Abstract
Download PDF
Full Article
Characterization of Willow Bast Fibers (Salix spp.) from Short-Rotation Plantation as Potential Reinforcement for Polymer Composites
Javane Oktaee,a,* Thea Lautenschläger,b Markus Günther,b Christoph Neinhuis,b André Wagenführ,a Mirko Lindner,c and Anja Winkler d
Short-rotation coppices have potential to be the future source of raw materials for many applications in the wood and paper industries. It is important to establish methods and products to handle their waste biomass. In this paper, the properties of bast fibers extracted from the bark of willow trees (Salix spp.) were evaluated for potential future use in the production of natural fiber-polymer composites. The anatomy of the fibers was investigated through optical and electron microscopy. The thermogravimetric analysis of these fibers showed that the major mass loss occurs at 257 °C. The density of the fibers was measured with a gas pycnometer (1.19 ± 0.2 g/cm3). The chemical analysis showed that willow bark fibers have a composition similar to willow wood. As an indicator of mechanical properties, single willow bast fibers were characterized by tensile tests. The results revealed values for tensile strength (307.6 ± 130.1 MPa) and Young’s modulus (16.9 ± 8.4 GPa) that are comparable to some commonly used natural fibers. The overall results showed that willow bast fibers have the required mechanical properties as well as thermal stability for application in reinforcement of polymers.
Keywords: Willow; Bark; Bast fibers; Natural Fibers; Short-rotation coppices
Contact information: a: Institute of Natural Materials Technology, Dresden University of Technology, P. O. Box 01307, Dresden, Germany; b: Institute for Botany , Dresden University of Technology, Zellescher Weg 20b, 01217 Dresden, Germany; c: Institute of Natural Materials Technology, Dresden University of Technology P. O. Box 01069, Dresden, Germany; d: Institute of Lightweight Engineering and Polymer Technology, Dresden University of Technology, P. O. 01307 Dresden, Germany;
* Corresponding author: javane.oktaee@tu-dresden.de
INTRODUCTION
During recent decades, natural fibers have gained a significant share in composite markets. Several favorable properties, such as good mechanical properties, low density, biodegradability, renewability, low cost, etc., make them quite competitive with synthetic or man-made fibers in many applications (Nishino 2004; Pickering et al. 2016). Natural fibers can be extracted from different parts of a plant, such as the seed, bast, leaf, or wood (Nishino 2004; Mohanty et al. 2005; Zimniewska and Wladyka-Przybylak 2016). In Europe, non-wood, natural fiber polymer composites are most commonly used in automotive industries (Carus et al. 2014). These same fibers can be used in the form of short or long fibers and as textile material in polymeric matrices.
Currently, most natural fibers are harvested from forests or agricultural feedstocks (Mohanty et al. 2005), whereas short-rotation coppices (SRCs) can also serve as a promising source. SRCs are defined as plantations of fast-growing species in high density, which are harvested every few years to produce sustainable raw material for the wood industry and for fuel (Christersson and Verma 2006; Wolbert-Haverkamp and Musshoff 2014). The total area of SRCs has been estimated at 32,000 ha in Europe (Pecenka et al. 2014), with 4,000 to 6,000 ha being in Germany alone (Klasnja et al. 2002; van Wühlisch 2012; Markoff 2016), and it is increasing continuously.
In plantations established for the purpose of energy production, trees are cut down in ages between 3 to 5 years and the entire stem (i.e. wood and bark) is converted to chips for combustion. Klasnja et al. 2002; Feng et al. 2013). The latter is related to the inorganic substances (Fengel and Wegener 1983), which can cause damage to wood boilers via corrosion or blockage of the burner gate (Feng et al. 2013; Guidi et al. 2013). Moreover, the particulate matter and NOx emissions produced by the combustion of bark is higher than with wood. Particulate matter and NOx are major sources of air pollution and are harmful to human health (Obernberger and Thek 2004; Caslin et al. 2015). In order to decrease the environmental footprint of energy production, and to increase the service life of the boilers; it favorable to separate the bark of the plantation trees for further utilization in some value-added products such as natural fiber polymer composites.
In Germany, the common species in SRCs are poplar (Populus spp.), robinia (Robinia pseudoacacia), and willow (Salix spp.) (Faasch and Patenaude 2012; Markoff 2016). The bark of willow trees is known to have strong fibers that have been extracted and used during ancient times to manufacture ropes and fishing nets (Bick 2012). Even today, the Wet´suwet´en people that inhabit northwestern British Colombia use willow bark (Salix scouleriana Baratt ex Hook.) for lashing, hanging fish, or twining into nets, fish lines, and ropes (Gottesfeld 1992).
A retting method is used to separate the bark fibers. Retting is a preferential rotting process that separates fibers by removing the cementing material that glues them together (Sain and Panthapulakkal 2004). The middle lamella in the inner most layer of the bark (i.e., the phloem and the cambium) consists mainly of pectin, which is more susceptible to microbial degradation than the lignified fibers (Ek et al. 2009). The middle lamella and parenchymal cells, which have thinner cell walls, decompose, leaving highly lignified bast fibers behind.
These fibers can be a potential candidate for reinforcement in polymer composites. Hence, the aim of the presented investigation is to use this old knowledge to extract willow bast fibers and to characterize their properties, for future use in the modern industry of the natural fiber polymer composites.
EXPERIMENTAL
Anatomy
Three- to four-year-old willow trees (Salix spp.) were cut in April 2015 from the bioenergy field Böhme GmbH in Saxony, Germany. The willow trees belonged to the clone “Inger” (S. triandra × S. viminalis), which is one of the most commonly planted willow hybrids in the SRCs in Germany (van Wühlisch 2012).
In order to investigate the structure of the willow bark, small discs were cut from trees, and microscopic sections of the bark for light microscopy (Olympus BX 52, Hamburg, Germany) were prepared using a freezing microtome (Model 1206, Leica Biosystems, Wetzlar, Germany) and stained with safranin/astra blue. Safranin and astra blue are indicators of lignin and cellulose, respectively. In a double staining method using these two colors, lignin is stained red, and cellulose is colored blue (Chaffey 2002).
Observation of the cross-section of the single extracted fibers was carried out by embedding the fibers in epoxy resin. Afterwards, the samples were polished with a polisher machine (Buehler AutoMet 250, Düsseldorf, Germany) to reach a smooth surface for light microscopy. Finally, a scanning electron microscope (SEM; Zeiss Supra 40VP, Ulm, Germany ) was used for a more precise view of the bast fibers.
Fiber Extraction
For the fiber extraction, first bark layers of 20 young willow trees was peeled off from the cut down trees. Peeling was performed by hand, with use of a simple cutter, while the trees were still fresh. Bark strips were tied into bundles and immersed in water for 6 weeks, according to the method described by Senwitz et al. (2016). Then the strips were cleaned with a fine brush to clean the fibers from the cementing materials. In the next steps, fibers were air-dried and later dried in a hot-air oven for 24 h at 103 °C to remove the moisture (Fig. 1).

Fig. 1. (a) SRC with young willow trees, (b) fresh bark bundle, and (c) extracted fiber bundle
Thermogravimetric Analysis
One of the major drawbacks for application of natural fibers in reinforcing polymers is their sensitivity to high temperature (Leong et al. 2014; Pandey et al. 2015; Pickering et al. 2016). During the composite manufacturing process, natural fibers are subjected to thermal stress that can lead to their thermal degradation and inferior mechanical properties of the produced composites (Monteirto et al. 2012a,b; Pandey et al. 2015).Therefore, it is essential to investigate the thermal behavior of the fibers under the elevated temperature for future use in the composite production design.
In this investigation, the thermal stability behavior of the fibers was assessed by thermogravimetric analysis (TG) using a Mettler Toledo GmbH TGA/SDTA841e (Giessen, Germany). The TG test was performed by heating 10 mg of bark fibers from 25 to 600 °C at a heating rate of 10 °C/min, under a 98% nitrogen atmosphere.
Chemical Composition Analysis
The chemical composition of natural fibers plays an important role in mechanical and physical properties of their polymer composites. Different fiber types have extensive variation in their chemical composition, but the main constituents in all of them are cellulose, hemicellulose, and lignin.
In order to estimate the amount of cellulose (Kürschner and Hoffer 1931), holocellulose (Poljak 1948), and lignin (Klason 1920), chemical analysis of the fibers was carried out. The content of hemicellulose was calculated by subtracting the amount of cellulose from holocellulose. To determine the ash content of the fibers, samples were dried in a crucible, preheated above the flame of a Bunsen burner, and placed in a muffle furnace at 525 °C for 5 h (Matissek et al. 2013).
Density and Mechanical Properties
One of the advantages of natural fibers over glass fibers is their lower density with comparable specific properties (Nabi Saheb and Jog 1999; Pandey et al. 2015). For measuring the real density of fibers, dried fibers were cut to the same length and their weight was measured with a precise balance (0.1 mg accuracy; Fisherbrand, PS-200). The fiber volumes were estimated with a gas pycnometer (Ultrapyc 1000, Quantachrome Instruments, FL, USA) using helium gas at 20 °C. In total 20 fibers were measured; for each fiber 5 measurements were taken.
Single fiber tensile tests were performed on 150 fibers to determine the tensile strength and the Young’s modulus of the fibers (ASTM D3822/D3822M – 14). For this test, a mini tensile machine was used that registers the displacement of the clamps and the load applied to the fibers (Zwickiline Z2.5, Zwick GmbH, Ulm, Germany). In order to keep the fibers straight and fixed during the test, the fiber ends were glued between two pieces of paper. The test speed was 1 mm/min, and a load cell of 10 kN was used. The distance between the two clamps were kept constant at 40 mm for all samples. After breaking the fibers, their cross-sectional area was measured under a microscope (Olympus BX 52) from at least 3 points, and the average was calculated.
RESULTS AND DISCUSSION
Anatomy
From a botanical point of view, bark is defined as the sum of those tissues produced by the vascular cambium oriented toward the outside of the stem. The vascular cambium is the meristematic layer of cells that produces wood (xylem) and bark (phloem). Bark itself is divided into two layers: inner and outer bark (Panshin and de Zeeuw 1980). The inner bark consists of the conducting, sclerenchymal, and parenchymal cells similar to the xylem (Fengel and Wegener 1983).
Light microscopy results, as shown in Fig. 2a and 2b, revealed tangential rows of fibers in the inner bark of willows. Because safranin colors only the lignin, the red color of the bundles is an indicator of high lignin content in their cell walls. These are the so-called bast fiber cells, which in most trees are the only lignified cells of the phloem (Panshin and de Zeeuw 1980). Bast fiber cells are thick-walled with overlapping ends, and they are located between parenchymal cells (Tsoumis 1968). They may be absent in the bark of some trees (Panshin and de Zeeuw 1980). These fibers have been observed in different willow species as well as hybrids (Arihan and Güvenç 2011; Dou et al. 2016).
As a result of staining cellulose with astra blue, the parenchymal cells were colored blue. Considering that the samples were taken in the beginning of the spring, the sieve tubes, which are the conducting elements in the bark of hardwoods, were not visible. These cells are functional for only one growth season; they die at the end of a vegetation period and collapse due to the pressure originating from cambial activity (Tsoumis 1968; Panshin and de Zeeuw 1980). The outer bark is composed of old phloem tissues (Panshin and de Zeeuw 1980) and is very thin in younger trees. There were no fibers observed in the outer bark of willow specimens.
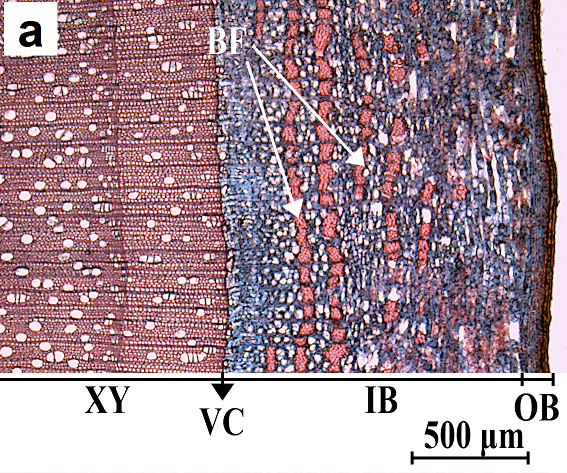
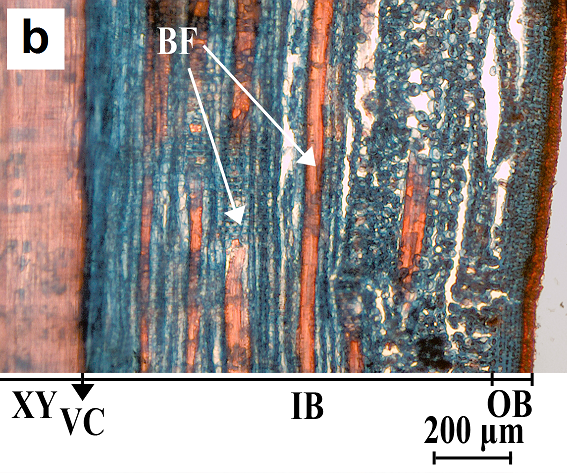
Fig. 2. Cross-section (a) and radial cut (b) of willow bark. XY, xylem or wood; VC, vascular cambium; IB, inner bark; OB, outer bark; and BF, bast fiber
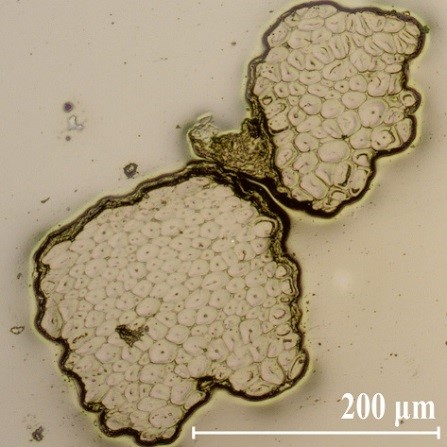
Fig. 3. Cross-section of bast fibers embedded in epoxy resin
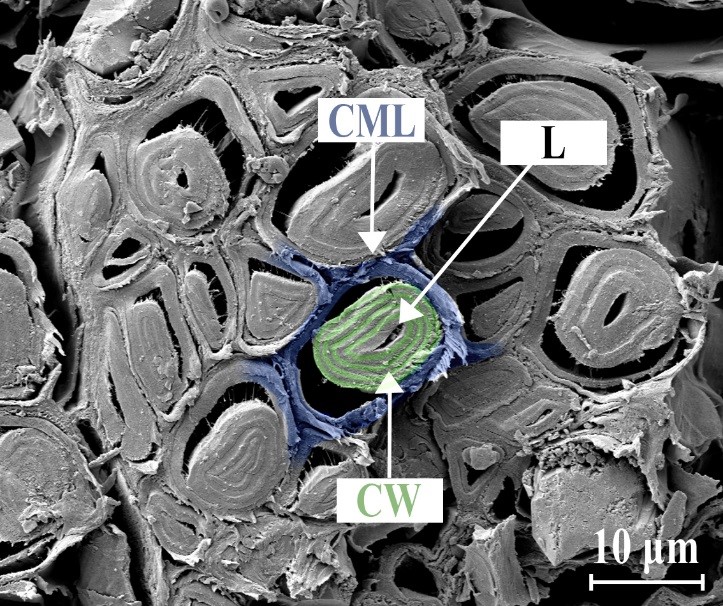
Fig. 4. SEM images of bast fibers, L, lumen; CML, compound middle lamella; and CW, secondary layers of cell wall. Colors were added for better visualization.
Figure 3 shows the cross-section of two bast fibers after extraction via retting. Each bast fiber is actually a bundle consisting of 10 to over 100 fiber cells that overlap at the end (Fig. 2) and form long bast fibers which seem to continue through the entire inner bark. Electron microscopy revealed the cell wall structure of the fibers (Fig. 4). The fibers have very thick cell walls, consisting of up to 8 layers. Because of additional secondary cell walls, the lumina were closed or narrowed. Furthermore, the fibers were partially separated from the compound middle lamella, which resulted from air-drying the fibers before sample preparation. The compound middle lamella was in fact two primary walls of the adjacent cells, together with the thin layer of the middle lamella (Tsoumis 1968; Fengel and Wegener 1983).
To roughly estimate the annual amount of bast fibers produced, 6 trees ranging from 3 to 4 years of age were cut down. Their breast height diameter (BHD) was around 3 cm, and about 12% of their surface was covered with bast fibers. Considering that Inger trees with a BHD of 3 cm have around 25% of their dried weight-based bark in their stems (Gallardo and Seymour 2011) and willow plantation annual yield is about 13 tons/ha with 25% moisture content (Caslin et al. 2015), the amount of bast fibers was estimated to be 13,000 × 0.75 × 0.25 × 0.12 = 290 kg/ha annually.
Thermogravimetric Analysis
In thermogravimetric (TG) curve of the willow bast fibers in Fig. 5, an initial mass loss below 110 °C is illustrated that is due to evaporation of the water and other volatile components in the sample. The main mass loss of the lignocellulosic material is reported to be around 220 to 240°C (Blezki and Gassan 1999; Nabi Saheb and Jog 1999) for the fiber investigated in this research this temperature was around 230 °C (Fig. 5). The temperature onset of decomposition (Ton), temperature for 20% wright loss (T20), and temperature of maximum rate of degradation (Tmax ) are recorded as 257, 305 , and 365°C, respectively.
The first derivative of the TG curve (DTG) reveals several steps of mass loss, as shown in Fig. 5. Thermogravimetric (TG) behavior of the natural fibers is correlated to thermal behavior of their chemical constituents (Monteirto et al. 2012b). The first peak (P. 1) corresponds to the evaporation of the volatiles at about 50 to 70 °C. This initial curve is observed in the TG analysis of other natural fibers as well (Yemele et al. 2010; Monteirto et al. 2012a).
The second one (P. 2) is the starting point of lignin decomposition. Lignin is a complex polymer with aromatic structure and various branches; this leads to a wide range of thermal decomposition temperatures with a very low rate (115 to 500 °C) (Randriamanantena et al. 2009; Yang et al. 2007).
Hemicellulose starts to decompose at 160 °C with the highest amount of decomposition occurring around 180 to 560 °C, whereas cellulose mainly degrades around 340 °C (Randriamanantena et al. 2009). Thus, the third (P. 3) and fourth (P. 4) peaks are respectively showing the superposition of degradation of lignin and hemicellulose (250 to 350 °C), and lignin and cellulose (320 to 400°C). The last peak (P. 5) is related to oxidative degradation of charred residues of samples (Fiore et al. 2011).
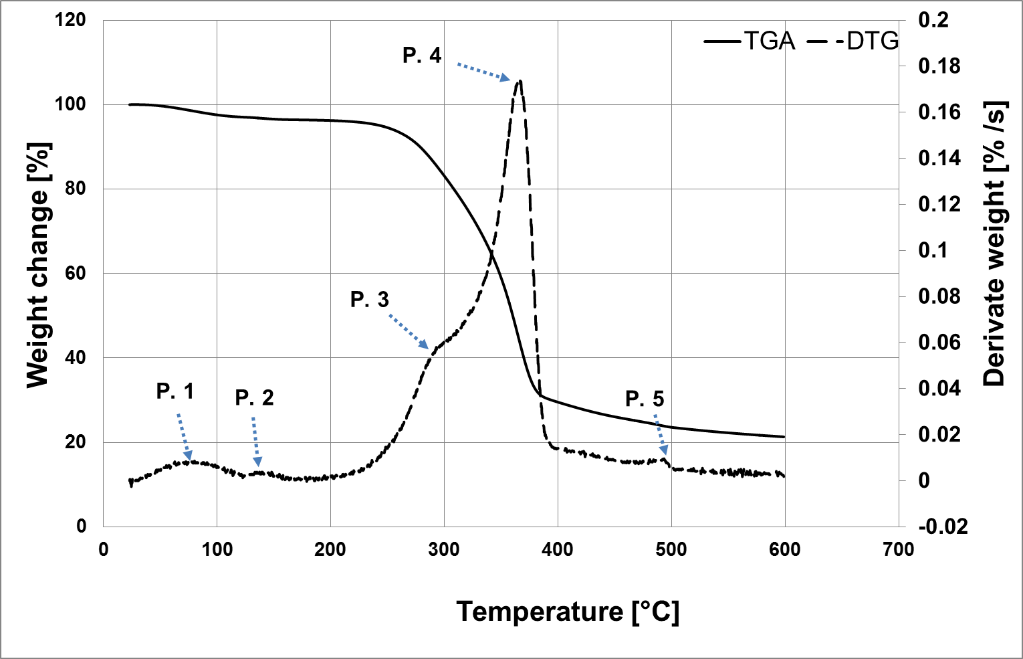
Fig. 5. TGA and DTG curves of willow bast fibers; P.1 to P.5 indicate peaks
Chemical Composition Analysis
Table 1 compares the chemical composition of some common bast fibers with the bast and wood of the examined willow hybrid (Inger). The composition of the willow bast fibers was similar to that of its wood, except for its relatively higher amount of ash in the bast fibers, which is due to the higher amount of inorganic extractives. The amount of ash in bast fibers was around 5 times higher than that of wood, which makes bark an undesirable material in combustion for energy production, as mentioned before.
Willow bast fibers contain about 44% cellulose, which is less than most common fibers (Table 1). It has been reported that the tensile strength of a fiber is directly proportional to its cellulose content (Pickering et al. 2016; Väisänen et al. 2016). Therefore, the lower amount of cellulose in willow bast fibers can be a disadvantage.
The hemicellulose content of willow bast fibers was more than that of common fibers (Table 1). Hemicellulose is the most sensitive to thermal and microbial effects, among the major fiber constituents (Arifur Rahman et al. 2015; Väisänen et al. 2016). Hemicellulose and to a lesser degree cellulose are considered hydrophilic materials, due to the presence of the hydroxyl (OH) groups in their structures (Stokke and Gardner 2003). Hydrophilicity of the natural fibers is one of their drawbacks in composite production, since it results in high moisture absorption and weak adhesion to hydrophilic polymers (Muthuraj et al. 2015; Väisänen et al. 2017).
Unlike cellulose and hemicellulose, lignin is a hydrophobic substance with a complex three-dimensional structure of aromatic monomers (Stokke and Gardner 2003). Therefore, when reinforced in polymer matrices, the high amount of lignin in willow bast fibers could lead to lower water absorption than other natural fibers.
Density and Mechanical Properties
The real density of the willow fibers was estimated at 1.2 g/cm3, which is lower than most industrial natural fibers such as: flax, hemp, and jute (Table 2). Lower density of the fibers is advantageous and could lead to production of the lighter composite panels.
A typical stress-strain curve of the examined fibers is illustrated in Fig. 6. The sudden load drop on the curve is a result of the brittle nature of the fibers. Willow bast fibers had a respective tensile strength and Young’s modulus of 307.6 ± 130.1 MPa and 16.9 ± 8.4 GPa, which is comparable to the lower range of the common natural fibers (Table 2). This can be explained by the fibers having a lower cellulose content, which is directly correlated with the mechanical performance of fibers. It is reported elsewhere that the fibers with higher amount of cellulose show higher mechanical properties (Arifur Rahman et al. 2015; Pickering et al. 2016).
Table 1. Chemical Composition of Willow Bast Fibers and Some Common and Novel Natural Fibers
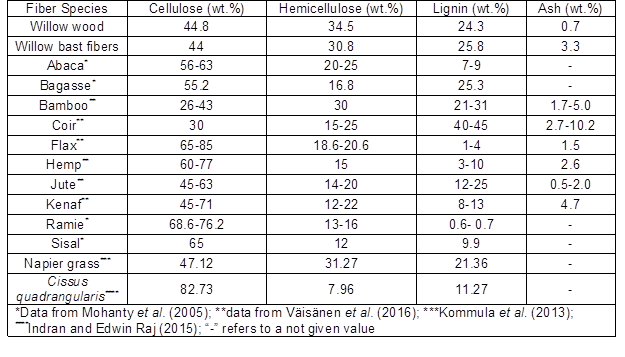
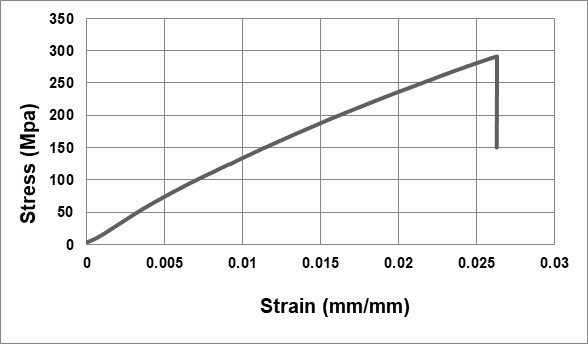
Fig. 6. Typical stress-strain curve of willow bast (Salix spp.) fibers
Table 2. Tensile Properties and Density of Willow Bast Fibers in Comparison to Other Common and Novel Natural Fibers
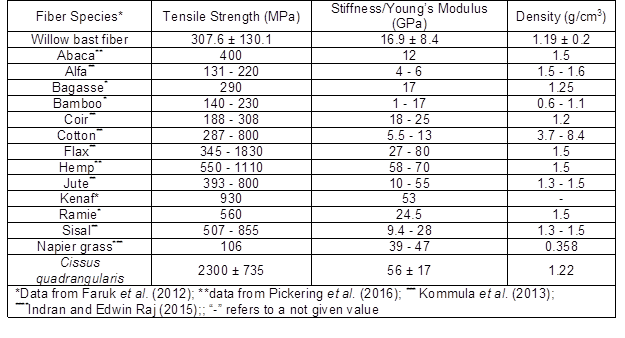
One of the important factors that affect mechanical properties of the natural fibers is their extraction method (Klysov 2007). It is possible to reach better mechanical properties by modifying parameters of the wet-retting process. In future work we will focus on adjusting these parameters and production of the willow bast fiber- polymer composites.
CONCLUSIONS
- This paper characterized the bast fibers of willow bark for future use in production of natural fiber-polymer composites. It was possible to extract the willow bast fibers with the common wet retting process for the application as reinforcement in polymer matrices.
- Willow bast fibers were found to be similar in chemical composition to its wood, with the exception of ash content, which is higher in bast fibers. Moreover, these fibers showed higher lignin and a lower cellulose content than other common natural fibers.
- Microscopic study of the fibers revealed that the lumen varied from being very small to being non-existent, and the secondary cell wall consisted of 6 to 8 layers.
- Willow bast fibers had a low density and showed promising tensile properties in single fiber testing. The physical and mechanical properties may be improved by modifying the fiber extraction and preparation process.
- The investigated fibers were considered by-products of SRCs for energy production. Separating bark from the willow stems before combustion will reduce the environmental pollution of the combustion process and facilitate maintenance of the combustors.
ACKNOWLEDGMENTS
The authors thank Thomas Böhme for providing the plant material for this research. The technical advice of Mr. Sebastian Siwek is also appreciated. Financial support from the Chair of Wood and Fibre Materials Technology (HFT) at the Technical University of Dresden is gratefully acknowledged.
REFRENCES CITED
Arifur Rahman, A. M., Parvin, F., Hasan, M., and Enamul Hoque, M. (2015). “Introduction to manufacturing of natural fibre-reinforced polymer composites,” in: Manufacturing of natural fibre reinforced polymer composites, M. S. Salit, M. Jawaid, N. B. Yusoff, and M. E. Hoque (eds.), Springer International Publishing, Switzerland, pp.17-43. DOI: 10.1007/978-3-319-07944-8_2
Arihan, O. and Güvenç, A. (2011). “Studies on the anatomical structure of stem of willow (Salix L.) species (Salicaceae) growing in Ankara province, Turkey,” Turkish Journal of Botany 35(5), 535-551. DOI: 10.3906/bot-1003-50
ASTM D3822/D3822M – 14 (2014). “Standard test method for tensile properties of single textile fibers,” ASTM International, West Conshohocken, PA.
Bick, A. (2012). Die Steinzeit (Theiss Wissen Kompakt) [The Stone Age (Theiss Knowledge Compact)], Theiss, Stuttgart, Germany.
Blezki, A.k., and Gassan, J. (1999).”Composites reinforced with cellulose based fibers,” Progress in Polymer Science 24, 221-274.
Carus, M., Eder, A., Dammer, L., Korte, H., Scholz, L., Essel, R., and Breitmayer, E. (2014). “WPC/NFC Market Study 2014-03,” (http://www.bio-based.eu/markets), Accessed 30 October 2016.
Chaffey, N. J. (2002), Wood Formation in Trees: Cell and Molecular Biology Techniques, Taylor and Francis, London, UK.
Christersson, L. and Verma, K. (2006). “Short-rotation forestry – A complement to ‘conventional’ forestry,” Unasylva 223(57), 34-39.
Caslin, B., Finnan, J., Johnston, C., McCracken, A., and Walsh, L. (eds.) (2015). Short Rotation Coppice Willow Best Practice Guidelines, Department of Agriculture and Rural Development (DARD), Northern Ireland.
Dou, J., Galvis, L., Holopainen-Mantila, U., Reza, M., Tamminen, T., and Vuorinen, T. (2016). “Morphology and overall chemical characterization of willow (Salix sp.) inner bark and wood: Toward controlled deconstruction of willow biomass,” ACS Sustainable Chemistry & Engineering 4(7), 3817-3876. DOI: 10.1021/acssuschemeng.6b00641
Ek, M., Gellerstedt, G., and Henriksson, G. (2009). Pulp and Paper Chemistry and Technology: Volume 1. Wood Chemistry and Wood Biotechnology, De Gruyter, Berlin, Germany.
Faasch, R. J. and Patenaude, G. (2012). “The economics of short rotation coppice in Germany,” Biomass Bioener. 45, 27-40. DOI: 1016/j.biombioe.2012.04.012
Faruk, O., Bledzki, A. K., Fink, H., and Sain, M. (2012). “Biocomposites reinforced with natural fibers: 2000-2010,” Prog. in Polym. Sci. 37(11), 1552-1596. DOI: 10.1016/j.progpolymsci.2012.04.003
Feng, S., Cheng, S., Yuan, Z., Leitch, M., and Xu, C. (2013). “Valorization of bark for chemicals and materials: A review,” Renew. Sust. Energ. Rev. 26, 560-578. DOI: 10.1016/j.rser.2013.06.024
Fengel, D. and Wegener, G. (1983). Wood: Chemistry, Ultrastructure, Reactions, De Gruyter, Berlin, Germany.
Fiore, V., Valenza, A., and Di Bella, G. (2011). “Artichoke (Cynaracardunculus L.) fibres as potential reinforcement of composite structures,” Compos. Sci. Technol. 71(8), 1138-1144. DOI: 10.1016/j.compscitech.2011.04.003
Gallardo, D. A. and Seymour, D. (2011). “Rindenanteile und Aschegehalte von Pappel- und Weidenhybriden,” in: Nordwestdeutsche Forstliche Versuchsanstalt Kompetenzzentrum HessenRohstoffe (HERO) e. V. Symposium, Hannoversch Münden, Germany.
Gottesfeld, L. M. J. (1992). “The importance of bark products in the aboriginal economies of northwestern British Columbia, Canada,” Economic Botany 46(2), 148-157.
Guidi, W., Pitre, F. E., and Labrecque, M. (2013). “Short-rotation coppice of willow for production of biomass in eastern Canada,” in: Biomass Now – Sustainable Growth and Use, M. D. Matovic (ed.), InTech, Rijeka, Croatia. DOI: 10.5772/51111
Indarin, S., and Edwin Raj, R. (2015). “Characterization of new natural cellulosic fiber from Cissus quadrangularis stem,” Carbohydrate Polymers 117, 302-399. DOI: 10.1016/j.carbpol.2014.09.072
Klasnja, B., Kopitovic, S., and Orlovic, S. (2002). “Wood and bark of some poplar and willow clones as fuelwood,” Biomass Bioenerg. 23(6), 427-432. DOI: 10.1016/S0961-9534(02)00069-7
Klason, P. (1920). “Über Lignin und Lignin-reaktionen [About lignin and lignin reactions],” Berichte der Deutschen Chemischen Gesellschaft [Report of the German Chemical Society]. 50, 706-711.
Klysov, A. A. (2007). Wood-Plastic Composites, John Wiley & Sons, New Jersey, USA.
Kürschner, K. and Hoffer, A. (1931). “Eine neue quantative Cellulose Bestimmung [A new quantitative cellulose determination],” Chemiker Zeitung [Chemist Newspaper] 17, 161-168.
Kommula, V. P., Obi Reddy, K., Shukla, M., Marwala, T., and Varada Rajulu, A. (2013). “Physico-chemical, tensile, and thermal characterization of napier grass (native African) fiber strands,” International Journal of Polymer Anal. Charact. 18, 303-314. DOI: 10.1080/1023666X.2013.784935
Leong, Y. W, Thitihanasarn, S., Yamada, K., and Hamada, H. (2014). “Compression and injection molding techniques for natural fiber composites,” in: Natural Fibre Composites: Materials, Processes and Properties, A. Hodzic and R. Shanks (ed.), Woodhead Publishing Limited, Philadelphia, USA, pp. 216-232. DOI: 10.1533/9780857099228.2.216
Markoff, I. (2016). Short Rotation Coppices in Germany (19/29.04.2016), Short Term Scientific Mission (STSM) supported by Eurocoppice.
Matissek, R., Steiner, G., and Fischer, M. (2013). Lebensmittelanalytik [Food Analysis], Springer Spektrum, Berlin, Germany.
Mohanty, A. K., Misra, M., Drzal, L. T., Selke, S. E., Harte, B. R., and Hinrichsen, G. (2005). “Natural fibers, biopolymers, and biocomposites: An introduction,” in: Natural Fibers, Biopolymers, and Biocomposites, A. K. Mohanty, M. Misra, and L. T. Drzal (eds.), Taylor & Francis, Boca Raton, FL, pp. 1-36.
Monteiro, S. N., Calado, V., Rodriguez, R. J .S., and Margem, F. M. (2012a). “Themogravimetric of natural fibers reinforced polymer composites – An overview,” Materials Science and Engineering: A 557, 17-28. DOI: 10.1016/j.msea.2012.05.109
Monteiro, S. N., Calado, V., Rodriguez, R. J. S., and Margem, F. M. (2012b). “Themogravimetric stability of polymer composites reinforced with less common lignocellulosic fibers – An overview,” Journal of Materials Research and Technology 1(2),117-126. DOI: 10.1016/S2238-7854(12)70021-2
Muthuraj, R., Mista, M., and Mohanty, A. K. (2015). “Studies on mechanical, thermal, and morphological characteristics of biocomposites from biodegradable polymers blend and natural fibers,” in: Biocomposites: Design and mechanical performance, M. Misra, J. K. Pandey, and A. K. Mohanty (eds.), Woodhead Publishing, Cambridge, UK, pp. 1-15. DOI: 10.1016/B978-1-78242-373-7.00014-7
Nabi Saheb, D., and Jog, J. P. (1999).” Natural fiber polymer composites: A review,” Advances in Polymer Technology 18(9), 351-363.
Nishino, T.(2004). “Natural fibre sources,” in: Green Composites: Polymer Composites and the Environment, C. Baillie (ed.), CRC Press, Boca Raton, FL, pp.49-80.
Obernberger, I., and Thek, G. (2004). “Physical characterisation and chemical composition of densified biomass fuel with regard to their combustion behavior,” Biomass Bioener. 27(6), 653-659. DOI: 10.1016/j.biombioe.2003.07.006
Pandey, J.K., Nagarajan, V., Mohanty, A.K., and Mirsa, M.(2015). “Commercial potential and competitiveness of natural fiber composites,” in: Biocomposites: Design and mechanical performance, Misra, M., Pandey, J.K., and Mohanty, A.K.(ed.), Woodhead Publishing, Cambridge, UK, pp. 1-15. DOI: 10.1016/B978-1-78242-373-7.00001-9
Panshin, A. J. and de Zeeuw, C. (1980). Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada, Volume 1, McGraw-Hill, New York, USA.
Pecenka, R., Lenz, H., Idler, C., Daries, W., and Ehlert, D. (2014). “Development of bio-physical properties during storage of poplar chips from 15 ha test fields,” Biomass Bioenerg. 65, 13-19. DOI: 10.1016/j.biombioe.2014.04.017
Pickering, K. L., Aruan Efendy, M. G., and Le, T. M. (2016). “A review of recent developments in natural fibre composites and their mechanical performance,” Compos. Part A – Appl. S. 83, 98-112. DOI: 10.1016/j.compositesa.2015.08.038
Poljak, A. (1948). “Holzaufschluß mit Peressigsäure [Wood pulp with peracetic acid],” Angewandte Chemie [Applied Chemistry] 60(2), 45-46.
Randriamanantena, T., Razafindramisa, F. L., Ramanantsizehena, G., Bernès, A., and Lacabane, C. (2009). Thermal Behaviour of Three Woods of Madagascar by Thermogravimetric Analysis in Inert Atmosphere, Laboratoire de Physique de la Matière et du Rayonnement [Laboratory of Physics of Matter and Radiation], Université D’Antananarivo [University of Antananarivo] and Laboratoire de Physique des Polymères [Laboratory of Polymer Physics], Institut Carnot CIRIMAT [Carnot Institute CIRIMAT], Université Paul Sabatier Toulouse III [University of Paul Sabatier Toulouse III].
Sain, M. and Panthapulakkal, S. (2004). “Green fibre thermoplastic composites,” in: Green Composites: Polymer Composites and the Environment, C. Baillie (ed.), CRC Press, Boca Raton, FL, pp.181-206.
Senwitz, C., Kempe, A., Neinhuis, C., Mandombe, J. L., Branquima, M. F., and Lautenschläger, T. (2016). “Almost forgotten resources – Biomechanical properties of traditionally used bast fibers from northern Angola,” BioResources 11(3), 7595-7607. DOI: 10.15376/biores.11.3.7595-7607
Stokke, D. D., and Gardner, D. J. (2003). “Fundamental aspects of wood as a component of thermoplastic composites,” J. Vinyl Addit. Techn. 9(2), 96-104.
Tsoumis, G. (1968). Wood as Raw Material: Source, Structure, Chemical Composition, Growth, Degradation and Identification, Pergamon Press, Oxford, UK.
Väisänen, T., Haapala, A., Lappalianen, R., and Tomppo, L. (2016). “Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review,” Waste Management 54, 62-73. DOI: 10.1016/j.wasman.2016.04.037
Väisänen, T., Das, O., and Tomppo, L. (2017). “A review on new bio-based constituents for natural fiber-polymer composites,” Journal of Cleaner Production 149, 582-596. DOI: 10.1016/j.jclepro.2017.02.132
Wolbert-Haverkamp, M., and Musshoff, O. (2014). “Is short rotation coppice economically interesting? An application to Germany,” Agroforestry Systems 88(3), 413-426. DOI: 10.1007/s10457-014-9697-2
van Wühlisch, G. (2012). Poplars and Willows in Germany: Report of the National Poplar Commission, Time Period: 2008-2011, Federal Ministry of Food, Agriculture, and Consumer Protection, Bonn, Germany.
Yang, H., Yan, R., Chen, H., Lee, D. H., and Zheng, C. (2007). “Characterization of hemicellulose, cellulose and lignin pyrolysis,” Fuel 86(12-13), 1781-1788. DOI: 10.1016/j.fuel.2006.12.013
Yemele, M. C. N., Kouba, A., Cloutier, A., and Soulounganga, P. (2010). “Effect of bark fiber content and size on the mechanical properties of bark/HDPE composites,” Composites Part A: Applied Science and Manufacturing 41(1), 131-137. DOI: 10.1016/j.compositesa.2009.06.005
Zimniewska, M., and Wladyka-Przybylak M. (2016). “Natural fibers for composite applications,” in: Fibrous and Textile Materials for Composite Application, S. Rana and R. Fangueiro (ed.), Springer Singapore, Singapore, pp. 171-204. DOI: 10.1007/978-p81-10-0234-2_5
Article submitted: November 21, 2016; Peer review completed: January 28, 2016; Revised version received and accepted: March 28, 2017; Published: April 28, 2016.
DOI: 10.15376/biores.12.2.4270-4282
