Abstract
Experimental binderless and adhesive-bonded particleboards were made from three different sample sizes, 0 to 103 µm, 104 to 210 µm, and 211 to 500 µm from Rhizophora spp. wood trunk at 1.0 g cm-3. The objective was to evaluate the physical and mechanical properties of the particleboards. The binderless and soy-lignin bonded particleboards were fabricated and studied based on the density, internal bonding, modulus of rupture, water absorption, and thickness swelling. Microstructure study using scanning electron microscopy (SEM) and elemental analysis by carbon hydrogen nitrogen (CHN) analyser were also performed. Particleboards with adhesives improved the internal bond strength. Smaller particle sizes also were shown to be able to improve the thickness swelling outcomes, with lower hygroscopic properties. The SEM images showed that smaller particle size allowed better bonding with adhesives and provided superior strength in the fabrication of tissue equivalent phantom material. The CHN ratio demonstrated by soy flour and lignin revealed no major difference when compared with the Rhizophora spp. samples, showing basic chemical composition of natural adhesives, which was crucial in the fabrication of tissue-mimicking phantom. The study revealed the potential of soy flour and lignin as adhesives for the fabrication of Rhizophora spp. particleboard as a tissue equivalent phantom material.
Download PDF
Full Article
Physical and Mechanical Properties of Soy-lignin Bonded Rhizophora spp. Particleboard as a Tissue-equivalent Phantom Material
Siti Hajar Zuber,a Nurul Ab. Aziz Hashikin,a,* Mohd Fahmi Mohd Yusof,b and Rokiah Hashim c
Experimental binderless and adhesive-bonded particleboards were made from three different sample sizes, 0 to 103 µm, 104 to 210 µm, and 211 to 500 µm from Rhizophora spp. wood trunk at 1.0 gcm-3. The objective was to evaluate the physical and mechanical properties of the particleboards. The binderless and soy-lignin bonded particleboards were fabricated and studied based on the density, internal bonding, modulus of rupture, water absorption, and thickness swelling. Microstructure study using scanning electron microscopy (SEM) and elemental analysis by carbon hydrogen nitrogen (CHN) analyser were also performed. Particleboards with adhesives improved the internal bond strength. Smaller particle sizes also were shown to be able to improve the thickness swelling outcomes, with lower hygroscopic properties. The SEM images showed that smaller particle size allowed better bonding with adhesives and provided superior strength in the fabrication of tissue equivalent phantom material. The CHN ratio demonstrated by soy flour and lignin revealed no major difference when compared with the Rhizophora spp. samples, showing basic chemical composition of natural adhesives, which was crucial in the fabrication of tissue-mimicking phantom. The study revealed the potential of soy flour and lignin as adhesives for the fabrication of Rhizophora spp. particleboard as a tissue equivalent phantom material.
Keywords: Physical properties; Mechanical properties; Fabrication; Rhizophora spp.
Contact information: a: School of Physics, Universiti Sains Malaysia, 11800 Penang, Malaysia; b: School of Health Sciences, Universiti Sains Malaysia, 15200 Kota Bharu, Kelantan, Malaysia; c: Division of Bio-resource, Paper and Coatings Technology, School of Industrial Technology, Universiti Sains Malaysia, 11800 Penang, Malaysia; *Corresponding author: hashikin@usm.my
INTRODUCTION
Rhizophora spp. is easily identified by their unique characteristics, abundantly growing in the tropical and subtropical coastal regions. The genus Rhizophora contains many species of mangrove (Abuarra et al. 2014b). Rhizophora spp. is generally processed and used mainly as charcoal and construction material, and previous studies proposed its propriety as phantom material mimicking human soft tissue (Bradley et al. 1991; Tajuddin et al. 1996; Banjade et al. 2001; Munem et al. 2004; Marashdeh et al. 2011; Tousi et al. 2014; Abuarra et al. 2014b; Mohd Yusof et al. 2017; Mohd Fahmi Mohd Yusof et al. 2017).
Phantom can be defined as a material that represents human soft tissue, exhibiting the properties that are comparable to human soft tissue relative to radiation, thus allowing it to be used as a tool in radiation dosimetry. A good phantom material for radiation dosimetry must resemble the human body and anatomy, and it needs to be manufactured with materials that are equivalent to human tissues when it comes to size, shape, positioning, density, and radiation’s interaction with matter (Ramos et al. 2017). This is due to its properties including the attenuation properties and effective atomic number that are closely approximated to those properties of human tissues. The use of ionizing radiation in the medical field has been well recognized for diagnostic and therapeutic purposes (Allison et al. 2014; Walsh and Craig 2016; Patel and Jackson 2018). However, due to its harmful effects towards the patients in the case of overexposure of or prolonged radiation, direct dosimetric studies on actual patients are commonly not practiced. Thus, phantoms are commonly being used as substitutes.
Numerous water-equivalent materials such as polystyrene, acrylics, and solid water have been developed (Banjade et al. 2001). However, the use of water as a phantom in dosimetric studies is not always practical due to its physical properties and the unavailability of radiation dosimeters to measure radiation in water. This study led to the use of Rhizophora spp. as a tissue-equivalent phantom material which refers to human soft tissue-equivalent, and it was acknowledged in previous studies in an attempt to replace the dominant polystyrene, Perspex, and epoxy resin-based phantom material (Sudin et al. 1988; Bradley et al. 1991).
However, due to the form an average circumference that the trunk of Rhizophora spp. can reach in a cycle of growth, a much more suitable approach utilizes the wood as a raw material source for the fabrication of human phantom. Instead of raw wood, an engineered wood-based particleboard was made to achieve the objective. More researchers are considering particleboard as an alternative to counter the size limitation of the wood trunk (Marashdeh et al. 2011; Mohd Fahmi Mohd Yusof et al. 2017). However, binderless particleboards resulted in low physical and mechanical properties (Marashdeh et al. 2011). Particleboard is a wood-based panel that is produced as a result of the application of various processes. The wood panel and particleboard industries heavily rely on the use of adhesives to allow adequate bonding.
Fabrication of particleboard often involves a petroleum-based adhesive. Such adhesives have proven to be strong and yet may cause health concerns. They account for 90% of the total wood panel adhesive in the market (Hashim et al. 2011). A petroleum-based adhesive containing formaldehyde has been declared as a pollutant to the environment and also harmful to human health despite its superior characteristics (Moubarik et al. 2010; Rokiah et al. 2009). Thus, significant efforts have been made by researchers to replace petroleum-based adhesives with a natural-made wood binder. The utilization of natural adhesive and bonding agent instead of petroleum-based adhesive has been investigated, and many proved capable of replacing the well-integrated formaldehyde adhesive. Since ancient time, the natural adhesives have been instinctively used in daily life as glue. for example, people used wood resin, a solid or highly viscous substance that can be obtained from birch trees, to attach the heads of spears and axes. The source of natural adhesive can be animal-based and plant-based, and it may include casein, blood, albumin, hide, bone, fish, starch, and wood resin (Landrock and Ebnesajjad 2008).
In this study, natural adhesive soy flour and lignin were used to fabricate the Rhizophora spp. particleboards with a target density of 1.0 gcm-3. Soy flour and lignin had been extensively used as adhesive in the production of medium-density fiberboard and more studies had demonstrated the suitability of both adhesives in the production of a much more firmer and sturdier particleboards (Nasir et al. 2014).
Soy flour is a protein-based adhesive in the fabrication of particleboard, and its capability has been demonstrated extensively by various studies (Frihart et al. 2010; Frihart and Lorenz 2013; Frihart and Satori 2013; Hojilla-Evangelista 2002; Khosravi et al. 2010). Soy protein adhesives are often applied in many industrial fields, such as paper coating, textiles, particleboard, fiberboard, and plywood, due to their favourable characteristics such as renewability, bio-degradability, low price, environment-friendly character, cured by either hot or cold pressing conditions, and easily modified functional properties.
In contrast, lignin is a complex chemical compound, cross-linked polymer that forms a large molecular structure. Lignin often is obtained from wood that provides mechanical strength to wood, supporting the tree’s structure. This particular property has roused interest for lignin to be used as a good wood adhesive that is non-toxic and environment-friendly. Lignin is renewable, non-toxic, easily available, and low cost as an adhesive and wood binder (Ferdosian et al. 2017). Because of the inferior properties of technical lignin as adhesive, formulation of lignin with other natural-based adhesives will improve the reactivity of lignin towards wood composites in the aspect of strength and water resistance.
Studies have stated that soy flour can be combined as an adhesive together with lignin to improve the strength of adhesion (Nasir et al. 2014). The outcomes of such study revealed that a sample of 2.0 g of soy flour, 80.0 mL of lignin with pH of 10.0 resulted in good internal bonding (IB), higher modulus of rupture (MOR) value, and better fiber bonding under scanning electron microscopy (SEM) analysis (Nasir et al. 2014). Phantom material needs to have basic water-resistant properties; however, due to the usage of phantom in a dry and temperature-controlled environment, the phantom rarely makes contact with water, thus reducing the need to scrutinize the impact of phantom immersion in water (Yusof et al. 2017).
Although soy protein and lignin have their own characteristics and potentials to be good adhesives, further investigation needs to be carried out to evaluate their suitability as wood adhesives, especially for the physical and mechanical properties of the particleboard. There is also hardly any research being done on producing an adhesive using these two components. In Malaysia, because they can be easily obtained and are cheap, producing adhesive through this method would be another great way to produce a greener and environmentally friendly adhesive.
In the present study, soy protein and lignin were used as natural adhesives for the fabrication of Rhizophora spp. particleboard. Soy-lignin based adhesive of varying percentages was used to prepare particleboard. Physical properties of the soy-lignin adhesive particleboard were studied through density determination by computed tomography (CT) number and gravimetric method, water absorption, and thickness swelling. The mechanical properties of the particleboard were tested through bending and internal bonding. Microstructure study using SEM and elemental analysis by carbon hydrogen nitrogen (CHN) analyser were performed for all the samples of Rhizophora spp., soy flour, and lignin.
EXPERIMENTAL
Materials
Rhizophora spp. was collected from a charcoal factory in Kuala Sepetang, Perak, Malaysia. The bark of the trunk was removed using bark spud before it was cut into two using a band saw. The wood trunk was chosen, cleaned, and planed using a surface planer machine (Model HP-20; Holytek Industrial Corporation, Taichung, Taiwan). Reduced size of wood chips was then ground into smaller wood particles using a grinder (Tai-yi, Retsch, Germany). A sieving machine with two different opening sizes (104 µm and 210 µm) was used to separate the particles into desired sizes. A digital moisture analyser (Radwag Moisture Analyzer 50/1; Radom, Poland) was used to measure the moisture content of the Rhizophora spp. particles according to the JIS A-5908 (2003). The moisture content of each sample and adhesive was maintained between 1.39%/L and 8.87%/L and measured before each step. This is because the moisture content is affected by various factors including humidity, type, size, and geometry of the sample particle (Tousi et al. 2014). Particleboard was fabricated using a hot press machine with a target density of 1.0 gcm-3. The samples were categorised according to their particle sizes and percentages of adhesives used. For adhesive, commercially available soy flour and lignin in powder form were used as adhesives in this study. For Sample A, particle sizes used were the biggest with the range of 211 µm to 500 µm, sample B was from 104 to 210 µm, and sample C consisted of particle with size lesser than 103 µm. Approximately 0%, 6%, and 12% of adhesives were added to the particleboard. The combination of soy flour and lignin as the adhesive material in the particleboard was based on a ratio of 3 to 1. The manufacturing conditions of the particleboards are explained in Table 1. These different adhesive levels with different particle sizes will be tested in the present study.
Table 1. Particleboard Manufacturing Condition
Parameters including mould dimension, percentage of water added, percentage of soy flour, percentage of lignin, moisture content of Rhizophora spp., soy flour, and lignin, as well as target density of 1.0 gcm-3 were taken into consideration in the fabrication of the particleboard. Nine particleboards with three different percentage of adhesives and three particle sizes of Rhizophora spp. were fabricated with dimensions of 23 × 23 × 0.5 cm3. The test specimens were cut into 5.0 × 0.5 cm2 dimensions. The length, width, and thickness of the specimens were measured using a digital caliper. The mass of each specimen was weighed, and the density was calculated. The calculation of the total amount of Rhizophora spp. particles, soy flour, lignin, and water for the fabrication of the particleboard was performed according to a previous study (Tousi et al. 2014) and defined by Eq. 1,
where ρ, ρt, M, and V are the density (g·cm-3), target density (g·cm-3), mass (g), and volume (cm3) of the fabricated particleboard, respectively. Parameters MR, MR%, and MC%R are the mass (g), weight percentage (%), and moisture content (%/L) of Rhizophora spp. wood particles. The variables MS, WS%, and MC%S are the mass (g), weight percentage (%), and moisture content (%/L) of soy flour adhesive. ML, ML%, and MC%L represented mass (g), weight percentage (%), and moisture content (%/L) of lignin adhesive. Variable MW is the mass (g) of water needed in the formulation.
Rhizophora spp. particle, soy flour, and lignin were carefully mixed to obtain a homogenous mixture. The mixture was poured into a customised mould and pressed for 3 min to form particleboard with no air gap. Then, hot pressing using a hot press machine under the temperature of 200 ℃ with the pressure of 20 MPa was applied for 20 min. The particleboard underwent conditioning after being removed from the frame and trimmed. Samples of the particleboards were prepared in the size of 5.0 × 5.0 × 0.5 cm3 using a band saw post-conditioning.
Methods
Density determination by gravimetric method and CT number
The average mass density of the particleboard samples was calculated using the gravimetric method based on the external dimension measurement of the particleboard samples and CT number obtained from the CT images. For the gravimetric method, a digital caliper was used to measure the external dimension of the particleboard sample that included length, thickness, and width. Electronic balance was used to measure the mass of the samples. The mass density of the particleboard samples was calculated and defined by Eq. 2:
The density calculation did not include air space consideration, as the sample underwent hot and cold pressing with the expectation of total air space removal from the particleboard.
For density determination using CT study, plug phantoms with customized densities were constructed from the particleboards with different adhesives percentages. The phantoms were made compatible with the CT electron density phantom. Several discs with diameter of 3.0 cm were cut out from each particleboard using a band saw, and were glued together vertically into a stack using polyvinyl acetate (PVA) adhesive. The test phantom was left for 24 h to allow proper conditioning before being trimmed to obtain an estimate diameter of 3.0 cm and length of 7.0 cm, compatible to the CIRS 062M phantom (Model 062M; Computerized Imaging Reference Systems Incorporation, Norfolk, VA, USA). The fabricated density plug phantoms are shown in Fig. 1.
The densities of all the phantoms were measured and recorded. The phantoms were scanned at the energy of 120 kVp with tube current of 187 mAs, which is the standard protocol for abdominal scan. Adaptive Iterative Dose Reduction 3D (AIDR 3D) was applied during the scanning procedure to reduce the radiation dose and noise, and to maximise the image quality. The images were reconstructed using filter kernel (FC18, without extra beam hardening correction) at 1.0-mm slice thickness. A computed tomography (CT) imaging system software (Aquilion LB, Toshiba Medical System Corporation, Tochigi, Japan) was used to determine the density distribution of the samples, as it is closely related to the CT number.
Fig. 1. Electron density phantom with interchangeable plugs with the customized CT plug phantom made of Rhizophora spp.
The density of each sample was determined by plotting the CT density calibration curve between the CT numbers and densities of the tissue-equivalent plug phantoms, i.e., lung (inhale), lung (exhale), liver, muscle, adipose tissue, breast (50/50), and water. Figure 3 shows the plug phantom scanned at tube voltage of 120 kVp and 187 mAs. From this curve, the density of adhesive-bonded Rhizophora spp. can be calculated using Eq. 3,
where CT is the CT number of the test sample in Hounsfield unit (HU). The density obtained from the calculation was compared with the density of test sample calculated via the gravimetric method. Statistical analysis, paired sample T-test using Microsoft Excel 2019 application (Intel ® Software; Microsoft Corporation, Redmond, WA, USA) were carried out to compare the density measured by gravimetric method and CT study.
Measurement and determination of internal bond strength
A testing system (Model UTM-5582; Instron, Norwood, MA, USA) with a load capacity of 1000 kg was used to determine the internal bond strength of every sample according to JIS A-5908 (2003) for particleboard. The length, width, and thickness of each sample were measured using digital caliper and recorded accordingly. Every sample was glued together with two aluminum blocks using hot melt adhesive and were left for 24 h under room temperature before further testing, as shown in Fig. 2.
A tension load of 2.0 mm/min was applied vertically to the sample surface area until the appearance of fracture, which had to occur in the middle of the sample. The maximum load (P) was measured at the time of failing force when the breaking load of perpendicular tensile strength to the particleboard occurred. The system gave a curve for each sample that showed a maximum load at fracture time (Pmax). The internal bond in MPa was calculated by dividing the maximum load per area of the board sample surface.
Fig. 2. Preparation of sample for internal bonding test with sample sandwiched between two metal blocks
MOR test for flexural strength
The modulus of rupture test was performed to determine the strength of the test sample before it reached its breaking point in flexion or torsion. The bending strength is based on the maximum fiber stress at failure, and it often refers to a 3-point flexure test on the particleboard. Test samples were prepared to 5.0 × 15.0 × 0.5 cm3, and the dimensions and weight were determined and recorded before the test. Before testing, the test pieces were conditioned following JIS A-5908 (2003). The test was conducted using a universal testing system (Model UTM-5582; Instron, Norwood, MA, USA) with a load capacity of 1000 kg. The load was applied at a test speed of 5.0 mm/min from the surface of the test piece, and the maximum load (P) was measured automatically by the system. The bending strength was calculated based on the formula defined by Eq. 4,
where P, L, b, and t are the maximum load (N), span (mm), width of test piece (mm), and thickness of test piece (mm).
Water absorption (WA) test and thickness swelling (TS) test
Water absorption and thickness swelling test were performed according to JIS A-5908 (2003) for particleboard to determine the dimensional stability of the test sample. The dimensions of 5.0 × 5.0 cm2 test pieces were recorded including the thickness, length, and width using digital caliper pre and post immersion in water. The weight of each test piece was also measured using an electronic balance. Each sample was immersed in distilled water at approximately 3.0 cm below the water surface, as shown in Fig. 3.
After 24 h, the test pieces were removed, and the excess water was wiped off. The water absorption and thickness swelling after the immersion are defined by Eqs. 5 and 6,
where t0 and t24 are the average thickness (mm) before and after 24 h immersion in water. Equation 5 is as follows,
where WA0 and WA24 are the weights (g) of the sample before and after 24 h immersion in water, respectively.
Fig. 3. Preparation of sample for thickness swelling and water absorption test
Morphological analysis of Rhizophora spp. sample, soy flour, and lignin adhesives
The surface morphology of Rhizophora spp. sample, soy flour, and lignin adhesives were obtained and studied using SEM. The SEM images were recorded using an SEM model (FEI Quanta FEG-650, The Netherlands) at the Centre for Global Archeological Research Earth Material Characterization Laboratory, Universiti Sains Malaysia. The samples were coated with gold to provide good conductivity. Rhizophora spp. sample was prepared with the dimensions of 0.5 × 0.5 × 0.5 cm3, whereas soy flour and lignin were prepared in powder form. The sample was fixed onto a specimen holder using double-face adhesive tape and the images were captured under the magnification of 1000×, 2000×, and 4000×.
CHN analysis
The elemental analyses of raw Rhizophora spp., soy flour, and lignin were performed using a carbon hydrogen nitrogen analyser (The Perkin Elmer 2400 Series II CHN Elemental Analyser; Perkin Elmer, Waltham, MA, USA) at the School of Chemical Sciences, Universiti Sains Malaysia, to discover the percentage of carbon, hydrogen, and nitrogen present in each sample. The percentage of oxygen was determined arithmetically by deducting the C, H, and N contents from 100%.
RESULTS AND DISCUSSION
Analysis of density using gravimetric method and CT number
The densities obtained using the gravimetric method were plotted against the measured CT number, where a linear regression was plotted. Table 2 shows the densities obtained using gravimetric method and the CT density calibration curve. Mass density is directly proportional to CT number. The CT calibration curve showed excellent linearity between CT number (HU) and the electron densities with linear regression value, R2 = 0.9992, as shown in Fig. 4.
Table 2. Density of Adhesive Bonded Rhizophora spp. Based on Gravimetric Method and CT Number
A = 211 to 500 µm, B = 104 to 210 µm, C = 0 to 103 µm particle size ranges;
0 = 0% soy flour and lignin, 6 = 4.5% soy flour and 1.5% lignin, 12 = 9% soy flour and 3% lignin
a Lung (inhale), b Lung (exhale), c Adipose tissue, d Breast (50/50), e Water, f Muscle, g Liver
Fig. 4. CT density calibration curve of the plug phantoms
Paired sample T-test statistical analysis was performed to determine the statistical significance of both gravimetric method and CT study in obtaining the density for each sample. Table 3 shows the statistical analysis performed and corresponding p-values.
Table 3. Paired Sample T-test for Density Measured Using Gravimetric Method and CT study
The p-value obtained from the paired sample T-test was 0.278, and revealed that the CT method to measure the density of particleboard did not statistically give significant differences toward the density of particleboard in comparison to the gravimetric method. This study deducted that the CT method was a potential way to measure accurate density of particleboard beside the usual gravimetric method.
The average densities of the particleboards were close to the value of water (1.0 gcm-3). The results were in good agreement with the required density for the fabrication of tissue-equivalent phantom, where the value is close to the density of water with the largest difference of 4% compared to water.
According to the International Commission on Radiation Units and Measurements (ICRU) Report 44 recommendation, the uncertainties to the absorbed dose for the tissue-equivalent phantom should not be greater than 1%; otherwise, correction factor may be required (White et al. 1992). Ideal materials, such as tissue or water equivalent materials, must have similar radiological properties as water or even closer to human soft tissue. These properties include mass density, effective atomic number, elemental composition, similar absorption, and scattering radiation within relevant energy range (Hill et al. 2008; Abuarra et al. 2014a). The attenuation properties of materials towards ionizing radiation are associated with the density, and since water density is close to human soft tissue, mass density of phantom material must be close to 1.0 gcm-3. Therefore, the use of board product with low density or Masonite board with high density will not provide the required density (Yusof et al. 2017).
Thus, every sample must reach the target density of 1.0 gcm-3 to fulfill the requirement for phantom radiological properties.
Analysis of Internal Bond Strength of the Particleboards
The internal bond strength was determined based on JIS A-5908 (2003). The average internal bond strength of different percentages adhesive bonded with Rhizophora spp. particleboard is recorded in Table 4 and Fig. 5.
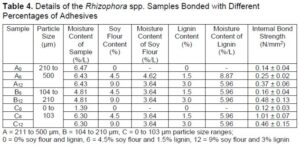
A = 211 to 500 µm, B = 104 to 210 µm, C = 0 to 103 µm particle size ranges;
0 = 0% soy flour and lignin, 6 = 4.5% soy flour and 1.5% lignin, 12 = 9% soy flour and 3% lignin
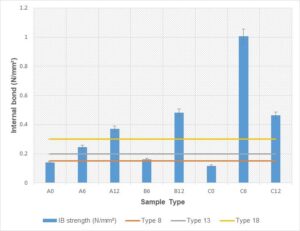
Fig. 5. The average internal bond strength of the fabricated Rhizophora spp. particleboard with different percentages of adhesives: A = 211 to 500 µm, B = 104 to 210 µm, C = 0 to 103 µm particle size; 0 = 0% soy flour and lignin, 6 = 6% soy flour and lignin, 12 = 12% soy flour and lignin
The minimum requirement according to JIS A-5908 (2003) includes classification of Type 8 (0.15 N/mm2), Type 13 (0.2 N/mm2), and Type 18 (0.3 N/mm2). All the particleboards with 12% adhesives (A12, B12, and C12) satisfied the requirements for all the particleboard classifications. One sample with a particle size of 0 to 103 µm and 6% adhesive also satisfied all the classifications by JIS A-5908 (2003). The result showed that sample with 0% adhesive did not satisfy any minimum requirement by JIS A-5908 (2003), despite their particle sizes. The internal bond strength increased as the adhesives added to the particleboards increased despite the particle sizes. The highest internal bond strength was achieved by sample C6, with a particle size of 0 to 103 µm and 6% adhesives. Internal bonding tests were used to evaluate the chemical bonding between the raw wood with adhesives added. A lot of factors influenced the performance of the particleboard, which included the heat and high pressure during hot pressing. Heat application during hot pressing activated the chemical component within the mixture. The addition of soy flour also activated the degradation of hemicellulose, thus producing simple sugar for self-bonding (Marashdeh et al. 2011; Iswanto et al. 2014). Formulation of soy-lignin adhesive introduced the presence of hemicellulose, free sugar, carbohydrate, or saccharides, which at high temperature created a natural bonding within the fabricated particleboard. Lumen voids between spaces were reduced due to high compression during cold and hot pressing. Good strength characteristics were also obtained due to the vessel element and parenchymatous cell of the wood that are closely attached by applying the pressure (Marashdeh et al. 2011).
Formulation of soy-lignin as adhesives improved the strength of fabricated particleboard and was consistent with previous study (Nasir et al. 2014). The addition of soy flour as part of adhesive formulation also was found to provide better strength compared to no soy flour (Frihart and Lorenz 2013). This is because soy flour, which contains protein, influences the bonding between particles, and at the same time carbohydrate serves as an inert diluent (Frihart et al. 2010; Frihart and Lorenz 2013). Other than that, the reactive groups of soy flour provided extra ability in term of coating and penetration of adhesive into the particleboard for improved strength and performance. The formulation of soy-lignin as adhesives provided enhanced strength and improved the bonding of fabricated particleboard.
Interactions between wood particle and adhesive were also influenced by the intermolecular forces (Hansen et al. 2004, 2007; Yemele et al. 2008; Frihart and Hunt 2010). Adhesion involves both mechanical and chemical factors that control the adhesive’s ability to hold together the wood particles. With many adhesives, the most durable, water-resistant bonds develop when the adhesive flows deeply into cell cavities and infiltrates inside the cell walls. Intermolecular forces refer to as the forces that mediate interaction between molecules, including forces of attraction or repulsion between molecules and other neighboring particles. Intermolecular attractive forces, such as Van der Waal’s forces, dipole–dipole forces, and hydrogen bonding, occur frequently to provide for bond strength between soy flour and lignin polymer in molecular form, especially given the high contact area of both adhesives with Rhizophora spp. wood particles.
The formulation of adhesives used in this study was natural-based adhesives, with lignin originated from the cell wall of wood itself and these characteristics contributed to great adhesion between adhesives and the wood particles. Particle sizes also played an important aspect in wood bonding (Frihart and Satori, 2013). Fine particle sizes resulted in greater adhesion and bonding as less empty void spaces within the mixture allowing better distribution.
Analysis of MOR or Flexural Strength
Table 5 shows the average modulus of rupture and the average flexural strength test of the fabricated Rhizophora spp. bonded with different percentages of adhesives. Almost all particleboards did not satisfy the minimum requirement of JIS A-5908 (2003). The results also showed that higher percentages of adhesive improved the modulus of rupture value, and the highest value of MOR was recorded by sample A12, with a particle size of 211 to 500 µm with 12% adhesives. The result was not consistent with the previous study by (Yusof et al. 2017b), which may have been due to the inhomogeneity of distribution of adhesive throughout the particleboard.
Table 5. Average MOR and Flexural Strength of Fabricated Rhizophora spp. Particleboards
Analysis of Thickness Swelling and Water Absorption
The average thickness swelling and water absorption values are recorded in Table 6.
Table 6. Average Thickness Swelling and Water Absorption of Fabricated Rhizophora spp. Particleboards
A = 211 to 500 µm, B = 104 to 210 µm, C = 0 to 103 µm particle size ranges;
0 = 0% soy flour and lignin, 6 = 4.5% soy flour and 1.5% lignin, 12 = 9% soy flour and 3% lignin
The minimum requirement according to JIS A 5908 (2003) is 12% for thickness swelling. The results showed that none of the samples fulfilled the minimum requirement, with the closest sample C0 with a particle size of 0 to 103 µm with 0% adhesive, followed by C6 and C12, each with 0 to 103 µm particle size and 6% and 12% adhesives, respectively. The results showed that with smaller particle sizes, recorded thickness swelling percentages were decreased. This may have been due to the smaller void within the particles, allowing the natural adhesive molecule to settle within the void. The highest percentage of thickness swelling was recorded by sample A0, with particle sizes of 211 to 500 µm with 0% adhesive, further proving that larger particle size increases the capillary effect and hygroscopic properties of the particleboards (Marashdeh 2013).
Morphological Analysis of Particleboards Using SEM
The surface morphology of Rhizophora spp. sample, soy flour and lignin were recorded. The SEM observation of the Rhizophora spp., soy flour, and lignin are shown in Figs. 6, 7, and 8, respectively.
Fig. 6. SEM image of 0 to 103 µm particle size Rhizophora spp. at 4000× magnification size with scale bar included in the image
Fig. 7. SEM image of soy flour at 4000× magnification size with scale bar included in the image
Fig. 8. SEM image of lignin at 4000× magnification size with scale bar included in the image
The microstructures of all the samples were observed at 4000× magnification size. The SEM images showed that the surface of soy flour was rougher than that of lignin, which may have been due to the particle sizes presented during scanning. The SEM of Rhizophora spp. displayed void spaces that can be reduced by hot pressing during the fabrication of particleboard. The void spaces were necessary to be filled in by adhesive to improve the strength and bond between them. Generally, SEM images of the sample with smaller particle size manifested a uniform homogenous merge of the cell when compressed together (Marashdeh et al. 2011). This particular characteristic improved the potential of Rhizophora spp. to be bonded with adhesives to improve the strength necessary as phantom material.
Carbon Hydrogen Nitrogen (Elemental) Analysis (CHN) Analysis
The elemental analysis of Rhizophora spp. sample, soy flour, and lignin were performed using the CHN analyser from School of Chemical Sciences, Universiti Sains Malaysia. Table 7 shows the elemental analysis of all the samples.
Table 7. Elemental Analysis of All the Samples Using CHN Analyser
The differences in the CHN element ratio between 0%, 6%, and 12% adhesive-bonded Rhizophora spp. were rather small, with an increase in adhesive that not only reduced the carbon and hydrogen content but reduced the nitrogen and oxygen content. Heat treatment of the sample resulted in the elimination of water, which is caused by dehydration of carbohydrates to yield aldehyde (Bobleter and Binder 1980). This resulted in the reduction in oxygen content in the samples that underwent hot pressing that were binderless Rhizophora spp. The decarboxylation also resulted from heat treatment, which allowed the reduction of oxygen content, and the condensation process contributed to the increase in carbon content during heat treatment as seen in the ratio of carbon content in binderless Rhizophora spp. samples (Boonstra and Tjeerdsma 2006). The CHN ratio demonstrated by soy flour and lignin showed no major difference when compared with Rhizophora spp. samples, which also portrayed the basic chemical composition for a natural adhesive. The knowledge of the elemental composition of each sample was vital in the fabrication of particleboard for phantom material. This is because the relative similarity between elemental composition of both human tissue and substitute material is important to mimic the characteristic that will provide the accurate response upon the usage of the tissue-equivalent phantom. Some might argue that human body composition is very different from wood-based particleboard. The human body has a high content of water and proportionally contain more nitrogen and less carbon. However, it was shown in previous study that percentages by weight for carbon in human soft tissue are approximately ten times more than the percentages of nitrogen, with oxygen at 63.55 %, carbon at 22.66 %, nitrogen at 2.49% and hydrogen at 10.45%, respectively (Bouchet et al. 2003). When the elemental composition of both tissue substitute material and human tissue were not so much different from one another, a properly designed tissue substitute phantom can be expected, which will provide accuracy to that of a tissue-equivalent material when used for dose measurements.
CONCLUSIONS
- Rhizophora spp. particleboard bonded with soy flour and lignin as adhesives showed great potential to be considered for base material in the fabrication of tissue-equivalent phantom material.
- The average densities of the particleboards were close to the value of water with no significant difference when compared between gravimetric method and CT number study. The results were in good agreement with the required density for the fabrication of tissue-equivalent phantom.
- The particleboards bonded with soy flour and lignin met the minimum requirement of JIS A-5908 (2003) for internal bond strength, with higher percentages of adhesive improving the bonding strength.
- Smaller particle sizes also showed the ability to improve the thickness swelling outcomes, with lower hygroscopic properties of the particleboards.
- The SEM images revealed that the void spaces displayed in Rhizophora spp. samples can be reduced by hot pressing during the fabrication of particleboard and adhesive bonding may improve the overall strength.
- The CHN analysis also revealed little difference between the elemental composition between Rhizophora spp. and the adhesives used, the soy flour and lignin thus established a similarity in terms of basic natural elemental composition between raw material and adhesives used for the fabrication of phantom material. Relative similarity between elemental composition of human soft tissue and the phantom material give rise to its potential as tissue-equivalent material for radiation dose measurement.
ACKNOWLEDGMENTS
The authors thank the School of Physics and School of Industrial Technology, Universiti Sains Malaysia for allowing this study to be conducted in the respective schools/institute. The authors also acknowledge the Universiti Sains Malaysia Short-Term Grant (304/PFIZIK/6315322), the School of Industrial Technology Grant (1001/PTEKIND/8014083) and the Universiti Sains Malaysia Bridging Grant (304.PPSK.6316324). Assistance in raw materials supplied by Kuala Sepetang is also acknowledged.
REFERENCES CITED
Abuarra, A., Bauk, S., Hashim, R., Kandaiya, S., Tousi, E.T., and Aldroobi, K. (2014a). “Microstructure examination, elemental composition analysis of gum arabic bonded Rhizophora spp. Particleboards and their potential as tissue equivalent material,” Int J Chem, Env. Biol Sci 2, 2320-4087.
Abuarra, A., Hashim, R., Bauk, S., Kandaiya, S., and Tousi, E.T. (2014b). “Fabrication and characterization of gum Arabic bonded Rhizophora spp. particleboards,” Mater. Des. 60, 108-115. DOI: 10.1016/j.matdes.2014.03.032
Allison, R. R., Patel, R. M., and McLawhorn, R. A. (2014). “Radiation oncology: Physics advances that minimize morbidity,” Futur. Oncol. 10, 2329-2344. DOI: 10.2217/fon.14.176
Banjade, D. P., Tajuddin, A. A., and Shukri, A. (2001). “A study of Rhizophora spp wood phantom for dosimetric purposes using high-energy photon and electron beams,” Appl. Radiat. Isot. 55, 297-302. DOI: 10.1016/s0969-8043(01)00057-4
Bobleter, O., and Binder, H. (1980). “Dynamic hydrothermal degradation of wood,” Holzforschung 34, 48-51. DOI: 10.1515/hfsg.1980.34.2.48
Boonstra, M. J., and Tjeerdsma, B. (2006). “Chemical analysis of heat treated softwoods,” Holz als Roh-und Werkst. 64, 204. DOI: 10.1007/s00107-005-0078-4
Bouchet, L., Bolch, W., Blanco, H., Wessels, B., Siegel, J., Rajon, D., Clairand, I., and Sgouros, G. (2003). “MIRD Pamphlet No. 19: Absorbed fractions and radionuclide s values for six age-dependent multiregion models of the kidney,” J. Nucl. Med. 44, 1113-1147.
Bradley, D. A., Tajuddin, A. A., Sudin, C. W. A. C. W., and Bauk, S. (1991). “Photon attenuation studies on tropical hardwoods,” Int. J. Radiat. Appl. Instrumentation. Part A. Appl. Radiat. Isot. 42, 771-773. DOI: 10.1016/0883-2889(91)90182-Z
Ferdosian, F., Pan, Z., Gao, G., and Zhao, B. (2017). “Bio-based adhesives and evaluation for wood composites application,” Polymers (Basel). 9, 70. DOI: 10.3390/polym9020070
Frihart, C. R., Birkeland, M. J., Allen, A. J., and Wescott, J. M. (2010). “Soy adhesives that can form durable bonds for plywood, laminated wood flooring, and particleboard,” in: Proceedings of the International Convention of Society of Wood Science and Technology and United Nations Economic Commission for Europe–Timber Committee, Geneva, Switzerland 20(12).
Frihart, C. R., and Hunt, C. G. (2010). Wood Handbook,” Adhesives with wood materials-bond formation and performance,” in: Rev. Process Informally Ref., Chapter 10.
Frihart, C. R., and Lorenz, L. (2013). “Protein modifiers generally provide limited improvement in wood bond strength of soy flour adhesives,” For. Prod. J. 63, 138-142. DOI: 10.13073/FPJ-D-13-00019
Frihart, C. R., and Satori, H. (2013). “Soy flour dispersibility and performance as wood adhesive,” J. Adhes. Sci. Technol. 27, 2043-2052. DOI: 10.1080/01694243.2012.696948
Hansen, C. M. (2007). Hansen Solubility Parameters: A User’s Handbook, CRC Press.
Hansen, C. M. (2004). “50 Years with solubility parameters—Past and future,” Prog. Org. Coatings 51, 77-84. DOI: 10.1016/j.porgcoat.2004.05.004
Hashim, R., Nadhari, W. N. A. W., Sulaiman, O., Kawamura, F., Hiziroglu, S., Sato, M., Sugimoto, T., Seng, T. G., and Tanaka, R. (2011). “Characterization of raw materials and manufactured binderless particleboard from oil palm biomass,” Mater. Des. 32, 246-254. DOI: 10.1016/j.matdes.2010.05.059
Hill, R. F., Brown, S., and Baldock, C. (2008). “Evaluation of the water equivalence of solid phantoms using gamma ray transmission measurements,” Radiat. Meas. 43, 1258-1264. DOI: 10.1016/j.radmeas.2008.01.019
Hojilla‐Evangelista, M. P. (2002). “Adhesive qualities of soybean protein‐based foamed plywood glues,” J. Am. Oil Chem. Soc. 79, 1145-1149. DOI: 10.1007/s11746-002-0618-z
Iswanto, A. H., Azhar, I., and Susilowati, A. (2014). “Effect of resin type, pressing temperature and time on particleboard properties made from sorghum bagasse,” Agriculture, Forestry and Fisheries 3(2), 62-66. DOI: 10.11648/j.aff.20140302.12
Khosravi, S., Khabbaz, F., Nordqvist, P., and Johansson, M. (2010). “Protein-based adhesives for particleboards,” Ind. Crops Prod. 32, 275-283. DOI: 10.1016/j.indcrop.2010.05.001
Landrock, A. H., and Ebnesajjad, S. (2008). Adhesives Technology Handbook, William Andrew.
Marashdeh, M. W., Hashim, R., Tajuddin, A. A., Bauk, S., and Sulaiman, O. (2011). “Effect of particle size on the characterization of binderless particleboard made from Rhizophora spp. Mangrove wood for use as phantom material,” BioResources 6, 4028-4044. DOI: 10.15376/biores.6.4.4028-4044
Marashdeh, M. W. M. (2013). “Fabrication, characterization and dosimetric evaluation of Rhizophora spp. binderless particleboard phantom for the mammography and diagnostic X-rays,” Universiti Sains Malaysia, Gelugor, Malaysia.
Moubarik, A., Charrier, B., Allal, A., Charrier, F., Pizzi, A. (2010). “Development and optimization of a new formaldehyde-free cornstarch and tannin wood adhesive,” Eur. J. Wood Wood Prod. 68, 167-177. DOI: 10.1007/s00107-009-0357-6
Munem, E. M. A., Shukri, A., Tajuddin, A.A. (2004). “Radiation dose distribution measurements around brachytherapy sources in water and Rhizophora spp. phantom,” Australas. Phys. Eng. Sci. Med. 27, 95.
Nasir, M., Gupta, A., Beg, M. D. H., Chua, G. K., and Kumar, A. (2014). “Physical and mechanical properties of medium-density fibreboards using soy—lignin adhesives,” J. Trop. For. Sci. 41-49.
Patel, A., and Jackson, B. (2018). “Low-dose radiation use in diagnostic imaging and cancer therapy settings,” Radiol. Med. 123, 618-619. DOI: 10.1007/s11547-018-0892-5
Ramos, S. M. O., Thomas, S., Berdeguez, M. B. T., Sá, L. V, and Souza, S. A. L. (2017). “Anthropomorphic phantoms-potential for more studies and training in radiology,” Int. J. Radiol. Radiat. Ther 2. DOI: 10.15406/ijrrt.2017.02.00033
Rokiah, H., Hazneza, A. H. S., Othman, S., Norli, I., Hakimi, I. M., Hasnah, M. J., and Salmiah, U. (2009). “Extractable formaldehyde from waste medium density fibreboard,” J. Trop. For. Sci. 25-33.
Sudin, C., Tajuddin, A. A., and Bradley, D. A. (1988). “Evaluation of tissue-equivalent media for dosimetric studies,” in: Proceeding of Local Seminar Activities on Radiation Physics, Biophysics and Medical Physics, Kuala Lumpur, Malaysia, pp. 71-80.
Tajuddin, A. A., Sudin, C. W. A. C. W., and Bradley, D. A. (1996). “Radiographic and scattering investigation on the suitability of Rhizophora spp. as tissue-equivalent medium for dosimetric study,” Radiat. Phys. Chem. 47, 739-740. DOI: 10.1016/0969-806X(95)00052-Y
Tousi, E. T., Hashim, R., Bauk, S., Jaafar, M. S., Abuarra, A. M. H., and Ababneh, B. (2014). “Some properties of particleboards produced from Rhizophora spp. as a tissue-equivalent phantom material bonded with Eremurus spp.,” Measurement 54, 14-21. DOI: 10.1016/j.measurement.2014.04.004
Walsh, R., and Craig, A. (2016). “Radiation therapists’ and diagnostic radiographers’ participation in continuing professional development and knowledge of regulatory body registration,” J. Radiother. Pract. 15, 150-160. DOI: 10.1017/S1460396916000054
White, D. R., Buckland-Wright, J. C., Griffith, R. V, Rothenberg, L. N., Showwalter, C. K., Williams, G., Wilson, I. J., and Zankl, M. (1992). “Report 48,” J. Int. Comm. Radiat. Units Meas. NP-NP. DOI: 10.1093/jicru/os25.1.Report48
Yemele, M. C. N., Cloutier, A., Diouf, P .N., Koubaa, A., Blanchet, P., and Stefanovic, T. (2008). “Physical and mechanical properties of particleboard made from extracted black spruce and trembling aspen bark,” For. Prod. J 58, 38-46.
Yusof, M. F., Mohd. Hamid, P. N. K. A., Tajuddin, A. A., Hashim, R., Bauk, S., Isa, N. M., and Isa, M. J. M. (2017). “Fabrication and characterisation of phantom material made of tannin-added Rhizophora spp. particleboards for photon and electron beams,” J. Phys. Conf. Ser. 851 (1).
Yusof, Mohd Fahmi Mohd, Hashim, R., Tajuddin, A. A., Bauk, S., and Sulaiman, O. (2017). “Characterization of tannin-added Rhizophora spp. particleboards as phantom materials for photon beams,” Ind. Crops Prod. 95, 467-474. DOI: 10.1016/j.indcrop.2016.10.057
Article submitted: April 3, 2020; Peer review completed: May 16, 2020; Revised version received and accepted: May 26, 2020; Published: May 29, 2020.
DOI: 10.15376/biores.15.3.5558-5576
