process requires large amounts of water. Therefore, the need to reuse water through the application of coagulation-flocculation processes, which is effective in the removal of solids and colloidal particles, has risen. In such processes, zeta potential (ZP) provides important information about the efficiency of the reagents used. The purpose of this study was to develop individual and combined tests of reagents to study turbidity and COD reduction based on ZP in the process of wastewater internal treatment for recirculation in the pulp and paper process. Factorial models were developed to explain the behavior of ZP depending on the different coagulants/flocculants. The statistical analyses showed that ZP had a positive correlation with parameters related to removal (COD and turbidity). It was demonstrated that innovate use of lentil extract (Lens esculenta) applied with aluminum sulfate favored the treatment, consistent with a coagulation-flocculation mechanism. The optimum doses of lentil extract were able to reduce the requirements of aluminum sulfate by almost 29%, providing an alternative strategy for water reuse processes in the pulp and paper industry.
Download PDF
Full Article
Zeta Potential as a Tool to Evaluate the Optimum Performance of a Coagulation-flocculation Process for Wastewater Internal Treatment for Recirculation in the Pulp and Paper Process
Luis A. Ordaz-Díaz,a Sergio Valle-Cervantes,b Josefina Rodríguez-Rosales,b Ana M. Bailón-Salas,b Maribel Madrid-Del Palacio,a Karla Torres-Fraga,a and Luis A. De la Peña-Arellano b,*
The pulp and paper mill process requires large amounts of water. Therefore, the need to reuse water through the application of coagulation-flocculation processes, which is effective in the removal of solids and colloidal particles, has risen. In such processes, zeta potential (ZP) provides important information about the efficiency of the reagents used. The purpose of this study was to develop individual and combined tests of reagents to study turbidity and COD reduction based on ZP in the process of wastewater internal treatment for recirculation in the pulp and paper process. Factorial models were developed to explain the behavior of ZP depending on the different coagulants/flocculants. The statistical analyses showed that ZP had a positive correlation with parameters related to removal (COD and turbidity). It was demonstrated that innovate use of lentil extract (Lens esculenta) applied with aluminum sulfate favored the treatment, consistent with a coagulation-flocculation mechanism. The optimum doses of lentil extract were able to reduce the requirements of aluminum sulfate by almost 29%, providing an alternative strategy for water reuse processes in the pulp and paper industry.
Keywords: Zeta potential; Efficiency; Evaluated; Recirculation; Pulp and paper; Lentil extract
Contact information: a: Environmental Engineering Technology, Universidad Politécnica de Durango, Carretera Dgo-México, km 9.5, Col. Dolores Hidalgo, Durango, Dgo. México; b: Chemical and Biochemical Engineering Department, Durango Institute of Technology (ITD), Blvd. Felipe Pescador 1830 Ote. Col. Nueva Vizcaya, 34080, Durango, Dgo., México;
* Corresponding author: herrdelapena@gmail.com
INTRODUCTION
Wastewater from different processes in pulp and paper mills generates various types of contaminants, which can adversely affect process efficiency and paper product quality (Ashrafi et al.2015). There is potential to reuse excess water from the paper machine process after it has been clarified by filtration or dissolved air flotation (DAF). The reduction of water use by industry has been a key topic in environmental legislation, which limits its use and consumption, increasing costs and its treatment due to the scarcity of fresh water. The recirculation and the internal treatment of water to employ the process more frequently and efficiently is one of the alternatives that the pulp and paper factories have sought in the pulping process. However, recirculation can generate an accumulation of soluble and colloidal substances, which can make it difficult or more costly to produce paper (Blanco et al. 2004; Abou-Elela et al.2008; Asghar et al. 2008; Hubbe et al. 2016).
Over the years, physicochemical processes have been developed to improve effluent quality (Kamali and Khodaparast 2015). In the past, the most common internal treatments of water used in papermaking was DAF (Ackermann et al. 2000), and that approach is still widely used in developing countries. The DAF system requires the addition of chemical substances, such coagulants and flocculants, which destabilize the colloidal material and promote the aggregation of particles for their removal. Aluminum-based products, ferric chloride, etc., as well as organic polymers – polyacrylamides (PAMs), minerals (bentonites or talcs), and polyamines have been used (Basta et al. 2004; Pelzer and Künzel 2006; Saarimaa et al. 2006; Hubbe et al.2016). Factors such as zeta potential (ZP), reagent dose, polyelectrolytes, etc., affect the coagulation-flocculation process. To achieve better efficiency, the optimal ranges should be found, as measured by the Willcomb index, when evaluating the consistency, size, and shape of the flocs produced in treated water (Cepeda and Cepeda 2005).
The use of plant extracts to purify groundwater has been investigated, achieving reduced turbidity (Pritchard et al. 2009). Natural polymers are an economically viable alternative to chemical coagulants (Sanghi et al. 2006; Choy et al. 2015). Natural polymers based on lentil extract have been used as treatment agents to reduce turbidity; however they have not been applied yet in coagulation and flocculation processes in the paper industry.
To promote the separation of suspended solids and colloids from the contaminated water, coagulation-flocculation methods are used, where the ZP and the electric charge provide relevant information on the system status. Also many wastewaters are composed of similarly charged particles that repel each other, creating a stable colloidal system. ZP can reduce the time needed to stability before settling. According to DLVO theory, in order for agglomeration to occur, two particles that are subject to Brownian diffusion must have experienced a sufficient sequence of random motions, due to collisions with adjacent water molecules, in order to pass over this energy barrier. When the dosage of polymer is progressively increased, the strength of the repulsive force between the particles decreases as the charge neutralization point is approached. Optimal flocculation will be achieved when the zeta potential has been reduced to zero, because then there will be no electrostatic repulsion (Bobrovnik and Popov 1982; Barany and Szepesszentgyörgyi 2004).
The electric charge of a surface identifies the anionic or cationic load of the sample, including how much of a charged coagulant may need to be added to reduce the zeta potential to zero. Typically a suspension of solid particles or air bubbles having a higher charge density at their surface will be more stable in suspension. Control of the ZP of the air bubbles during DAF treatment of process water can help with the adhesion of contaminants such as oil droplets to the bubble, as well as the coalescence of the bubbles, which is why the ZP is an important parameter (Fukui and Yuu 1980).
Sjöholm and Norman (2000) determined a negative ZP when measuring the mobility of lignin particles that were present in black liquor after reducing the pH. It was found that such lignin can be precipitated by using a cationic polycyclic agent (Belosinschi and Bobu 2008). Indirect methods are sometimes used when measuring the ZP of such systems, because the lignin tends to agglutinate as a stick mass when the ZP approaches zero (Li and Tian 2002).
The main objective of this work was to study zeta potential and evaluate the optimum performance of the coagulation-flocculation process for wastewater internal treatment for recirculation in the pulp and paper process. Aluminum sulfate, a low-charge, high-mass cationic acrylamide flocculant, and a lentil extract coadjuvant were evaluated.
EXPERIMENTAL
Materials
The metal coagulants used are Aluminum Sulfate (Al2(SO4)3) and a commercial cationic acrylamide flocculant (Technics 1704-PGI®, Queretaro, Mexico). Also, lentil extract modified (coadjuvant) was prepared using the technique reported by Schultz and Okun (1990). Four alternatives were analyzed (Table 1).
Table 1. Treatment Alternatives
Sampling
The samples were collected before their arrival at the wastewater treatment plant (WWTP) and were stored at 4 °C before use. The effluent used for this study was the same studied by (Ordaz-Díaz et al. 2014; Ordaz-Díaz et al. 2016). Three types of wastewater samples were analyzed, dependent upon the type of fibrous formulation (Table 2).
Table 2. Wastewater Samples and Composition Analyzed
Physicochemical characterization of the water samples is shown in Table 3.
Table 3. Physicochemical Characterization of samples
Jar tests for paper and pulp industry effluent
To evaluate the functionality of the four alternatives raised in this study, jar tests were carried out, which allowed comparison of the effectiveness of different treatment conditions. A jar test apparatus was used for coagulation-flocculation and the settling of the paper industry effluent.
Coagulation and flocculation experiments
The coagulation and flocculation experiments were conducted by jar tests at room temperature to determine which alternatives were most effective in removing color, turbidity, and chemical oxygen demand (COD). COD was determined using the closed reflux (colorimetric) method, and turbidity was determined by a spectrophotometric method to analyze water and wastewater (Rice et al. 2012). The experiments were designed to determine optimal doses based on the neutral charge of ZP. A pH adjustment was made to the raw water, prior to the addition of the reagent, in a range of 8 to 8.5 with hydrated lime that made a subsequent removal of fine particles.
Two liters of sample were added to each jar test. First, coagulation velocity and time were established based on the literature, considering this type of wastewater and the efficient removal of organic matter and total suspended solids. Thus, the following conditions were established: coagulation rate = 50 rpm, coagulation time = 5min, flocculation rate = 25 rpm, flocculation time = 5 min, and sedimentation time = 10 min. The experiments were repeated three times.
One of the implementations that was proposed in this research was the development of individual and combined tests, one of which used minimal doses of the polymer plus the coagulant, and one of which used minimal doses of the coagulant plus the coadjuvant, until finding the maximum removal of the parameters. The goal was to determine whether it is possible to reduce the optimal doses of coagulants or flocculants (used separately). The treatment alternatives were performed according to Table 1.
Methods
After settling, 20 mL of the sample was taken from each vessel, and the measurement was performed. The COD, color, and TSS concentrations were measured according to standard methods (APHA 2005). The zeta potential was measured by a Zeta meter 3.0 (Zeta-Meter, Inc. Staunton, USA). The determination of zeta potential (ZP) was performed by taking 100 mL of sample from each jar.
Statistical analysis
The effectiveness of alternative A and C was evaluated by comparing the results obtained by the response variables ZP, turbidity removal, and effect of COD removal. A correlation between the alternatives and the response variables was performed. For the alternative B and D, statistical models were generated to predict the behavior of ZP in three samples (M1, M2, and M3). Finally, the alternatives were analyzed according to the type of sample. Data analysis was followed by a least significant difference (LSD) test to determine the significant difference influences, using Statistica 7 StatSoft, Inc. (Quest, Oklahoma City, OK, USA).
RESULTS AND DISCUSSION
In paper production processes, the need arises to produce different varieties (different composition). This generates wastewater with high organic loads. If one wishes to apply a coagulation-flocculation process, it will be necessary to test each of the effluents constantly for each of the different types of paper. The optimal dose can be expected to vary over time due to process variability. Therefore, it is necessary to generate models capable of predicting these optimal doses, which makes the treatment process more efficient. Then, the alternatives to generate the models are presented.
Alternative A
The correlation of variables of the preliminary experiments for the level of cPAM (Technics) employed are shown in Table 4, where it was possible to observe that there was a high inverse correlation (-69%) between COD versus ZP. In addition, the sample type was related to the percentage of turbidity removal and COD.
Table 4. Correlation of cPAM, Correlations are Significant at p < 0.05
Negative charges are mainly caused by colloidal matter from hemicellulose and extractives components (Jaycock and Pearson 1976). With respect to Fig. 1, for M1, M2, and M3, the doses that approached the isoelectric point were 8.5 ppm, 3 ppm, and 6.5 ppm, respectively. In the case of the sample M3 with doses between 7 ppm and 15 ppm, the isoelectric point could not be reached. This suggested it was required to use greater doses of cPAM. It is worth noting, in Fig. 1 and some of the later figures, that vertical positions on the graphs not corresponding to the exact values of M1, M2, and M3 can be regarded as corresponding to proportional mixtures of the respective wastewater batches.
Fig. 1. Zeta potential as function of cPAM dose and its influence on the samples
Using the low-charge, very high mass cationic acrylamide flocculant for M1 and M3, with doses of 7.6 ppm and 10 ppm resulted in removal of 60% in turbidity and COD removal of 20% and 40%, respectively (Figs. 2 and 3). The M2 wastewater exhibited the best removal of 70% in turbidity, using a dose of 10 ppm, and COD removal of 50%. Though synthetic flocculants are effective and convenient, they have been reported to have unstable performance (Sanghi et al. 2006). The optimum values of turbidity and COD were affected by the increase of the doses of the cPAM.
Fig. 2. Effect of turbidity removal
Fig. 3. Effect of COD removal
According to Subramanian et al. (1999), it has been accepted that one of the governing factors of a water treatment process is charge neutralization. Increasing repeating units will increase the effectiveness of a positively charged additive, thus improving the charge neutralization or destabilization of negative particles. Increasing the molecular weight also allows for larger loops and tails of polymers to develop, and therefore yields more space to attract suspended particles (Shatat et al. 2008). This phenomenon was consistent with the study by Zhang et al.(2010), who studied reed pulp suspensions. Zhang observed that increasing the positive charge increased flocculation efficiency.
In the tests performed with the cPAM, its reaction mechanism was shown to be that of interparticle bridging, as the polymer adhered to the surface of the particle and interlaced to another by connecting to each other. Also, the ZP remained in the negative region, even though high removals of the analyzed parameters were achieved. Since flocculants tend to be more resistant to shear, successful use of flocculants without the addition of coagulants is possible even when there is a net surface charge (La Mer and Healy 1963; Ghosh et al. 1985).
Alternative B
Since flocculants are more expensive, it is generally advantageous to previously add a coagulant to reduce the surface load, neutralize the anionic charge and coagulating the sample. The select doses were obtained from alternative A (doses constant cPAM at 3 ppm). In some cases, partial neutralization is preferred rather than a fully neutralized system, to allow for the efficient action of flocculant. Leaving a small percentage of negative charge, the cationic copolymer is able to find suitable points of adsorption (Hubbe et al. 2012). The analysis performed for the ZP behavior models using cPAM-aluminum sulfate showed a correlation coefficient of 0.84 (Table 5).
Table 5. Test of SS Whole Model vs. SS Residual (cPAM-aluminum Sulfate)
With a significance level of 0.05, the univariate significance test (Table 6) showed that the sample type and the dose of cPAM-aluminum sulfate were important factors for the neutral charge of ZP.
Table 6. Univariate Tests of Significance for ZP (mV) (cPAM-aluminum Sulfate)
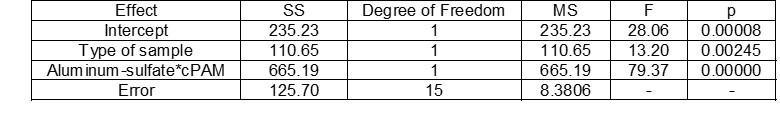
With α = 0.05 in the mean difference test (Tables 7 and 8), the authors note that the sample type and aluminum sulfate doses showed significant statistical differences. As for the effect of the type of sample (Table 6), the test of means showed that in M3 and M2, similar ZP’s and in M1 a ZP closer to zero was achieved. For the different doses of aluminum sulfate (Table 7), all of them presented mean differences between the three doses used, it can be observed that the dose of 80 ppm presented a ZP close to the isoelectric point.
Table 7. Fisher LSD Test; Variable ZP (mV), Difference in Means between Doses of cPAM-aluminum Sulfate
Table 8. Fisher LSD Test; Variable ZP (mV), Difference in Means between Doses of cPAM-aluminum Sulfate
In the response surface graph (Fig. 4), here the area of the surface where the ZP is close to zero is shown in doses of aluminum sulfate between 70 and 85 ppm. Over-dosage is observed with doses greater than 75 ppm aluminum sulfate. Such over-dosing causes the redispersion of the components. By contrast, efficient heterocoagulation (loaded oppositely) of the positive and negative components can be expected when the net ZP is near to zero (Alince and Van de Ven 1993).
Fig 4. Response surface graph for ZP behavior in three water samples of pulp and paper industry using alternative B
The model that predicted the behavior of ZP (Eq. 1) was,
(1)
where Y = sample, X = cPAM × aluminum sulfate, and Z = ZP. Using Eq. 1, the optimal doses to find the isoelectric point using 3 ppm of cPAM, were for the M1, M2, and M3 71.007, 84.017, and 84.079, respectively. It follows that coagulant doses can be reduced, due optimum flocculants concentrations generate larger flocs sizes which facilitates the sedimentation process.
Alternative C
The correlation of variables using aluminum sulfate are shown in Table 9, where it was possible to observe that doses of aluminum sulfate were highly correlated with ZP, turbidity removal, color, and COD removal. The ZP was correlated with turbidity, sample type, and COD removal. The correlation of turbidity was 0.40 with the removal of color; also the COD was 0.53 with respect to color.
Table 9. Correlation of Aluminum Sulfate, Correlations are Significant at p < 0.05
The ZP was close to the neutral charge at 660 ppm aluminum sulfate for samples M1. Otherwise for M2 and M3, doses above 550 ppm and 650 ppm were required to reach charges (-10 and -5) of ZP (Fig. 5), which was far from the isoelectric point, and meant the necessary dosage was more than 700 ppm.
Fig. 5. Zeta potential as function of aluminum sulfate dose and its influence on the samples
The maximum turbidity removal (80%) was achieved in M1 using 680 ppm doses using aluminum sulfate. In M2 and M3, 40% was reached at 470 ppm and 80% was reached at 550 ppm, respectively (Fig. 6). Minor removals of turbidity were found compared to Ahmad et al. (2008) because in the cited report, levels of removal above 92% were achieved when using polyaluminum chloride and aluminum sulfate as coagulants.
For COD removal, it was observed that M1, M2, and M3 with doses above 620 ppm achieved 50% removal (Fig. 7). It can be observed that the turbidity removals were not the same. Optimum conditions for COD or color removal are not always the same as those for turbidity removal using aluminum sulfate (Zhou et al. 2008).
Fig. 6. Effect of turbidity removal
Fig. 7. Effect of COD removal
Alternative D
In alternative D, a constant concentration of 470 ppm of aluminum sulfate was maintained. The analysis performed for the ZP behavior models using aluminum sulfate in combination with lentil extract showed a correlation coefficient of 0.79 (Table 10).
Table 10. Test of SS Whole Model vs. SS Residual (Aluminum Sulfate-lentil Extract)
With a significance level of 0.05, the univariate significance test (Table 11) for the behavior of ZP using aluminum sulfate-lentil extract showed that lentil extract, sample, and sample2 all were important factors.
Table 11. Univariate Tests of Significance for ZP (mV) (Aluminum Sulfate-lentil Extract)
The mean difference test (Table 12) in the sample type did not show significant statistical differences. However, for the doses of lentil extract (Table 13), there were significant minimal differences; concentrations of 40 ppm were the closest to the isoelectric point.
Table 12. Fisher LSD Test; Variable ZP (mV) Using Aluminum Sulfate-lentil Extract, Difference of Means between Sample Types
Table 13. Fisher LSD Test; Variable ZP (mV) Using Aluminum Sulfate-lentil Extract, Difference in Means Between Doses of Lentil Extract
In the response surface graph (Fig. 8), in the area in which lead concentrations near zero were obtained, the doses were 40 ppm lentil extract.
Fig. 8. Response surface graph for ZP behavior in three water samples of pulp and paper industry using alternative D
The model that predicted the behavior of ZP (Eq. 2) was,
(2)
where Y = sample, X = aluminum sulfate + lentil extract modified, and Z = ZP. Using Eq. 2, the optimal doses to find the isoelectric point using 470 ppm of aluminum sulfate for the M1, M2, and M3 were 31.66, 45.8, and 24.96 ppm of lentil extract, respectively. Optimum doses to achieve neutral charge, using the lentil extract, were able to reduce aluminum sulfate requirements at M1, M2 and M3 by 29, 15 and 28% respectively. The extract behaves as an effective coadjuvant, achieving decrease alum requirements by almost 50% (Schultz and Okun, 1990).
Comparison of Alternatives vs. Selected Samples
The Analysis of Least Significant Difference for the data indicated highly significant differences (P < 0.05) between treatments. When analyzing the effect of the four alternatives in M1, the influence of alternatives B and D with LSD0.05 value of 3.84 and 1.45, without significant minimum differences, respectively (Table 14), was observed. Either B or D can be used. In the case of M2 with LSD0.05 value of 2.32 and M3 with LSD0.05 value of 0.66 (Table 15 and Table 16), it is shown that alternative D was the most efficient. Lentil extract are a viable alternative to favor coagulation-flocculation for wastewater internal treatment for recirculation in the pulp and paper process. Using the modified lentil extract as a coadjuvant of aluminum sulfate in the coagulation-flocculation process showed importance, because the amount of aluminum sulfate was decreased.
In the case of alternative B, although high doses of cPAM were used, the isoelectric point was not reached. To achieve this in alternative C, doses greater than 700 ppm were required. Comparing the B and D alternatives, it was demonstrated that alternative D most favored the treatment of coagulation-flocculation.
Table 16. Fisher LSD Test to M3, Using Four Alternatives
From this investigation, it was possible to affirm that chemical coagulation/ flocculation was particularly effective in removing turbidity and COD. An optimum performance of the flocculation/coagulation process was achieved by measuring ZP in the treated water as an indicator of the destabilization of the particles. The neutralization of loads created a greater number of flocs, that is to say, the highest flocculation occurred at low PZ values, the PZ decreases of repulsion between particles and more flocs were created (Razali et al. 2011)
CONCLUSIONS
- This research represented the opportunity to evaluate the potential use of organic coagulants or low cost mixtures as an alternative to the traditional use of inorganic coagulants and flocculants.
- The ZP had a positive correlation with the removal of parameters (COD and turbidity). When modifying the dose of reagent, it was possible to reach zero ZP values. The ZP has been shown to be a reliable tool for evaluating water reuse processes.
ACKNOWLEDGMENTS
The authors are thankful for the support of the Science and Technology National Council (CONACyT) by the SNI appointment received by the principal author and to the (PFCE) 2016 Educational Quality Strengthening Program.
REFERENCES CITED
Abou-Elela, S. I., Nasr, F. A., Ibrahim, H. S., Badr, N. M., and Askalany, A. R. M. (2008). “Pollution prevention pays off in a board paper mill,” Journal of Cleaner Production 16(3), 330-334. DOI: 10.1016/j.jclepro.2006.07.045
Ackermann, C., Gottsching, L., Pakarinen, H. (2000). “Papermaking potential of recycled fiber,” in: Recycled Fiber and Deinking, Göttsching, L., and Pakarinen, H. (eds.), Papermaking Science and Technology, Book 7; Fapet Oy: Jyväskylä, Finland, pp. 359-439.
Alince, B., and Van de Ven, T. G. M. (1993). “Kinetics of colloidal particle deposition on pulp fibers 2. Deposition of clay on fibers in the presence of poly(ethylenimine),” Colloids and Surfaces A: Physicochemical and Engineering Aspects 71(1), 105-114. DOI: 10.1016/0927-7757(93)80033-B
Ahmad, A. L., Wong, S. S., Teng, T. T., and Zuhairi, A. (2008). “Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill wastewater,” Chemical Engineering Journal 137(3), 510-517. DOI: 10.1016/j.cej.2007.03.088
APHA (2005). “Standard methods for the examination of water and wastewater,” American Public Health Association (APHA), Washington, DC, USA.
Asghar, M. N., Khan, S., and Mushtaq, S. (2008). “Management of treated pulp and paper mill effluent to achieve zero discharge,” Journal of Environmental Management 88(4), 1285-1299. DOI: 10.1016/j.jenvman.2007.07.004
Ashrafi, O., Yerushalmi, L., and Haghighat, F. (2015). Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission,” Journal of Environmental Management 158, 146-157. DOI: 10.1016 / j.jenvman.2015.05.010
Barany, S., and Szepesszentgyörgyi, A. (2004). “Flocculation of cellular suspensions by polyelectrolytes,” Advances in Colloid and Interface Science 111(1), 117-129. DOI: 10.1016/j.cis.2004.07.003
Basta, A. H., Zhan, H., He, B., Wang, X., Zao, G., and Chen, J. (2004). “Cleaning efficiency of process water in newsprint mill,” Progress in Paper Recycling 13(3), 13-22.
Beloşinschi, D., and Bobu, E. (2008). “Effects of coagulants on the DCS accumulation in process water of papermaking,” Environmental Engineering and Management Journal 7(3), 269-276.
Blanco, A., Negro, C., Monte, C., Fuente, H., and Tijero, J. (2004). “The challenges of sustainable papermaking,” Environmental Science Technology 38, 414A-420A. DOI: 10.1021/es040654y
Bobrovnik, V. M., and Popov, A. G. (1982). “Evaluation of flocculant efficiency in wastewater treating,” Chemistry and Technology of Fuels and Oils 18(9), 477-479. DOI: 10.1007/BF00725998
Cepeda, Z., and Cepeda, E. (2005). “Application of generalized linear models to data analysis in drinking water treatment,” Revista Colombiana de Estadistica 28(2), 233-242.
Choy, S. Y., Prasad, K. M. N., Wu, T. Y., and Ramanan, R. N. (2015). “A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification,” International Journal of Environmental Science and Technology, 12(1), 367-390. DOI: 10.1007/s13762-013-0446-2
Fukui, Y., and Yuu, S. (1980). “Collection of submicron particles in electro-flotation,” Chemical Engineering Science 35(5), 1097-1105.
Ghosh, M. M., Cox, C. D., and Prakash, T. M. (1985). “Polyelectrolyte selection for water treatment,” Journal American Water Works Association 77(3), 67-73.
Hubbe, M. A., Metts, J. R., Hermosilla, D., Blanco, M. A., Yerushalmi, L., Haghighat, F., Lindholm-Lehto, P., Khodaparast, Z., Kamali, M., and Elliott, A. (2016). “Wastewater treatment and reclamation: A review of pulp and paper industry practices and opportunities,” BioResources 11(3), 7953-8091. DOI: 10.15376/biores.11.3.Hubbe
Hubbe, M. A., Sundberg, A., Mocchiutti, P., Ni Y., and Pelton, R. (2012). “Dissolved and colloidal substances (DCS) and the charge demand of papermaking process water and suspensions: A review,” BioResources 7(4), 6109-6193. DOI: 10.15376/biores.7.4.6109-6193
Jaycock, M. J., and Pearson, J. L. (1976). “A study of the retention of pigment during paper formation,” J. Colloid Interface Sci. 55(1), 181-190. DOI: 10.1016/0021-9707(76)90024-2
Kamali, M., and Khodaparast, Z. (2015). “Review on recent developments on pulp and paper mill wastewater treatment,” Ecotoxicology and Environmental Safety 114, 326-342.
La Mer, V. K., and Healy, T. W. (1963). “Absorption-flocculation reactions of macromolecules at the solid -liquid interface,” Review of Pure and Applied Chemistry,” 13, 112-33.
Li, L. C., and Tian, Y. (2002). “Zeta potential,” in: Encyclopedia of Pharmaceutical Technology, 2nd Ed., Marcel Dekker, Inc., New York.
Ordaz-Díaz, L. A., Rojas-Contreras, J. A., Flores-Vichi, F., Flores-Villegas, M. Y., Álvarez-Álvarez, C., Velasco-Vázquez, P., and Bailón-Salas, A. M. (2016). “Quantification of endoglucanase activity based on carboxymethyl cellulose in four fungi isolated from an aerated lagoon in a pulp and paper mill,” BioResources 11(3), 7781-7789. DOI: 10.15376/biores.11.3.7781-7789
Ordaz-Díaz, L. A., Rojas-Contreras, J. A., Rutiaga-Quiñones, O. M., Moreno-Jiménez, M. R., Alatriste-Mondragón, F., and Valle-Cervantes, S. (2014). “Microorganism degradation efficiency in BOD analysis formulating a specific microbial consortium in a pulp and paper mill effluent,” BioResources 9(4), 7189-7197. DOI: 10.15376/biores.9.4.7189-7197
Pelzer, R., and Künzel, U. (2006). “Polyacrylamides (PAM) for process water treatment in the production of mechanical printing papers,” Wochenblatt fur Papierfabrikation 134(22), 1320-1323.
Pritchard, M., Mkandawire, T., Edmondson, A., O’Neill, J. G., and Kululanga, G. (2009). “Potential of using plant extracts for purification of shallow well water in Malawi,” Physics and Chemistry of the Earth, Parts A/B/C, 34(13), 799-805. DOI: 10.1016/j.pce.2009.07.001
Razali, M. A. A., Ahmad, Z., Ahmad, M. S. B., and Ariffin, A. (2011). “Treatment of pulp and paper mill wastewater with various molecular weight of polyDADMAC induced flocculation,” Chemical Engineering Journal 166(2), 529-535. DOI: 10.1016/j.cej.2010.11.011
Rice, E. W., Baird, R. B., Eaton, A. D., and Clesceri, L. S. (2012). Standard Methods for the Examination of Water and Wastewater (22nd Ed.), American Public Health Association, New York.
Saarimaa, V., Sundberg, A., Holmbom, B., Blanco, A., Fuente, E., and Negro, C. (2006). “Monitoring of dissolved air flotation by focused beam reflectance measurement,” Industrial and Engineering Chemistry Research 45(21), 7256-7263. DOI: 10.1021/ie060250+
Sanghi, R., Bhattacharya, B., Dixit, A., and Singh, V. (2006). “Ipomoea dasysperma seed gum: An effective natural coagulant for the decolorization of textile dye solutions,” Journal of Environmental Management 81(1), 36-41. DOI: 10.1016/j.jenvman.2005.09.015
Schultz, C. R., and Okun, D. A. (1990). Surface Water Treatment for Developing Countries, 1st Ed., Wiley-Interscience, pp. 375-382.
Shatat, R., Ariffin, A., Rahman, N. N. A., and Kadir, M. O. A. (2008). “The effect of molecular weight and charge density on floc size distribution of palm oil mill effluent flocculated with cationic polyelectrolytes,” Journal of Basic and Applied Sciences 4(2), 95-103.
Sjöholm, E., and Norman, E. (2000). “Charge density of lignin samples from kraft cooking of birch wood,” Journal of Wood Chemistry Technology 20(4), 337-356. DOI: 10.1080/02773810009351888
Subramanian, R., Zhu, S., and Pelton, R. H. (1999). “Synthesis and flocculation performance of graft and random copolymer microgels of acrylamide and diallyldimethylammonium chloride,” Colloid and Polymer Science 277(10), 939-946. DOI: 10.1007/s003960050473
Zhang X., Gu, W. J., Li H., and Chen, L. (2010). “Flocculation of reed pulp suspensions by quaternary chitosan-nanoparticle SiO2 retention aid systems,” Journal of Applied Polymer Sciencie 117(1), 742-749. DOI: 10.1002/app.30230
Zhou, Y., Liang, Z., and Wang, Y. (2008). “Decolorization and COD removal of secondary yeast wastewater effluents by coagulation using aluminum sulfate,” Desalination 225(1-3), 301-311. DOI: 10.1016/j.desal.2007.07.010
Article submitted: March 9, 2017; Peer review completed: June 2, 2017; Revised version received: June 21, 2017; Accepted June 23, 2017; Published: July 5, 2017.
DOI: 10.15376/biores.12.3.5953-5969
