Abstract
Silica (SiO2) from rice husk was coated with magnetic nanoparticles (MNPs) as an adsorbent to adsorb phenol from river water. The structure of SiO2 and SiO2-MNPs were characterized by Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD). Field emission scanning electron microscopy (FESEM) showed a rod shape, with a rough surface area in the range of 2 to 3 µm. Transmission electron microscopy (TEM) and energy dispersive x-ray spectroscopy (EDX) were used to examine the resulting spherical shape of the synthesized SiO2-MNP. The results showed a range of 1.3 to 1.5 nm for SiO2 and 4.2 to 6.4 nm for SiO2-MNP. Vibrating-sample magnetometer (VSM) showed an Fe value of 45.1% in SiO2-MNP (VSM); for MNP, SiO2, SiO2-MNP 104.12, 4.72, and 8.01 emu/g, respectively. Response surface methodology (RSM) was used to study the parameters and responses to obtain an optimized condition in SiO2-MNPs usage. The optimized parameters were extraction time selected at 5 min, pH 8, 8 mL acetonitrile as solvent and 15 min as sonication time. The application of SiO2-MNPs was applied to real water samples, with recovery of 84% of phenol. Thus, the synthesized adsorbent, SiO2-MNPs, was developed successfully for phenol removal from water samples.
Download PDF
Full Article
Synthesis of Silica from Rice Husk as Coating Material on Magnetic Nanoparticle for Efficient Adsorption of Phenol from Water Samples
Nik Nur Atiqah Nik Wee,a Noorashikin Md Saleh,a,* Tanusha Devi,a Alinda Samsuri,b and Muhammad Zulhaziman Mat Salleh a
Silica (SiO2) from rice husk was coated with magnetic nanoparticles (MNPs) as an adsorbent to adsorb phenol from river water. The structure of SiO2 and SiO2-MNPs were characterized by Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD). Field emission scanning electron microscopy (FESEM) showed a rod shape, with a rough surface area in the range of 2 to 3 µm. Transmission electron microscopy (TEM) and energy dispersive x-ray spectroscopy (EDX) were used to examine the resulting spherical shape of the synthesized SiO2-MNP. The results showed a range of 1.3 to 1.5 nm for SiO2 and 4.2 to 6.4 nm for SiO2-MNP. Vibrating-sample magnetometer (VSM) showed an Fe value of 45.1% in SiO2-MNP (VSM); for MNP, SiO2, SiO2-MNP 104.12, 4.72, and 8.01 emu/g, respectively. Response surface methodology (RSM) was used to study the parameters and responses to obtain an optimized condition in SiO2-MNPs usage. The optimized parameters were extraction time selected at 5 min, pH 8, 8 mL acetonitrile as solvent and 15 min as sonication time. The application of SiO2-MNPs was applied to real water samples, with recovery of 84% of phenol. Thus, the synthesized adsorbent, SiO2-MNPs, was developed successfully for phenol removal from water samples.
DOI: 10.15376/biores.18.3.6204-6220
Keywords: Magnetic nanoparticles (MNPs); Magnetic solid phase extraction (MPSE); Phenol extraction; River water; Response surface methodology (RSM); Rice husk; Silica; UV-Vis
Contact information: a: Department of Chemical and Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43650 Bandar Baru Bangi, Selangor, Malaysia; b: Department of Chemistry and Biology, Centre for Defence Foundation Studies, National Defence, University of Malaysia, Kem Sungai Besi, 57000 Kuala Lumpur Malaysia;
* Corresponding author: noorashikin@ukm.edu.my
GRAPHICAL ABSTRACT

INTRODUCTION
Phenol is a common environmental pollutant because it is used in many industries (Norseyrihan et al. 2016; Hazrina et al. 2018). The waste produced by industries becomes a challenge for pollutants removal and disposal process, thus requiring further removal steps, which involve high costs. Contamination of water sources with endocrine-disrupting organic micropollutants, such as phenolic compounds, affects aquatic life and human health (Ahmed et al. 2010). The phenolic group has the highest pollutant toxicity, causing severe, chronic harm even at low concentrations (Mecha et al. 2016; Frasetya et al. 2019). Therefore, the separation or extraction of wastewater containing phenol is essential.
Sustainable and green chemistry could be used to convert agro-industrial solid wastes into valuable products. This is aligned with global plans to reduce waste and environmental contamination. For example, the by-products of sugarcane bagasse, rice husk, and straw have a high silicon content. Rice husk and ash are used frequently as renewable sources to produce value-added materials, such as silica magnetic nanoparticles (SiO2-MNP).
Rice husk contains materials as the main constituent, and the ash comprises 87% to 98%, including a small percentage of metallic elements. It is a by-product of rice milling and is used for poultry, brick industry fuel, planting medium, and compost. According to Ariffin et al. (2019), the silicone content of husk is 18.8% to 22.3%, and the silicone content of the straw is 9.30% (Mecha et al. 2016).
Due to their high surface area, increased magnetic capabilities, and low cost, magnetic nanoparticles (MNPs) synthesized from ferrous compounds (Fe3O4) are well suited for use as a magnetic adsorbent (Zhang and Shi. 2012; Ul-Islam et al. 2017). However, it has drawbacks that limit its use for phenol extraction, including the ease with which it oxidizes when exposed to air, agglomerates in aqueous media, is unstable in an acidic environment, and is very hydrophilic (Jones and Clarke 2005). Finding a solution for these issues, such as materials to functionalize the surface of MNPs, is imperative to overcoming these limitations.
The effectiveness of the phenol extraction method in water samples has been demonstrated in numerous investigations. There are many complicated techniques, including liquid-liquid extraction (LLE), solid phase extraction (SPE), micro-solid phase extraction (MSPE), and supercritical fluid extraction (SFE) (Labat et al. 2000; Lee et al. 2005; Lee et al. 2006; Dan et al. 2022). However, these approaches have several drawbacks, including their time requirement, intricate procedures, heavy reliance on organic solvents, and low sensitivity.
Fig. 1. MSPE method
The methodology used in this study is known for removing selected analytes from aqueous media based on magnetic adsorbents (MNPs) (Begum et al. 2021). The adsorbent is not tightly wrapped in the cartridge. It can be placed directly in a large volume of water sample solution or suspension, and the extraction time is shortened. Other benefits of MSPE include the use of low-volume, non-toxic organic solvents, good repeatability, quick sample processing, and compatibility with most analytical instruments (Noushad et al. 2012; Rafiee et al. 2012; Boudesocque et al. 2019; Ram Talib et al. 2019). Figure 1 summarizes the MSPE approach.
In addition, the response surface methodology (RSM) was used to determine the concentration of phenol in water using the optimum condition of parameters studied. RSM is a mathematical and statistical method that is widely used in industry to determine the optimal conditions in the experiment. It is helpful for maximizing or minimizing various variables because it evaluates the effect of multiple factors and their respective interactions on one or more variables moving silently. It also reduces the impact of noise to improve the convergence of the optimization process (Boudesocque et al. 2019).
This study established the viability of using rice husk silica as a coating material for MNPs (SiO2-MNP) to extract phenols from river water. When compared to other paddy plants debris, the rice husk has the highest content of silica. Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), field emission scanning electronic microscopy (FESEM), transmission electron microscope (TEM), energy dispersive X-ray analysis (EDX), and vibrating sample magnetometer (VSM) studies were used to look at the morphology, structure, and composition of SiO2-MNP. The findings indicate that silica has been successfully coated on the MNPs core-shell. The effects of different experimental parameters, including sample volume, extraction time, desorption time, pH value, amounts of sorbent, and kinds and amount of desorption solvents, were examined.
The phenol recovery in water samples (84.8% to 92.4%) is considered high with simple and quick steps without the usage of the complex apparatus. At the same time, the results on water samples without phenol were detected in the concentration range of 3.82 ppm to 7.58 ppm.
EXPERIMENTAL
Reagents
RandM Chemicals Company provided the ferric (II) chloride tetrahydrate (FeCl2.4H2O), and ferric (III) chloride hexahydrate (FeCl3.6H2O) (Essex, UK). Phenol was provided by Merck (Darmstadt, Germany). Acetonitrile was obtained from J.T. Baker (Philipsburg, New Jersey). Methanol and ethanol from HmbG Company (Hamburg, Germany). Stocks of the standard analytical solution were created using acetonitrile at concentration of 1000 g/mL and kept at 4 °C. Prior to usage, acetonitrile was brought to room temperature. Sodium hydroxide and hydrochloric acid were provided by Merck (Germany) and were used to regulate the pH of the solution throughout experiment. Stock solutions were thoroughly diluted with acetonitrile or Milli-Q ultrapure water to create working solutions.
Instrumentation
FT-IR Spectrometer (Thermo Nicolet, Nexus 670) by ATR technique in the absorption mode with 32 scans and a resolution of ±4 cm-1, and a wavenumber range of 4000 to 450 cm-1 with diamond as a detector was used to confirm the functional group’s as-synthesized nanoparticles. Using a Bruker D8 Advance X-ray powder diffraction (XRD) diffractometer (CuK, radiation, α at 40 kV/30mA), at a scanning speed of 1°/min and 0.02°/min from 10° to 80° (2θ), it was determined how the produced nanoparticles behaved in terms of crystallinity. Field emission scanning electronic microscopy (FESEM), model VP (ZEISS SUPRA 55VP, evolution 0.2 nm@120 kV), outfitted with energy dispersive X-ray spectrometry, was used for the elemental analysis. Talos L120C evolution 0.2 nm @ 120 kV transmission electron microscope (TEM) was utilized. The magnetic behaviour of the prepared nano-adsorbents was characterized by using a vibrating sample magnetometer (VSM Lake Shore 7404 Series, Tokyo, Japan). Data were recorded with 80 points/loop with speed of 10s/drop. The separation and quantification of the tested phenol was achieved by using an UV-Vis analysis.
Preparation of Silica (SiO2) from Rice Husk
The main component was rice husk, and examples came from Tanjung Karang Bernas Company in Kuala Selangor. To remove sand and dirt stuck to the surface of the rice husk, it was first scrubbed and washed with distilled water (Rafiee et al. 2012). The samples took 10 min to dry in the oven. After that, 1.0 M of hydrochloric acid (HCl) was added, and the process to remove metal impurities was continued by soaking at 90 °C for 1 h in water bath. Prior to filtering and overnight oven drying, samples were washed with distilled water (Chakraverty and Kaleemullah 1991; Noushad et al. 2012). In addition, this technique was undertaken to boost the purity of silica (Norsuraya et al. 2016).
The dried rice husk was steeped in 1.0 M NaOH to form a sodium silicate solution. The silica component in the rice husk dissolved as a result of the reaction between the rice husk and NaOH, creating a sodium silicate solution (Patil et al. 2014). The solution was immersed in water bath for 1 h while being agitated with stirrer. The colour of the solution altered after 1 h and took on a blackish hue as a result of the sodium silicate reaction.
Whatman filter paper No. 41 was used to filter the solution, separating the rice husk residue from the dark-coloured solution. The silica supernatant solution was collected. HCl was drip-fed while swirling on a heated plate until pH 7 to 8 was achieved. After 45 min of stirring, the solution was allowed to mature for around 2 weeks. The precipitate was centrifuged, and the precipitate collected was calcined for 4 h at 800 °C. A white silica powder was produced. A white silica powder was produced and stored for use in the procedure that followed. The approximate yields of white silica powder produced was 0.7 gram.
Preparation of SiO2 Coated with Magnetic Nanoparticle (MNPs)
First, 450 mL of deionized water was used to dilute 0.7 g of extracted SiO2 powder. In the meantime, a 2:1 aqueous solution of ferric chloride (Fe3+) and ferrous chloride (Fe2+) were made. The solution was gradually incorporated into a room temperature SiO2. The pH of the solution was raised to 10 by slowly adding ammonia solution. The solution was continuously stirred while being heated at 90 °C for 4 h. The solution was filtered after being cooled to room temperature. The dark precipitate was repeatedly washed with distilled water and acetonitrile. The precipitate was dried overnight at 70 °C.
Extraction Procedure
SiO2-MNPs of 0.1 g was placed in a vial, and 8 mL of prepared spiked phenol solution (pH 8) was transferred into the same vial. The solution was shaken for 10 min. The adsorbent was isolated from the sample solution using an external magnet, and then the sample solution was decanted. The adsorbed phenol on SiO2-MNP was eluted by sonication method with 8.0 mL of desorption solvent (acetonitrile) for 15 min. The eluent was collected and stored in a vial for UV-Vis analysis.
Optimization of MSPE
Several factors that affect extraction efficiency, such as solution pH value, adsorbent amount, types of desorption solvent, volume of desorption solvent, extraction time, desorption time and sample volume, were studied.
Samples
River water and lake water were selected as real water samples for investigation. River water samples were collected from Selangor, Malaysia. The specific river water locations were Sungai Kuyoh, Sungai Buah, Sungai Kantan, and Sungai Langat (in the area of Universiti Kebangsaan (UKM). Lake waters were collected from Selangor, Malaysia. The specific location of lake water was collected from Taman Idaman, Tasik KBG (FRIM), Taman Tasik Cempaka, Tasik Sri Serdang, Tasik Faculty of Engineering (FKAB, UKM), and Tasik Bandar Tun Hussein Onn. All water samples were filtered through a 0.25 µm membrane and stored at 4 °C.
RESULTS AND DISCUSSION
Characterization of SiO2 and SiO2-MNP
Morphology analysis
Figure 2 shows the particle size of SiO2 and SiO2-MNP. SiO2 was depicted as having a rod-like shape and SiO2-MNP as having an erratic arrangement. The SiO2 shown here was rice-husk silica. The samples were irregular in organization and ranged in size 3 nm to 10 nm. According to earlier research, the solvent utilized significantly impacted the molecules’ size and arrangement (Ariffin et al. 2019). The size of the magnetic silica nanoparticles increases with the length of the alcohol chain, resulting in a rougher and bigger surface on the sample, as seen in Fig. 2(b). Blobs result from the high surface area and the resulting high surface energy area.
Fig. 2. Analysis FESEM of (a) Silica (rice-husk silica), (b) Silica magnetic nanoparticle (silica from rice-husk)
The outcomes of the study using transmission electron microscopy (TEM) are shown in Fig. 3. The structure of SiO2-MNP was spherical, with SiO2 covering the exterior of Fe3O4 nanoparticles and Fe3O4 nanoparticles inside (Lee et al. 2018). These findings demonstrated that SiO2-MNPs were spherical, contained Fe3O4 nanoparticles, and had a SiO2 covering shell around them. In addition, two independent electron density areas confirmed the production of silica-coated nanoparticles. The magnetic core was represented by the electron-dense region, while the silica coating was represented by the less dense translucent zone surrounding the core (Helmi Rashid Farimani et al. 2014).
Fig. 3. Analysis TEM of (a) Silica(rice-husk silica), (b) Silica magnetic nanoparticle (silica from rice-husk)
Elemental study
To identify the composition of components including O, Fe, Si, and C in prepared MNPs, SiO2 and SiO2-MNPs, energy dispersive X-ray analysis (EDX) was carried out. According to Fig. 4(a) samples of MNPs, only 35.8% of Si, 40.3% of O, and 22.0% of C were presented in the MNPs. However, following coating with SiO2, new components were presented, including 45.1% of Fe, 33.2% of O, 7.9% of C, and 13.8% of Si. The large percentages of C (22.0%), Si (2.46%) and O (40.3%) in SiO2-MNP demonstrated that MNP was successfully introduced onto SiO2-MNP surface, as shown in Fig. 4(b).
Fig. 4. Analysis EDX of (a) Silica (rice-husk silica), (b) Silica magnetic nanoparticle (silica from rice-husk)
Functional group analysis
Fourier Transform Infrared Spectroscopy (FTIR) was used to characterize the surface chemicals structures of SiO2-MNP. The results are depicted in Fig. 5. The appearance of additional peaks at 3405.2 cm-1, 1064.8 cm-1, and 470.6 cm-1 were attributed to O-H stretching vibration, anti-symmetric Si-O-Si, symmetric Si-O-Si, and Fe3O stretching, respectively. This suggests the linker SiO2 was added to the surface of MNP. These outcomes demonstrated the functionalization of MNP onto SiO2-MNP surface.
Fig. 5. Analysis FTIR of silica magnetic nanoparticle (silica from rice-husk)
Crystallinity analysis
Figure 6 displays the XRD patterns of SiO2 and SiO2-MNPs. Broad spectral curves with peak values between 2θ = 15° up to 30° are known as amorphous forms (Park and Lee, 2004; Ghorbani et al. 2015). Figure 6(b) show peaks 2θ = 27.44°, 31.71°, 45.42°, and 56.41° are the resulting nanoparticle diffraction angles. These results demonstrated the creation of highly pure crystals (Gopal et al. 2020; Nodeh et al. 2015).
Fig. 6. Analysis XRD of (a) Silica (rice-husk silica), (b) Silica magnetic nanoparticle(silica from rice-husk)
Magnetic behavior
Vibrati ng-sample magnetometry (VSM) analysis was carried out to determine the concentration of SiO2 and SiO2-MNPs. The magnetic behaviour of MNPs, SiO2 and SiO2-MNP. SiO2 and SiO2-MNP were found to be 104.1, 4.718, and 8.008 emu/g, respectively. Figure 7 illustrates the superparamagnetic behaviour of the different sorbents. The absence of hysteresis, low remanence, and coercivity demonstrates it. SiO2 coating on the outer layer of MNP surface, which reduced interaction between the nanoparticles was evidence by the decreased magnetic value of SiO2-MNPs as compared to MNP.
Fig. 7. Analysis VSM of (a) Silica, (b) magnetic nanoparticle, and (c) Silica magnetic nanoparticle
Optimization of MSPE conditions
To find ideal conditions for SiO2-MNPs through the MSPE technique, key experimental parameters including pH value, amounts of sorbent, types of solvent, types of desorption solvent, extraction, and desorption time, as well as sample volume were optimized in this study.
Fig. 8. Effect of pH on phenol extraction
Effect of sample solution pH
The impact of sample solution was examined from pH 6 to 12, as shown in Fig. 8. Low recovery rates were seen regardless of the pH. Thus, SiO2-MNPs charge density and phenol charge state were considered. In terms of adsorbent charge, the chosen phenol produced a neutral molecular state under acidic circumstances. Phenol extraction at pH 8 displayed the most significant peak area, so pH 8 was selected as the best pH value (Zhang et al. 2013). When the pH is less than 5, the solution is acidic, and the metal-organic framework may not be able to support the sorption (Wang et al. 2014). Phenols are more likely to ionize at high pH. As a result of the increasing quantity of charges, the absorption force weakens (Gamonchuang and Burakham 2021). Therefore, using sample solutions of 0.1 M HCl and 0.1 M NaOH, the impact of pH on extraction performances was investigated in the pH range of 6 to 12. From pH 6 to 7, the adsorption declined, and it grew more at pH 8. It started to deteriorate when the value was further raised to pH 12. This pH change may be closely correlated with the surface charge of the phenolic compound, whereby phenol is a weak acid and tend to exist in its protonated state (Gopal et al. 2020).
Effect of sorbent amounts
The amount of sorbent used limits the amount of phenol that can be extracted before an equilibrium is reached. According to Nodeh et al. (2015), an increase in the adsorbent mass results in an increase in adsorption efficiency. This is because an increase in adsorbent will result in a rise in adsorption sites, which would allow for the occupancy of more analytes. In this experiment, various dosages of the SiO2-MNP sorbents, ranging from 0.04 to 0.3 g, were applied. Figure 9 shows that when the amount of sorbent grew, the peak regions climbed sequentially from 0.04 g to 0.1 g, and the recovery plateaued after 0.1 g due to the active sites on the adsorbent SiO2-MNP being saturated, which was similar to results by Dolaksiz et al. (2018). Therefore, the ideal quantity of adsorbents used in subsequent tests was 0.1 g SiO2-MNP.
Fig. 9. Effect on amount adsorbent of phenol extraction
A similar study by Gopal et al. (2020) stated that dosage of adsorbent was a vital factor related to the extraction capacity. An increase in adsorbent dosage would increase the active binding site of magnetic to interact toward phenolic compound, eventually enhancing the extraction efficiency. Therefore, an increasing amount of adsorbent dosage resulted in saturation and aggregation of the active binding site, which resulted in further adsorption and caused the extraction efficiency to remain constant (Gopal et al. 2020).
Effect of desorption solvent
Acetone, acetonitrile, methanol, and ethanol were employed as desorption solvents. Acetonitrile yielded better recoveries for nearly all the targeted phenols and was the best eluting solvent, as demonstrated in Fig. 10. When employed as an extraction solvent, acetonitrile is an aprotic polar solvent that is unlikely to react. Acetonitrile is an aprotic polar solvent that might break the hydrogen chain between phenol and SiO2-MNP, following the earlier work. Additional investigations showed the ability to solubilize acetonitrile as a spray adsorbent solution (Van Berkel and Kertesz 2007). Therefore, acetonitrile was chosen for subsequent experiments.
Fig. 10. Effect of desorption solvent on phenol extraction
Application to Real Water Sample
The proposed method was applied to determine the targeted phenols in selected water samples with different water matrices (river water and lake water). Results ranged from 84.8% to 92.4%. Results from water samples that were unspiked with phenol showed that it could be detected at concentration between 3.82 ppm to 7.58 ppm. Table 1 examines the adsorption results from the study’s use of UV-Vis spectroscopic analysis on lake and river water. Figure 11 displays the outcomes of UV-Vis phenol extraction on actual water samples. A UV-Vis spectrum from one of the outcomes from water samples is shown in Fig. 12.
Table 1. Phenol Analysis on River Water and Lake Water
A weight of 0.2 g SiO2-MNP with 4 mL volume of desorption solution produced high adsorption. In contrast, a volume content of 10 mL at the same adsorbent weight produced a lower adsorption result. This phenomenon suggested that the removal efficiency increased rapidly and significantly when more surface area and adsorption sites were used. It also remained stable to achieve saturation and a compatible balance (Mandal et al. 2019).
Fig. 11. Results of phenol extraction on real water samples using UV-Vis
Fig. 12. UV-Vis spectrum on real water sample
Table 2 shows results from previous studies. There are many different techniques that can extract phenol from wastewater. The results of the current investigation demonstrated the superiority of the developed silica-based approach for phenol extraction from wastewater.
Table 2. Previous Studies on Phenolic Compound with Different Techniques
Mechanism of Interaction between Silica Magnetic Nanoparticles towards Phenol in Water
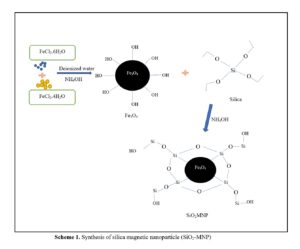
A series of nanocomposites based on Fe3O4 MNP obtained by co-precipitation and coated with SiO2 to form SiO2-MNP were synthesized, as shown in Figs. 13 and 14. The target molecules interact with functional groups on the silica surface of the absorbents to improve their efficiency, capacity, and selectivity. Due to their dual functionality, amino groups remove both cationic and anionic contaminants by electrostatic attraction. The amino group removes cationic contaminants such as heavy metals when the pH is higher (Hazrina et al. 2018; Asab et al. 2020).
By using a modified co-precipitation approach, magnetite (Fe3O4), nanoparticles and silica coated magnetic nanoparticles (SiO2-MNP) were created as adsorbents for phenol elimination in water. Magnetic nanoparticles were created to produce SiO2-MNP by carefully hydrolyzing aqueous solutions of FeCl2 and FeCl3 with ammonium hydroxide (Fig. 13). Silica was attached to create the SiO2-MNP molecule (Fig. 13).
Due to the low isoelectric point of silica surfaces, silanol groups (-Si-O-) exist even if silanol groups (-Si-OH-) are the prevalent type (Hozhabr and Entezari 2015). The expected H-bonds (-Si-OH) are between the SiO2-MNP and phenolic hydroxyl groups (Fig. 14). The expected H-bonds (-Si-OH) are between the SiO2-MNP and phenolic hydroxyl group (Fig. 14). The adsorption of phenol onto SiO2-MNP involves hydrogen bonding interaction (H-bonding) through the MSPE technique (Ding et al. 2012). Phenol forms hydrogen bonds readily in solution (Norseyrihan et al. 2016). Additionally, this molecule organizes water-bridge dimers interactions and engaging assembly wherein all the H-bonding sites. Meanwhile, SiO2 is very polar because of the presence of the -OH groups that form hydrogen bonds. The presence of ℼ-ℼ bonds in silica magnetic nanoparticles indicates successful interaction between ℼ-conjugated phenol and SiO2-MNP.
Fig. 13. Synthesis of SiO2-MNP
Fig. 14. Interaction of mechanism of phenol from water using SiO2-MNP
CONCLUSIONS
- The deposition approach can be used in this study to create silica that has been extracted from rice husk to recover phenol from water samples. In addition, magnetic nanoparticles functionalized with SiO2-magnetic nanoparticles (MNP) were also created. First, information regarding the sedimentation method of silica extraction from rice husk was gathered. Second, the micro-solid phase extraction (MSPE) approach was successfully used to synthesize the covering silica on magnetic nanoparticles. The SiO2-MNP were employed as adsorbents for phenol extraction in ambient water samples through hydrophobic interaction and hydrogen bonding.
- The optimized parameters for adsorption were selected as 5 min and pH 8. These parameters were used to treat real water samples and the recoveries obtained ranged from 84.8% to 92.4% of phenol uptake. This approach is quicker, easier to use, more effective, and less time-consuming than other available methods.
ACKNOWLEDGMENTS
The authors are grateful for Fundamental Research Grant Scheme (FRGS), grant number FRGS/1/2022/STG04/UKM/02/7, funded by the Ministry of Higher Education (MOHE) Malaysia.
REFERENCES CITED
Ahmed, S., Rasul, M. G., Martens, W. N., Brown, R., and Hashib, M. A. (2010). “Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments,” Desalination 261(1-2), 3-18. DOI: 10.1016/j.desal.2010.04.062
Ariffin, M. M., Sohaimi, N. M., Yih, B. S., and Saleh, N. M. (2019). “Magnetite nanoparticles coated with surfactant Sylgard 309 and its application as an adsorbent for paraben extraction from pharmaceutical and water samples,” Analytical Methods 11(32), 4126-4136.
Asab, G., Zereffa, E. A., and Abdo Seghne, T. (2020). “Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method: characterization and evaluation of antimicrobial activity,” International Journal of Biomaterials, 2020. DOI: 10.1155/2020/4783612
Begum, S., Yuhana, N. Y., Saleh, N. M., Kamarudin, N. H. N., and Sulong, A. B. (2021). “Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties,” Carbohydrate Polymers 259, article 117613. DOI: 10.1016/j.carbpol.2021.117613
Boudesocque, S., Mohamadou, A., Conreux, A., Marin, B., and Dupont, L. (2019). “The recovery and selective extraction of gold and platinum by novel ionic liquids,” Separation and Purification Technology 210, 824-834. DOI: 10.1016/j.seppur.2018.09.002
Chakraverty, A., and Kaleemullah, S. (1991). “Conversion of rice husk into amorphous silica and combustible gas,” Energy Conversion and Management 32(6), 565-570.
Dan, S. F. A. M., Jaafar, J. A., Saleh, N. M., Timmiati, S. N., and Kamarudin, N. H. N. (2022). “Temperature variation on doxorubicin adsorption by mesoporous silica nanoparticles and its effect towards release rate,” Journal of Chemical Engineering and Industrial Biotechnology 8(1), 8-13.
De Morais, P., Stoichev, T., Basto, M. C. P. and Vasconcelos, M.T.S.D. (2012). “Extraction and preconcentration techniques for chromatographic determination of chlorophenols in environmental and food samples,” Talanta 89, 1–11.
Deng, Z. H., Wang, X., Wang, X. L., Gao, C. L., Dong, L., Wang, M. L. and Zhao, R. S. (2019). “A core-shell structured magnetic covalent organic framework (type Fe3O4@COF) as a sorbent for solid-phase extraction of endocrine-disrupting phenols prior to their quantitation by HPLC,” Microchimica Acta 186(2), 0–8.
Dolaksiz, Y. E., Temel, F., and Tabakci, M. (2018). “Adsorption of phenolic compounds onto calix [4] arene-bonded silica gels from aqueous solutions,” Reactive and Functional Polymers 126, 27-35. DOI: 10.1016/j.reactfunctpolym.2018.03.003
Ding, H. L., Zhang, Y. X., Wang, S., Xu, J. M., Xu, S. C., and Li, G. H. (2012). “Fe3O4@ SiO2 core/shell nanoparticles: The silica coating regulations with a single core for different core sizes and shell thicknesses,” Chemistry of Materials 24(23), 4572-4580.
Frasetya, B., Harisman, K., Sudrajat, D. and Subandi, M. (2019). “Utilization of rice husk silicate extract to improve the productivity of paddy Ciherang cultivar,” Bulgarian Journal of Agricultural Science 25, 499-505.
Gamonchuang, J., and Burakham, R. (2021). “Amino-based magneto-polymeric-modified mixed iron hydroxides for magnetic solid phase extraction of phenol residues in environmental samples,” Journal of Chromatography A 1643, article 462071. DOI: 10.1016/j.chroma.2021.462071
Gopal, K., Ibrahim Al deeb, Raaov, M., Suah, F. B. M., Samad, N. A., Yahaya, N., Lim, V., and Zain, N. N. M. (2020). “Supramolecular solvent combined with dispersive solid phase extraction based magnetic silicone surfactant activated charcoal adsorbent for extraction of phenolic compounds from industrial wastewater,” Microchemical Journal 157, 105110. DOI: 10.1016/j.microc.2020.105110
Hazrina, H. Z., Noorashikin, M. S., Beh, S. Y., Loh, S. H., and Zain, N. N. M. (2018). “Formulation of chelating agent with surfactant in cloud point extraction of methylphenol in water,” Royal Society Open Science 5(7), article 180070. DOI: 10.1098/rsos.180070
Helmi Rashid Farimani, M., Shahtahmassebi, N., Rezaee Roknabadi, M., and Ghows, N. (2014). “Synthesis and study of structural and magnetic properties of superparamagnetic Fe3O4@ SiO2 core/shell nanocomposite for biomedical applications,” Nanomedicine Journal 1(2), 71-78.
Jones, R. P., and Clarke, J. U. (2005). “Analytical chemistry detection limits and the evaluation of dredged sediment,” Technical Notes: Dredging Operations Technical Support Program, ERDC/TN EEDP-04-36, 1-11.
Lee, D. W., Fatima, H., and Kim, K. S. (2018). “Preparation of silica coated magnetic nanoparticles for bioseparation,” Journal of Nanoscience and Nanotechnology 18(2), 1414-1418. DOI: 10.1166/jnn.2018.14888
Lee, H. B., Peart, T. E., and Svoboda, M. L. (2005). “Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography-mass spectrometry,” Journal of Chromatography A 1094(1-2), 122-129.
Lee, M. R., Lin, C. Y., Li, Z. G., and Tsai, T. F. (2006). “Simultaneous analysis of antioxidants and preservatives in cosmetics by supercritical fluid extraction combined with liquid chromatography-mass spectrometry,” Journal of Chromatography A 1120(1-2), 244-251.
Mandal, A., Mukhopadhyay, P., and Das, S. K. (2019). “The study of adsorption efficiency of rice husk ash for removal of phenol from wastewater with low initial phenol concentration,” SN Applied Sciences 1, 1-13.
Mecha, A. C., Onyango, M. S., Ochieng, A., Fourie, C. J., and Momba, M. N. (2016). “Synergistic effect of UV-vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study,” Journal of Catalysis 341, 116-125. DOI: 10.1016/j.jcat.2016.06.015
Noushad, M., Rahman, I. A., Husein, A., Mohamad, D., and Ismail, A. R. (2012). “A simple method of obtaining spherical nanosilica from rice husk,” International Journal on Advanced Science, Engineering and Information Technology 2(2), 141-143.
Nodeh, H. R., Ibrahim, W. A. W., Kamboh, M. A., and Sanagi, M. M. (2015). “Dispersive graphene-based silica coated magnetic nanoparticles as a new adsorbent for preconcentration of chlorinated pesticides from environmental water,” RSC Advances 5(93), 76424-76434. DOI: 10.1039/C5RA13450A
Norsuraya, S., Fazlena, H., and Norhasyimi, R. (2016). “Sugarcane bagasse as a renewable source of silica to synthesize Santa Barbara Amorphous-15 (SBA-15),” Procedia Engineering 148, 839-846. DOI: 10.1016/j.proeng.2016.06.627
Norseyrihan, M. S., Noorashikin, M. S., Adibah, M. S. N., and Yusoff, F. (2016). “Cloud point extraction of methylphenol in water samples with low viscosity of non-ionic surfactant Sylgard 309 coupled with high-performance liquid chromatography,” Separation Science and Technology 51(14), 2386-2393.
Patil, R., Dongre, R., and Meshram, J. (2014). “Preparation of silica powder from rice husk,” Journal of Applied Chemistry 27, 26-29.
Park, S. B., and Lee, B. C. (2004). “Studies on expansion properties in mortar containing waste glass and fibers,” Cement and Concrete Research 34(7), 1145-1152.
Ram Talib, N. S., Halmi, M. I. E., Abd Ghani, S. S., Zaidan, U. H., and Shukor, M. Y. A. (2019). “Artificial neural networks (ANNs) and response surface methodology (RSM) approach for modelling the optimization of chromium (VI) reduction by newly isolated Acinetobacter radioresistens strain NS-MIE from agricultural soil,” BioMed Research International 2019. DOI: 10.1155/2019/5785387
Rafiee, E., Shahebrahimi, S., Feyzi, M., and Shaterzadeh, M. (2012). “Optimization of synthesis and characterization of nanosilica produced from rice husk (a common waste material),” International Nano Letters 2(29), 1-8.
Soltani, R., Shahvar, A., Dinari, M. and Saraji, M. (2018). “Environmentally-friendly and ultrasonic-assisted preparation of two-dimensional ultrathin Ni/Co-NO3 layered double hydroxide nanosheet for micro solid-phase extraction of phenolic acids from fruit juices,” Ultrasonics Sonochemistry 40(A), 395-401.
DOI: 10.1016/j.ultsonch.2017.07.031
Ul-Islam, M., Ullah, M. W., Khan, S., Manan, S., Khattak, W. A., Ahmad, W., Shah, N. and Park, J. K. (2017). “Current advancements of magnetic nanoparticles in adsorption and degradation of organic pollutants,” Environmental Science and Pollution Research 24, 12713-12722. DOI: 10.1007/s11356-017-8765-3
Van Berkel, G. J., and Kertesz, V. (2007). “Using the electrochemistry of the electrospray ion source,” Analytical Chemistry 79(15). DOI: 10.1021/ac071944a
Wang, G. H., Lei, Y. Q., and Song, H. C. (2014). “Evaluation of Fe3O4@ SiO2-MOF-177 as an advantageous adsorbent for magnetic solid-phase extraction of phenols in environmental water samples,” Analytical Methods 6(19), 7842-7847.
Yang, J., Si, L., Cui, S. and Bi, W. (2015). “Synthesis of a graphitic carbon nitride nanocomposite with magnetite as a sorbent for solid phase extraction of phenolic acids,” Microchimica Acta 182(3–4), 737–744.
Zhang, J., Shao, J., Guo, P., and Huang, Y. (2013). “A simple and fast Fe3O4 magnetic nanoparticles-based dispersion solid phase extraction of Sudan dyes from food and water samples coupled with high-performance liquid chromatography,” Analytical Methods 5(10), 2503-2510.
Zhang, H. F., and Shi, Y. P. (2012). “Preparation of Fe3O4 nanoparticle enclosure hydroxylated multi-walled carbon nanotubes for the determination of aconitines in human serum samples,” Analytica Chimica Acta 724, 54-60. DOI: 10.1016/j.aca.2012.02.039
Article submitted: May 18, 2023; Peer review completed: July 15, 2023; Revised version received and accepted: July 20, 2023; Published: July 28, 2023.
DOI: 10.15376/biores.18.3.6204-6220
